Introduction
Lung cancer is regarded as one of the leading causes
of cancer-associated mortality worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for 80% of lung cancers (2,3).
Epidermal growth factor (EGFR) is an important therapeutic target
in NSCLC (4). Selective EGFR
tyrosine kinase inhibitors (TKIs) such as gefitinib have been
developed, and are highly effective for the treatment of
EGFR-mutated NSCLC (5). Notably,
the efficacy duration of these drugs is short, and patients who
initially respond to TKIs inevitably relapse, suggesting that
resistance may easily emerge (6–8).
Gefitinib has shown measurable efficacy at early stages of
treatment, but disease progression usually occurs following 6–8
months of therapy, which eventually leads to treatment failure
(9). Radiation is one of the most
effective therapeutic strategies for patients with NSCLC who are
not eligible for a surgical procedure following chemotherapy
failure (10). Although
chemotherapies inhibit cancer cell growth when combined with
appropriate radiotherapy, this type of treatment leads to severe
side effects, including irradiation pneumonitis and suppression of
the hemopoietic system (11,12).
Therefore, increasing tumor response to irradiation with targeted
sensitizers has become the focus of numerous studies in patients
with NSCLC that relapse following treatment with TKIs (13). Natural products are suitable
alternatives that may be used in the treatment of cancer. In past
decades, an increasing number of investigations have focused on
finding anti-tumor agents from natural resources (13–35).
In recent years, steroidal saponins have attracted
scientific attention for their structural diversity and significant
anti-tumor bioactivities (14–18).
Steroidal saponins belong to a family of glycosides with a chemical
structure that contains either a steroid or a triterpenoid attached
via C3 and an ether bond to a sugar side chain (14–18).
In addition, numerous studies have been designed to evaluate the
anti-tumor effects of Paris Saponins (PSs), which are derived from
the roots and rhizome of Paris polyphylla (19–25).
PSI is a potent anti-tumor agent that inhibits cell proliferation
and acts as a radiosensitizer for gefitinib-resistant NSCLC cells
(13,26). Although PSI has been extensively
studied for its ability to inhibit tumor growth in various types of
cancer (13,26–29),
PSII, PSVI and PSVII have only recently emerged as potential
anti-tumor agents (30–35). To the best of our knowledge, the
radiosensitization potential of PSII, PSVI, and PSVII in
TKI-resistant NSCLC has yet to be investigated. Therefore, the
present study aimed to investigate the radiosensitization effects
of PSII, PSVI, and PSVII in NSCLC with acquired in vitro
gefitinib resistance, and also the potential mechanisms underlying
their function.
Materials and methods
Drugs and reagents
PSII, PSVI and PSVII were obtained from the Zhejiang
Institute for Food and Drug Control (Hangzhou, China; batches no.
111591, 111592, and 111593, respectively; >99% purity). PSII,
PSVI and PSVII (100 µg) were each dissolved in 100 µl
dimethyl sulfoxide (DMSO) as a 100 µg/µl stock
solution and stored at −20°C. PSII, PSVI and PSVII were then
diluted in Dulbecco's modified Eagle's medium (DMEM) to achieve the
final concentration of 0.5 µg/ml for each experiment, with a
final DMSO concentration of 0.25% (v/v). DMEM and 10% fetal bovine
serum were purchased from GE Healthcare Life Sciences (Logan, UT,
USA). A Cycletest™ Plus DNA Reagent kit and fluorescein
isothiocyanate-Annexin V Apoptosis Detection kit were purchased
from BD Biosciences (Franklin Lakes, NJ, USA). Rabbit anti-rat B
cell lymphoma 2 (Bcl-2; cat. no. 3498), Bcl-2-associated X protein
(Bax; cat. no. 5023), caspase-3 (cat. no. 9665) and p21/Waf1/Cip1
(cat. no. 2947) monoclonal primary antibodies at 1:1,000 dilution
were purchased from Cell Signaling Technology, Inc., (Danvers, MA,
USA), and mouse anti-rat glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) monoclonal antibody (sc-365062) from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (heavy & light chain)
polyclonal antibody (611–1302) was purchased from Rockland, Inc.
(Limerick, PA, USA).
Cell culture
A PC-9-ZD NSCLC cell line (36) resistant to gefitinib following
long-term exposure to the drug was obtained from the Laboratory of
Biochemistry and Molecular Biology, Tongji University (Shanghai,
China). The PC-9-ZD cells were cultured to 80% confluence in DMEM
supplemented with 10% FBS, 100 µg/ml penicillin and 100
µg/ml streptomycin (both Sigma-Aldrich, St. Louis, MO, USA)
for 2 weeks at 37°C in a humidified atmosphere containing 5%
CO2.
Clonogenic assay
The PC-9-ZD cells were divided into four
experimental groups, as follows: i) The control group; ii) the PS
group; iii) the radiation group; and iv) the PS + radiation group.
The control group received no treatment, whereas the PS group was
subdivided into three groups that were treated with 0.5
µg/ml PSII, PSVI or PSVII for 3 h. The radiation group was
irradiated at 4 Gy with a 6-MV X-ray, and the PS + radiation group
was treated with PSII, PSVI or PSVII for 3 h, then irradiated at 4
Gy with a 6-MV X-ray. After 24 h, the cells were trypsinized
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and then counted
under the Olympus CKX41 inverted light microscope (Olympus
Corporation, Tokyo, Japan). Subsequently, the cells were seeded at
1,000 cells/flask and cultured for 14 days at 37°C in a humidified
atmosphere containing 5% CO2. The colonies were then
fixed using a mixture of methanol and glacial acetic acid (3:1;
Sigma-Aldrich), stained with crystal violet (Sigma-Aldrich), and
counted under the Olympus CKX41 inverted light microscope. Only
colonies containing >50 cells were scored. The experiments were
performed in triplicate.
