Introduction
Over the past three decades, the number of patients
with diabetes mellitus has increased rapidly; 90% of these patients
suffer from type 2 diabetes mellitus, rendering type 2 diabetes one
of the most serious public health challenges worldwide (1). Type 2 diabetes mellitus is an
endocrine system disease that results from β-cell dysfunction.
β-cell dysfunction is characterized by the specific absence of the
first phase of glucose-induced insulin secretion (2). Pancreatic β-cells are responsible for
abnormal glucose metabolism due to defects in insulin secretion or
due to a loss of β-cell mass resulting from cell death (3,4).
Apoptosis constitutes the primary form of β-cell death (5–7);
however, the underlying mechanisms remain unclear.
The transcription factor, forkhead box O1 (Foxo1),
is a key regulator of pancreatic β-cell mass; however, the role of
Foxo1 in the maintenance of β-cell function remains controversial
(8). A previous study provided a
mechanism linking glucose- and growth factor receptor-activated
pathways to protect β-cells against oxidative damage via Foxo1
(9). Kitamura et al
(10) reported that Foxo1 could
inhibit the expression of the β-cell-specific transcription factor
Pdx1 and that this led to the impairment of β-cell neogenesis,
which should be responsible for a reduction in β-cell mass. Other
studies have also reported that the suppression of Foxo1 expression
reduces the expression of apoptotic markers and promotes β-cell
survival in type 2 diabetes (9–12).
However, further studies are required to determine the role of
Foxo1 in β-cells.
Cluster of differentiation (CD)24 is a glycoprotein
expressed in a wide variety of human malignancies, such as renal
cell carcinoma, β-cell lymphoma, small cell and non-small cell lung
carcinoma, epithelial ovarian cancer, and breast cancer (13–16).
However, little is known regarding the correlation between CD24
expression and β-cell function.
The aim of the present study was two-fold, to
determine whether Foxo1 could promote β-cell apoptosis and to
examine the association between Foxo1 and CD24, and the effect of
CD24 expression on β-cell function. The results of this study may
provide a novel approach for the treatment and prevention of type 2
diabetes.
Materials and methods
Materials
RPMI-1640, HEPES, fetal bovine serum (FBS),
L-glutamine, Lipofectamine 2000 transfection reagent, TRIzol
reagent, a PureLink RNA Mini kit, and a High Capacity cDNA Reverse
Transcription kit were obtained from Invitrogen, Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Sodium pyruvate,
β-mercaptoethanol and Cell counting kit-8 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). pcDNA3-Foxo1, pcDNA3-Foxm1,
pcDNA3-Foxp, pcDNA3-Foxa1, pcDNA3-Foxc and pcDNA3-Foxb1 were
purchased from Fujian Funeng Co., Ltd. (Shanghai, China). Rat INS-1
pancreatic β-cells were obtained from the China Center for Type
Culture Collection (Shanghai, China). An Apoptosis Detection kit
was purchased from KeyGEN Biotech (Shanghai, China). Real-time PCR
primers, which included primers against CD24, ZAP70, PTAFR, TMEM14
and SPOCK2, were custom-synthesized by Invitrogen, Thermo Fisher
Scientific, Inc. Rabbit polyclonal anti-rat CD24 antibodies were
purchased from Santa Cruz Biotechnology, Inc., Dallas, TX, USA
(cat. no. sc-11406; dilution, 1:200) and mouse monoclonal anti-rat
β-actin primary antibodies were purchased from Abcam, Cambridge, UK
(cat. no. ab6276; dilution, 1:10,000). The secondary antibodies
were mouse anti-rabbit IgG (dilution, 1:100; cat. no. 211-005-109)
and rabbit anti-mouse IgG (dilution, 1:200; cat. no. 315-0005-003)
horseradish peroxidase (HRP)-conjugated antibodies, which were
purchased from Jackson ImmunoResearch Laboratories, Inc. (West
Grove, PA, USA). The present study was performed according to the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (17) and the
guidelines for animal experiments and associated activities by the
ethics committee of Shanghai First Central Hospital, and was
approved by the ethics committee of Shanghai First People's
Hospital, Shanghai Jiao Tong University School of Medicine
(Shanghai, China).
INS-1 cell culture
Rat INS-1 pancreatic β-cells were cultured in
RPMI-1640 medium containing 11 mM glucose, 1 mM sodium pyruvate, 10
mM HEPES, 10% FBS, 2 mM glutamine, and 50 µM
β-mercaptoethanol at 37°C in a 5% CO2 incubator. The
medium was refreshed every 2 days.
INS-1 cell transfections
Prior to transfection, INS-1 cells were seeded in
6-well plates at a density of 2×106 cells/well until the
cells grew to >80% confluence. Then, the cells were transfected
with 2 µg pcDNA3-Foxo1, pcDNA3-Foxm1, pcDNA3-Foxp,
pcDNA3-Foxa1, pcDNA3-Foxc or pcDNA3-Foxb1 using Lipofectamine 2000
transfection reagent according to the manufacturer's instructions.
Opti-MEM I (50 µl; Invitrogen; Thermo Fisher Scientific,
Inc.) containing 2 µg plasmid was mixed with Opti-MEM I
containing 2 µl Lipofectamine 2000 and incubated for 20 min
at room temperature. The medium was placed in a 6-well plate (100
µl/well) and cultured at 37°C in a 5% CO2
inhibitor for 72 h. pcDNA3 was used as a control. The transfection
medium was replaced with regular growth medium (RPMI-1640 medium
with 11 mmol/l D-glucose supplemented with 10% FBS, 100 U/ml
penicillin, 10 µg/ml streptomycin, 10 mmol/l HEPES, 2 mmol/l
L-glutamine, 1 mmol/l sodium pyruvate and 50 µmol/l
β-mercaptoethanol) after 5 h, and the cells were observed at each
indicated time point using an inverted fluorescence microscope (DMI
6000B; Leica Microsystems GmbH, Wetzlar, Germany).
Cell counting kit (CCK)-8 tests
NS-1 cells transfected with Foxo1 were seeded in
96-well plates at a density of 1×105 cells per well for
24 h. Then, 10, 20 or 40 mM Foxo1 was added, and the cells were
continuously cultured for 24 or 72 h. Untreated cells were used as
a negative control, and dimethyl sulfoxide-treated cells were used
as a positive control. At the indicated times, 10 µl CCK-8
(Sigma-Aldrich) was added, and the plates were incubated for 3 h.
After this period, the absorbance was measured at 450 nm using a
microplate reader (Multiskan® Spectrum; Thermo Fisher
Scientific, Inc.).
