Introduction
Lung cancer is one of the most common types of
occupational cancer, and is the leading cause of cancer-associated
mortality worldwide (1). According
to its histological characteristics, lung cancer is defined as
either non-small cell lung cancer (NSCLC) or small cell lung
cancer. Approximately 80% of lung cancer cases are considered to be
NSCLC, which comprises adenocarcinoma, squamous cell carcinoma and
large cell carcinoma. It is well known that air pollution and
tobacco smoke, as well as numerous other factors, increase the
incidence of lung cancer; however, the mechanisms underlying lung
cancer formation remain largely unknown (2). Furthermore, in the majority of
clinical cases, lung cancer is metastatic, which is typically
incurable. Metastasis is one of the most lethal characteristics of
cancer, and is responsible for ~90% of human cancer-associated
mortality (3). The metastasis of
lung cancer is a complex process, which includes cellular
migration, local invasion and dissemination. Blocking one of the
steps of metastasis may effectively prevent secondary tumors from
spreading in the body (4,5).
Receptor tyrosine kinases (RTKs), which are well
known as the major regulators of cellular growth, are associated
with the regulation of cell proliferation, differentiation and
migration (6). The ephrin (Eph)
receptors represent the largest family of RTKs and interact with
ligands known as ephrins. Previous pre-clinical and laboratory
studies have demonstrated that the function of RTKs is associated
with tumor growth, metastasis and neovascularization (7,8).
Based on sequence homology, structure and binding affinity, Eph
receptors are subdivided into two types: A and B (9). Class A receptors are tethered to
glycosylphosphatidylinositol-linked class A ephrins, whereas class
B receptors are tethered to ephrin-B ligands, which comprise a
transmembrane domain and a short cytoplasmic tail. Overall, 14
receptors (nine class A and five class B) and eight ligands (five
class A and three class B) have been detected in the human genome.
Previous studies have demonstrated that Eph receptor and ephrin
genes are differentially expressed in human tumors, including
neuroblastoma, prostate cancer and breast cancer (10–12).
Eph receptor A7 (EphA7), which was formerly known as
Mdk1/Ebk/Ehk, is highly conserved in vertebrates from fish to
humans (13). Hafner et al
(14) reported that EphA7 is
highly expressed in kidney vasculature. In addition, the mRNA
expression levels of EphA7 are markedly upregulated in
hepatocellular carcinoma, as compared with in healthy liver tissue,
and EphA7 is transcriptionally activated in lung cancer (15). Furthermore, overexpression of EphA7
is frequently detected in younger patients, and in patients with
advanced gastric carcinoma (16).
However, little is currently known regarding the role of EphA7 in
the development of NSCLC. The present study silenced EphA7
expression using EphA7-specific small interfering (si)RNA, and
examined the effects on cell viability and apoptosis. In addition,
alterations in cell migration and invasion were detected. The
results of the present study provided evidence suggesting that
siEphA7 exerted anticancer activity in A549 human NSCLC cells via
proapoptotic mechanisms.
Materials and methods
Cell culture and siRNA transfection
The A549 human NSCLC cell line was purchased from
the Institute of Biochemistry and Cell Biology, Chinese Academy of
Science (Shanghai, China). The cells were cultured at 37°C in
Dulbecco's modified Eagle's medium (DMEM; Shanghai Huiying
Biotechnology Co., Ltd., Shanghai, China) supplemented with 10%
fetal bovine serum (FBS; Shanghai Huiying Biotechnology Co., Ltd.),
100 U/ml penicillin and 100 µg/ml streptomycin (Shanghai
Huiying Biotechnology Co., Ltd.). Subsequently, 70–80% of the cells
were transferred to 96-well plates and cultured in fresh medium
without antibiotics. X-treme™ GENE siRNA Transfection Reagent
(Roche Diagnostics, Basel, Switzerland) was used to transfect the
cells with siEphA7 (Shanghai GenePharma Co., Ltd., Shanghai, China;
sequence, 5′-GUG GGA AGU UAU GUC UUA UTT AUA AGA CAU AAC UUC CCA
CTT-3′) or negative control siRNA (Shanghai GenePharma Co., Ltd.;
sequence, 5′-UUC UCC GAA CGU GUC ACG UTT ACG UGA CAC GUC CGG AGA
ATT-3′). The transfection concentration determined for use by
transfection efficiency testing was 50 nM siEphA7, transfection was
conducted for 24 h at 37°C. A549 cells were used as the
untransfected control group.