Apoptosis assay
Apoptosis levels were measured using a fluorescein
isothiocyanate-Annexin V Apoptosis Detection kit (eBioscience,
Inc., San Diego, CA, USA). The cells in all groups were harvested
at 24 h following treatments (or no treatments for the control) and
stained with 5 µl PI (2.5 µg/ml) and 5 µl
Annexin V. Apoptosis levels were detected by flow cytometry
(Beckman Coulter, Inc., Brea, CA, USA).
Cell cycle assay
The radiation group received 4 Gy irradiation
treatment and the PS + radiation group received 4 Gy irradiation
followed by treatment with PSII, PSVI and PSVII. The cells were
harvested at 12, 24 and 48 h prior to being fixed with 70% ethanol
and stored overnight at −20°C. The cells were then centrifuged at
300 × g for 5 min at 20°C, and washed twice with phosphate-buffered
saline. The cells were labeled with PI (50 mg/ml) and protected
from the light for 30 min prior to analysis by flow cytometry and a
Kaluza software, version 1.20 (Beckman Coulter, Inc.). The
experiments were performed in triplicate.
Western blot analysis
The cells were treated with PSII, PSVI and PSVII for
3 h prior to being irradiated at a dose of 4 Gy and incubated for
24 h. The cells were lysed with lysis buffer containing 50 mM
Tris-HCl (pH 8.0) and 150 mM 1% Triton X-100 (Sigma-Aldrich). The
concentration of protein in the cell lysate was determined using
the Bradford Protein Assay (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Equal amounts of protein (100 µg) were separated
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to nitrocellulose membranes (Thermo Fisher
Scientific). The membranes were blocked using bovine serum albumin
(GE Healthcare Life Sciences) to prevent non-specific binding,
prior to incubation overnight at 4°C with rabbit anti-rat Bcl-2,
Bax, caspase-3 and p21/Waf1/Cip1 monoclonal antibodies and mouse
anti-rat GAPDH monoclonal antibody (all 1:1,000). Subsequently, the