Apoptosis assays
Apoptosis was analyzed using an Annexin V-FITC
Apoptosis Detection kit (KeyGEN Biotech) according to the
manufacturer's instructions. Foxo1-transfected INS-1 cells were
cultured in 6-well plates at a density of 1×105 cells
per well for 2 days. Adherent INS-1 cells at 80% confluence were
passaged with 0.125% trypsin-0.02% EDTA (Invitrogen; Thermo Fisher
Scientific, Inc.) and inoculated at a density of 2×104
in 24-well culture dishes in growth medium as described above. At
the indicated times, the cells were digested with 0.25% trypsin and
washed with pre-cooled phosphate-buffered saline (PBS; Gibco;
Thermo Fisher Scientific, Inc.). Binding buffer (300 µl)
containing 5 µl Annexin V-fluorescein isothiocyanate (FITC)
was added and incubated at room temperature in the dark for 15 min.
Subsequently, 5 µl propidium iodide (PI) solution was added
and incubated at room temperature in the dark for 15 min. The
stained cells were measured and analyzed in a BD FACSCalibur™ (BD
Biosciences, Franklin Lakes, NJ, USA by CellQuest software (version
1.1; BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Rat pancreases were obtained from male Goto-Kakizaki
(GK) rats (n=8; age, 7 weeks; weight, 260–300 g) and Sprague Dawley
rats (n=8; age, 7 weeks; weight, 260–300 g) serving as a control,
which were obtained from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). These animals were maintained in a standard
animal laboratory with free activity and free access to water and
food. They were maintained in a temperature-controlled environment
at 22–24°C, relative humidity of 40–60%, with a 12-h light/dark
cycle. The rats were fasted for 8 h prior to surgery and were
sacrificed by exsanguination under anesthesia with 40 mg/kg sodium
pentobarbital (Sigma-Aldrich), and maximal efforts were made to
minimize suffering. Rat islet cells were digested using collagenase
P (Roche Diagnostics, Basel, Switzerland) and purified by Ficoll
density separation with Eurocollins (Mediatech, Inc., Herndon, VA,
USA) using a previously described method (18). The cells released were then
resuspended in growth medium as described above. The pancreatic
islet cells obtained from GK rats (age, 7 weeks) were ground to a
powder with liquid nitrogen. Then, the powders of INS-1 cells that
received different treatments were lysed using TRIzol reagent, and
total RNA was purified using a PureLink RNA Mini kit. The purified
RNA (0.5 µg) was reverse-transcribed into cDNA using a High
Capacity cDNA Reverse Transcription kit at 42°C for 1 h, followed
by 85°C for 5 sec. The cDNA produced was diluted 5-fold and used as
the PCR template. PCR was performed with SYBR® Premix Ex
Taq kit (containing No AmpErase UNG, 0.4 µl primer mixture
(each 10 µM) and 7.6 µl double distilled water) to
detect CD24, ζ-chain-associated protein kinase 70 (ZAP70),
platelet-activating factor receptor (PTAFR), transmembrane protein
14 (TMEM14) and SPOCK2 mRNA as previous studies have shown these
genes are associated with the proliferation and viability of tumor
cells (19–23). Reactions were conducted on a DNA
Engine Opticon 2 system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The thermocycling conditions were as follows: Initiation,
95°C for 2 min; 40 cycles at 95°C for 15 sec and 60°C for 30 sec;
and a melting curve stage of 60°C for 20 sec and 95°C for 10 sec.
β-actin served as a control to normalize relative expression of the
target genes which was calculated using the 2−ΔΔCq
method (24).
The primers used were as follows:
5′-TGCTCCTACCCACGCAGATT-3′ (sense) and 5′-GGCCAACCCAGAGTTGGAA-3′
(antisense) for CD24; 5′-GTTGACTCATCCTCAGAGACGAATC-3′ (sense) and
5′-AGGTTATCGCGCTTCAGGAA-3′ (antisense) for ZAP70;
5′-GCTGCTCATTGGAGGGTAGA-3′ (sense) and 5′-TGTGTCTCTGTCTGGGTCCT-3′
(antisense) for PTAFR; 5′-GATAGTCAGCCCGTACG-3′ (sense) and
5′-CGCATCGCCTTATGCGAT-3′ (antisense) for TMEM14;
5′-GAGACGAAGTGGAGGATGACTA-3′ (sense) and
5′-CTTGCAGATGGAGTCTTTGTTT-3′ (antisense) for SPOCK2;
5′-GACTCATCGTCGTACTCCTGCTTGCTG-3′ (sense) and
5′-GGAGATTACTGCCCTGGCTCCTA-3′ (anti-sense) for β-actin.
Western blot analysis
Proteins were extracted from the pancreatic islets
as previously described (11),
concentration was determined using the Bradford assay and equal
quantities of protein (20 µg) were resolved by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins
were transferred to nitrocellulose membranes and blocked in PBS
with Tween 20 (Invitrogen; Thermo Fisher Scientific, Inc.; PBST)
containing 5% non-fat milk. The membranes were then incubated with
CD24 primary antibodies for 12 h at 4°C. The membranes were washed
three times with PBST and incubated with HRP-conjugated secondary
antibodies and washed a further three times with PBST. The protein
bands were visualized using SuperSignal Pico ECL reagent (Pierce
Biotechnology, Inc., Rockford, IL, USA). The membranes were
reprobed with anti-β-actin antibody as loading controls and
immunocomplexes were detected with Amersham™ ECL Prime Western
Blotting Detection reagent (GE Healthcare Life Sciences, Chalfont,
UK). The immunoblots were scanned and quantified using ImageTool
3.0 software (compdent.uthscsa.edu/dig/itdesc.html), the relative
expression level of CD24 was normalized to β-actin expression.
Lentiviral vector carrying CD24 gene and
infection
The lentiviral vector carrying the CD24 gene was
purchased from Fujian Funeng Co., Ltd. (cat no. 20120810). The
lentiviral system was used to produce INS-1 cells that stably
overexpressed CD24. INS-1 cells were cultured in 24-well plates.
When the cell confluence reached 90%, the lentiviral vector (20
MOI) carrying the CD24 gene was added, and the plates were
incubated for the indicated time at 37°C in a 5% CO2
incubator.
Role of CD24 in β-cells on Gene Set
Enrichment Analysis (GSEA)
The dataset (E-GEOD-14668.CD24) used in this study
was downloaded from the Broad Institute website (http://www.broadinstitute.org/gsea/index.jsp), and the
entire data set with expression values was uploaded to the GSEA
software (25) to explore the role
of CD24 in β-cells and interpret the enrichment results.