Cell viability assay
The colorimetric
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay (Beyotime Institute of Biotechnology, Haimen, China) was used
to assess the viability of cells cultured in 96-well plates by
measuring mitochondrial dehydrogenase activity. The MTT assay is
based on the notion that viable cells, but not dead cells, can
reduce MTT. Following siRNA transfection, the cells were incubated
with 10 µl MTT (0.5 mg/ml) at 37°C for 4 h. The purple
formazan crystals were then dissolved using 100µl dimethyl
sulfoxide. The absorbance was subsequently measured at 490 nm using
an 340 AT Easy Microplate Reader (SLT Lab Instruments GmbH,
Salzburg, Austria).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Cellular mRNA expression levels of EphA7 were
determined using RT-qPCR. Total RNA (1 µg) was extracted
from each sample of cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and was used to generate cDNA
using M-MLV Reverse Transcriptase (Promega Corporation, Madison,
WI, USA). The cycling conditions were as follows: Initial
denaturation at 25°C for 10 min; 37°C for 120 min; 85°C for 5min;
the temperature was dropped to 4°C and the product was reached at
20°C. qPCR was conducted using SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.), and the PCR cycling
conditions were as follows: Initial denaturation at 95°C for 10
min, followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and
72°C for 30 sec, followed by a final extension step at 72°C for 10
min. The PCR primers used were as follows: Sense, 5′-CTA ATG TTG
GAT TGT TGG CAA AAG-3′ and antisense, 5′-TTG ATC CAG AAG AGG GCT
TATTG-3′ for EphA7 and sense, 5′-AAG AAG GTG GTG AAGCAGGC-3′ and
antisense, 5′-TCC ACC ACC CAG TTG CTGTA-3′ for GAPDH. A 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used for cDNA amplification, with routine product
checking using dissociation curve software (QuantStudio™ Real-Time
PCR software, version 2.0.6; Applied Biosystems; Thermo Fisher
Scientific, Inc.). Transcript quantities were compared using a
relative quantification method. The amount of detected mRNAs was
normalized to the amount of the endogenous control GAPDH. The
relative value to the control sample was determined using the
2−ΔΔCq method (17).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining assay
The cells were fixed in 4% paraformaldehyde (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) and
pretreated with ethanol, proteinase K (Tiangen Biotech Co., Ltd.,
Beijing, China), Triton X-100 (Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China), and pepsin (Shanghai Haoran
Bio Technologies Co., Ltd., Shanghai, China). Following washing in
PBS three times, the samples were treated with the TUNEL reaction
mixture and TUNEL Dilution buffer (In Situ Cell Death
Detection kit; Roche Diagnostics, Basel, Switzerland) at a ratio of
1:9, and incubated in a humidified chamber for 1 h at 37°C. After
washing in phosphate-buffered saline (PBS), anti-digoxigenin
peroxidase conjugate (Roche Diagnostics) was added, and incubation
continued in a humidified chamber for 30 min at room temperature.
The samples were washed with PBS and the nuclei were stained with
DAPI (Roche Molecular Diagnostics, Pleasanton, CA, USA) for 5 min
at room temperature. The number of total cells and TUNEL-positive
cells were automatically counted using Image-Pro Plus (Media
Cybernetics, Inc., Rockville, MD, USA), in order to calculate the
apoptotic rate, which was defined as the ratio of apoptotic cells
to total cells.
Transwell migration and invasion
assays
The migration and invasion assays were carried out
using Transwell plates (EMD Millipore, Billerica, MA, USA). The
filter surfaces (8 µm pore size) of the Transwell plates
were uniformly coated with 25 mg Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA) overnight at 4°C prior to experimentation. The
lower chamber was filled with culture medium supplemented with 10%
FBS. The A549 cells were carefully transferred onto the
matrigel-coated (BD Biosciences, Franklin Lakes, NJ, USA) upper
surface of the chamber at a seeding density of 1–4×105
cells per flask (Corning Incorporated, Corning, NY, USA). Following
a 24 h incubation at 37°C, the filter was gently removed, and the
upper surface was wiped to remove the attached cells. The cells
that had invaded through the Matrigel and attached to the lower
surface of the filter were fixed with 4% paraformaldehyde and
stained with Giemsa (GefanBio Co. Ltd., Shanghai, China). Three
replicates were conducted for each condition. A total of 15 random
fields in each replicate were selected and the number of cells was
counted using an Olympus CKX41 inverted microscope (Olympus
Corporation, Tokyo, Japan). The results are presented as the ratio
of invasive cells relative to the invasive cells in the control
conditions (cells seeded in serum-free media, which invaded towards
DMEM containing 10% FBS). Cells that had invaded to the lower sides
of the Transwell were fixed with 4% (w/v) paraformaldehyde and
stained using 0.05% crystal violet (Amresco, LLC, Solon, OH, USA)
for cell counting as described above. Cells on the upper side of
the membrane were scraped off and cells that had migrated to the
lower side of the membrane were fixed in 4% paraformaldehyde,
stained with 0.1% crystal violet for 30 min at room temperature,
and washed 3 times with PBS.
Western blot analysis
Total protein was extracted from the cells for
protein immunoblotting using radioimmunoprecipitation buffer
(Beyotime Institute of Biotechnology) and protease inhibitors
(Shenergy Biocolor, Shanghai, China) and the protein concentration
was quantified using a bicinchoninic acid assay (Beyotime Institute
of Biotechnology). Briefly, protein samples (80 µg) were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and blotted onto nitrocellulose membranes (Beyotime
Institute of Biotechnology). The blots were blocked with 5% non-fat
milk for 2 h at room temperature, and then incubated with the
following primary antibodies: Rabbit polyclonal anti-EphA7 IgG
(1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no.
sc-918), rabbit polyclonal anti-B-cell lymphoma 2 (Bcl-2; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA; cat. no. 2876),
rabbit polyclonal anti-Bcl-2-associated X protein (Bax; 1:1,000;
Cell Signaling Technology, Inc.; cat. no. 2774), rabbit polyclonal
anti-caspase-3 (1:1,000; Cell Signaling Technology, Inc.; cat. no.
