Introduction
Acute liver failure (ALF), which is characterized by
coagulopathy and encephalopathy, is associated with a high
mortality rate (1,2). ALF may be induced by alcohol, viral
hepatitis, bacteria or hepatotoxic drugs (3), and there is currently no available
therapy for ALF other than liver transplantation (4).
D-galactosamine (D-GalN) and lipopolysaccharide
(LPS) are often used to generate hepatitis test models (5). Animal models are used in
hepatoprotective drug screening, and to elucidate the mechanisms
underlying clinical liver dysfunction (6). D-GalN induces a loss of uridine
triphosphate via the galactose pathway, and inhibits RNA and
protein synthesis (7), thus
resulting in hepatic necrosis and apoptosis due to metabolic
changes (8). LPS activates liver
macrophages, which secrete diverse proinflammatory cytokines, thus
inducing hepatic necrosis and reducing the production of
antioxidant enzymes (9).
The D-GalN/LPS hepatotoxic model induces
inflammatory reactions and oxidative stress within the liver
(10,11), due to increased inflammation and
expression of inducible nitric oxide synthase (iNOS) and
cyclooxygenase-2 (COX-2) (12).
iNOS has an important role in drug-induced liver injury (13), and COX-2 has an essential role in
D-GalN/LPS-induced inflammation (14). Inflammation leads to the production
of reactive oxygen species (ROS), including
H2O2, O2− and
OH− (15). ROS attack
polyunsaturated fatty acids in the cell membrane via lipid
peroxidation, and trigger various pathological states, including
oxidative stress (16).
Mitogen-activated protein kinases (MAPKs) comprise
three major proteins: C-jun NH2-terminal kinase (JNK),
p38 MAPK and extracellular signal-regulated kinase (ERK).
Phosphorylated MAPK proteins have various roles in oxidative stress
and inflammatory diseases (17).
In particular, activated JNK has an important role in hepatic
injury via activation of the caspase cascade and induction of liver
cell necrosis (18).
Pyropia yezoensis is a type of red algae,
which has long been considered an important food source in Korea,
Japan and China (19). Various
previous studies have demonstrated the therapeutic effects of P.
yezoensis, including chemoprotec-tive (20), anticancer (21) and anti-inflammatory activities
(22). There are currently no
studies regarding the antioxidative activities of P.
yezoensis glycoprotein (PYGP) against D-GalN/LPS-induced
hepatotoxicity. The present study aimed to investigate the
anti-inflammatory effects of PYGP against D-GalN/LPS in
vivo.
Materials and methods
Preparation of PYGP
P. yezoensis was purchased in 2014 (Suhyup,
Seoul, South Korea). P. yezoensis powder (40 g) was diluted
with 1 L distilled water and stirred for 4 h at room temperature.
The solution was then centrifuged at 3,000 × g and 4°C for 20 min,
and vacuum filtered. Triple the volume of ethanol was added to the
solution (total quantity of filtrate x 3). After 24 h, the solution
was filtered and concentrated using rotary evaporation at 40°C. The
concentrated solution was divided into 1.5 ml tubes, freeze-dried,
and stored at −70°C until further use.
Experimental animals
Male Sprague-Dawley rats (6 weeks old) were
purchased from Samtaco (Osan, South Korea). Animal studies were
conducted in accordance with the Animal Ethics Committee of the
Pukyong National University (Busan, South Korea). The rats were
maintained in the following laboratory conditions: 23±3°C, 12 h
light/12 h dark cycle and 50% humidity, with ad libitum
access to food and water.
Experimental design
The rats were randomly divided into four groups
(n=5/group): Group 1, control rats received distilled water only;
group 2, rats received 500 mg/kg/body weight (BW) D-GalN + 10
µg/kg/BW LPS; group 3, rats received 500 mg/kg/BW D-GalN +
10 µg/kg/BW LPS + 150 mg/kg/BW PYGP; and group 4, rats
received 500 mg/kg/BW D-GalN + 10 µg/kg/BW LPS + 300
mg/kg/BW PYGP. PYGP was administered orally once a day for 7 days.