Statistical analysis
Data processing and statistical analysis were
performed using SPSS 19 (IBM SPSS, Armonk, NY, USA). Data are
presented as the mean ± standard error of the mean. Data from two
groups were compared using Student's t-test and continuous
variables between several groups were compared with one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
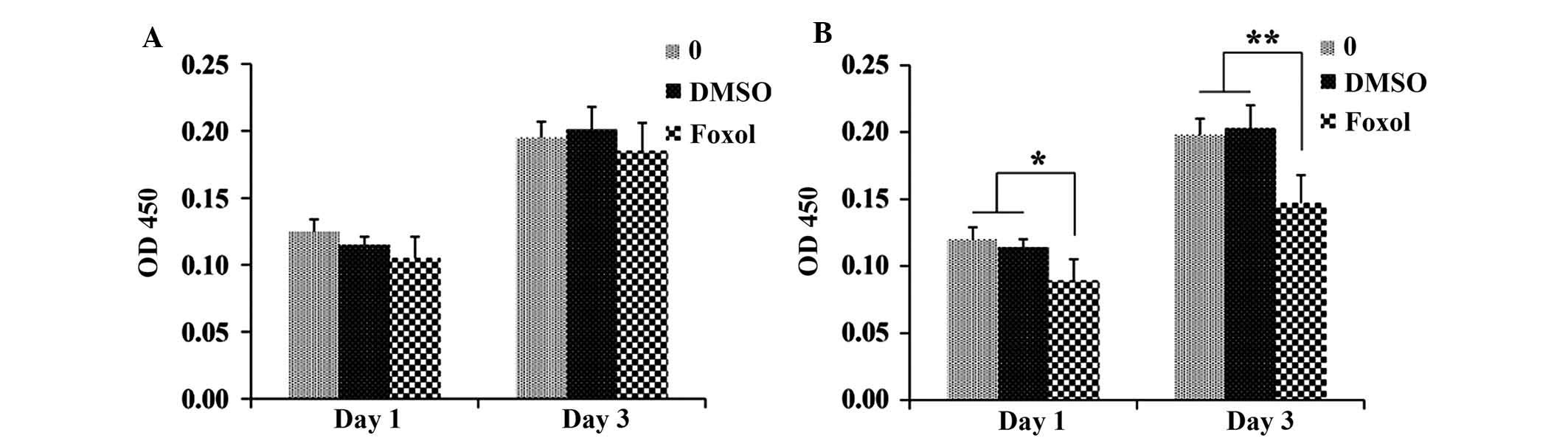
Foxo1 inhibits INS-1 proliferation
INS-1 cells were transfected with Foxo1, foxm1,
foxp, foxa1, foxc or foxb1 to explore which of these factors
effectively inhibited proliferation; pcDNA3 treatment served as the
control. In all of the six tested FOX family genes, only Foxo1
significantly downregulated the expression of CD24 and inhibited
INS-1 cell proliferation (P<0.01). As shown in Fig. 1, it was demonstrated that Foxo1
could effectively suppress the proliferation of INS-1 cells after 3
days of incubation, whereas the foxm1-, foxp-, foxa1-, foxc-, and
foxb1-transfected cells did not show similar results. Moreover, the
correlation between the concentration of Foxo1 and the
proliferation rate of INS-1 cells was investigated as shown in
Fig. 1B. The proliferation rate of
INS-1 cells decreased with increases in the concentration of Foxo1
on day 1 and day 3, and the proliferation rate of INS-1 cells in
the 10 mM Foxo1-treated group was significantly lower than those of
