Introduction
Neurological disorders affect ~30,000,000
individuals in China, leading to disability and contributing to
mortality rates (1). These
disorders are characterized by pathological changes in
disease-specific areas of the brain, and the degeneration of
distinct neural subsets (2). It
has been well reported that neurological disorders are linked to
elevated levels of oxidative stress, which is involved in
modulating the biochemical changes resulting in neurological
disorders (3). The supplementation
of natural antioxidants is regarded as a prophylactic strategy
against diseases caused by oxidative stress (4).
Ginseng, the root of Panax species (5,6), is
one of the most frequently used herbs in China due to its potential
as a general tonic or chemopreventive agent (7,8). The
antioxidant action of ginseng is an area of interest in scientific
investigations, which provides information for dietary
supplementation and the pharmacological usage of ginseng products
(9). Ginseng has been shown to
have several beneficial effects in a wide range of pathological
conditions, including cardiovascular disease, cancer,
immunodeficiency and hepatotoxicity in vivo and in
vitro. Of note, ginsenosides are the most biologically active
substances found in ginseng (10).
There are >30 different types of ginsenosides, which have been
isolated from ginseng and classified into three major types:
Panaxadiol, including Rb1, Rb2, Rg3, Rd, Rc, Rg3, Rh2 and Rs1);
panaxatriol, including Rg1, Rg2, Re, Rf and Rh1; and oleanolic acid
type ginsenosides, including Ro (9). Among these, the most commonly
investigated ginsenosides are Rb1, Rd, Rg1 and Re, as these four
compounds are relatively more abundant in ginseng and have a wide
range of actions in the central nervous system (CNS), including
promoting neural survival, extending neurite growth and rescuing
neurons from pathological conditions (11).
Several studies have provided evidence that
ginsenoside Rb1 possesses potent neuroprotective effects on
cortical neurons and dopaminergic neurons against glutamate
toxicity, protects against cerebral ischemia by promoting
neurogenesis, prevents MPP+-induced apoptosis in PC12
cells, improves spatial learning, and increases levels of
hippocampal synaptophysin in mice (12–16).
In the CNS, Rd has been shown to be effective in decreasing the
formation of reactive oxygen species (ROS) in cultured astrocytes,
protecting PC12 cells from hydrogen peroxide-induced oxidative
damage, mitigating neuroinflammation and nitric oxide
overproduction, and attenuating neuronal oxidative damage induced
by oxygen-glucose deprivation (17). Rg1 has been shown to possess
neurotrophic and neuroprotective effects on dopaminergic cells
against glutamate injury and MPP+ toxicity, inhibit the
mitochondrial apoptotic pathway and increase the survival of
primary cultured nigral neurons against rotenone toxicity (18). It has also been demonstrated that
Rg1 exerts neuroprotective effects through ameliorating amyloid
pathology, modulating the production of APP and activating the
protein kinase A/cAMP response element binding protein signaling
pathways (19). Re has been
reported to protect mouse nigral neurons from mitochondrial
permeability transition pore-induced apoptosis in a Parkinson's
disease model, and this effect was considered to be attributable to
upregulation in the protein expression of B cell lymphoma (Bcl)-2,
downregulation in the expression levels of Bcl2-associated X
protein and inducible nitric oxide synthase, and subsequent
inhibition of the activation of caspase-3 (20). These previous reports suggest that
the Rb, Rd, Rg1 and Re ginsenosides offer therapeutic potential in
the treatment of neurological disorders.
In the present study, the anti-oxidative effects of
four ginsenosides (Rb1, Rd, Rg1 and Re) on NPCs were investigated
and compared. NPCs can be utilized for functional tissue
engineering as a potential treatment for neurologic diseases
(21). They are defined by their
ability to self-renew through mitotic cell division and
differentiate into neurons, astrocytes and oligodendrocytes
(22,23). The results of the present study may
provide evidence on the optimal ginsenoside for use as a potent
antioxidant in the treatment of neurological disorders.
Materials and methods
Chemicals and reagents
Ginsenosides Rb1, Rg1, Rd and Re were provided in
powder form (>98% purity) by Chengdu Must Bio-technology Co.,
Ltd. (Chengdu, China). The powder was dissolved in saline.
Dulbecco's modified Eagle's medium (DMEM) nutrient mix F12, goat
serum, fetal bovine serum (FBS), 0.05% (w/v) trypsin/EDTA,
phosphate-buffered saline (PBS) powder and N2 and B27 supplements
were supplied by Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Poly-l-lysine (PLL), laminin,
4′,6-diamidino-2-phenylindole (DAPI), bovine serum albumin (BSA),
5-Bromo-2-deoxyuridine (BrdU), tert-Butylhydroperoxide
(t-BHP), paraformaldehyde, mouse anti-BrdU (B8434), rabbit
anti-glial fibrillary acidic protein (GFAP; SAB4501162) and mouse
anti-β-tubulin III (Tuj-1; T8578) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). A Lactate Dehydrogenase (LDH) Cytotoxicity
Assay kit (cat. no. 11644793001) and In Situ Cell Death Detection
kit (cat. no. 11684817910) were obtained from Roche (Basel,
Switzerland). The goat anti-mouse 488 antibody, goat anti-rabbit
568 antibody, Click-iT EdU Alexa Fluor® 594 Imaging kit
(cat. no. C10339) and the Qubit® RNA BR Assay kit (cat.
no. Q10210) were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). The RNeasy® Mini kit (cat. no. 74134)
was purchased from Qiagen (Hilden, Germany), and the primers,
PrimeScript™ RT Master Mix (Perfect Real Time) kit (cat. no.
RR036A) and SYBR® Premix Ex Taq™ II (Tli RNase H Plus;
cat. no. RR820A) were supplied by Takara Biotechnology, Co., Ltd.
(Dalian, China). The MicroAmp® Optical 96-well reaction
plates with barcode were obtained from Applied Biosystems (Thermo
Fisher Scientific, Inc.). Mouse anti-Nestin (MAB353) was purchased
from EMD Milipore (Billerica, MA, USA); epidermal growth factor
(EGF) and basic fibroblast factor (bFGF) were purchased from
Peprotech (Rocky Hill, NJ, USA); and mouse anti-receptor
interacting protein (Rip) was provided by Dr Xiaoming Xu of the
University of Louisville (Louisville, USA). All other chemicals and
reagents were of analytical grade.