9662), rabbit polyclonal anti-phosphatase and tensin homolog (PTEN;
1:1,000; Cell Signaling Technology, Inc.; cat. no. 9552), rabbit
polyclonal anti-AKT (1:1,000; Cell Signaling Technology, Inc.; cat.
no. 9272) and anti-phosphorylated (p)-AKT (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 9271), and anti-GAPDH (1:1,000; Santa
Cruz Biotechnology, Inc.; cat. no. sc-48166) in PBS at 4°C
overnight. Subsequently, the membranes were washed with PBS
containing 0.1% Tween and incubated with Alexa Fluor®
700-conjugated goat anti-mouse IgG (1:8,000; Invitrogen; Thermo
Fisher Scientific, Inc.; cat. no. A-21036) and IRDye®
800CW goat anti-rabbit IgG (1:8,000; LI-COR Biosciences, Lincoln,
NE, USA) for 1 h at room temperature. The bands were visualized
using an imaging system (LI-COR Biosciences), and semi-quantified
using Odyssey v1.2 software (LI-COR Biosciences) by measuring
intensity (area x optical density). GAPDH was used as an internal
control. The results were expressed as fold changes by normalizing
the data to the control values.
Statistical analysis
All data are expressed as the mean ± standard error
of mean. Statistical analyses, including an unpaired two-tailed
Student's t-test and one-way analysis of variance followed by the
Bonferroni's multiple comparison post hoc test were carried out
using GraphPad Prism v6.0 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Silencing EphA7 with siEphA7 suppresses
the viability of A549 cells
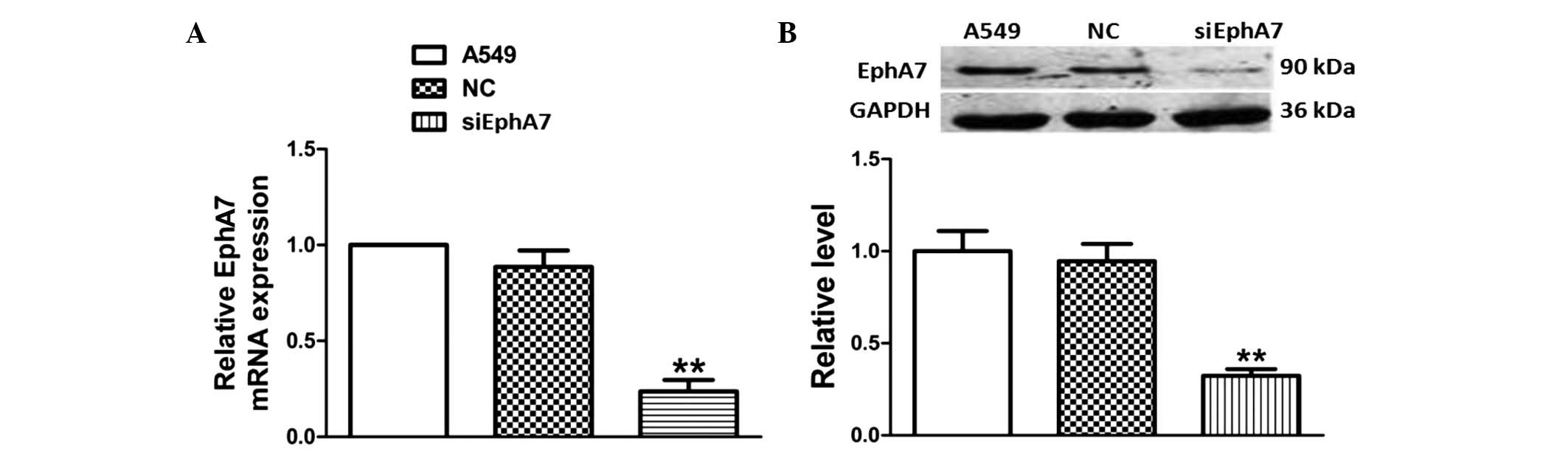
To confirm the silencing effect of siEphA7 on EphA7
expression, the EphA7 mRNA expression levels were detected using
RT-qPCR. As shown in Fig. 1A, as
compared with the control group, there were no significant
alterations in the non-specific siRNA-transfected control group,
however the EphA7 mRNA expression levels were significantly
decreased following transfection with siEphA7 for 24 h.
Furthermore, western blotting demonstrated that siEphA7 also
downregu-lated the protein expression levels of EphA7 (Fig. 1B). These results indicate that
EphA7 expression was significantly silenced post-transfection with
siEphA7.