Hepatotoxicity was induced in the rats by intraperitoneal injection
of D-GalN/LPS (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 500
mg/kg/BW D-GalN and 10 µg/kg/BW LPS. The rats were
sacrificed under mild ether anesthesia (Duksan Pure Chemicals Co.,
Ltd., Ansan, South Korea) by decapitation for blood and liver
sample collection 6 h after induction of hepatotoxicity.
GOT/GPT measurement
The blood samples were centrifuged at 3,000 × g for
20 min at 4° to collect serum and stored at −20°C until analysis.
The activities of glutamic-oxaloacetic transaminase (GOT) and
glutamic-pyruvic transaminase (GPT) in the serum samples were
determined using an enzymatic analysis kit (Asan Pharmaceuticals,
Hwasung, South Korea), according to the manufacturer's protocols.
The absorbance was measured at 505 nm using a microplate reader
(Benchmark Plus 10730; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Lipid peroxidation measurements
The liver tissues were added to 1X butyl hydroxyl
toluene (Cell Biolabs, Inc., San Diego, CA, USA) and homogenized on
ice at 10,000 × g for 5 min to collect the supernatant. According
to the Thiobarbituric Acid Reactive Substances (TBARS) Assay kit
protocol (Cell Biolabs), 100 µl of sample or malondialdehyde
(MDA) standard was added to microcentrifuge tubes and then 100
µl SDS lysis solution was added, mixed thoroughly, incubated
for 5 min at room temperature, and 250 µl of TBA reagent
added. Each tube was closed, incubated at 95°C for 60 min, removed
and then cooled to room temperature in an ice bath for 5 min. All
the sample tubes were centrifuged at 842 x g for 15 min, the
supernatant removed, 200 µl was transferred, along with 200
µl of MDA standard, to a 96-well microplate compatible with
a microplate reader (Benchmark Plus 10730) and the absorbance read
at 532 nm.
Antioxidant enzyme measurements
Antioxidant enzyme activities, including catalase
(CAT), glutathione (GSH) and glutathione S-transferase (GST), were
measured in the liver samples using appropriate kits, according to
the manufacturer's protocols (Catalase Assay kit, Glutathione Assay
kit and Glutathione S-Transferase Assay kit; all Cayman Chemical
Company, Ann Arbor, MI, USA). The absorbance was measured using a
microplate reader (Benchmark Plus 10730).
Western blot analysis
Liver tissue samples were homogenized in lysis
buffer [150 mM sodium chloride, 50 mM Tris-HCl (pH 7.5), 0.5%
sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1% Triton
X-100 and 2 mM ethylenediaminetetra-acetic acid; Intron
Biotechnology, Inc., Seongnam, South Korea] containing inhibitors
(1 mM Na3 VO4, 1 µg/ml aprotinin, 1
µg/ml leupeptin, 1 µg/ml pepstatin A and 1 mM
phenylmethylsulfonyl fluoride; Sigma-Aldrich). Protein
concentration was determined using the Bichinchoninic Acid Assay
kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Equal protein
quantities (20 µg) from each sample were separated by 10–15%
SDS-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 1% bovine serum albumin (BSA)
in TBST [10 mM Tris-HCl (pH 7.5), 150 mM NaCl and 0.1% Tween 20;
USB Corporation, Cleveland, OH, USA]. Subsequently, the membrane
was incubated for 4 h at room temperature with the following
primary immunoglobulin G antibodies, diluted to 1:1,000 in
BSA/TBST: Rabbit anti-rat ERK polyclonal antibody (cat. no. sc-94),
rabbit anti-rat phosphorylated (p)-ERK polyclonal antibody (cat.
no. sc-7383), mouse anti-rat JNK monoclonal antibody (cat. no.
sc-7345), mouse anti-rat p-JNK monoclonal antibody (cat. no.
sc-6254), rabbit anti-rat p38 polyclonal antibody (cat. no.
sc-7149), mouse anti-rat p-p38 monoclonal antibody (cat. no.
sc-7973), mouse anti-rat iNOS polyclonal antibody (cat. no.