Primary culture of cortical NPCs
A total of eight pregnant female Sprague-Dawley (SD)
rats (weight, 300–350 g; age, 3–4 months) were obtained from the
Animal Unit at the University of Macau (Macau, China). The rats
were maintained in a temperature-controlled room under a 12-h
light/dark cycle, with ad libitum access to food and water. The
present study was approved by the Committee on the Care and Use of
Laboratory Animals at the University of Macau (Macau, China). The
primary rat embryonic cortical NPCs were prepared from E14.5
embryos derived from the SD rats using a modified protocol
(24,25). Briefly, the cortex was separated
from the surrounding tissue following removal of the meninges. The
cortex was transferred into a 15 ml centrifuge tube containing
culture medium (10 µl/ml N2, 20 µl/ml B27, 20 ng/ml
EGF and 20 ng/ml bFGF in DMEM/F12) and dissociated into a
single-cell suspension (5×106 cells/ml) by gentle
mechanical trituration through a fire-polished Pasteur pipette. The
dissociated cells were filtered through a cell strainer and then
cultured in a T25 flask in suspension. The cells were incubated in
a humidified incubator at 37°C in 5% CO2. Half of the
culture medium was replaced every 2–3 days. After 5–6 days, the
cells had grown in neurospheres with the diameter of ~150
µm. The cells in the neurospheres were passaged at the ratio
of 1:6. These sub-cultured cells were designated as 'first passage'
(P1) cells. The third passage (P3) cells were used for all
subsequent experiments.
Establishment of the oxidative injury
model
t-BHP is commonly used as a model substance
for evaluating the mechanisms of cellular alterations resulting
from oxidative stress in cells and tissues (26). In the present study, the P3 NPCs
were dissociated into single cells, and then seeded into 96-well
plates coated with PLL (25 µg/ml) and laminin (13.3
µg/ml) at a density of 1×104 cells per well. The
cultures were grown at 37°C humidified CO2 incubator for
36 h and then treated with 50, 100, 200 and 300 µM
t-BHP for 2.5 h at 37°C. The cytotoxicity of t-BHP in
the whole cells culture was determined using an LDH cytotoxicity
assay. A toxicity rate of ~35–45% induced by t-BHP at
specific concentrations was considered to be an optimal
t-BHP-induced oxidative injury model.
Drug treatment
To determine the neuroprotective effects of the four
ginsenosides, the cultured NPCs were pre-treated with 0, 0.1, 1, 10
and 100 ìM Rb1, Rg1, Rd or Re, respectively, for 24 h at 37°C,
followed by drug washout with 0.01 M PBS. The cells were then
treated with 300 ìM t-BHP for another 2.5 h. The cell
viability was measured using the LDH assay and further confirmed
using a terminal deoxynucleotidyl transferase dUTP nick-end
labeling (TUNEL) assay.
TUNEL assay
The free 3-OH DNA ends were detected in situ
using an In Situ Cell Death Detection kit, according to the
manufacturer's protocol. Briefly, after washing the cells three
times with ice-cold PBS, the cells were fixed by incubation with
fixation solution (Sigma-Aldrich) for 1 h, followed by incubation
with permeabilization solution (Sigma-Aldrich) for 2 min on ice.
The fixed cell samples were incubated in the TUNEL reaction medium
for 1 h at 37°C in the dark. Following completion of the reaction,
the cells were washed using PBS, transferred into 2 µg/ml
DAPI solution, and mounted on slides. The number of apoptotic
nuclei and the total number of nuclei were determined under a
fluorescence microscope (Axio Imager A2; Carl Zeiss AG, Oberkochen,
Germany).
Lactate dehydrogenase (LDH) release
assay
Cell death in the NPCs was quantified by measuring
the release of LDH into the medium. As the enzyme is released from
cells with damaged membranes, the efflux of LDH is closely
associated with the extent of damage or destruction of the NPCs
(27). To confirm cortical NPC
injury, the activity of LDH in the medium following oxidative
injury was determined using the Cytotoxicity Detection kit,
according to the manufacturer's protocol. Briefly, the treated
cells were lysed for 45 min at 37°C in PBS supplemented with 1X
Triton X-100 (0.1%; Invitrogen; Thermo Fisher Scientific, Inc.),
followed by centrifugation at 1,000 × g for 10 min at 37°C. The
sample supernatants were transferred to a 96-well enzymatic assay
plate and reacted with the substrate mix from the Cytotoxicity
Detection kit in the dark for 30 min at room temperature. The
absorbance of the samples was measured at 490 nm, according to the
filter of the SpectraMaxR M5 Multi-Mode microplate
reader (Molecular Devices LLC, Sunnyvale, CA, USA). Each experiment
was repeated three times independently.
Immunocytochemical analysis
The P3 NPCs in a single cell suspension were
cultured on cover slips, which were coated with PLL/laminin (1:1
ratio), at a density of 1×104 cells/cm2 in a
24-well plate. For differentiation experiments, growth factors were
removed from the culture medium and 1% FBS was added. The cultures
were allowed to differentiate for up to 5 days.
The cells on the cover slips were then fixed with
freshly prepared 4% paraformaldehyde solution in PBS at room
temperature for 20 min. Following several washes with 0.01 M PBS,
the cells were processed for immunocytochemistry. The following
primary antibodies were used to stain the cells: Monoclonal
anti-Nestin antibody (1:500) for NPCs; monoclonal anti-Tuj-1
antibody (1:500) for neurons; polyclonal anti-GFAP antibody
(1:1,000) for astrocytes; and monoclonal anti-Rip antibody (1:50)
for oliogodendrocytes. The cultures were incubated with the primary
antibodies in PBS with 1% BSA, 10% normal goat serum and 0.3%
Triton X-100 overnight at 4°C. The samples were then washed twice
with PBS and incubated in secondary antibody conjugated to
fluorescent Alexa 568 or 488 (1:500; Thermo Fisher Scientific,
Inc.) for 45 min at room temperature. To visualize the nuclei, the
cells were mounted in anti-fade solution containing DAPI for 10
min. The fluorescence images were captured using a fluorescence
microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was used to evaluate the mRNA expression
levels of the antioxidant gene in response to oxidative injury. The
cells were pretreated with 10 µM Rb1 for 24 h, after which
total RNA was extracted using the RNeasy® Mini kit. The
RNA concentrations were determined using a NanoDrop 2000 (Thermo
Fisher Scientific, Inc.) with a Qubit® RNA BR Assay kit. Total RNA
was reverse transcribed into cDNA using the PrimeScript™ RT Master
Mix kit, according to the manufacturer's protocol. Amplifications
were performed in duplicate in 20 µl reaction volumes
containing 1X SYBR® Premix Ex Taq™ II (Tli RNase H
Plus), 0.2 µM of each primer and 2 µl target DNA, to
quantitatively detect the gene expression levels of nuclear factor
(erythroid-derived 2)-like 2 (Nrf2), heme oxygenase-1 (HO-1),
superoxide dismutase 2 (SOD2), NAD(P)H: quinone oxidoreductase 1
(NQO1) and catalase (CAT). The relative expression level of each
target gene was normalized to the housekeeping gene, β-actin. All
primer sequences used are listed in Table I. Subsequently, qPCR was performed
using the 7500 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The reaction conditions were as follows:
Initial denaturation at 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 34 sec, followed by melting curve
analysis. Each sample was assessed in triplicate and the
2−ΔΔCq method was used to analyze the relative
transcription data (28).