membranes were washed three times with Tris-buffered saline
supplemented with Tween-20 (Sigma-Aldrich), prior to incubation for
2 h at room temperature with the HRP-conjugated goat anti-rabbit
IgG secondary antibody (1:10,000). The membranes were visualized
using an enhanced chemiluminescence system (Immun-Star™AP
Chemiluminescence kit; Bio-Rad Laboratories, Inc.) and X-ray films
(Santa Cruz Biotechnology Inc.). The blots were analyzed using
Quantity One software, version 4.6 (Bio-Rad Laboratories).
Statistical analysis
The results of the present study were compared by
one-way analysis of variance using SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA). The experimental data are presented
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
PSII, PSVI and PSVII enhance the
radiosensitivity of PC-9-ZD cells
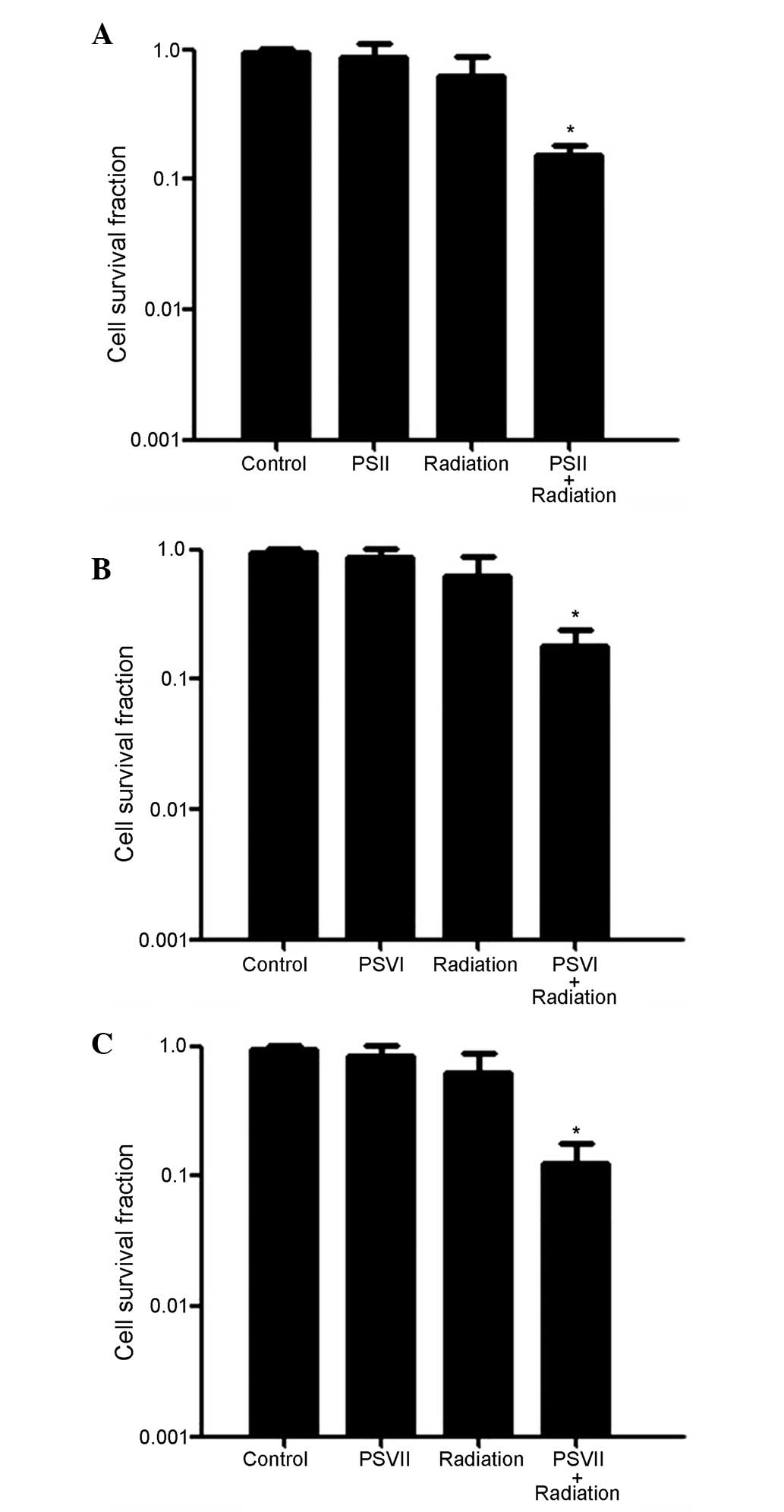
To investigate the effects of PS on radiosensitivity
in gefitinib-resistant lung adenocarcinoma cells, PC-9-ZD cells
were exposed to radiation (4 Gy) either with or without PSII, PSVI,
and PSVII (0.5 µg/ml each), and cell survival was determined
using a colony formation assay. As shown in Fig. 1A–C, the cell survival rates were
significantly reduced in the combined treatment groups, as compared
with the radiation only group (*P<0.01). These
results suggest that PC-9-ZD cells are more sensitive to the
combination treatment than to either treatment alone.
PSII, PSVI and PSVII induce apoptosis of
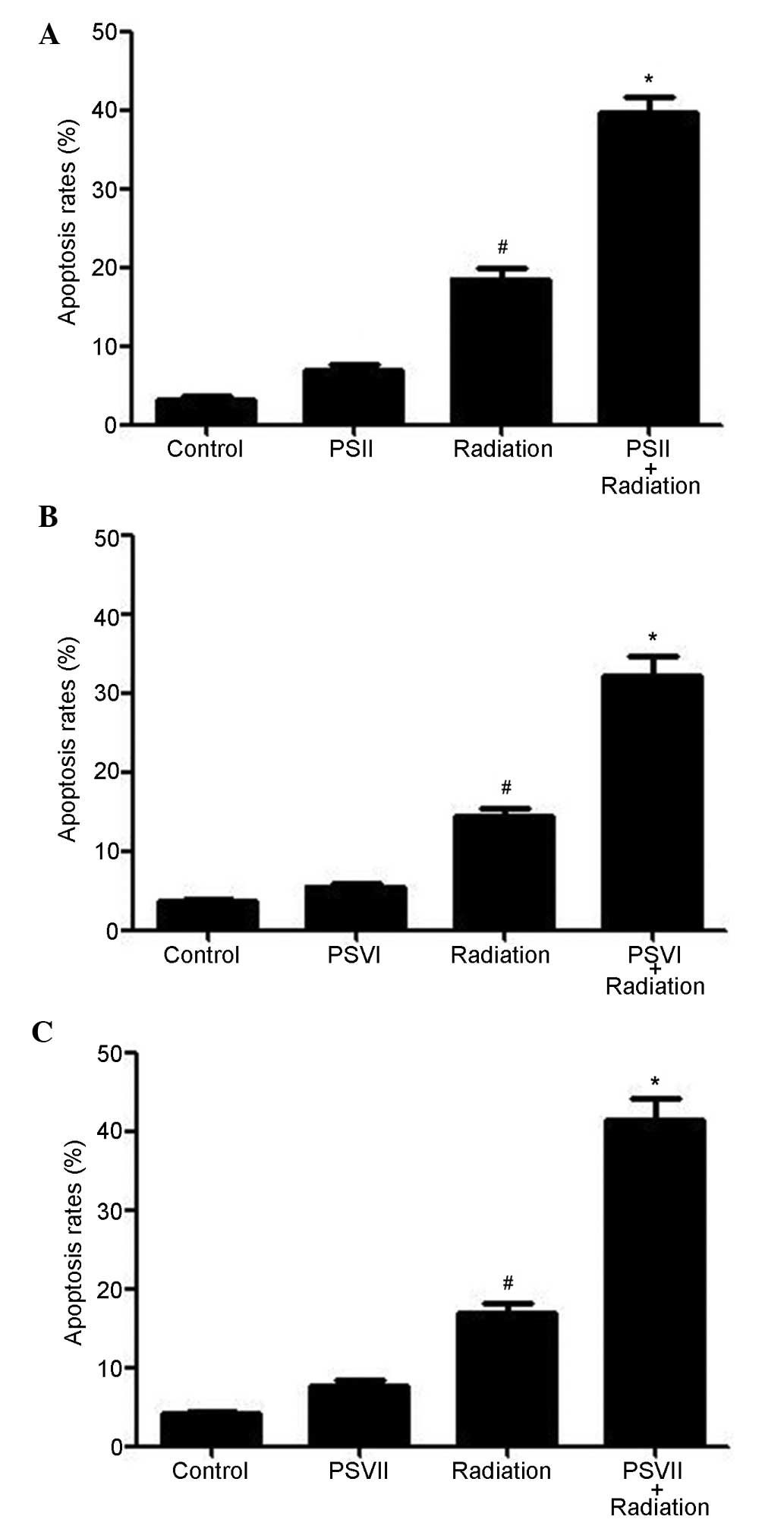
irradiated PC-9-ZD cells
Annexin V/PI double staining was used to evaluate
the apoptosis induced by PSII, PSVI, and PSVII (0.5 µg/ml
each) in irradiated PC-9-ZD cells. As shown in Fig. 2A–C, irradiation increased apoptosis
levels at 24 h; however, combined treatment with PS (II, VI or VII)
further increased apoptosis levels (P<0.01). These results
suggested that treatment with PSs significantly increases
radiation-induced apoptosis.