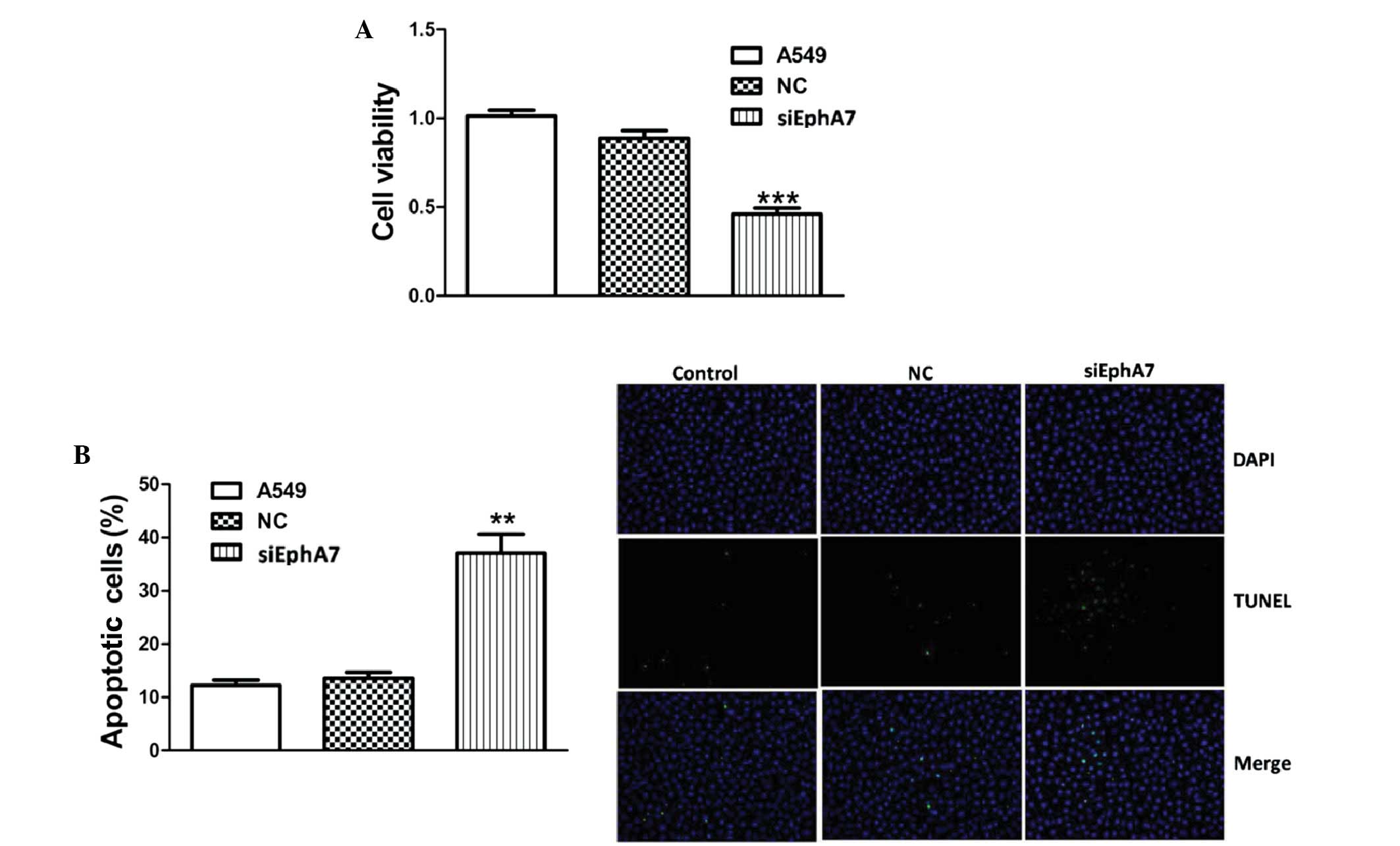
An MTT viability assay was conducted to determine
the effects of siEphA7 on cell viability. As shown in Fig. 2A, transfection with siEphA7 for 24
h resulted in a ~50% reduction in proliferation rate, as compared
with the control group. Furthermore, the results of the MTT assay
were confirmed by TUNEL assay. Transfection with siEphA7 markedly
induced apoptosis in A549 cells (Fig.
2B). These results suggest that EphA7 has a key role in the
regulation of lung cancer cell growth.
siEphA7 alters the migration and invasion
of A549 cells
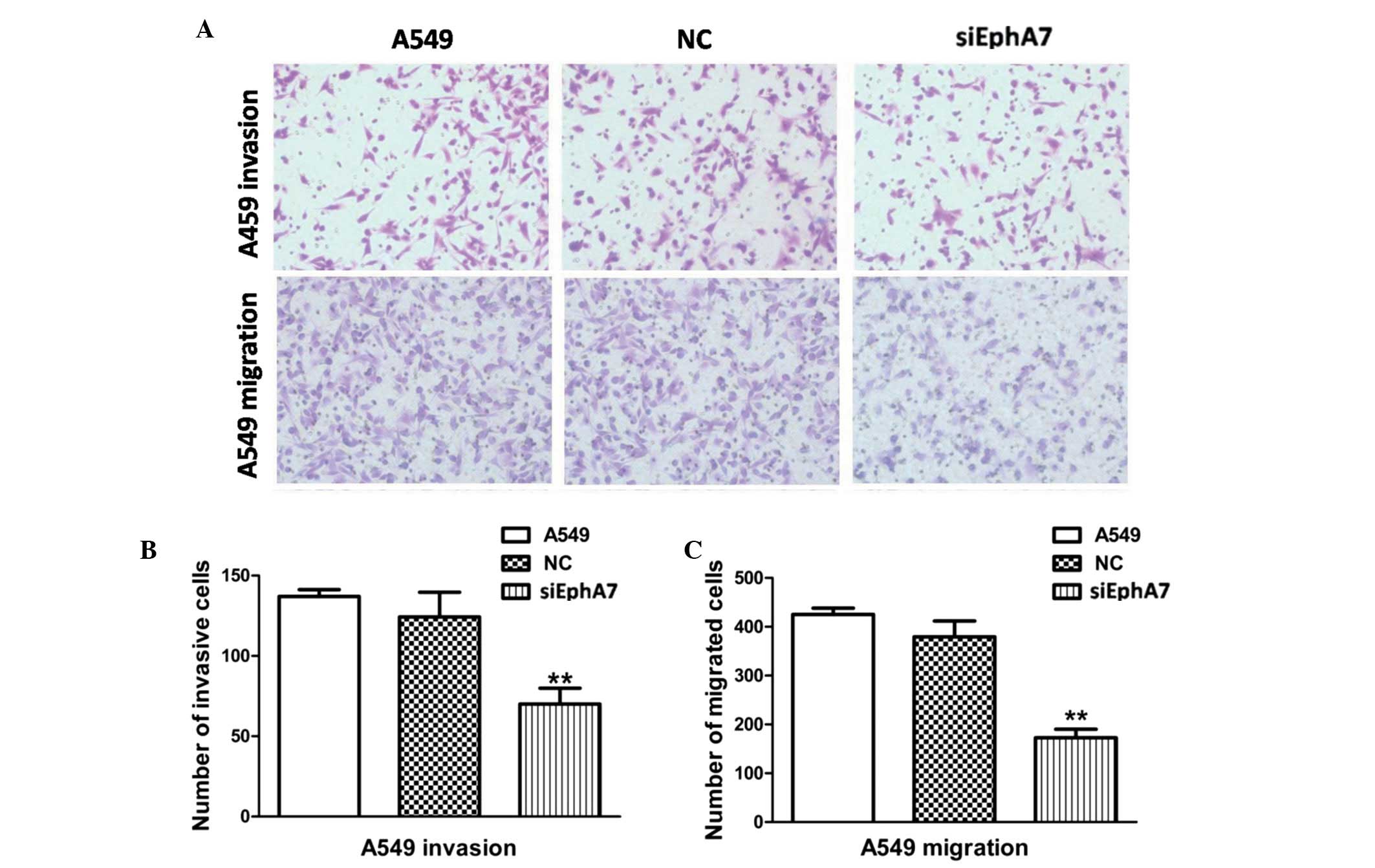
An important factor in tumor metastasis and
progression is the ability of tumor cells to invade beyond the
limitations of the primary tumor environment. Therefore, a
Transwell assay was conducted, in order to investigate the ability
of cells to invade through a biological matrix. As shown in
Fig. 3A and B, transfection with
siEphA7 markedly inhibited the ability of A549 cells to invade
through the Matrigel-coated filter; the rate of invasion of A549