the control and DMSO-treated groups on day 1 (P<0.05) and day 3
(P<0.01). A significant difference was also observed between the
groups treated with 20 and 40 mM Foxo1 and the control and DMSO
groups on day 1 (P<0.01), and similar results were observed on
day 3. These results suggested that Foxo1 was able to inhibit INS-1
cell proliferation.
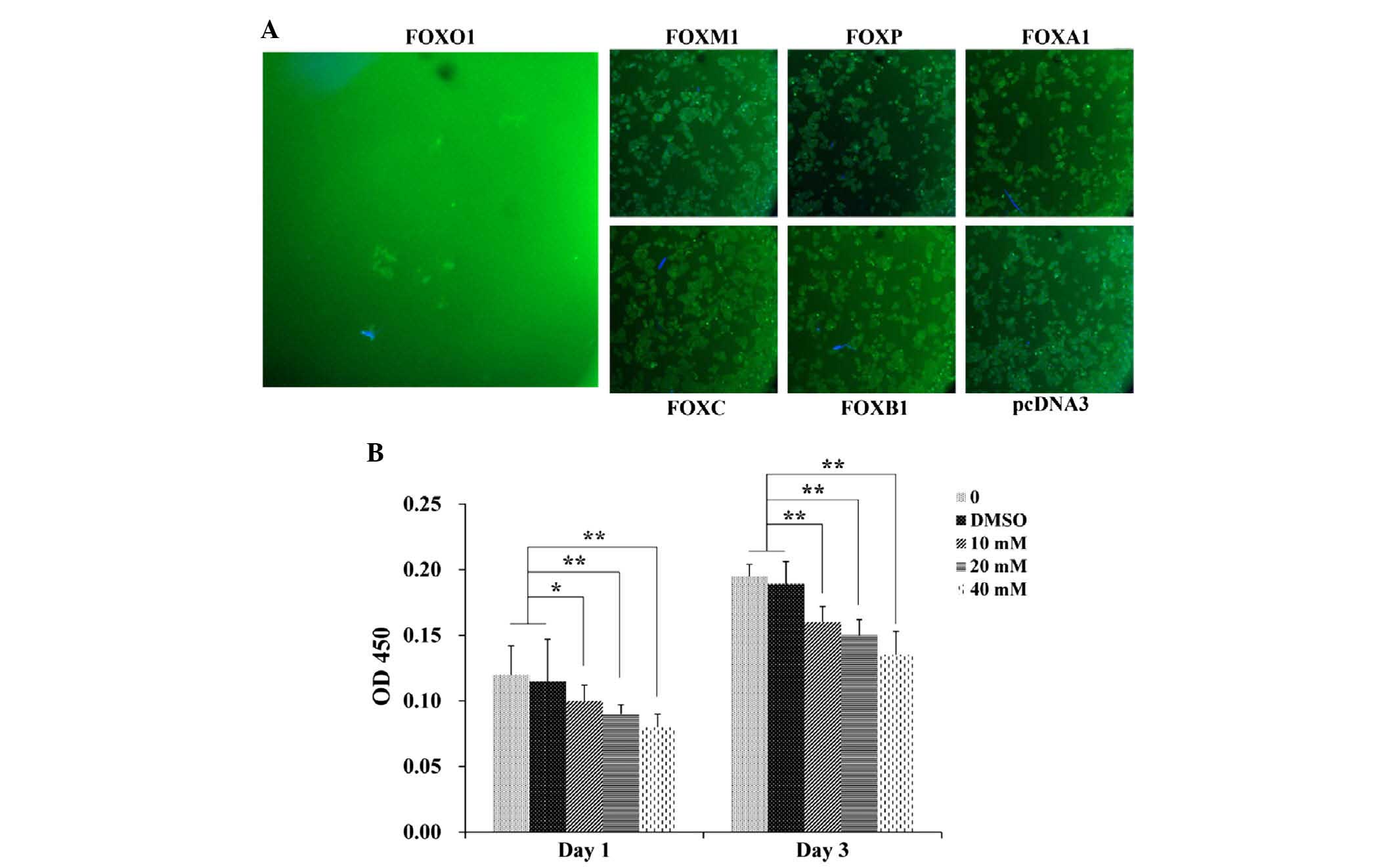 | Figure 1Foxo1 inhibits INS-1 cell
proliferation. (A) INS-1 cell proliferation 3 days after treatment
with Foxo1, foxm1, foxp, foxa1, foxc, or foxb1 using
immunofluorescent assay. Magnification, ×100. (B) Proliferation
tests of INS-1 cells treated with 10, 20 or 40 mM Foxo1 on days 1
and 3. *P<0.05, **P<0.01. DMSO,
dimethyl sulfoxide; OD, optical density; fox, forkhead box. |
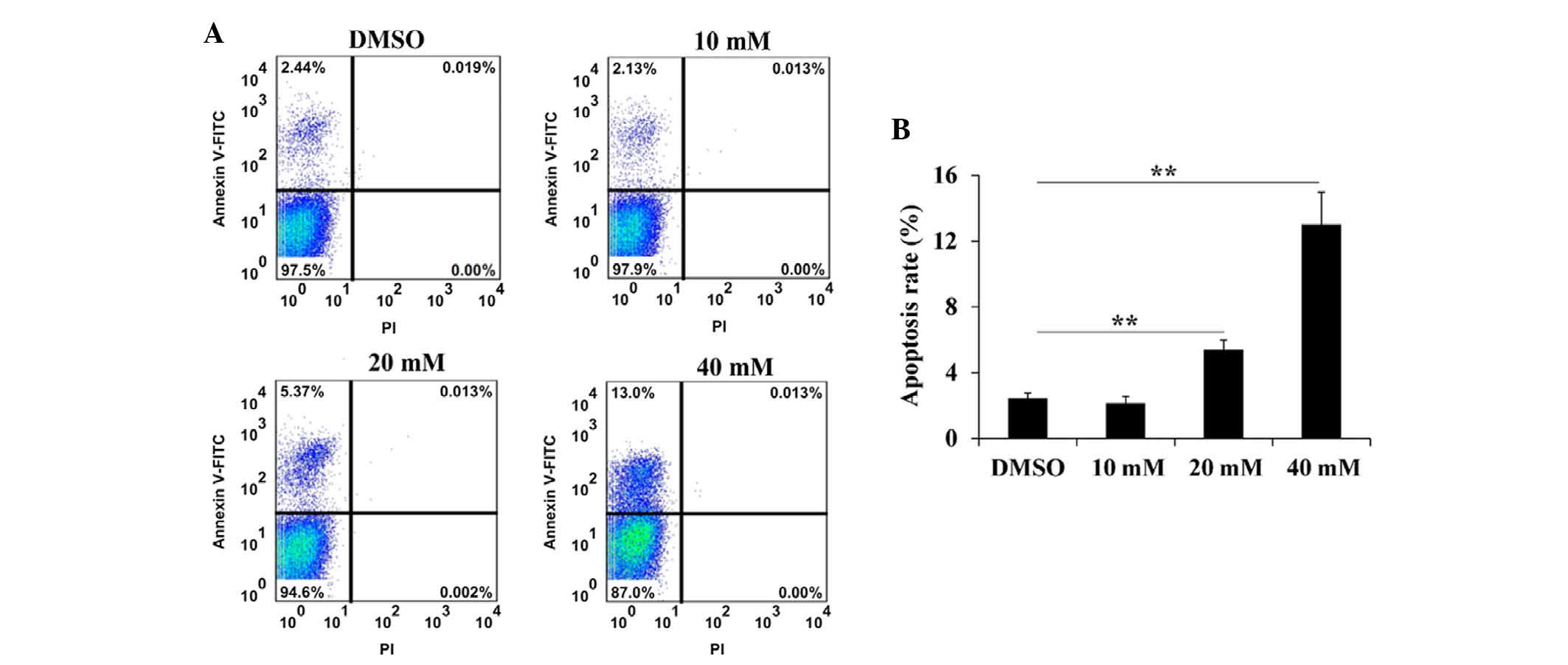
Foxo1 promotes INS-1 cell apoptosis
It was demonstrated that Foxo1 was able to inhibit
INS-1 cell proliferation, and whether Foxo1 can promote INS-1 cell
apoptosis. As shown in Fig. 2, the
apoptosis rate of INS-1 cells increased with increasing
concentrations of Foxo1. The apoptosis rate of INS-1 cells treated
with 10 mM Foxo1 was lower than that of the DMSO-treated group;
however, this difference was not statistically significant. The
apoptosis rate of INS-1 cells treated with 20 and 40 mM Foxo1 was
significantly higher than that of the DMSO-treated group
(P<0.01). These results revealed that Foxo1 could promote INS-1
cell apoptosis.