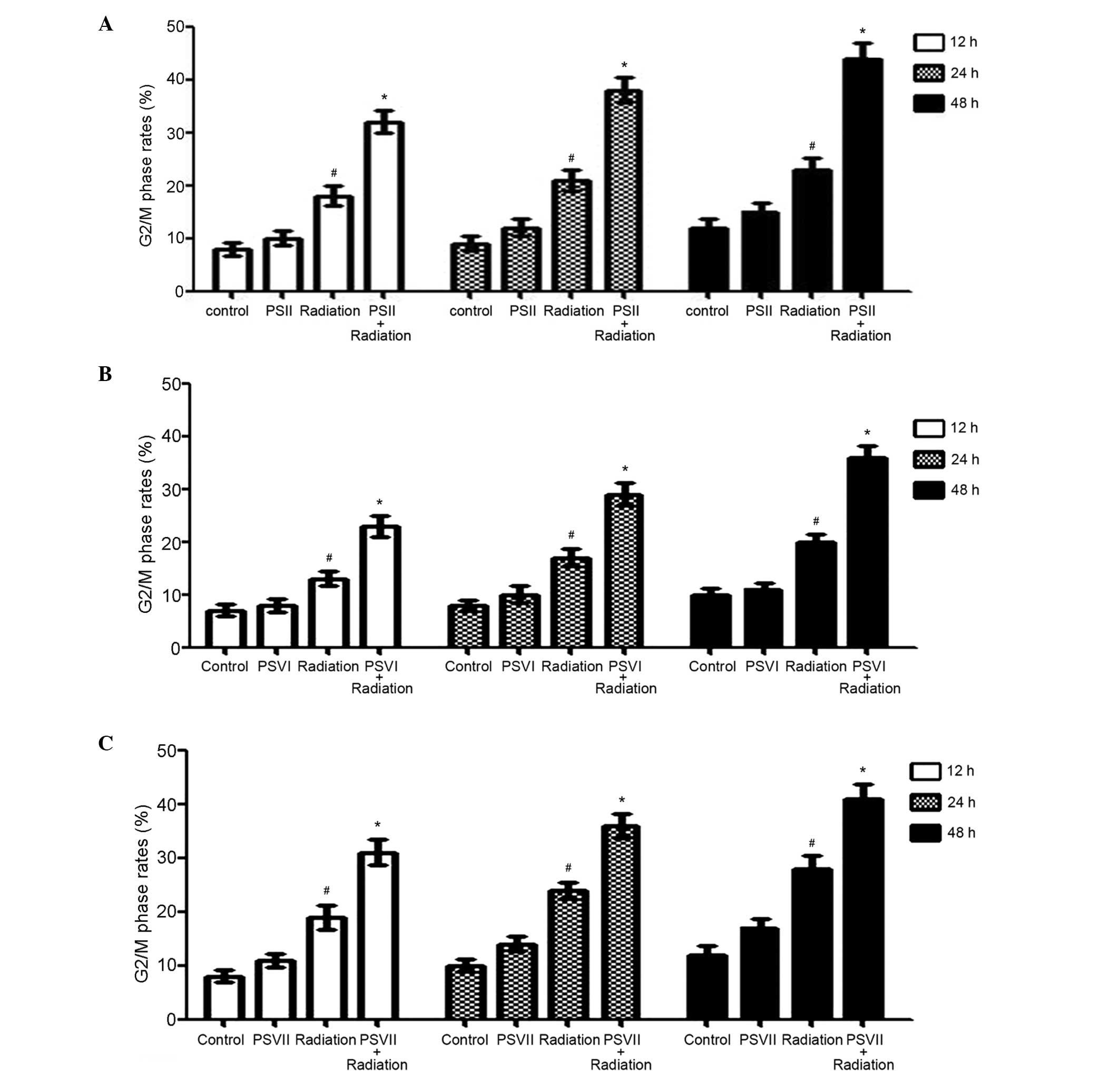
PSII, PSVI, and PSVII induce
G2/M arrest of irradiated PC-9-ZD cells
In order to determine whether the radiosensitivity
induced by PSs were due to cell cycle arrest, the effects of PSII,
PSVI and PSVII (0.5 µg/ml each) on cell cycle distribution
were observed. Irradiation alone induced G2/M phase
arrest in a time-dependent manner, as compared with the control
group (P<0.01). However, treatment with PSII, PSVI and PSVII
following irradiation further changed the cycle distribution of
irradiated cells, leading to a significant increase in cell cycle
arrest at the G2/M phase in a time-dependent manner, as
compared with the radiation group (P<0.01; Fig. 3A–C).