cells was decreased by >50%, as compared with the control
group.
To explore whether the decreased invasiveness
mediated by siEphA7 was associated with cell motility, the effects
of siEphA7 on cell migration were detected. As shown in Fig. 3A and C, the migratory capabilities
of the A549 cells transfected with siEphA7 were reduced by ~62.9%,
as compared with the control group. These results indicate that
knockdown of EphA7 significantly inhibits cell invasion and
migration in vitro.
Effects of siEphA7 on the expression
levels of apoptosis-associated proteins
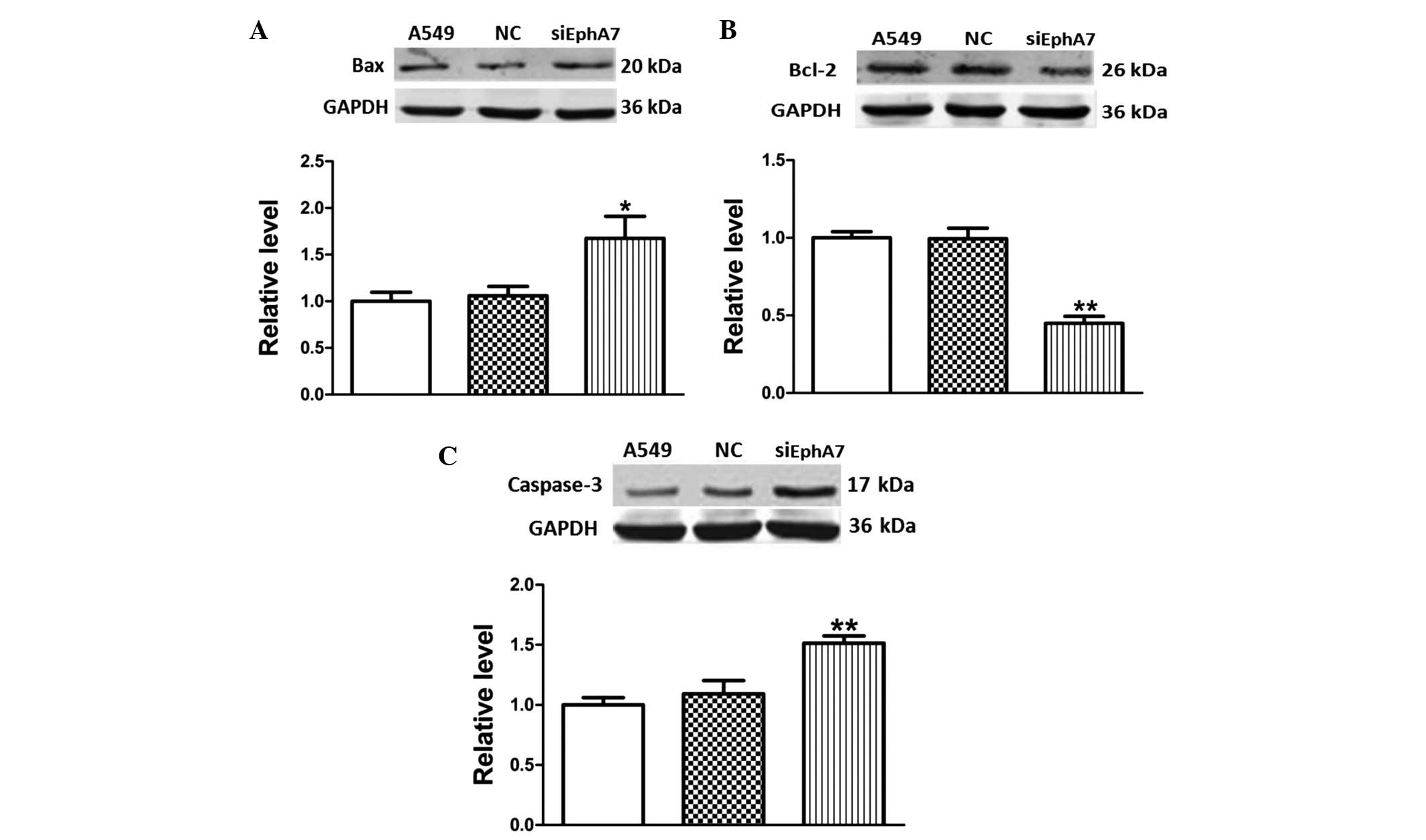
Alterations in the expression levels of
apoptosis-inducing signaling molecules were evaluated in A549 cells
post-transfection with siEphA7 for 24 h. The Bcl-2 family proteins,
which include pro-apoptotic Bax and anti-apoptotic Bcl-2, are known
regulators of apoptotic pathways (18). Therefore, in the present study,
Bcl-2 and Bax protein expression levels were detected, in order to
investigate whether these proteins were associated with
siEphA7-induced apoptosis. As compared with the control group,