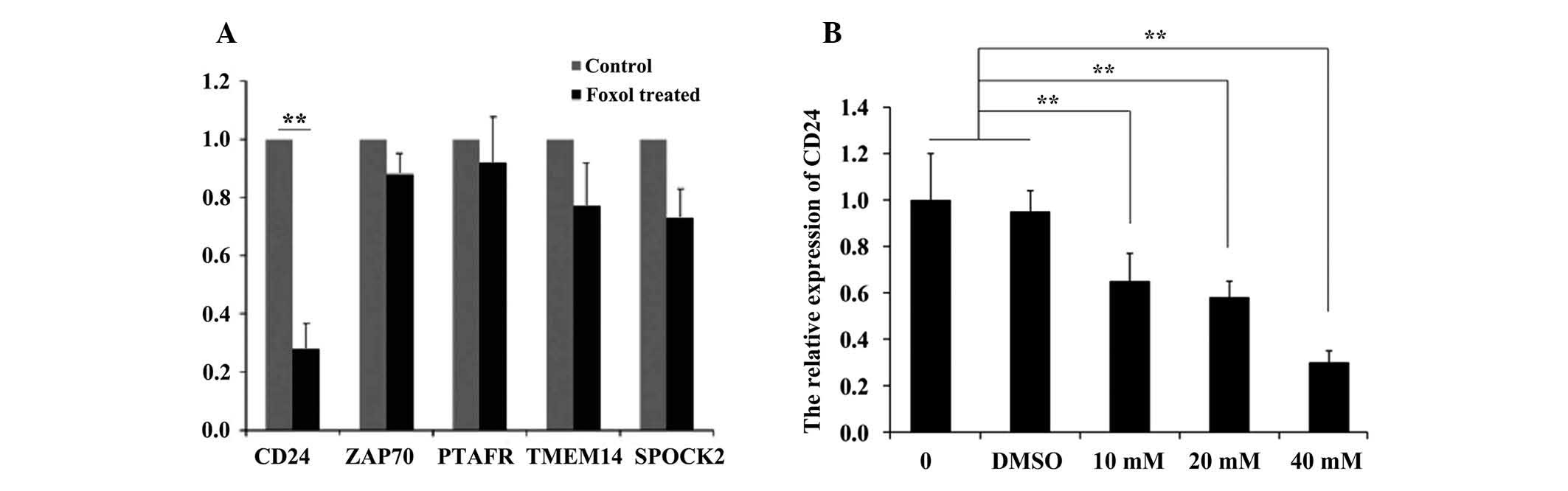
Foxo1 suppresses CD24 expression in INS-1
cells
PCR arrays were used to screen the key signaling
molecules that participate in this process. Five genes (CD24,
ZAP70, PTAFR, TMEM14 and SPOCK2) were selected for analysis, and it
was demonstrated that CD24 expression significantly decreased in
the Foxo1-treated group compared with that of the DMSO-treated
group (Fig. 3A). However, there
was no significant decrease in the relative expression of ZAP70,
PTAFR, TMEM14 or SPOCK2 (P=0.88, P=0.92, P=0.77 and P=0.73,
respectively) CD24 expression was also measured in INS-1 cells
after treatment with different concentrations of Foxo1, as shown in
Fig. 3B. A negative correlation
was identified between the expression of CD24 and the concentration
of Foxo1. CD24 expression in the 10, 20 and 40 mM Foxo1-treated
groups was significantly lower than that of the control and
DMSO-treated groups (P<0.01). These results indicated that Foxo1
could inhibit CD24 expression.
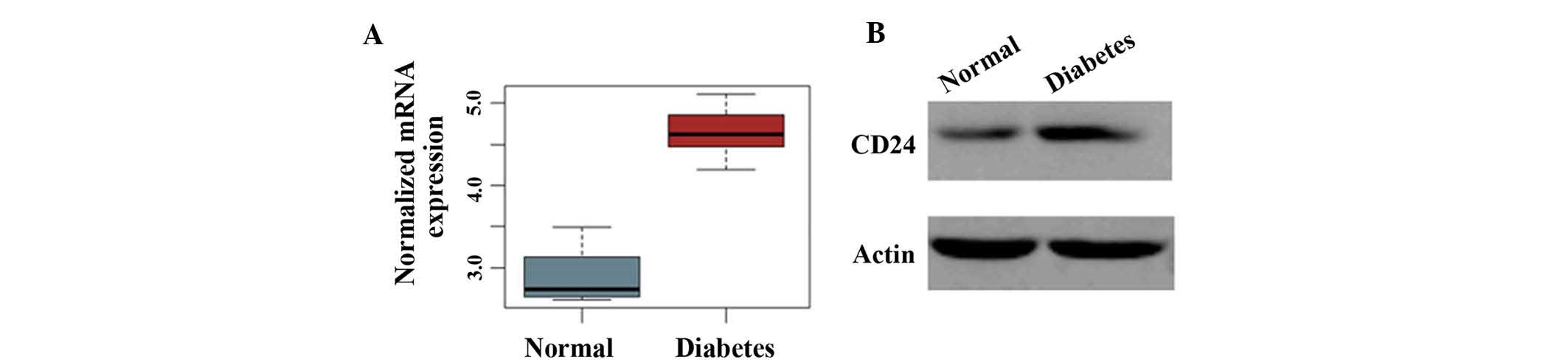
CD24 is highly expressed in a diabetes
model
Then, the expression level of CD24 was measured in
the pancreatic islets of normal rats and of diabetic GK rats. As
shown in Fig. 4, the expression
levels of CD24 mRNA and protein were markedly higher in the
diabetes model than in normal animals. This finding suggested that
CD24 is highly expressed in the diabetic pancreas islet.
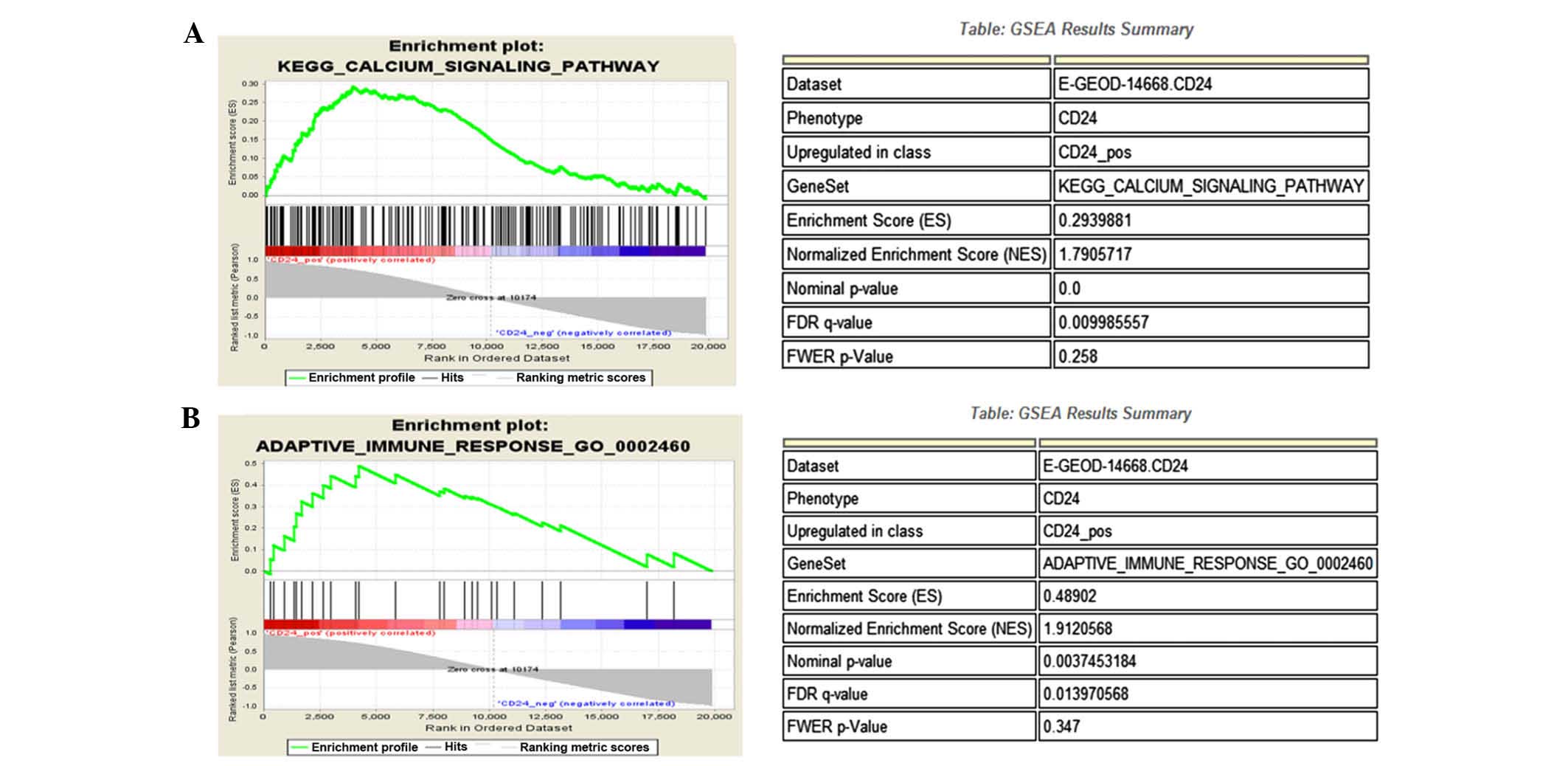
Bioinformatic analysis