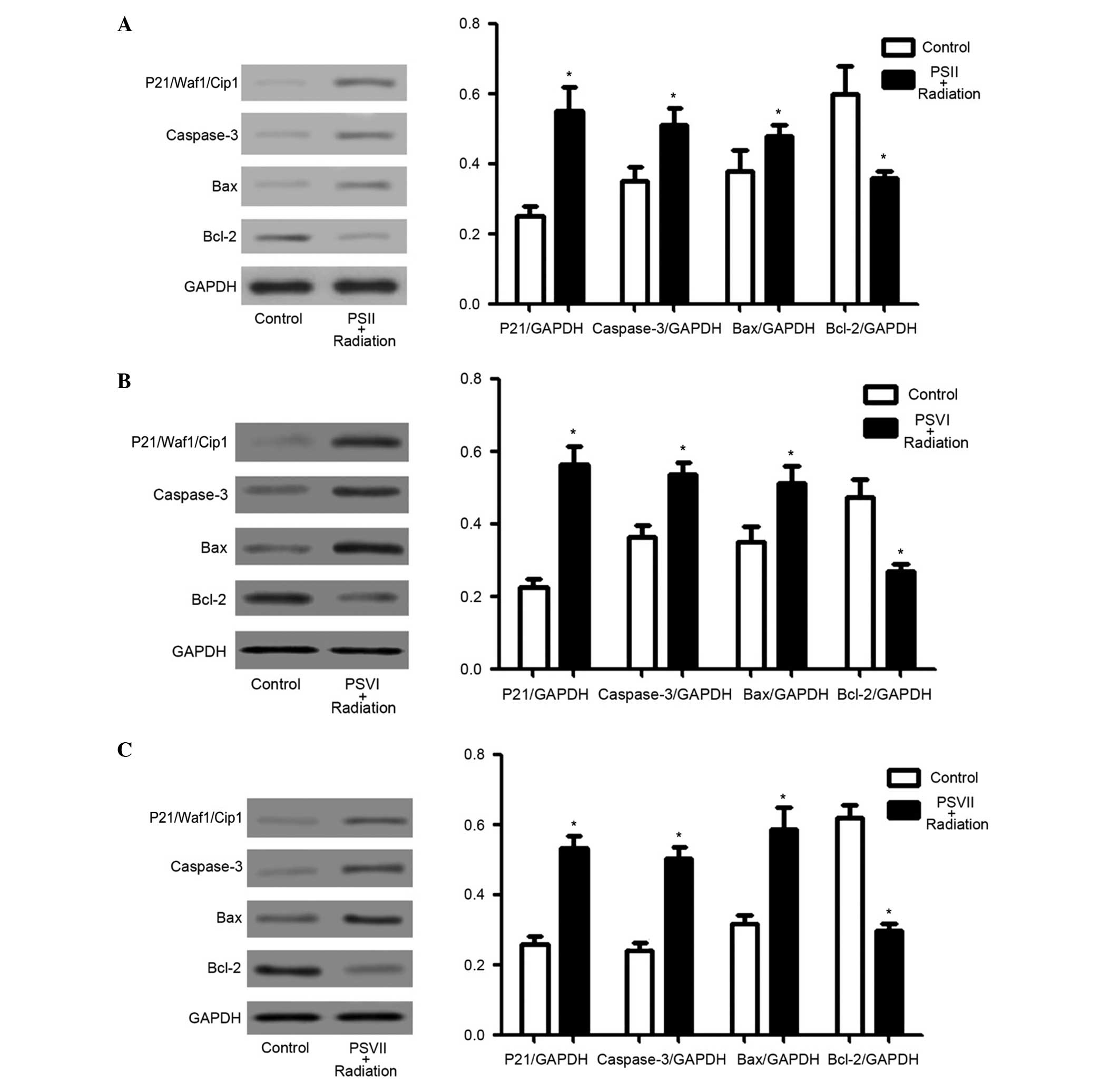
PSII, PSVI, and PSVII upregulate
p21/Waf1/Cip1, caspase-3, and Bax protein expression levels, and
downregulate Bcl-2 protein expression levels in irradiated PC-9-ZD
cells
The expression levels of p21/Waf1/Cip1, which are
the most important regulators of the cell cycle checkpoint
(37), and caspase-3, Bax and
Bcl-2, which are important apoptosis regulators (38–41),
were investigated to determine which molecules were involved in
PS-induced cell cycle arrest and apoptosis in irradiated PC-9-ZD
cells. PSII, PSVI, and PSVII (0.5 µg/ml) significantly
increased the expression levels of p21/Waf1/Cip1, caspase-3 and Bax
in irradiated cells, and significantly decreased the expression
levels of Bcl-2 in irradiated cells (P<0.01; Fig. 4A–C). These results suggest that
increased p21/Waf1/Cip1 expression levels may contribute to
G2/M phase arrest, and increased caspase-3 and Bax
expression levels, as well as decreased Bcl-2 expression levels,
may contribute to PS-induced apoptosis in irradiated PC-9-ZD
cells.