transfection with siEphA7 upregulated Bax protein expression levels
(Fig. 4A), and significantly
inhibited Bcl-2 protein expression levels (Fig. 4B). The inhibition rate was ~50%.
These results indicate that siEphA7 induced apoptosis via
regulation of the Bcl-2 family protein-mediated mitochondrial
pathway.
Since caspase-3 is well established as a major
caspase, and its activation ultimately leads to cell death
(19), it is often used to detect
apoptosis. The present study evaluated whether siEphA7-induced
apoptosis was associated with alterations in caspase-3 protein
expression. Caspase-3 protein expression levels were significantly
upregulated (~1.5-fold) in A549 cells post-transfection with
siEphA7 (Fig. 4C). These results
indicate that siEphA7 induced apoptosis via upregulation of
caspase-3 protein expression.
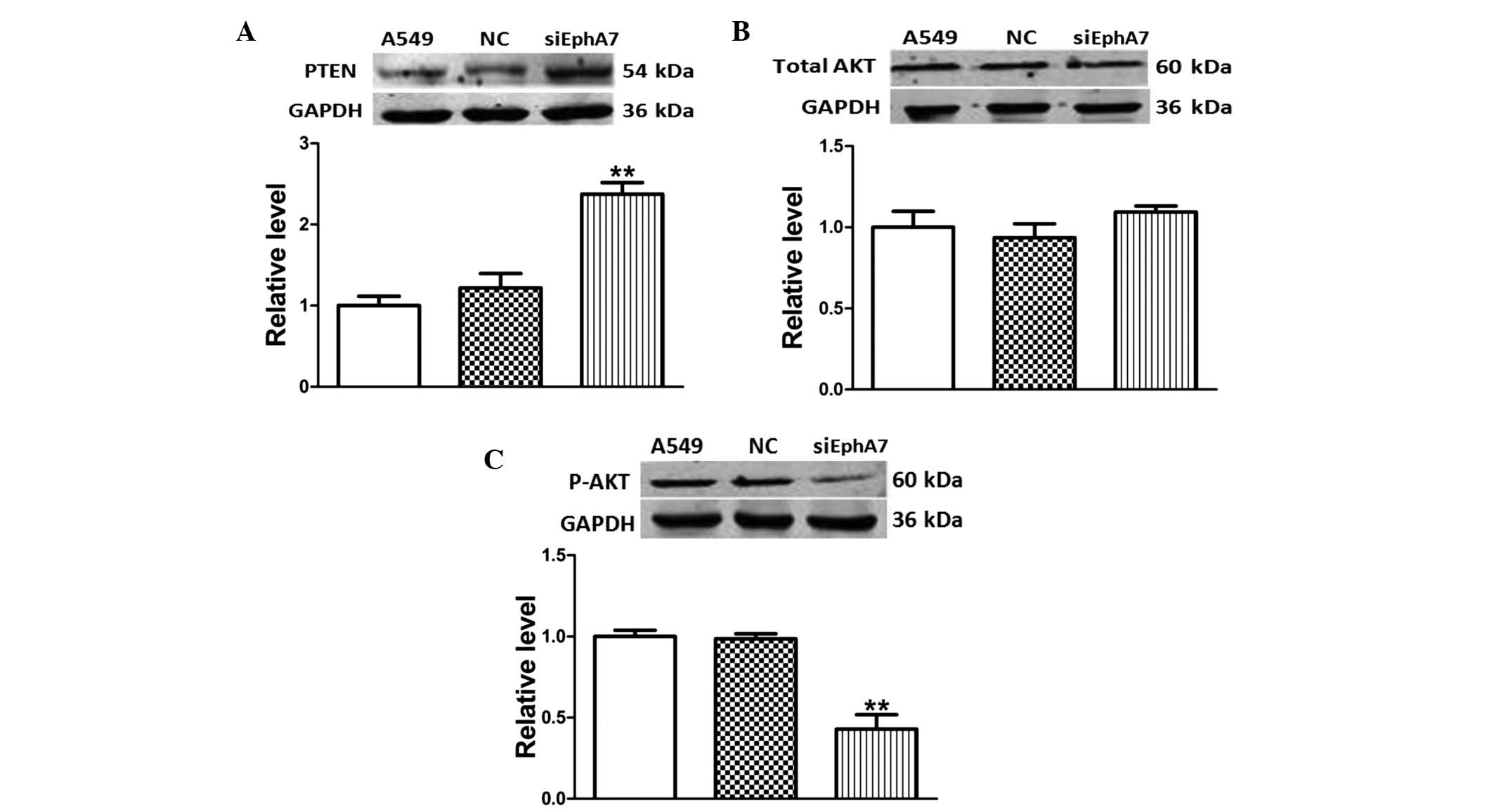
Silencing EphA7 regulates the PTEN/AKT
signaling pathway in A549 cells
PTEN, which is an important tumor suppressor that is
mutated in various malignancies, is a central negative regulatory
factor of the AKT pathway (20).