sc-650), goat anti-rat COX-2 polyclonal antibody (cat. no. sc-1745)
and rabbit anti- rat GAPDH polyclonal antibody which served as a
loading control (cat. no. sc-25778; all Santa Cruz Biotechnology
Inc., Dallas, TX, USA). The membrane was then incubated with
peroxidase-conjugated anti-goat (cat. no. 81-1620), anti-mouse
(cat. no. 62-6520) and anti-rabbit (cat. no. 65-6120) secondary
antibodies (1:10,000; Bethyl Laboratories, Inc., Montgomery, TX,
USA) for 1 h at room temperature. Antibody binding was visualized
using the Super Signal West Pico Stable Peroxide solution and the
Super Signal West Pico Luminol/Enhancer solution (Thermo Fisher
Scientific, Inc., Rockford, IL, USA). The signal was developed on
Kodak X-ray film (Kodak, Rochester, NY, USA) using a developer and
fixer twin pack (Kodak).
Statistical analysis
The results of the present study are presented as
the mean ± standard deviation. Data were analyzed using SPSS
version 10.0 software (SPSS, Inc., Chicago, IL, USA). Results were
validated using analysis of variance and Duncan's multiple range
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
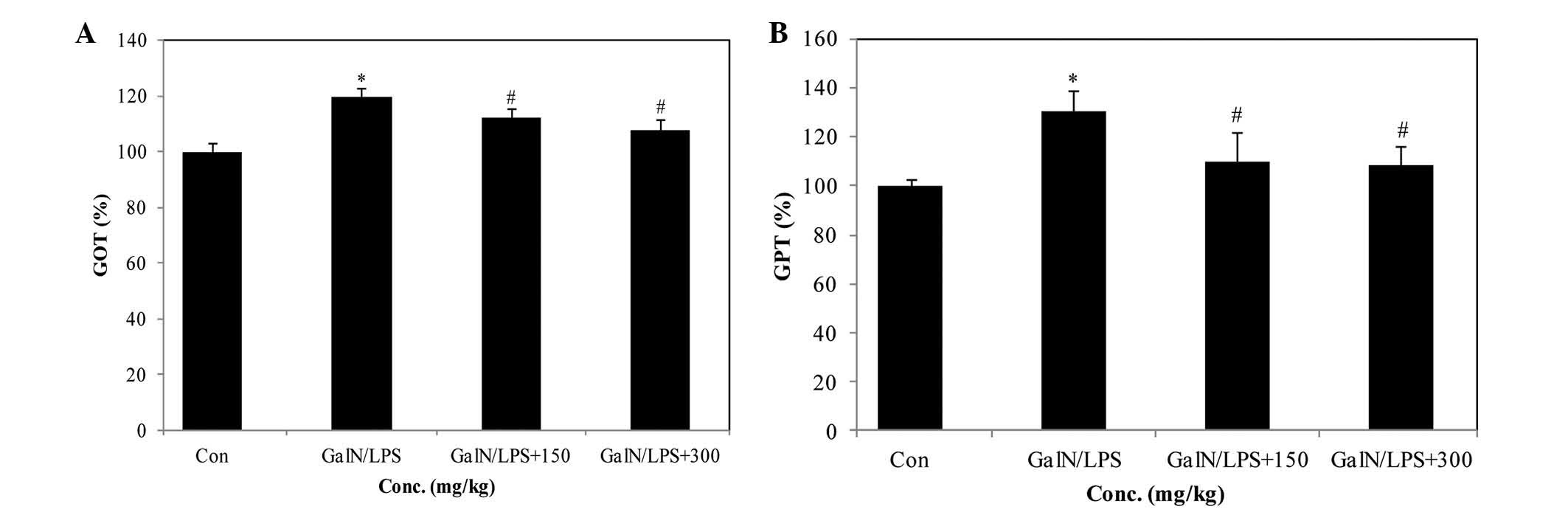
PYGP suppresses GOT and GPT levels in the
serum of D-GalN/LPS-treated rats
GOT and GPT serum levels are important indicators of
liver function (23). Injection
with D-GalN/LPS elevated the levels of GOT and GPT; however,
treatment with 300 mg/kg/BW PYGP significantly reduced these levels
(Fig. 1A and B).