Discussion
Inhibition of EGFR has emerged as a promising cancer
therapy approach for the treatment of EGFR-mutated lung cancer over
the last decade (5). Previous
studies have reported that the majority of patients who initially
responded to EGFR inhibition, eventually exhibited tumor recurrence
(6–8). These results suggested the existence
of mechanisms underlying acquired resistance to EGFR inhibitors.
These include mutations in EGFR or V-Ki-ras2 Kirsten rat sarcoma
viral oncogene homolog, or the activation of other receptor
tyrosine kinases, such as ErbB3 or c-Met (42). A comparative analysis revealed that
acquired resistance to EGFR inhibitors was associated with
cross-resistance to radiation (43). Therefore, radiation is less
effective in EGFR-TKI-resistant lung cancer.
Previous findings have demonstrated that PSs are
able to induce cell death, reverse multidrug resistance, and
inhibit angiogenesis and tumor cell migration by modulating various
signaling pathways (30–32,35).
PSII suppresses the growth of human ovarian cancer xenografts by
modulating VEGF-mediated angiogenesis (30) and tumor cell migration by elevating
the expression levels of pro-apoptotic elements including Bax,
cytosolic cytochrome c, activated caspase-3, and activated
caspase-9, and by reducing extracellular signal-regulated kinase
(ERK)1/2 phosphorylation and anti-apoptotic Bcl-2 expression levels
(31). PSVII induces cell
apoptosis and cell cycle arrest in the G1 phase, and
triggers apoptosis in a caspase-3-dependent manner by
downregulating mitogen-activated protein kinase kinase 1/2
expression, ERK1/2 phosphorylation, and by suppressing the protein
kinase B signaling pathway (32).
PSVII reverses multidrug resistance in MCF-7/ADR
adriamycin-resistant cells via P-glycoprotein inhibition and
apoptosis augmentation (35). In
our previous study, the results demonstrated that PSI was able to
enhance the radiosensitivity of gefitinib-resistant PC-9-ZD lung
adenocarcinoma cells, which was associated with cell cycle arrest
at the G2/M phase and apoptosis via increased caspase-3,
Bax and p21/Waf1/Cip1 expression levels, and decreased Bcl-2
expression levels (13). PSI,
PSII, PSVI, and PSVII exhibit chemical structural similarities;
however, to the best of our knowledge, no studies have yet to
explore the efficacy and mechanisms underlying the radiosensitivity
of PSs in EGFR-TKI resistance cells.
In the present study, the mechanism underlying the
radiosensitivity induced by PSII, PSVI, and PSVII in
EGFR-TKI-resistant cells was examined in order to develop PSII,
PSVI, and PSVII radiosensitization agents for the treatment of
EGFR-TKI-resistant lung cancer. The results demonstrated that PSII,
PSVI, and PSVII significantly increased radiosensitivity in PC-9-ZD
cells. These data provided reasonable evidence that addition of PS
treatment to radiation may improve patient response to radiotherapy
in EGFR-TKI-resistant lung cancer. It is widely-accepted that
cellular response to radiation depends on the phase of the cell
cycle the cells were in at the time of irradiation (44). Cells in the G2/M phase
are the most sensitive to irradiation (45). In the present study, arrest in the
G2/M phase was achieved by treatment with PSII, PSVI,
and PSVII. The results demonstrated that PSII, PSVI, and PSVII
induced marked changes in cell cycle distribution, leading to cell
cycle arrest in the G2/M phase in a time-dependent
manner. p21/Waf1/Cip1 is considered to be the most important cell
cycle checkpoint regulator (37).
The results obtained from the present study showed that treatment
with PSII, PSVI, and PSVII significantly increased the expression
levels of p21/Waf1/Cip1, which resulted in cell cycle progression
through G2/M phase arrest in the PC-9-ZD cells. This
suggested that p21/Waf1/Cip1 has an important role in mediating
cell growth through G2/M phase arrest in
gefitinib-resistant cell lines.
Furthermore, investigations analyzing apoptosis by
fluorescence-activated cell sorting demonstrated significantly
increased cell apoptosis levels following treatment with PSII,
PSVI, and PSVII. In the present study, apoptosis was the primary
pathway to cell death induced by PSII, PSVI, and PSVII in the
irradiated cells. The results also demonstrated that PSII, PSVI,
and PSVII significantly increased apoptosis levels, as compared
with radiation alone in PC-9-ZD cells. Caspases are important
mediators of apoptosis (38).
Among them, caspase-3 is a frequently activated death protease,
catalyzing the specific cleavage of numerous cellular proteins
(39,40). The Bcl-2 family, which comprises
anti-apoptotic (including Bcl-2 and Bcl-extra large) and
pro-apoptotic members (including Bax and Bcl-2-antagonist/killer
1), is the predominant regulator and mediator of cell apoptosis
(41). To investigate the roles of
PSII, PSVI, and PSVII in radiation-induced apoptosis in
gefitinib-resistant PC-9-ZD cells, the expression levels of Bcl-2
family proteins and caspase-3 were analyzed in the present study.
The results indicated that Bcl-2 expression levels were decreased,
and those of Bax and caspase-3 were increased following treatment
with PSII, PSVI, and PSVII. Therefore, PSII, PSVI, and PSVII
promoted radiation-induced apoptosis via Bcl-2, Bax, and caspase-3,
eventually leading to enhanced radiosensitivity.
In conclusion, the results of the present study
demonstrated that PSII, PSVI, and PSVII induced radiosensitivity in
gefitinib-resistant cells by arresting cells in the G2/M
phase and by enhancing the apoptosis response via the modulation of
caspase-3, Bax, Bcl-2 and p21/Waf1/Cip1 expression levels, proteins
which are involved in apoptosis and cell cycle signaling pathways.