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Target gene | Direction | Sequence | Association
no. |
|---|
| β-actin | Forward |
5′-GTCGTACCACTGGCATTCTG-3′ | NM_031144 |
| Reverse |
5′-CTCTCAGCTGTGGTGGTGAA-3′ | |
| NRF-2 | Forward |
5′-GCAACTCCAGAAGGAACAGG-3′ | NM-031789.1 |
| Reverse |
5′-CAGTGAGGGGATCGATGAGT-3′ | |
| HO-1 | Forward |
5′-TGCTCGCATGAACACTCTG-3′ | NM_012580.2 |
| Reverse |
5′-TCCTCTGTCAGCAGTGCCT | |
| SOD2 | Forward |
5′-GGCCAAGGGAGATGTTACAA-3′ | NM_001274771 |
| Reverse |
5′-GCTTGATAGCCTCCAGCAAC-3′ | |
| NQO1 | Forward |
5′-GCCCGGATATTGTAGCTgAA-3′ | NM_017000.3 |
| Reverse |
5′-GTGGTGATGGAAAGCAAGGT-3′ | |
| CAT | Forward |
5′-TTATGGCCTCCGAGATCTTTTC-3′ | NM_012520 |
| Reverse |
5′-ACCTTGGTCAGGTCAAATGGAT-3′ | |
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). The two-tailed
Student's t-test was used to make comparisons between two groups
and one-way analysis of variance followed by Tukey's post-hoc test
was used to analyze differences among multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
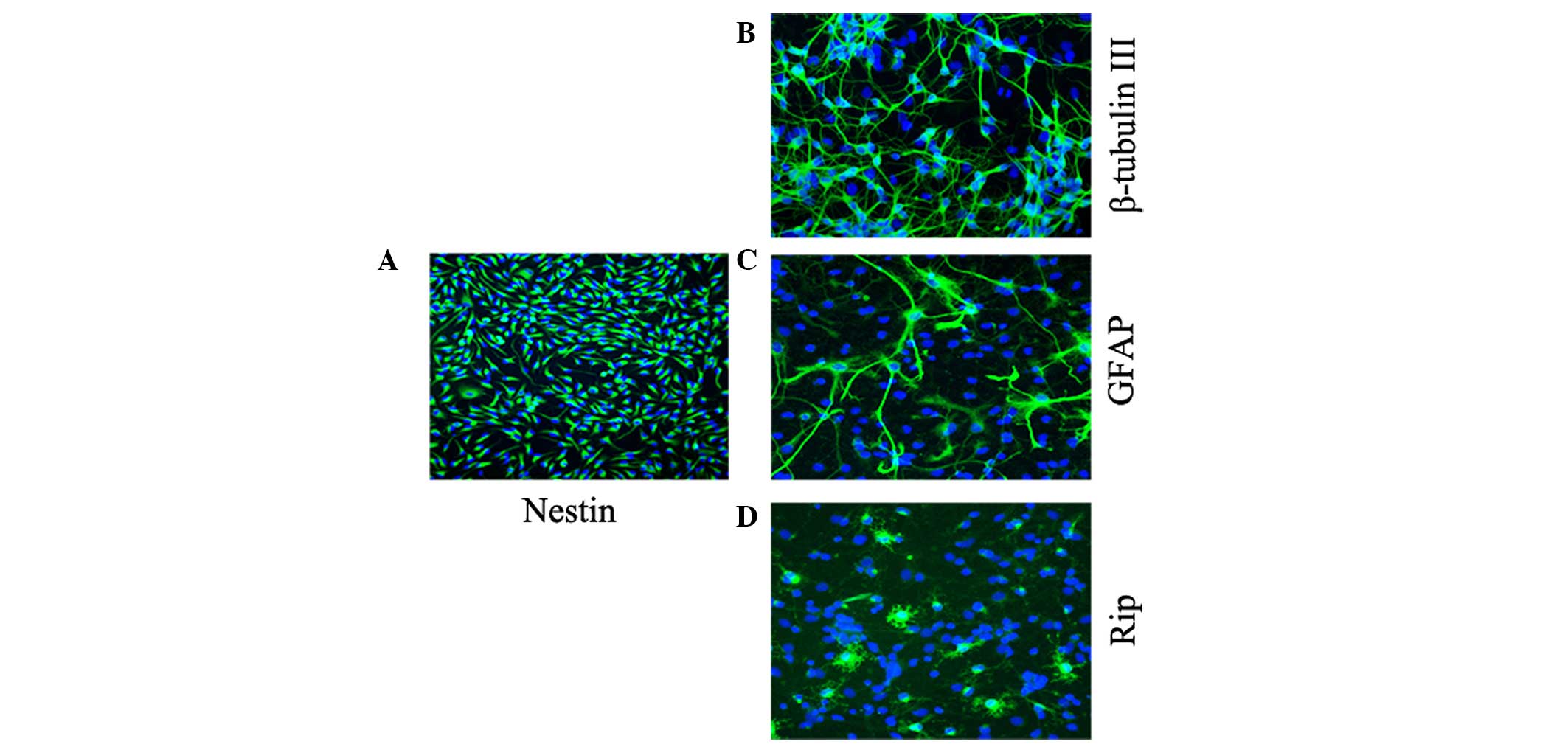
Characterization of NPCs
In the presence of the EGF and bFGF mitogens, the
majority of cells showed bipolar or multipolar morphology with
small cell bodies, and were immunoreactive for Nestin, an effective
marker for NPCs (Fig. 1A),
confirming that the cells remained in an immature stage. Following
replacement of the mitogens with 1% FBS, the NPCs began to
differentiate. At day 5 of culture in the differentiating medium,
the NPCs had successfully differentiated into Tuj-1-positive
neurons (Fig. 1B), GFAP-positive
astrocytes (Fig. 1C) and
Rip-positive oligodendrocytes (Fig.
1D).