Bioinformatic analysis indicated that CD24
participates in the calcium signaling pathway (Fig. 5A and B) and is involved in the
adaptive immune response of β-cells (Fig. 5C and D).
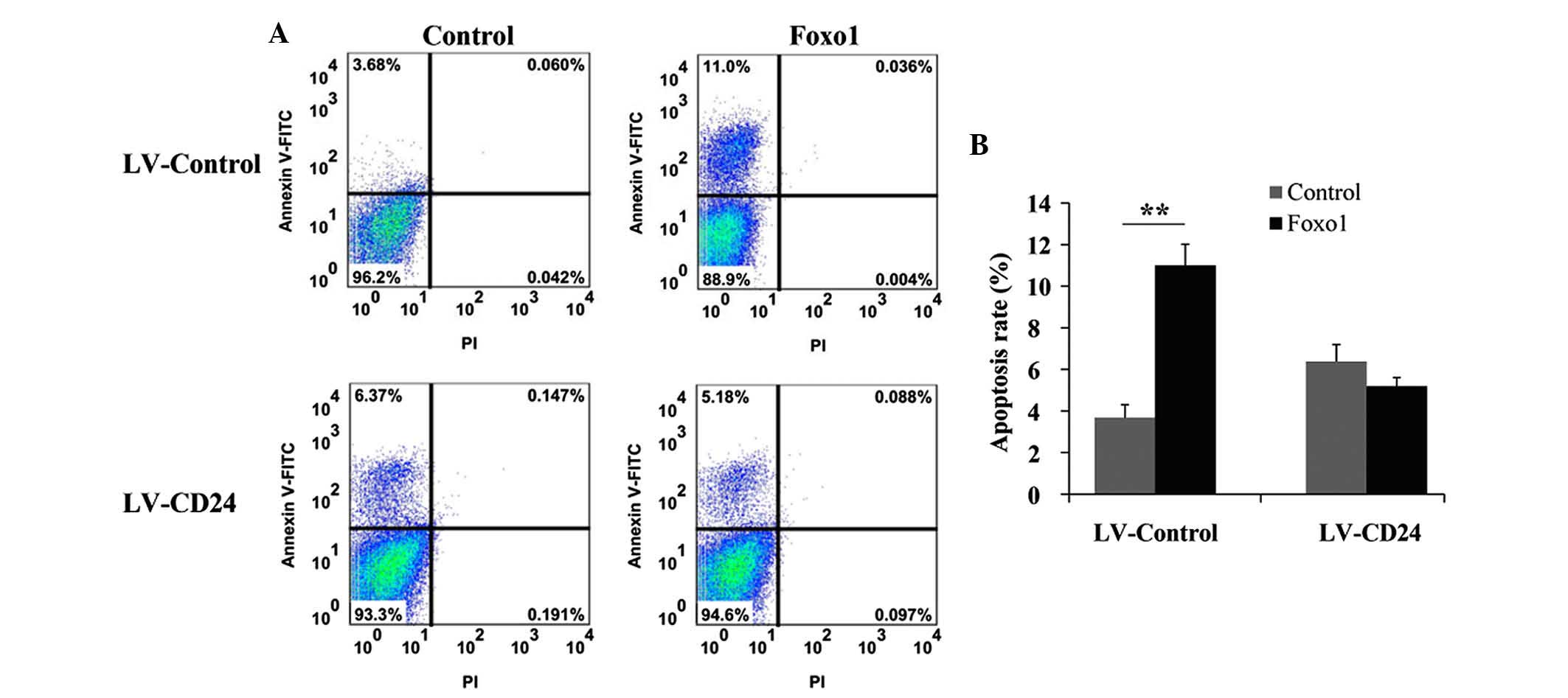
Overexpression of CD24 promotes INS-1
cell proliferation
Having demonstrated that Foxo1 inhibits INS-1 cell
proliferation by suppressing CD24 expression, further studies were
performed to evaluate the role of CD24 in INS-1 cell proliferation
by reverse-transcribing CD24. Following overexpression of CD24, no
significant difference in cell proliferation was observed in the
Foxo1-treated group compared with the control and DMSO-treated
groups on days 1 and 3. Whereas the proliferation rate was lower in
the LV-Control-treated group than in the control and DMSO-treated
groups on day 1 (P<0.05), a significant difference in cell
proliferation was also observed in the LV-Control-treated group and
in the control and DMSO-treated groups on day 3 (P<0.01;
Fig. 6). These results revealed
that CD24 is important in INS-1 cell proliferation.
Overexpression of CD24 inhibits INS-1
cell apoptosis
The apoptosis rate of INS-1 cells overexpressing
CD24 following treatment with Foxo1 was also determined. As shown
in Fig. 7, the apoptosis rate in
the Foxo1-treated group was significantly higher than that of the
control reverse-transcribed group on day 3 (P<0.01). However, no
obvious difference in the apoptosis rate was observed between the
Foxo1-treated group and the control group overexpressing CD24.