Therefore, PSII, PSVI, and PSVII may serve as radiosensitizers in
gefitinib-resistant lung cancer. However, studies are required for
further clinical evaluation.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81303274
and 81202947) and the Huzhou Science Project (grant no.
2015GY39).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sordella R, Bell DW, Haber DA and
Settleman J: Gefitinib-sensitizing EGFR mutations in lung cancer
activate anti-apoptotic pathways. Science. 305:1163–1167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Workman P: Altered states: Selectively
drugging the Hsp90 cancer chaperone. Trends Mol Med. 10:47–51.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi N, Toyooka S, Soh J, Yamamoto H,
Dote H, Kawasaki K, Otani H, Kubo T, Jida M, Ueno T, et al: The
anti-proliferative effect of heat shock protein 90 inhibitor,
17-DMAG, on non-small-cell lung cancers being resistant to EGFR
tyrosine kinase inhibitor. Lung cancer. 75:161–166. 2012.
View Article : Google Scholar
|
|
5
|
Langer CJ: Epidermal growth factor
receptor inhibition in mutation-positive non-small-cell lung
cancer: Is afatinib better or simply newer? J Clin Oncol.
31:3303–3306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arcila ME, Nafa K, Chaft JE, Rekhtman N,
Lau C, Reva BA, Zakowski MF, Kris MG and Ladanyi M: EGFR exon 20
insertion mutations in lung adenocarcinomas: Prevalence, molecular
heterogeneity, and clinicopathologic characteristics. Mol Cancer
Ther. 12:220–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adeno-carcinomas to gefitinib or erlotinib is associated with
a second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar
|
|
8
|
Gainor JF and Shaw AT: Emerging paradigms
in the development of resistance to tyrosine kinase inhibitors in
lung cancer. J Clin Oncol. 31:3987–3996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lutz ST, Jones J and Chow E: Role of
radiation therapy in palliative care of the patient with cancer. J
Clin Oncol. 32:2913–2919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Socinski MA, Rosenman JG, Halle J, Schell
MJ, Lin Y, Russo S, Rivera MP, Clark J, Limentani S, Fraser R, et
al: Dose-escalating conformal thoracic radiation therapy with
induction and concurrent carboplatin/paclitaxel in unresectable
stage IIA/B nonsmall cell lung carcinoma: A modified phase I/II
trial. Cancer. 92:1213–1223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santana-Davila R, Devisetty K, Szabo A,
Sparapani R, Arce-Lara C, Gore EM, Moran A, Williams CD, Kelley MJ
and Whittle J: Cisplatin and etoposide versus carboplatin and
paclitaxel with concurrent radiotherapy for stage III
non-small-cell lung cancer: an analysis of Veterans Health
Administration data. J Clin Oncol. 33:567–574. 2015. View Article : Google Scholar :
|
|
13
|
Jiang H, Zhao P, Feng J, Su D and Ma S:
Effect of Paris saponin I on radiosensitivity in a
gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett.
7:2059–2064. 2014.PubMed/NCBI
|
|
14
|
Yan L, Gao W, Zhang Y and Wang Y: A new
phenylpropanoid glycosides from Paris polyphylla var. yunnanensis.
Fitoterapia. 79:306–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Negi JS, Bisht VK, Bhandari AK, Bhatt VP,
Singh P and Singh N: Paris polyphylla: Chemical and biological
prospectives. Anticancer Agents Med Chem. 14:833–839. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He H, Zheng L, Sun YP, Zhang GW and Yue
ZG: Steroidal saponins from Paris polyphylla suppress adhesion,
migration and invasion of human lung cancer A549 cells via
down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev.
15:10911–10916. 2014. View Article : Google Scholar
|
|
17
|
Cheng ZX, Liu BR, Qian XP, Ding YT, Hu WJ,
Sun J and Yu LX: Proteomic analysis of anti-tumor effects by
Rhizoma Paridis total saponin treatment in HepG2 cells. J
Ethnopharmacol. 120:129–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Man S, Gao W, Zhang Y, Yan L, Ma C, Liu C
and Huang L: Anti-tumor and antimetastatic activities of Rhizoma
Paridis saponins. Steroids. 74:1051–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma DD, Lu HX, Xu LS and Xiao W:
Polyphyllin D exerts potent anti-tumour effects on Lewis cancer
cells under hypoxic conditions. J Int Med Res. 37:631–640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shuli M, Wenyuan G, Yanjun Z, Chaoyi M,
Liu Y and Yiwen L: Paridis saponins inhibiting carcinoma growth and
metastasis in vitro and in vivo. Arch Pharm Res. 34:43–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
GuangLie C, WeiShi G, GaiLing H and
JianPing C: Effect of Paris saponin on antitumor and immune
function in U14 tumor-bearing mice. Afr J Tradit Complement Altern
Med. 10:503–507. 2013.PubMed/NCBI
|
|
22
|
Wen F, Yin H, Chen C, Liu X, Xue D, Chen
T, He J and Zhang H: Chemical characteristics of saponins from
Paris fargesii var. brevipetala and cytotoxic activity of its main
ingredient, Paris saponin H. Fitoterapia. 83:627–635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao Y, Kang LP, Liu YX, Liang YG, Tan DW,
Yu ZY, Cong YW and Ma BP: Steroidal saponins from the rhizome of
Paris poly- phylla and their cytotoxic activities. Planta Med.