Effects of PYGP on D-GalN/LPS-induced
oxidative stress and antioxidant enzyme activity
A total of 6 h post-D-GalN/LPS injection, the TBARS
levels were determined, which indicate liver tissue lipid
peroxidation. As shown in Fig. 2A,
TBARS increased significantly following treatment with D-GalN/LPS.
Conversely, TBARS levels in the D-GalN/LPS+PYGP 150 and
D-GalN/LPS+PYGP 300 groups were markedly decreased. Furthermore,
antioxidant enzyme activities were markedly decreased in the
D-GalN/LPS group, as compared with in the control group. CAT levels
were decreased by ~20%, as compared with the control group. In
addition, GST and GSH levels were decreased following D-GalN/LPS
treatment, however, levels were restored to control group levels
following treatment with PYGP (Fig.
2B–D).
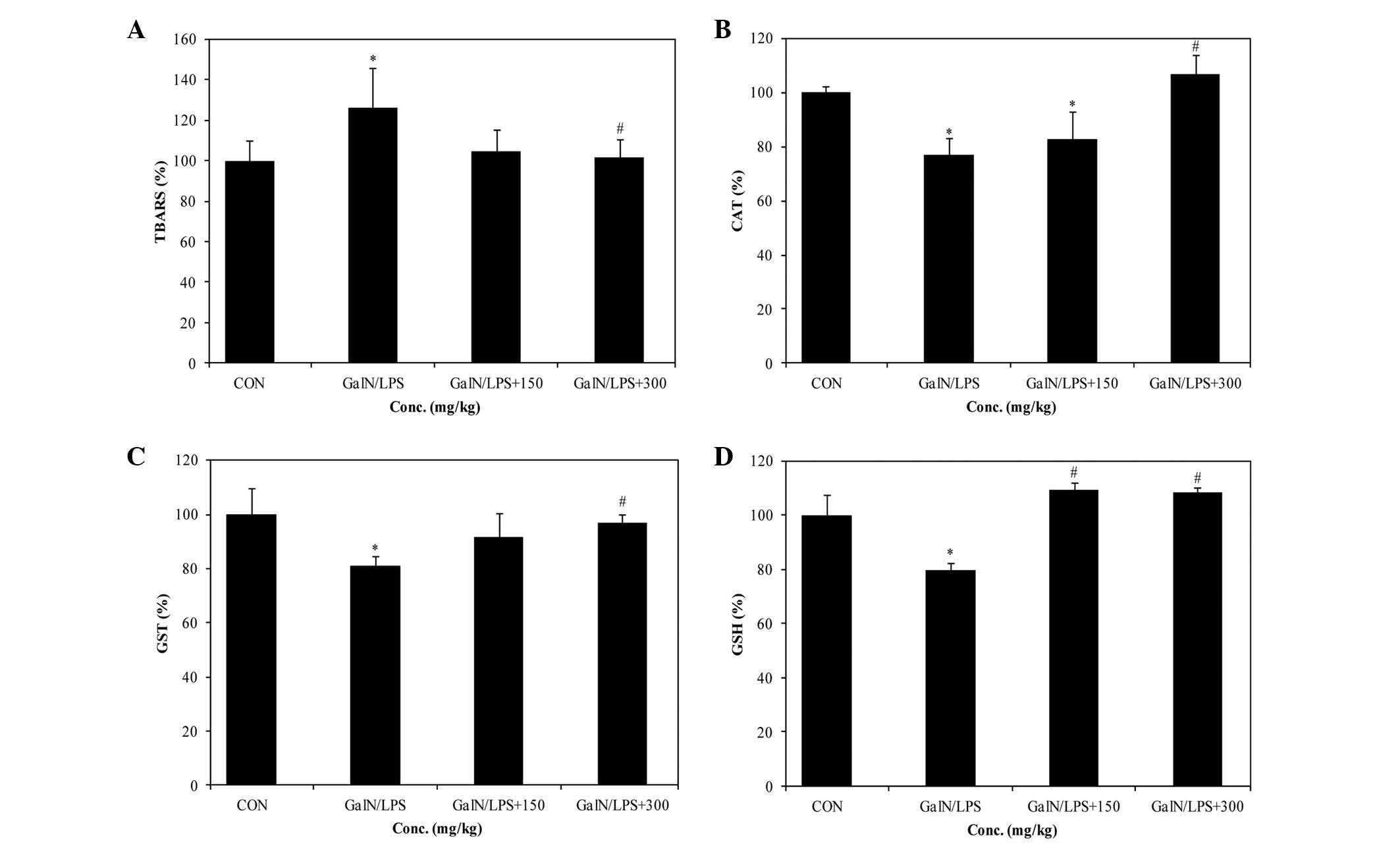 | Figure 2Levels of TBARS and antioxidant enzyme
activity (CAT, GST and GSH) in the livers of control and
experimental rat groups. (A) TBARS, (B) CAT, (C) GST and (D) GSH.