These results indicated that CD24 is important in INS-1 cell
apoptosis.
Discussion
The prevalence of diabetes mellitus is rapidly
increasing worldwide. The global number of patients with diabetes
mellitus is projected to rise to 439 million by 2030, and 90% of
these patients will have type 2 diabetes mellitus (26). Excessive apoptosis of β-cells is
the primary cause of type 2 diabetes; however, the mechanism
underlying this apoptosis remains unclear. Foxo1 is a transcription
factor that is a member of the FOX family. It is important in
multiple biological processes including oxidative stress, apoptosis
and cell cycle arrest. Furthermore, Foxo1 is a tumor suppressor,
which is downregulated in multiple types of tumor (27). Recent studies showed that the
expression of the other five genes (CD24, ZAP70, PTAFR, TMEM14 and
SPOCK2) are correlated with the proliferation and viability of
tumor cells (19–23). A widely used cell line for islet
β-cell function studies is the INS-1 cell line derived from the
original radiation-induced tumor described by Chick et al
(28) In the present study, a
possible apoptotic mechanism in β-cells was identified, where Foxo1
overexpression promotes apoptosis by reducing CD24 expression.
Therefore, this study demonstrated the important roles of Foxo1 and
CD24 in β-cell apoptosis.
In the adult pancreas, Foxo1 is exclusively
expressed in the islet β-cells (29). Foxo1 is a negative regulator of the
transcription factor Pdx1, which is crucial in β-cell growth and
function (30,31). Foxo1 inactivation leads to
increased Pdx1 expression and β-cell proliferation (10). By contrast, Foxo1 activation
promotes apoptosis in β-cells. Roy et al (32). reported that the suppression of the
PI3K/AKT and MEK/ERK pathways activated foxo transcription factors,
leading to cell cycle arrest and apoptosis in pancreatic cancer
(32). Moreover, McLoughlin et
al (33) found that Foxo1
enhances skeletal muscle atrophy by promoting skeletal muscle cell
apoptosis via DNA binding-dependent and DNA binding-independent
mechanisms. These results indicated that Foxo1 is key in the
apoptosis of β-cells and of other cells. The results of the present
study are consistent with this conclusion; Foxo1 overexpression
promoted apoptosis in β-cells, and the inhibitory effects were
enhanced with increasing Foxo1 concentrations.
CD24 is a glycoprotein that is expressed at the
surface of the majority of β lymphocytes and is essential in the
immune system (34). In INS-1
cells treated with Foxo1 following overexpression of the control,
proliferation was significantly reduced on day 1 (P<0.05) and
day 3 (P<0.01), and the apoptosis rate was significantly
increased (P<0.01. However, no significant differences were
observed following Foxo1 treatment of cells overexpressing CD24.
This suggests overexpression of CD24 may block the effects of Foxo1
to promote INS-1 cell proliferation and inhibit apoptosis. The
present results also confirmed this model and it was demonstrated
that CD24 was associated with the adaptive immune response of
β-cells. Furthermore, a previous study demonstrated that the
expression of genes involved in the final steps of insulin
secretion is reduced in patients with type 2 diabetes (35). One of the final steps in insulin
secretion is the influx of Ca2+ through
voltage-dependent Ca2+ channels, which triggers the
exocytosis of insulin (36), and
this calcium-triggered exocytosis results in the release of insulin
from the secretory granules, which follows the Ca2+
influx through voltage-gated channels (37). Notably, it was demonstrated that
CD24 was involved in the calcium signaling pathway, where this
protein may regulate β-cell function.
In conclusion, in the present study, it was
demonstrated that Foxo1 overexpression was able to promote β-cell
apoptosis by decreasing CD24 expression, and the role of CD24 in
β-cell function was preliminarily discussed. CD24 is involved in
the calcium signaling pathway and in the adaptive immune response
of β-cells. However, additional studies are required to clarify the
role of CD24 in β-cell function.
Acknowledgments
The study was supported by the National Natural
Science Foundation of China (nos. 81370904 and 81400785), Key
Projects of Shanghai Municipal Health Bureau Research Fund (no.
2014Y015), Shanghai Jiao Tong University Research Funding on
Medical and Engineering Interdisciplinary Projects (YG2015ZD08 and
YG2015MS30), Wang Kuancheng Prize Fund for Medicine, Excellent
Physician Fund Project of Shanghai General Hospital and Songjiang
District Health Bureau of Climbing Medical Program (2014).
Abbreviations:
|
Foxo1
|
forkhead box O1
|
|
Foxm1
|
forkhead box M1
|
|
Foxp
|
forkhead box p2
|
|
Foxa1
|
forkhead box A1
|
|
Foxc
|
forkhead box C
|
|
Foxb1
|
forkhead box b1
|
|
CD24
|
cluster of differentiation 24
|
|
ZAP70
|
ζ-chain-associated protein kinase
70
|
|
PTAFR
|
platelet-activating factor
receptor
|
|
TMEM14
|
transmembrane protein 14
|
References
|
1
|
Chen L, Magliano DJ and Zimmet PZ: The
worldwide epidemiology of type 2 diabetes mellitus-present and
future perspectives. Nat Rev Endocrinol. 8:228–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poitout DV and Robertson RP: An integrated
view of beta-cell dysfunction in type-II diabetes. Annu Rev Med.
47:69–83. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahrén B: Type 2 diabetes, insulin
secretion and beta-cell mass. Curr Mol Med. 5:275–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karaskov E, Scott C, Zhang L, Teodoro T,
Ravazzola M and Volchuk A: Chronic palmitate but not oleate
exposure induces endoplasmic reticulum stress, which may contribute
to INS-1 pancreatic beta-cell apoptosis. Endocrinology.
147:3398–3407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Butler AE, Janson J, Bonner-Weir S, Ritzel
R, Rizza RA and Butler PC: Beta-cell deficit and increased
beta-cell apoptosis in humans with type 2 diabetes. Diabetes.
52:102–110. 2003. View Article : Google Scholar
|
|
6
|
Donath MY and Halban PA: Decreased
beta-cell mass in diabetes: Significance, mechanisms and
therapeutic implications. Diabetologia. 47:581–589. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rhodes CJ: Type 2 diabetes-a matter of
beta-cell life and death? Science. 307:380–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi M, Kikuchi O, Sasaki T, Kim HJ,
Yokota-Hashimoto H, Lee YS, Amano K, Kitazumi T, Susanti VY,
Kitamura YI and Kitamura T: FoxO1 as a double-edged sword in the
pancreas: Analysis of pancreas- and β-cell-specific FoxO1 knockout
mice. Am J Physiol Endocrinol Metab. 302:E603–E613. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitamura YI, Kitamura T, Kruse JP, Raum
JC, Stein R, Gu W and Accili D: FoxO1 protects against pancreatic
beta cell failure through NeuroD and MafA induction. Cell Metab.