Neuroprotective effects of the four
ginsenosides on t-BHP-induced cytotoxicity in NPCs
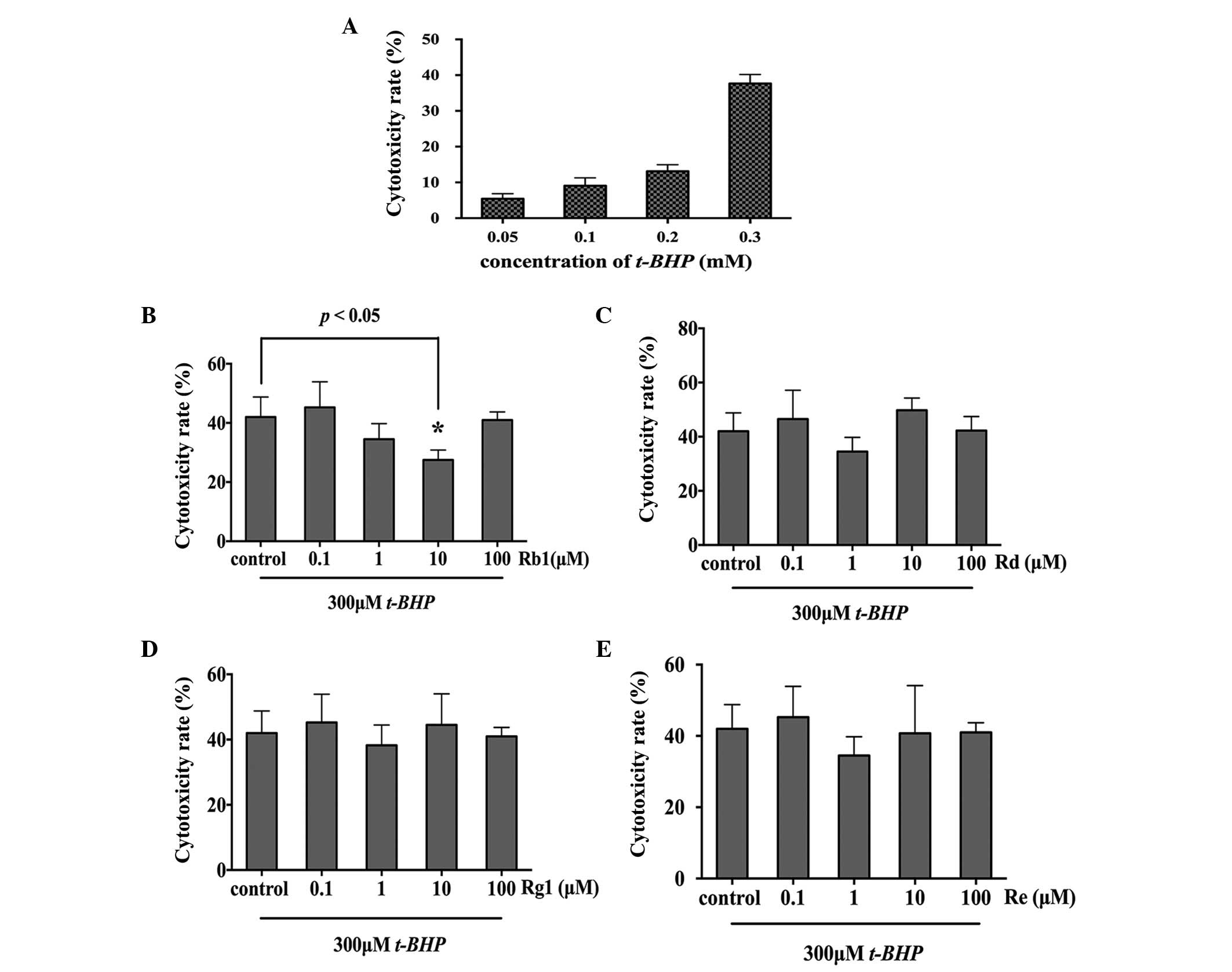
The present study used t-BHP to establish a
model of oxidative injury. As shown in Fig. 2A, t-BHP treatment induced
cell toxicity in a concentration-dependent manner. The NPCs treated
with 50, 100, 200 and 300 µM for 2.5 h exhibited a
cytotoxicity rate of 5.43±1.40, 9.07±2.20, 13.13±1.80 and
37.67±2.52%, respectively. As a toxicity rate of 35–45% induced by
t-BHP was considered to be an optimal oxidative stress
model, the oxidative injury induced by 300 µM t-BHP
for 2.5 h was selected for the subsequent experiments to
investigating the anti-oxidative effect of the four
ginsenosides.
The NPCs were pretreated with different
concentrations of Rb1, Rd, Rg1, Re (0.1, 1, 10 and 100 µM)
for 24 h, followed by treatment with 300 µM t-BHP for
2.5 h, respectively. Cell viability was measured using an LDH assay
and TUNEL staining. The results of the LDH assay suggested that
only 10 µM Rb1 showed a protective effect against oxidative
stress, with a cytotoxicity rate of 27.5±2.87% in the 10 µM
Rb1-pretreated group, compared with 42±5.87% in the control group
(P=0.0208; Fig. 2B). Rd, Rg1 and
Re had no neurooprotective effects against oxidative injury
(Fig. 2C–E). The neuroprotective
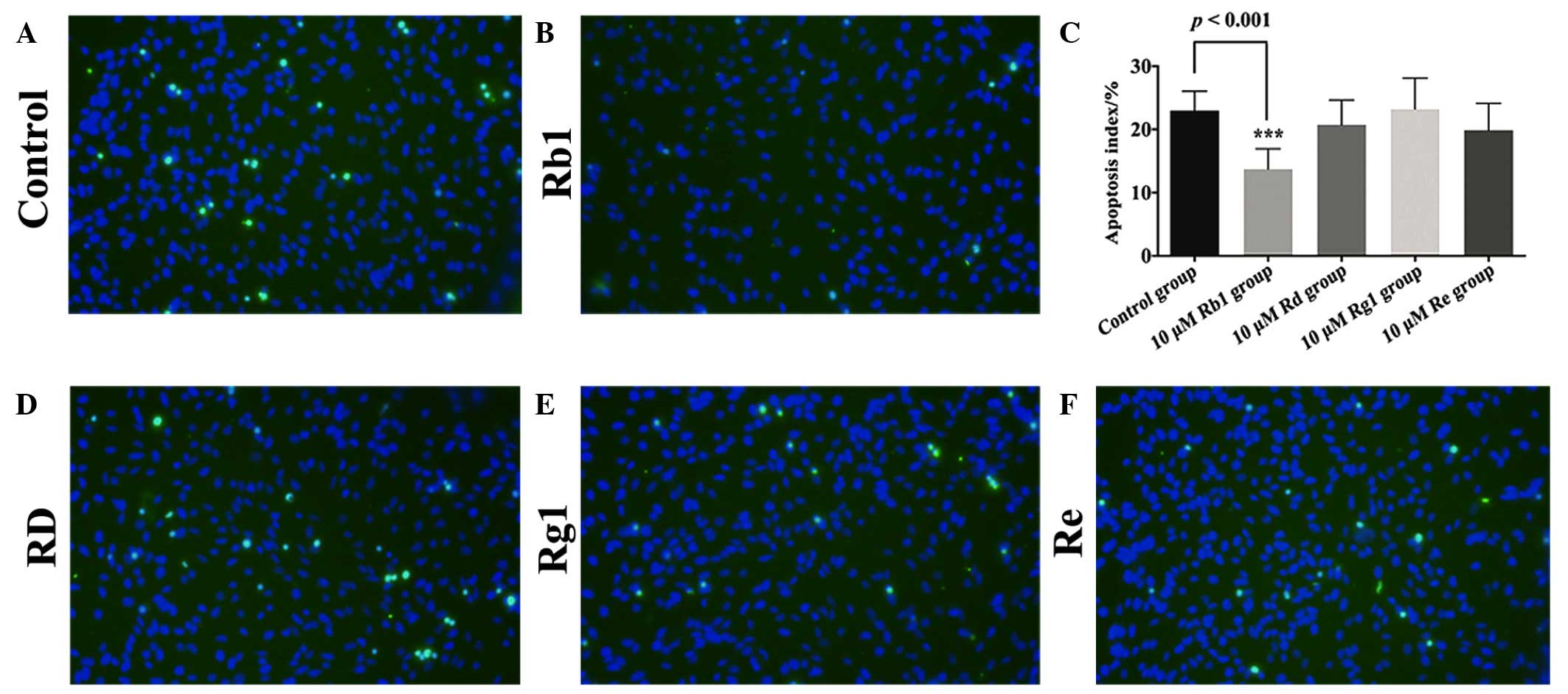
effects of Rb1 were confirmed by the TUNEL assay, with an apoptotic
index of 12.5±2.20% in the 10 µM Rb1-pretreated group,
compared with 23±3.02% in the control group (P=0.0003; Fig. 3A–C). The TUNEL staining
demonstrated that the remaining three ginsenosides, Rd, Rg1 and Re,
exhibited no neuroprotective effects on the NPCs against oxidative
injury (Fig. 3D–F).