Data are presented as the mean ± standard deviation.
*P<0.05 vs. the control group, #P<0.05
vs. the GaIN/LPS group. GalN, D-galactosamine; LPS,
lipopolysaccharide; 150, 150 mg/kg/body weight PYGP; 300, 300
mg/kg/body weight PYGP; PYGP, Pyropia yezoensis
glycoprotein; TBARS, thiobarbituric acid reactive substances; CAT,
catalase; GST, glutathione S-transferase; GSH, glutathione; Con,
control. |
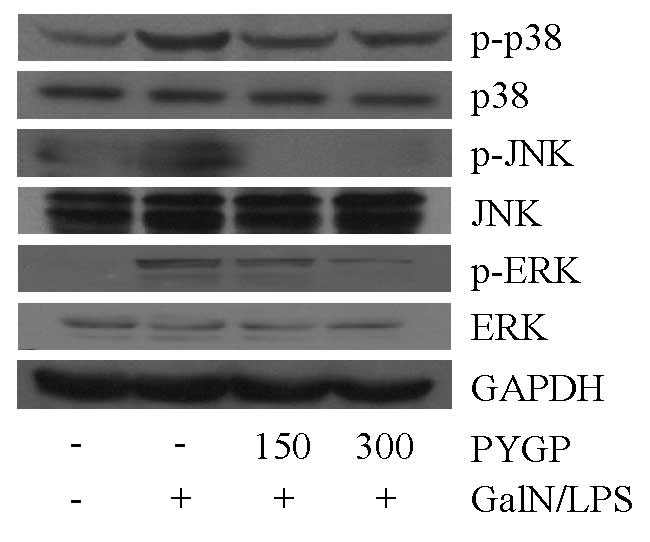
PYGP suppresses D-GalN/LPS-induced MAPK
phosphorylation
To investigate whether PYGP was able to modulate
MAPK signaling, MAPK protein expression and phosphorylation levels
were detected by western blot analysis. The protein expression
levels of ERK, JNK and p38 did not differ between the groups.
However, the phosphorylation of these proteins increased in the
D-GalN/LPS-treated group, as compared with in the control group. In
the D-GalN/LPS + PYGP co-treated groups, the phosphorylation levels
of these proteins were downregulated (Fig. 3). These results suggest that PYGP
may inhibit D-GalN/LPS-induced MAPK phosphorylation.
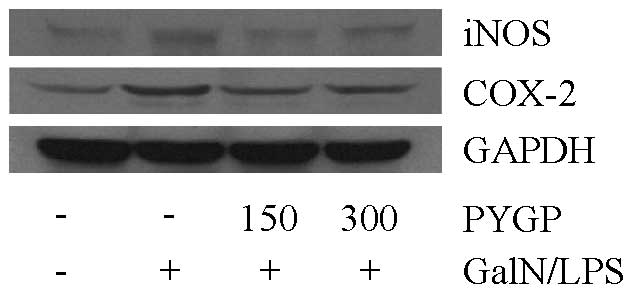
Effects of PYGP on iNOS and COX-2 protein
expression
To confirm the effects of PYGP on inflammation in
the rat liver, D-GalN/LPS-induced iNOS and COX-2 protein expression
levels were detected. Following treatment with D-GalN/LPS, the
protein expression levels were markedly increased; however,
treatment with PYGP prior to injection with D-GalN/LPS inhibited
D-GalN/LPS-induced iNOS and COX-2 protein expression (Fig. 4). These results indicate that PYGP
has an important role in the suppression of D-GalN/LPS-induced iNOS
and COX-2 protein expression.