In order to investigate the effects of EphA7 knockdown on the
activity of the PTEN/AKT signaling pathway in A549 cells, the
protein expression levels of PTEN, AKT and P-AKT were measured
using western blotting. The protein expression levels of PTEN were
significantly higher in A549 cells transfected with siEphA7, as
compared with the control group (Fig.
5A). In addition, the protein expression levels of total AKT
were unchanged between the groups, whereas the expression levels of
P-AKT were reduced (Fig. 5B and
C). These results suggest that the inhibition of EphA7 may
inhibit the AKT signaling pathway via upregulation of PTEN in A549
cells.
Discussion
The present study, to the best of our knowledge, is
the first to demonstrate that silencing EphA7 with siEphA7 inhibits
the viability, invasion and migration of A549 cells. In addition,
the expression levels of the apoptosis-inducing signaling molecules
Bax and caspase-3 were increased, whereas the expression levels of
Bcl-2 were decreased. Furthermore, the AKT signaling pathway was
inhibited via upregulation of PTEN in A549 cells. These findings
indicated that the proliferation and metastasis of NSCLC cells may
be altered at the molecular level. The results of the present study
help improve our understanding regarding the proapoptotic
mechanisms underlying the anticancer effects of siEphA7, thus
suggesting that knockdown of EphA7 has potential therapeutic
value.
Previous studies have demonstrated that the
regulation of Eph receptors and ephrin ligands is associated with
tumor growth, metastasis and adverse outcomes (21–23).
It has been reported that EphA7 is highly expressed in kidney
vascula-ture (14), and the
expression of EphA7 is transcriptionally activated in lung cancer
(15). However, few studies have
clarified the role of EphA7 in tumor pathogenicity. Therefore, in
the present study, EphA7 expression was silenced using siEphA7, and
the effects on cell viability and apoptosis were detected. Results
of the MTT and TUNEL assays demonstrated that knockdown of EphA7
with siEphA7 significantly decreased cell viability and promoted
apoptosis. Therefore, the present study aimed to further
investigate the underlying molecular mechanisms associated with
siEphA7-induced cell apoptosis.
Bcl-2 is an antiapoptotic protein, whereas Bax is a
proapoptotic protein, both of which belong to the Bcl-2 family, a
group of proteins that regulate the permeability of the outer
mitochondrial membrane (24). In
addition, downregulation of Bcl-2 or upregulation of Bax may induce
cell apoptosis and subsequently activate caspase-3 (25). Therefore, the present study
examined the expression levels of apoptosis-associated proteins,
including Bcl-2, Bax and caspase-3 in A549 NSCLC cells.
Transfection with siEphA7 significantly downregulated the protein
expression levels of Bcl-2 and upregulated the protein expression
levels of Bax, which led to the activation of cell apoptosis.
Furthermore, caspase-3 protein expression levels were increased.
These results indicated that knockdown of EphA7 induced A549 cell
apoptosis predominantly via the Bcl-2/Bax-dependent mitochondrial
pathway. In addition, caspase-3 was also involved in the cell
apoptosis.
In the present study, PTEN, AKT and P-AKT protein
expression levels were detected by western blotting, in order to
investigate whether silencing EphA7 led to inhibition of the
activity of the PTEN/AKT signaling pathway. The results
demonstrated that the expression levels of PTEN were markedly
increased. In addition, a low phosphorylation level of AKT was
observed. These results indicated that silencing EphA7 may
downregulate the PTEN/AKT signaling pathway in NSCLC cells.
Furthermore, it was hypothesized that the anticancer effects of
EphA7 knockdown may not only by due to cell apoptosis, but also be
due to effects on tumor invasion and metastasis. Transwell assays
were performed in A549 NSCLC cells, and the results indicated that
siEphA7 was able to regulate cell invasion and migration as a tumor
suppressor. These results provide initial insights into the
function of siEphA7 in regulating cell migration and invasion of
NSCLC cells.