Rb1 pretreatment activates anti-oxidative
genes in cultured NPCs
The present study subsequently investigated the
potential mechanism underlying the anti-oxidative effect induced by
Rb1. Firstly, the changes in the mRNA expression levels of Nrf2
were measured using RT-qPCR analysis. Nrf2 belongs to the
basicleucine zipper family and coordinately upregulates the
constitutive and inducible transcription of a wide array of genes
involved in drug metabolism, detoxification and antioxidant
defenses (29). The mRNA
expression level of Nrf2 was increased 2-fold following
pretreatment of the cultured NPCs with 10 µM Rb1 for 24 h.
However, pretreatment of the NPCs with the other three ginsenosides
did not elevate the expression of Nrf2 (Fig. 4A). The mRNA expression levels of
the Nrf2-responsive genes, HO-1, SOD2, NQO1 and CAT were then
examined. A 1.5-fold increase in the expression of HO-1 was
observed in the 10 µM Rb1-pretreated cells, whereas the
other three ginsenosides had no effects on the activation of the
downstream HO-1, SOD2, NQO1 or CAT genes (Fig. 4B).
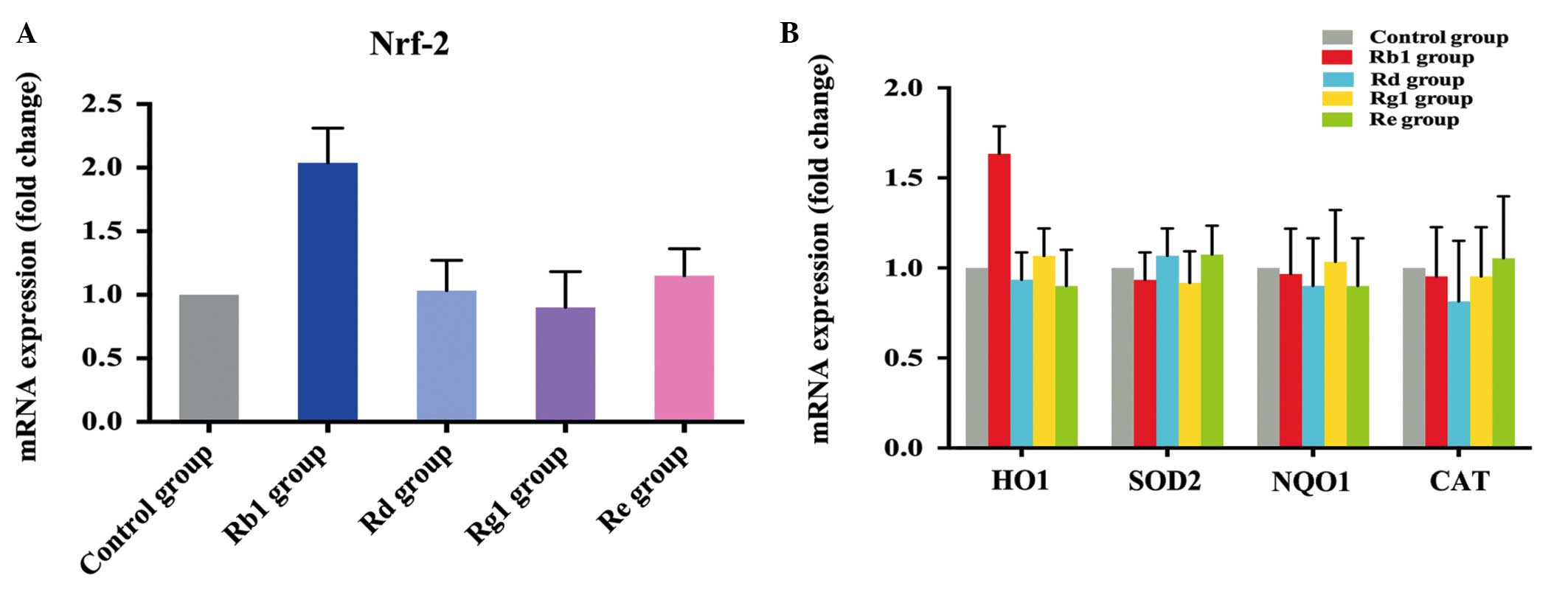 | Figure 4mRNA expression levels of Nrf2, HO-1,
SOD2, NQO1 and CAT in NPCs following pretreatment with Rb1, Rd, Rg1
and Re. (A) RT-qPCR analysis demonstrated that the expression of
Nrf2 was increased 2-fold in NPCs following pre-treatment with 10
µM Rb1 for 24 h, compared with the control, whereas no
changes in the expression of Nrf2 were observed following
pre-treatment with 10 µM Rd, 10 µM Rg1 or 10
µM Re. (B) RT-qPCR measurement of levels of HO-1, SOD2, NQO1
and CAT in NPCs following pre-treatment with 10 µM Rb1, 10
µM Rd, 10 µM Rg1 and 10 µM Re for 24 h,
followed by incubation with 300 µM t-BHP for 2.5 h.
Rb1 treatment significantly increased the expression of HO-1,
compared with the control group, but did not increase the
expression levels of SOD2, NQO1 or CAT. Rd, Rg1 and Re had no
effect on the expression levels of HO-1, SOD2, NQO1 or CAT in the
NPCs. NPCs, neural progenitor cells; t-BHP;
tert-Butylhydroperoxide; Nrf-2, nuclear factor
(erythroid-derived 2)-like 2; HO-1, heme oxygenase-1; SOD2,
superoxide dismutase 2; NQO1, NAD(P)H: quinone oxidoreductase 1;
CAT, catalase; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
Discussion
Ginseng is reported to have a wide range of
therapeutic and pharmacological applications, and has been widely
used to treat various diseases and improve health for thousands of
years in Asia (7). Accumulating
evidence has indicated that ginsenosides are the principle
pharmacologically active ingredients of ginseng. An increasing
number of studies are being performed to investigate purified
ginsenoside alone to examine the mechanism of function of ginseng,
rather than using whole ginseng root (30–35).
Each ginsenoside is suggested to have distinct effects in
pharmacology and distinct mechanisms due to their unique structures
(36). At present, ~40 ginsenoside
compounds have been identified, among which Rb1, Rd, Rg1 and Re are
the most commonly investigated ginsenosides due to their
quantitative abundance in ginseng root (9). The present study investigated and
compared the neuroprotective effects of four types of ginsenosides
on NPCs against oxidative stress. The results showed that only Rb1
exhibited a protective effect on the NPCs, whereas the Rd, Rg1 and
Re ginsenosides exhibited no protective effects towards NPCs under
oxidative stress.