2:153–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitamura T, Nakae J, Kitamura Y, Kido Y,
Biggs WH III, Wright CV, White MF, Arden KC and Accili D: The
forkhead transcription factor Foxo1 links insulin signaling to Pdx1
regulation of pancreatic beta cell growth. J Clin Invest.
110:1839–1847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buteau J, Spatz ML and Accili D:
Transcription factor FoxO1 mediates glucagon-like peptide-1 effects
on pancreatic beta-cell mass. Diabetes. 55:1190–1196. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martinez SC, Tanabe K, Cras-Méneur C,
Abumrad NA, Bernal-Mizrachi E and Permutt MA: Inhibition of Foxo1
protects pancreatic islet beta-cells against fatty acid and
endoplasmic reticulum stress-induced apoptosis. Diabetes.
57:846–859. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristiansen G, Pilarsky C, Pervan J,
Stürzebecher B, Stephan C, Jung K, Loening S, Rosenthal A and
Dietel M: CD24 expression is a significant predictor of PSA relapse
and poor prognosis in low grade or organ confined prostate cancer.
Prostate. 58:183–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kristiansen G, Schlüns K, Yongwei Y,
Denkert C, Dietel M and Petersen I: CD24 is an independent
prognostic marker of survival in nonsmall cell lung cancer
patients. Br J Cancer. 88:231–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu W and Vadgama JV: Identification and
characterization of amino acid starvation-induced CD24 gene in
MCF-7 human breast cancer cells. Int J Oncol. 16:1049–1103.
2000.PubMed/NCBI
|
|
16
|
Welsh JB, Zarrinkar PP, Sapinoso LM, Kern
SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA and Hampton GM:
Analysis of gene expression profiles in normal and neoplastic
ovarian tissue samples identifies candidate molecular markers of
epithelial ovarian cancer. Proc Natl Acad Sci USA. 98:1176–1181.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: The 1996 guide for the care and use of laboratory
animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar
|
|
18
|
Dong W, Ding X, Cai J, Peng Y, Wang Y and
Tan J: Study on the standardization of islet isolation method in
rats. Chin J Cell Stem Cell (Electronic Edition). 2:237–240.
2012.In Chinese.
|
|
19
|
Pei Z, Zhu G, Huo X, Gao L, Liao S, He J,
Long Y, Yi H, Xiao S, Yi W, et al: CD24 promotes the proliferation
and inhibits the apoptosis of cervical cancer cells in vitro. Oncol
Rep. Dec 24–2015.Epub ahead of print. View Article : Google Scholar
|
|
20
|
Krenn PW, Hofbauer SW, Pucher S, Hutterer
E, Hinterseer E, Denk U, Asslaber D, Ganghammer S, Sternberg C,
Neureiter D, et al: ILK induction in lymphoid organs by a
TNFα-NF-κB-regulated pathway promotes the development of chronic
lymphocytic leukemia. Cancer Res. Feb 2–2015.Epub ahead of
print.
|
|
21
|
Sahu RP: Expression of the
platelet-activating factor receptor enhances benzyl
isothiocyanate-induced apoptosis in murine and human melanoma
cells. Mol Med Rep. 12:394–400. 2015.PubMed/NCBI
|
|
22
|
Woo IS, Jin H, Kang ES, Kim HJ, Lee JH,
Chang KC, Park JY, Choi WS and Seo HG: TMEM14A inhibits
N-(4-hydroxyphenyl) retinamide-induced apoptosis through the
stabilization of mitochondrial membrane potential. Cancer Lett.
309:190–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren F, Wang DB, Li T, Chen YH and Li Y:
Identification of differentially methylated genes in the malignant
transformation of ovarian endometriosis. J Ovarian Res. Jul
10–2014.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acac Sci USA. 102:15545–15550. 2005. View Article : Google Scholar
|
|
26
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu DA, Yoon J, Ko YS, Park J, Kim SY, Kim
MA, Kim JH, Jung J, Cheon Y, Lee HS, et al: Forkhead transcription
factor FOXO1 inhibits nuclear factor-κB in gastric cancer. APMIS.
122:848–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chick WL, Warren S, Chute RN, Like AA,
Lauris V and Kitchen KC: A transplantable insulinoma in the rat.
Proc Natl Acad Sci. 74:628–632. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buteau J and Accili D: Regulation of
pancreatic Beta-cell function by the forkhead protein FoxO1.
Diabetes Obes Metab. 9(Suppl 2): S140–S146. 2007. View Article : Google Scholar
|
|
30
|
Ahlgren U, Jonsson J, Jonsson L, Simu K
and Edlund H: Beta-cell-specific inactivation of the mouseIpf1/Pdx1
gene results in loss of the beta-cell phenotype and maturity onset
diabetes. Genes Dev. 12:1763–1768. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jonsson J, Carlsson L, Edlund T and Edlund
H: Insulin-promoter-factor 1 is required for pancreas development
in mice. Nature. 371:606–609. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McLoughlin TJ, Smith SM, DeLong AD, Wang
H, Unterman TG and Esser KA: FoxO1 induces apoptosis in skeletal
myotubes in a DNA-binding-dependent manner. Am J Physiol Cell
Physiol. 297:C548–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi D, Lee HW, Hur KY, Kim JJ, Park GS,
Jang SH, Song YS, Jang KS and Paik SS: Cancer stem cell markers
CD133 and CD24 correlate with invasiveness and differentiation in
colorectal adenocarcinoma. World J Gastroenterol. 15:2258–2264.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andersson SA, Olsson AH, Esguerra JL,
Heimann E, Ladenvall C, Edlund A, Salehi A, Taneera J, Degerman E,
Groop L, et al: Reduced insulin secretion correlates with decreased
expression of exocytotic genes in pancreatic islets from patients
with type 2 diabetes. Mol Cell Endocrinol. 364:36–45. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eliasson L, Abdulkader F, Braun M,
Galvanovskis J, Hoppa MB and Rorsman P: Novel aspects of the
molecular mechanisms controlling insulin secretion. J Physiol.
586:3313–3324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ammälä C, Eliasson L, Bokvist K, Larsson
O, Ashcroft FM and Rorsman P: Exocytosis elicited by action
potentials and voltage-clamp calcium currents in individual mouse
pancreatic B-cells. J Physiol. 472:665–688. 1993. View Article : Google Scholar : PubMed/NCBI
|