Discussion
Treating rats with a combination of D-GalN and LPS
is widely used in studies researching the mechanisms underlying
human ALF (24). D-GalN and LPS
co-treatment induces greater critical hepatic damage, accompanied
by apoptotic and necrotic changes in the liver, which closely
resembles human viral hepatitis (25,26).
In the present study, administration of D-GalN/LPS increased GOT
and GPT serum levels; however, oral administration of PYGP
attenuated these levels. These results suggested that D-GalN/LPS
was able to induce severe damage to hepatic membranous tissues, and
PYGP may prevent this hepatotoxicity.
D-GalN/LPS hepatotoxicity induces ROS production and
reduces antioxidant enzyme activity in the liver (27). Furthermore, ROS may cause cell
membrane lipid peroxi-dation (28). Oxidative stress is a well-known
factor in D-GalN/LPS-induced liver injury. Increased TBARS and
conjugated dienes have previously been detected following treatment
with D-GalN/LPS (29). In the
present study, the levels of TBARS increased in response to
D-GalN/LPS treatment; however, co-treatment with D-GalN/LPS and
PYGP suppressed hepatic TBARS levels. Antioxidant enzymes,
including CAT, GST and GSH are important in D-GalN/LPS
hepatotoxicity. CAT catalyzes the dismutation reaction of
H2O2, resulting in the formation of
H2O and O2 (26). GSH is a substrate of GST, and GST
catalyzes the conjugation of GSH with drugs and chemicals (30). In the present study, treatment with
D-GalN/LPS significantly reduced the activities of CAT, GST and
GSH, as compared with in the control group. Conversely, increased
CAT and GST activities, and GSH levels were detected following PYGP
treatment. These results suggested that PYGP exerts antioxidative
effects against D-GalN/LPS-induced liver injury.
MAPKs comprise ERK, JNK and p38 proteins, which are
phosphorylated by D-GalN/LPS (6).
These proteins are involved in cell proliferation, differentiation,
metabolism, survival and apoptosis (31). In particular, these proteins
regulate cytokine production, and the expression of tumor necrosis
factor-α and transcription factors (32,33).
In the present study, treatment with PYGP significantly suppressed
the GalN/LPS-induced phosphorylation of ERK, JNK and p38. These
results indicated that GalN/LPS and PYGP co-treatment may reduce
MAPK phosphorylation.
Inflammation occurs via various biological pathways.
Nitric oxide (NO) production occurs via the iNOS pathway (34); in the cell, increased iNOS protein
expression produces large amounts of NO, which increases the
prevalence of inflammation (35).
In addition, overexpression of NO induces hepatic dysfunction and
hepatotoxicity (36). COX-2 is
associated with the patho-physiology of inflammatory dysfunction,
and the production of prostaglandins and thromboxanes (37), which may lead to hepatic injury
(12). In the present study, PYGP
pretreatment inhibited GalN/LPS-induced iNOS and COX-2
overexpression.
In conclusion, the present study demonstrated that
PYGP may exert protective effects against D-GalN/LPS-induced ALF
via inhibition of MAPK phosphorylation and iNOS and COX-2
expression. In addition, PYGP increased the activity of antioxidant
enzymes.
Acknowledgments
The present study was supported by the Fishery
Commercialization Technology Development Program through the Korean
Institute of Planning and Evaluation of Technology in Food,
Agriculture, Forestry and Fisheries (iPET) funded by the Ministry
of Oceans and Fisheries (grant no. 2012300734).
References
|
1
|
Zhang L, Kang W, Lei Y, Han Q, Zhang G, Lv
Y, Li Z, Lou S and Liu Z: Granulocyte colony-stimulating factor
treatment ameliorates liver injury and improves survival in rats
with D-galactosamine-induced acute liver failure. Toxicol Lett.
204:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gunning K: Hepatic failure. Anaesthesia
& Intensive Care Medicine. 10:124–126. 2009. View Article : Google Scholar
|
|
3
|
Lee WM: Acute liver failure. Semin Respir
Crit Care Med. 33:36–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumoto K, Mizumoto H, Nakazawa K, Ijima
H, Funatsu K and Kajiwara T: Hepatic differentiation of mouse
embryonic stem cells in a three-dimensional culture system using
polyurethane foam. J Biosci Bioeng. 105:350–354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakama T, Hirono S, Moriuchi A, Hasuike S,
Nagata K, Hori T, Ido A, Hayashi K and Tsubouchi H: Etoposide
prevents apoptosis in mouse liver with
D-galactosamine/lipopolysaccharide-induced fulminant hepatic
failure resulting in reduction of lethality. Hepatology.
33:1441–1450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Ren F, Zhang H, Wen T, Piao Z,
Zhou L, Zheng S, Zhang J, Chen Y, Han Y, et al: Inhibition of
glycogen synthase kinase 3β ameliorates D-GalN/LPS-induced liver
injury by reducing endoplasmic reticulum stress-triggered
apoptosis. PLoS One. 7:e452022012. View Article : Google Scholar
|
|
7
|
Wang Y, Gao LN, Cui YL and Jiang HL:
Protective effect of Danhong injection on acute hepatic failure
induced by lipo-polysaccharide and D-galactosamine in mice. Evid
Based Complement Alternat Med. 2014:1539022014. View Article : Google Scholar
|
|
8
|
Wilhelm EA, Jesse CR, Roman SS, Nogueira
CW and Savegnago L: Hepatoprotective effect of 3-alkynyl
selenophene on acute liver injury induced by D-galactosamine and
lipopoly-saccharide. Exp Mol Pathol. 87:20–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong YI, Jung ID, Lee CM, Chang JH, Chun
SH, Noh KT, Jeong SK, Shin YK, Lee WS, Kang MS, et al: The novel
role of platelet-activating factor in protecting mice against
lipopoly-saccharide-induced endotoxic shock. PLoS One. 4:e65032009.
View Article : Google Scholar
|
|
10
|
Jin Q, Jiang S, Wu YL, Bai T, Yang Y, Jin
X, Lian LH and Nan JX: Hepatoprotective effect of cryptotanshinone
from Salvia milt- iorrhiza in
D-galactosamine/lipopolysaccharide-induced fulminant hepatic
failure. Phytomedicine. 21:141–147. 2014. View Article : Google Scholar
|
|
11
|
Wei L, Ren F, Zhang X, Wen T, Shi H, Zheng
S, Zhang J, Chen Y, Han Y and Duan Z: Oxidative stress promotes
D-GalN/LPS-induced acute hepatotoxicity by increasing glycogen
synthase kinase 3β activity. Inflamm Res. 63:485–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang CC, Lin KJ, Cheng YW, Hsu CA, Yang
SS and Shyur LF: Hepatoprotective effect and mechanistic insights
of deoxyele-phantopin, a phyto-sesquiterpene lactone, against
fulminant hepatitis. J Nutr Biochem. 24:516–530. 2013. View Article : Google Scholar
|
|
13
|
Wen T, Wu ZM, Liu Y, Tan YF, Ren F and Wu
H: Upregulation of heme oxygenase-1 with hemin prevents
D-galactosamine and lipopolysaccharide-induced acute hepatic injury
in rats. Toxicology. 237:184–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liong EC, Xiao J, Lau TY, Nanji AA and
Tipoe GL: Cyclooxygenase inhibitors protect
D-galactosamine/lipopoly-saccharide induced acute hepatic injury in
experimental mice model. Food Chem Toxicol. 50:861–866. 2012.
View Article : Google Scholar
|
|
15
|
Jaeschke H: Reactive oxygen and mechanisms
of inflammatory liver injury. J Gastroenterol Hepatol. 15:718–724.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaeschke H: Reactive oxygen and mechanisms
of inflammatory liver injury: Present concepts. J Gastroenterol
Hepatol. 26(Suppl 1): 173–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lian LH, Wu YL, Wan Y, Li X, Xie WX and
Nan JX: Anti-apoptotic activity of gentiopicroside in
D-galactosamine/lipopolysac-charide-induced murine fulminant
hepatic failure. Chem Biol Interact. 188:127–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wullaert A, Heyninck K and Beyaert R:
Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK
activation in hepa-tocytes. Biochem Pharmacol. 72:1090–1101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Kim HC, Vitek L and Nam CM: Algae
consumption and risk of type 2 diabetes: Korean National Health And
Nutrition Examination Survey in 2005. J Nutr Sci Vitaminol (Tokyo).