Oxidative stress is defined as the general principle
of imbalance between the formation and detoxification of ROS. When
not sufficiently scavenged, these small molecules may cause DNA
damage, or mutations and lipid peroxidation, leading to membrane
damage (37). Substantial evidence
has indicated that oxidative stress is a major contributor to the
pathophysiology of a variety of neurodegenerative disorders,
including Alzheimer's disease, Parkinson's disease and acute CNS
injuries, including spinal cord injury and traumatic brain injury
(2,38,39).
Ginsenosides have been confirmed to exert protective effects,
attributed to their antioxidant ability through increasing internal
antioxidant enzymes and acting as a free-radical scavenger
(40–42). It has been suggested that the
administration of 100 or 200 mg/kg/day of ginsenosides through
drinking water improves memory loss in senescence accelerated
(SAMP8) mice, and increases serum antioxidant levels (43).
The association between the structure of ginsenoside
and its anti-oxidative or pro-oxidative activity has been
investigated in free radical-induced hemolysis of human
erythrocytes (44,45). The exact mechanisms underlying the
differences in protective effects of ginsenosides on NPCs against
oxidative stress remain to be elucidated. A number of studies have
suggested that Rb1 has beneficial effects in the treatment of
oxidative stress (46–51). The present study also demonstrated
that pretreatment with Rb1 significantly protected NPCs against
oxidative injury, and upregulated Nrf2 and its downstream
antioxidant-responsive gene, HO-1. It is generally considered that
the activation of the Nrf2 may further upregulate the transcription
of multiple antioxidant response element (ARE)-controlled genes,
and finally initiate the expression of a variety of antioxidant
enzymes and phase II drug-metabolizing enzymes (52,53).
HO-1, which belongs to the heat shock protein family, is an
inducible enzyme, which catalyzes the first and rate-limiting step
in oxidative degradation (54).
Evidence has indicated the critical role of HO-1 and its enzymatic
by-products in anti-inflammation, anti-oxidation, and more diverse
biological functions (55,56).
The mechanism underlying the response of the
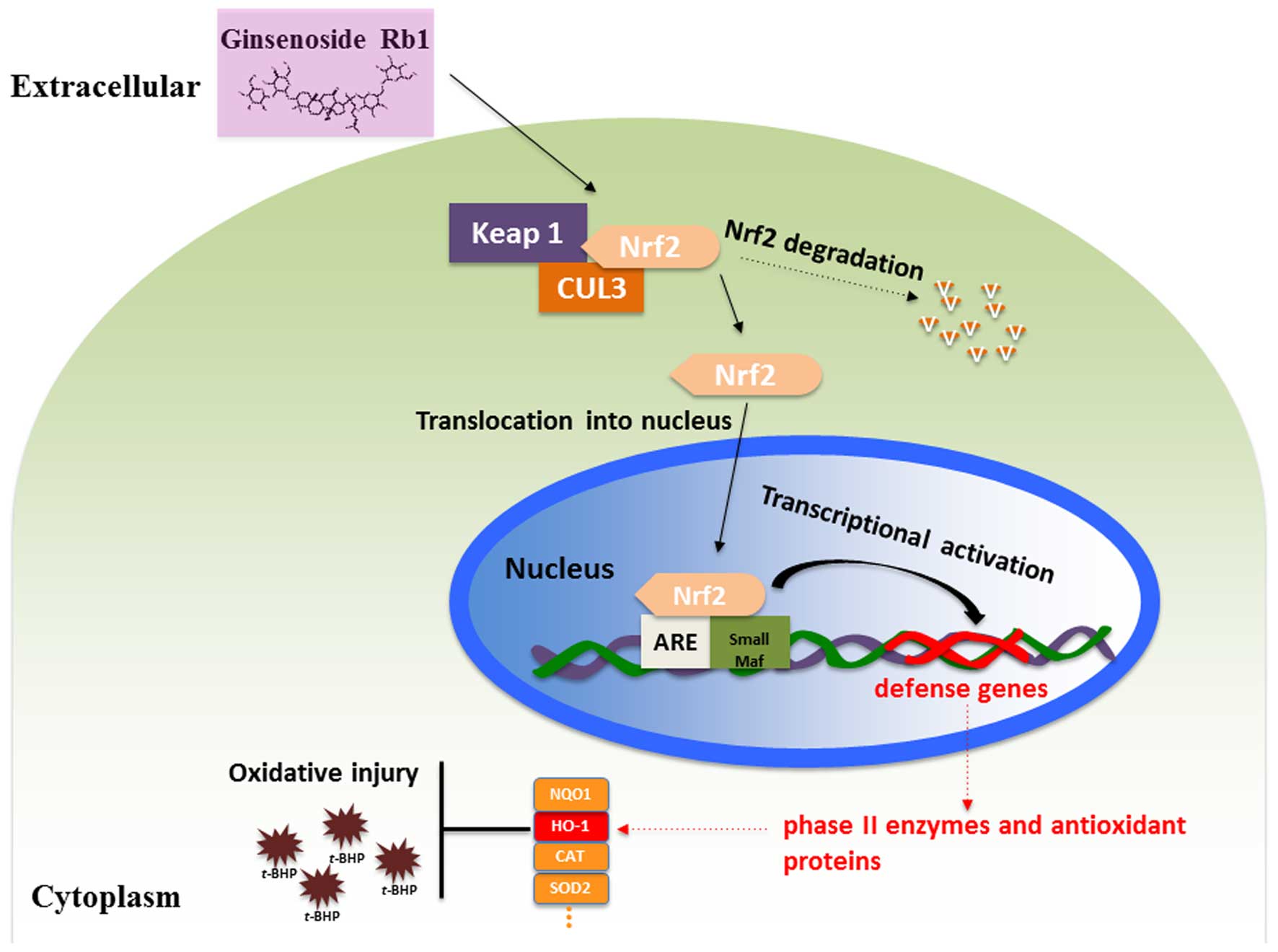
Nrf2/HO-1 signaling pathway to oxidative stress on NPCs by
pretreatment with Rb1 is shown in Fig.
5. Under homeostatic conditions, Nrf2 signaling is repressed by
Kelch-like ECH-associated protein 1 (Keap1), which has been
identified as a Cullin3-dependent substrate adaptor protein. Nrf2
is found to bind to Keap1 and be sequestered in the cytoplasm,
where it is ubiquitinated and subsequently degraded (57). When treated with ginsenoside Rb1,
Nrf2 is activated and triggered to translocate into the nucleus,
where it elicits a series of anti-oxidative responses. A complex,
which consists of Nrf2 protein, a group of small musculoaponeurotic
fibrosarcoma proteins and a cis-acting enhancer, ARE, is
then formed, which is essential for the anti-oxidative response to
cell injury induced by t-BHP by activating the transcription
of the downstream anti-oxidative gene, HO-1 (58,59).