75:356–363. 2009. View Article : Google Scholar
|
|
24
|
Yan LL, Zhang YJ, Gao WY, Man SL and Wang
Y: In vitro and in vivo anticancer activity of steroid saponins of
Paris polyphylla var. yunnanensis. Exp Oncol. 31:27–32.
2009.PubMed/NCBI
|
|
25
|
He H, Sun YP, Zheng L and Yue ZG:
Steroidal saponins from Paris polyphylla induce apoptotic cell
death and autophagy in A549 human lung cancer cells. Asian Pac J
Cancer Prev. 16:1169–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014.PubMed/NCBI
|
|
27
|
Xiao X, Bai P, Bui Nguyen TM, Xiao J, Liu
S, Yang G, Hu L, Chen X, Zhang X, Liu J and Wang H: The antitumoral
effect of Paris Saponin I associated with the induction of
apoptosis through the mitochondrial pathway. Mol Cancer Ther.
8:1179–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao M, Dai X, He X, Zhou R, Zhang B, Hu
G, Huang Z and Fan X: Paris saponin I induces G2/M cell
cycle arrest and apoptosis in human gastric carcinoma SGC7901
cells. J Huazhong Univ Sci Technolog Med Sci. 31:768–772. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao P, Jiang H, Su D, Feng J, Ma S and
Zhu X: Inhibition of cell proliferation by mild hyperthermia at
43°C with Paris Saponin I in the lung adenocarcinoma cell line
PC-9. Mol Med Rep. 11:327–332. 2015.
|
|
30
|
Xiao X, Yang M, Xiao J, Zou J, Huang Q,
Yang K, Zhang B, Yang F, Liu S, Wang H and Bai P: Paris Saponin II
suppresses the growth of human ovarian cancer xenografts via
modulating VEGF-mediated angiogenesis and tumor cell migration.
Cancer Chemother Pharmacol. 73:807–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao X, Zou J, Bui-Nguyen TM, Bai P, Gao
L, Liu J, Liu S, Xiao J, Chen X, Zhang X and Wang H: Paris saponin
II of Rhizoma Paridis - a novel inducer of apoptosis in human
ovarian cancer cells. Biosci Trends. 6:201–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Sun Y, Fan L, Zhang F, Meng J, Han
J, Guo X, Zhang D, Zhang R, Yue Z and Mei Q: Paris saponin VII
inhibits growth of colorectal cancer cells through Ras signaling
pathway. Biochem Pharmacol. 88:150–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Zhang D, Ma X, Liu Z, Li F and Wu
D: Paris saponin VII suppressed the growth of human cervical cancer
Hela cells. Eur J Med Res. 19:412014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fan L, Li Y, Sun Y, Yue Z, Meng J, Zhang
X, Zhang R, Zhang D, Zhang F and Mei Q: Paris saponin VII inhibits
metastasis by modulating matrix metalloproteinases in colorectal
cancer cells. Mol Med Rep. 11:705–711. 2015.
|
|
35
|
Li Y, Fan L, Sun Y, Miao X, Zhang F, Meng
J, Han J, Zhang D, Zhang R, Yue Z and Mei Q: Paris saponin VII from
trillium tschonoskii reverses multidrug resistance of
adriamycin-resistant MCF-7/ADR cells via P-glycoprotein inhibition
and apoptosis augmentation. J Ethnopharmacol. 154:728–734. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji Y, Ma SL, Zhang YP, Tang JJ, Wu YM and
Lu YJ: Combined treatment with TNF-α/gefitinib alleviates the
resistance to gefitinib in PC-9 cells. Anticancer Drugs.
20:832–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deckbar D, Jeggo PA and Löbrich M:
Understanding the limitations of radiation-induced cell cycle
checkpoints. Crit Rev Biochem Mol Biol. 46:271–283. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu Q, Peng J, Liu W, He X, Cui L, Chen X,
Yang M, Liu H, Liu S and Wang H: Elevated cleaved caspase-3 is
associated with shortened overall survival in several cancer types.
Int J Clin Exp Pathol. 7:5057–5070. 2014.PubMed/NCBI
|
|
40
|
Dhar R, Persaud SD, Mireles JR and Basu A:
Proteolytic cleavage of p70 ribosomal S6 kinase by caspase-3 during
DNA damage-induced apoptosis. Biochemistry. 48:1474–1480. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shroff EH, Snyder C and Chandel NS: Bcl-2
family members regulate anoxia-induced cell death. Antioxid Redox
Signal. 9:1405–1409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Engelman JA and Jänne PA: Mechanisms of
acquired resistance to epidermal growth factor receptor tyrosine
kinase inhibitors in non-small cell Lung cancer. Clin Cancer Res.
14:2895–2899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang S, Benavente S, Armstrong EA, Li C,
Wheeler DL and Harari PM: p53 modulates acquired resistance to EGFR
inhibitors and radiation. Cancer Res. 71:7071–7079. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chetty C, Bhoopathi P, Rao JS and Lakka
SS: Inhibition of matrix metalloproteinase-2 enhances
radiosensitivity by abrogating radiation-induced FoxM1-mediated
G2/M arrest in A549 lung cancer cells. Int J Cancer. 124:2468–2477.
2009. View Article : Google Scholar : PubMed/NCBI
|