In conclusion, the results of the present study
suggested that silencing EphA7 may exert potential tumor
suppressive effects by targeting PTEN/AKT. In addition, siEphA7 had
a crucial role in regulating the migration and invasion of NSCLC
cells. The precise molecular mechanisms require further study;
however, silencing EphA7 expression provides a novel insight into
potential therapeutic strategies for the treatment of patients with
lung cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Xiong Y, Sun Y, Fang Z, Li L, Ji H
and Shi T: HLungDB: An integrated database of human lung cancer
research. Nucleic Acids Res. 38(Database Issue): D665–D669. 2010.
View Article : Google Scholar :
|
|
3
|
Inamura K and Ishikawa Y: Lung cancer
progression and metastasis from the prognostic point of view. Clin
Exp Metastasis. 27:389–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mihaljevic AL, Michalski CW, Friess H and
Kleeff J: Molecular mechanism of pancreatic cancer - understanding
proliferation, invasion, and metastasis. Langenbecks Arch Surg.
395:295–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoon DS, Ferris R, Tanaka R, Chong KK,
Alix-Panabières C and Pantel K: Molecular mechanisms of metastasis.
J Surg Oncol. 103:508–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Geer P, Hunter T and Lindberg RA:
Receptor protein-tyrosine kinases and their signal transduction
pathways. Annu Rev Cell Biol. 10:251–337. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumura Y, Umemura S, Ishii G, Tsuta K,
Matsumoto S, Aokage K, Hishida T, Yoshida J, Ohe Y, Suzuki H, et
al: Expression profiling of receptor tyrosine kinases in high-grade
neuroendocrine carcinoma of the lung: A comparative analysis with
adenocarcinoma and squamous cell carcinoma. J Cancer Res Clin
Oncol. 141:2159–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eph Nomenclature Committee: Unified
nomenclature for Eph family receptors and their ligands, the
ephrins. Cell. 90:403–404. 1997. View Article : Google Scholar
|
|
10
|
Kiyokawa E, Takai S, Tanaka M, Iwase T,
Suzuki M, Xiang YY, Naito Y, Yamada K, Sugimura H and Kino I:
Overexpression of ERK, an EPH family receptor protein tyrosine
kinase, in various human tumors. Cancer Res. 54:3645–3650.
1994.PubMed/NCBI
|
|
11
|
Tang XX, Zhao H, Robinson ME, Cohen B,
Cnaan A, London W, Cohn SL, Cheung NK, Brodeur GM, Evans AE and
Ikegaki N: Implications of EPHB6, EFNB2, and EFNB3 expressions in
human neuroblastoma. Proc Natl Acad Sci USA. 97:10936–10941. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walker-Daniels J, Coffman K, Azimi M, Rhim
JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ and Kinch MS:
Overexpression of the EphA2 tyrosine kinase in prostate cancer.
Prostate. 41:275–280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taneja R, Thisse B, Rijli FM, Thisse C,
Bouillet P, Dollé P and Chambon P: The expression pattern of the
mouse receptor tyrosine kinase gene MDK1 is conserved through
evolution and requires Hoxa-2 for rhombomere-specific expression in
mouse embryos. Dev Biol. 177:397–412. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hafner C, Schmitz G, Meyer S, Bataille F,
Hau P, Langmann T, Dietmaier W, Landthaler M and Vogt T:
Differential gene expression of Eph receptors and ephrins in benign
human tissues and cancers. Clin Chem. 50:490–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Surawska H, Ma PC and Salgia R: The role
of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev.
15:419–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakanishi H, Nakamura T, Canaani E and
Croce CM: ALL1 fusion proteins induce deregulation of EphA7 and ERK
phos-phorylation in human acute leukemias. Proc Natl Acad Sci USA.
104:14442–14447. 2007. View Article : Google Scholar
|
|
17
|
Wu Z, Liu K, Wang Y, Xu Z, Meng J and Gu
S: Upregulation of microRNA-96 and its oncogenic functions by
targeting CDKN1A in bladder cancer. Cancer Cell Int. 15:1072015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, -6, and -7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar
|
|
20
|
Liu S, Cheng L, Bi X, Zhang X, Liu S, Bai
X, Li F and Zhao AZ: Elevation of ω-3 polyunsaturated fatty acids
attenuates PTEN-deficiency induced endometrial cancer development
through regulation of COX-2 and PGE2 production. Sci Rep.
5:14958
|
|
21
|
Kinch MS, Moore MB and Harpole DH Jr:
Predictive value of the EphA2 receptor tyrosine kinase in lung
cancer recurrence and survival. Clin Cancer Res. 9:613–618.
2003.PubMed/NCBI
|
|
22
|
Kataoka H, Igarashi H, Kanamori M, Ihara
M, Wang JD, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Maruyama K,
et al: Correlation of EPHA2 overexpression with high microvessel
count in human primary colorectal cancer. Cancer Sci. 95:136–141.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura R, Kataoka H, Sato N, Kanamori M,
Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, et
al: EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci.
96:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martinou JC and Green DR: Breaking the
mitochondrial barrier. Nat Rev Mol Cell Biol. 2:63–67. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arnoult D, Gaume B, Karbowski M, Sharpe
JC, Cecconi F and Youle RJ: Mitochondrial release of AIF and EndoG
requires caspase activation downstream of Bax/Bak-mediated
permeabilization. EMBO J. 22:4385–4399. 2003. View Article : Google Scholar : PubMed/NCBI
|