56:13–18. 2010. View Article : Google Scholar
|
|
20
|
Choi YH, Kim EY, Mikami K and Nam TJ:
Chemoprotective effects of a recombinant protein from Pyropia
yezoensis and synthetic peptide against acetaminophen-induced Chang
liver cell death. Int J Mol Med. 36:369–376. 2015.PubMed/NCBI
|
|
21
|
Zhang LX, Cai CE, Guo TT, Gu JW, Xu HL,
Zhou Y, Wang Y, Liu CC and He PM: Anti-cancer effects of
polysaccharide and phycocyanin from Porphyra yezoensis. J Mar Sci
Technol. 19:377–382. 2011.
|
|
22
|
Shin ES, Hwang HJ, Kim IH and Nam TJ: A
glycoprotein from Porphyra yezoensis produces anti-inflammatory
effects in liposaccharide-stimulated macrophages via the TLR4
signaling pathway. Int J Mol Med. 28:809–815. 2011.PubMed/NCBI
|
|
23
|
Maiti R, Jana D, Das UK and Ghosh D:
Antidiabetic effect of aqueous extract of seed of Tamarindus indica
in streptozotocin-induced diabetic rats. J Ethnopharmacol.
92:85–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gilani AH, Yaeesh S, Jamal Q and Ghayur
MN: Hepatoprotective activity of aqueous-methanol extract of
Artemisia vulgaris. Phytother Res. 19:170–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu LL, Gong LK, Wang H, Xiao Y, Wu XF,
Zhang YH, Xue X, Qi XM and Ren J: Baicalin inhibits macrophage
activation by lipopolysaccharide and protects mice from endotoxin
shock. Biochem Pharmacol. 75:914–922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vimal V and Devaki T: Hepatoprotective
effect of allicin on tissue defense system in
galactosamine/endotoxin challenged rats. J Ethnopharmacol.
90:151–154. 2004. View Article : Google Scholar
|
|
27
|
Wang H, Xu DX, Lv JW, Ning H and Wei W:
Melatonin attenuates lipopolysaccharide (LPS)-induced apoptotic
liver damage in d-galactosamine-sensitized mice. Toxicology.
237:49–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bindhumol V, Chitra KC and Mathur PP:
Bisphenol A induces reactive oxygen species generation in the liver
of male rats. Toxicology. 188:117–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lekić N, Cerný D, Hořínek A, Provazník Z,
Martínek J and Farghali H: Differential oxidative stress responses
to D-galactosamine/lipopolysaccharide hepatotoxicity based on real
time PCR analysis of selected oxidant/antioxidant and apoptotic
gene expressions in rat. Physiol Res. 60:549–558. 2011.
|
|
30
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
32
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishizaki M, Kaibori M, Uchida Y, Hijikawa
T, Tanaka H, Ozaki T, Tokuhara K, Matsui K, Kwon AH, Kamiyama Y, et
al: Protective effect of FR183998, a Na+/H+
exchanger inhibitor, and its inhibition of iNOS induction in
hepatic ischemia-reperfusion injury in rats. Shock. 30:311–317.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia X, Su C, Fu J, Zhang P, Jiang X, Xu D,
Hu L, Song E and Song Y: Role of α-lipoic acid in LPS/d-GalN
induced fulminant hepatic failure in mice: Studies on oxidative
stress, inflammation and apoptosis. Int Immunopharmacol.
22:293–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li R, Yuan C, Dong C, Shunang S and Choi
MM: In vivo anti-oxidative effect of isoquercitrin on
cadmium-induced oxidative damage to mouse liver and kidney. Naunyn
Schmiedebergs Arch Pharmacol. 383:437–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Serhan CN and Oliw E: Unorthodox routes to
prostanoid formation: New twists in cyclooxygenase-initiated
pathways. J Clin Invest. 107:1481–1489. 2001. View Article : Google Scholar : PubMed/NCBI
|