The present study provided an overview on the
pharmacological activity of ginsenoside Rb1, Rd, Rg1 and Re, in
terms of the neuroprotective effects on NPCs against oxidative
injury. Only Rb1 was shown to have protective effects, by
activating Nrf2/HO-1 pathway, in an experimental model of oxidative
injury, whereas Rd, Rg1 and Re had no protective effects on the
NPCs against oxidative injury. Future investigations are warranted
to further examine the mechanisms underlying the protective actions
of ginsenoside Rb1 against oxidative injury, and to investigate the
therapeutic potential of Rb1 in animal models.
Acknowledgments
The present study was supported by a multi-year
research grant from the University of Macau [grant nos. MYRG122
(Y1-L3)-ICMS12-SHX and MYRG110 (Y1-L2)-ICMS13-SHX], a matching
project grant (grant no. MRG003/SHX/2014/ICMS) and the Macao
Science and Technology Development Fund (grant no.
018/2013/A1).
Abbreviations:
|
Keap1
|
kelch-like ECH-associated protein1
|
|
ARE
|
antioxidant response element
|
|
CAT
|
catalase
|
|
HO-1
|
heme oxygenase-1
|
|
LDH
|
lactate dehydrogenase
|
|
Nrf2
|
nuclear factor (erythroid-derived
2)-like 2
|
|
NQO1
|
NAD(P)H dehydrogenase (quinone 1)
|
|
SOD2
|
superoxide dismutase2
|
|
PLL
|
Poly-l-lysine
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
t-BHP
|
tert-Butylhydroperoxide
|
|
CNS
|
central nervous system
|
|
NPCs
|
neural progenitor cells
|
|
BrdU
|
5-Bromo-2-deoxyuridine
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick-end labeling
|
|
GFAP
|
glial fibrillary acidic protein
|
References
|
1
|
Pei JJ, Giron MS, Jia J and Wang HX:
Dementia studies in Chinese populations. Neurosci Bull. 30:207–216.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radi E, Formichi P, Battisti C and
Federico A: Apoptosis and oxidative stress in neurodegenerative
diseases. J Alzheimers Dis. 42(Suppl 3): S125–S152. 2014.PubMed/NCBI
|
|
3
|
Ghaffari H, Venkataramana M, Jalali
Ghassam B, Chandra Nayaka S, Nataraju A, Geetha NP and Prakash HS:
Rosmarinic acid mediated neuroprotective effects against HO-induced
neuronal cell damage in N2A cells. Life Sci. 113:7–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diao Y, Lin XM, Liao CL, Tang CZ, Chen ZJ
and Hu ZL: Authentication of Panax ginseng from its adulterants by
PCR-RFLP and ARMS. Planta Med. 75:557–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin JH, Leung FC, Fung Y, Zhu D and Lin B:
Rapid authentication of ginseng species using microchip
electrophoresis with laser-induced fluorescence detection. Anal
Bioanal Chem. 381:812–819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu JM, Yao Q and Chen C: Ginseng
compounds: An update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rausch WD, Liu S, Gille G and Radad K:
Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars).
66:369–375. 2006.
|
|
9
|
Liu ZQ: Chemical insights into ginseng as
a resource for natural antioxidants. Chem Rev. 112:3329–3355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ru W, Wang D, Xu Y, He X, Sun YE, Qian L,
Zhou X and Qin Y: Chemical constituents and bioactivities of Panax
ginseng (C. A. Mey.). Drug Discov Ther. 9:23–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YF, Fan XJ, Li X, Peng LL, Wang GH,
Ke KF and Jiang ZL: Ginsenoside Rg1 protects neurons from
hypoxic-ischemic injury possibly by inhibiting Ca2+ influx through
NMDA receptors and L-type voltage-dependent Ca2+ channels. Eur J
Pharmacol. 586:90–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni N, Liu Q, Ren H, Wu D, Luo C, Li P, Wan
JB and Su H: Ginsenoside Rb1 protects rat neural progenitor cells
against oxidative injury. Molecules. 19:3012–3024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Lu T, Yue X, Wei N, Jiang Y, Chen
M, Ni G, Liu X and Xu G: Neuroprotective effect of ginsenoside Rb1
on glutamate-induced neurotoxicity: With emphasis on autophagy.
Neurosci Lett. 482:264–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu
J, Ge P and Liang J: Inhibition of autophagy via activation of
PI3K/Akt pathway contributes to the protection of ginsenoside Rb1
against neuronal death caused by ischemic insults. Int J Mol Sci.
15:15426–15442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, He J, Huang L, Dou L, Wu S and Yuan
Q: Neuroprotective effects of ginsenoside Rb1 on hippocampal
neuronal injury and neurite outgrowth. Neural Regen Res. 9:943–950.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hashimoto R, Yu J, Koizumi H, Ouchi Y and
Okabe T: Ginsenoside Rb1 Prevents MPP(+)-Induced Apoptosis in PC12
Cells by Stimulating Estrogen Receptors with Consequent Activation
of ERK1/2, Akt and Inhibition of SAPK/JNK, p38 MAPK. Evid Based
Complement Alternat Med. 2012:6937172012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye R, Yang Q, Kong X, Han J, Zhang X,
Zhang Y, Li P, Liu J, Shi M, Xiong L and Zhao G: Ginsenoside Rd
attenuates early oxidative damage and sequential inflammatory
response after transient focal ischemia in rats. Neurochem Int.
58:391–398. 2011. View Article : Google Scholar
|
|
18
|
Leung KW, Yung KK, Mak NK, Chan YS, Fan TP
and Wong RN: Neuroprotective effects of ginsenoside-Rg1 in primary
nigral neurons against rotenone toxicity. Neuropharmacology.
52:827–835. 2007. View Article : Google Scholar
|
|
19
|
Fang F, Chen X, Huang T, Lue LF, Luddy JS
and Yan SS: Multi-faced neuroprotective effects of Ginsenoside Rg1
in an Alzheimer mouse model. Biochim Biophys Acta. 1822:286–292.
2012. View Article : Google Scholar :
|
|
20
|
Xu BB, Liu CQ, Gao X, Zhang WQ, Wang SW
and Cao YL: Possible mechanisms of the protection of ginsenoside Re
against MPTP-induced apoptosis in substantia nigra neurons of
Parkinson's disease mouse model. J Asian Nat Prod Res. 7:215–224.
2005. View Article : Google Scholar
|
|
21
|
Leipzig ND, Wylie RG, Kim H and Shoichet
MS: Differentiation of neural stem cells in three-dimensional
growth factor-immobilized chitosan hydrogel scaffolds.
Biomaterials. 32:57–64. 2011. View Article : Google Scholar
|
|
22
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez-Buylla A and Garcia-Verdugo JM:
Neurogenesis in adult subventricular zone. J Neurosci. 22:629–634.
2002.PubMed/NCBI
|
|
24
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su HX, Zhang W, Guo J, Guo A, Yuan Q and
Wu W: Neural progenitor cells enhance the survival and axonal
regeneration of injured motoneurons after transplantation into the
avulsed ventral horn of adult rats. J Neurotrauma. 26:67–80. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kučera O, Endlicher R, Roušar T, Lotková
H, Garnol T, Drahota Z and Cervinková Z: The effect of tert-butyl
hydroperoxide-induced oxidative stress on lean and steatotic rat
hepatocytes in vitro. Oxid Med Cell Longev. 2014:7525062014.
View Article : Google Scholar
|
|
27
|
Koh JY and Choi DW: Quantitative
determination of glutamate mediated cortical neuronal injury in
cell culture by lactate dehydrogenase efflux assay. J Neurosci
Methods. 20:83–90. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
29
|
Aueviriyavit S, Phummiratch D and
Maniratanachote R: Mechanistic study on the biological effects of
silver and gold nanoparticles in Caco-2 cells-induction of the
Nrf2/HO-1 pathway by high concentrations of silver nanoparticles.
Toxicol Lett. 224:73–83. 2014. View Article : Google Scholar
|
|
30
|
Buettner C, Yeh GY, Phillips RS, Mittleman
MA and Kaptchuk TJ: Systematic review of the effects of ginseng on
cardiovascular risk factors. Ann Pharmacother. 40:83–95. 2006.
View Article : Google Scholar
|
|
31
|
Gillis CN: Panax ginseng pharmacology: A
nitric oxide link? Biochem Pharmacol. 54:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hofseth LJ and Wargovich MJ: Inflammation,
cancer and targets of ginseng. J Nutr. 137(Suppl 1): 183S–185S.
2007.
|
|
33
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q
and Chen CJ: Molecular mechanisms and clinical applications of
ginseng root for cardiovascular disease. Med Sci Monit.
10:RA187–RA192. 2004.PubMed/NCBI
|
|
35
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Murthy HN, Georgiev MI, Kim YS, Jeong CS,
Kim SJ and Park SY: Ginsenosides: Prospective for sustainable
biotechnological production. Appl Microbiol Biotechnol.
98:6243–6254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alfadda AA and Sallam RM: Reactive oxygen
species in health and disease. J Biomed Biotechnol.
2012:9364862012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith JA, Park S, Krause JS and Banik NL:
Oxidative stress, DNA damage and the telomeric complex as
therapeutic targets in acute neurodegeneration. Neurochem Int.
62:764–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernández-Gajardo R, Matamala JM, Carrasco
R, Gutiérrez R, Melo R and Rodrigo R: Novel therapeutic strategies
for traumatic brain injury: Acute antioxidant reinforcement. CNS
Drugs. 28:229–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie JT, Shao ZH, Hoek TL, Chang WT, Li J,
Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ and Yuan CS:
Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J
Pharmacol. 532:201–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda
N, Peng H, Aburaya J, Ishihara K and Sakanaka M: Protection of
ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient
of ginseng root. Neurosci Res. 28:191–200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tian J, Fu F, Geng M, Jiang Y, Yang J,
Jiang W, Wang C and Liu K: Neuroprotective effect of
20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci Lett.
374:92–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao H, Li Q, Zhang Z, Pei X, Wang J and
Li Y: Long-term ginsenoside consumption prevents memory loss in
aged SAMP8 mice by decreasing oxidative stress and up-regulating
the plasticity-related proteins in hippocampus. Brain Res.
1256:111–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC
and Sun YX: In vitro study of the relationship between the
structure of ginsenoside and its antioxidative or prooxidative
activity in free radical induced hemolysis of human erythrocytes. J
Agric Food Chem. 51:2555–2558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu ZQ, Luo XY, Sun YX, Chen YP and Wang
ZC: Can ginsenosides protect human erythrocytes against
free-radical-induced hemolysis? Biochim Biophys Acta. 1572:58–66.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu D, Zhang H, Gu W, Liu Y and Zhang M:
Neuroprotective effects of ginsenoside Rb1 on high glucose-induced
neurotoxicity in primary cultured rat hippocampal neurons. PLoS
One. 8:e793992013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Z, Chen J, Huang W, Zeng Z, Yang Y and
Zhu B: Ginsenoside Rb1 protects rat retinal ganglion cells against
hypoxia and oxidative stress. Mol Med Rep. 8:1397–1403.
2013.PubMed/NCBI
|
|
48
|
Tan SJ, Yu Z and Dong QT: Effects of
ginsenoside Rb1 on the oxidative stress in the skeletal muscles of
rats with postoperative fatigue syndrome. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 32:1535–1538. 2012.In Chinese.
|
|
49
|
Wu Y, Xia ZY, Dou J, Zhang L, Xu JJ, Zhao
B, Lei S and Liu HM: Protective effect of ginsenoside Rb1 against
myocardial ischemia/reperfusion injury in streptozotocin-induced
diabetic rats. Mol Biol Rep. 38:4327–4335. 2011. View Article : Google Scholar
|
|
50
|
Migliore L and Coppedè F:
Environmental-induced oxidative stress in neurodegenerative
disorders and aging. Mutat Res. 674:73–84. 2009. View Article : Google Scholar
|
|
51
|
Li J, O W, Li W, Jiang ZG and Ghanbari HA:
Oxidative stress and neurodegenerative disorders. Int J Mol Sci.
14:24438–24475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xie Y, Zhao QY, Li HY, Zhou X, Liu Y and
Zhang H: Curcumin ameliorates cognitive deficits heavy ion
irradiation-induced learning and memory deficits through enhancing
of Nrf2 antioxidant signaling pathways. Pharmacol Biochem Behav.
126:181–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li W and Kong AN: Molecular mechanisms of
Nrf2-mediated antioxidant response. Mol Carcinog. 48:91–104. 2009.
View Article : Google Scholar :
|
|
54
|
Li B, Choi HJ, Lee DS, Oh H, Kim YC, Moon
JY, Park WH, Park SD and Kim JE: Amomum tsao-ko Suppresses
lipopoly-saccharide-induced inflammatory responses in RAW264.7
macrophages via Nrf2-dependent heme oxygenase-1 expression. Am J
Chin Med. 42:1229–1244. 2014. View Article : Google Scholar
|
|
55
|
Was H, Dulak J and Jozkowicz A: Heme
oxygenase-1 in tumor biology and therapy. Curr Drug Targets.
11:1551–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu ML, Ho YC, Lin CY and Yet SF: Heme
oxygenase-1 in inflammation and cardiovascular disease. Am J
Cardiovasc Dis. 1:150–158. 2011.
|
|
57
|
Tkachev VO, Menshchikova EB and Zenkov NK:
Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry
(Mosc). 76:407–422. 2011. View Article : Google Scholar
|
|
58
|
Zenkov NK, Menshchikova EB and Tkachev VO:
Keap1/Nrf2/ARE redox-sensitive signaling system as a
pharmacological target. Biochemistry (Mosc). 78:19–36. 2013.
View Article : Google Scholar
|
|
59
|
Dinkova-Kostova AT, Holtzclaw WD and
Kensler TW: The role of Keap1 in cellular protective responses.
Chem Res Toxicol. 18:1779–1791. 2005. View Article : Google Scholar : PubMed/NCBI
|