Introduction
Metastasis is the most common cause of cervical
cancer-associated mortality. The American Cancer Society estimated
that in 2015, ~12,900 novel cases and 4,100 fatalities will result
from cervical cancer in the United States (1). Screening and prevention programs have
contributed to declining mortality rates, and surgical resection is
available for the treatment of early stage tumors; however, the
current prognosis for patients with cervical cancer remains poor,
due to high rates of tumor metastasis (2–4).
Improved understanding regarding the precise molecular mechanisms
underlying metastasis remains a challenge in cancer research, in
addition to identifying key targets against which therapeutic
strategies may be directed.
Tumor metastasis is a complex, multistep process
that includes dysregulation of tumor cell adhesion, cytoskeletal
remodeling, and invasion of the surrounding organs or tissues. An
essential factor of this morphogenetic transformation is referred
to as epithelial-mesenchymal transition (EMT). During EMT,
epithelial cells actively lose their cell-cell adhesive capacity
and polarity, and acquire highly motile mesenchymal
characteristics, as well as reduced intracellular interactions,
which are essential for metastasis (5,6).
Notably, EMT is considered a crucial event that contributes to
cancer progression, due to its ability to facilitate invasion and
metastasis. Numerous studies have reported the importance of EMT,
particularly with regards to the cell junction molecule E-cadherin,
which can be functionally overexpressed in order to establish
stable intracellular adhesion; therefore, loss of E-cadherin
expression is considered a hallmark of EMT. Loss of E-cadherin
expression and gain of vimentin expression (another marker of EMT)
are common alterations that occur during human cancer invasion and
metastasis (7,8).
Various molecular processes are associated with the
initiation and progression of EMT, including alterations in the
expression of specific microRNAs (miRNAs). miRNAs are an abundant
class of endogenous, small, non-coding regulatory RNA molecules,
which are 17-25 nucleotides long. It is widely accepted that miRNAs
regulate gene expression by recog-nizing imperfect complementary
sites in the 3′-untranslated regions (3′-UTR) of target mRNA, which
may lead to suppression of the target gene expression, or mRNA
degradation (9). Previous studies
have demonstrated that miRNAs have roles in almost all aspects of
cancer biology, including invasion and metastasis (10–12).
The human miRNA (miR)-200 family comprises five members: miR-200a,
miR-200b, miR-200c, miR-429 and miR-141, which are able to regulate
EMT by targeting the E-box-binding transcription factors, zinc
E-box-binding homeobox 1 (ZEB1) and Smad-interacting protein 1
(SIP1/ZEB2) (13–15). In addition, ZEB1 and ZEB2 are
essential suppressors of E-cadherin transcription and have been
implicated in EMT. Previous studies have identified miR-200b as a
powerful regulator of EMT, which is associated with cancer
metastasis, via regulating the expression of genes, including
E-cadherin and vimentin (14–16).
Therefore, miR-200b is considered an important factor in the
metastatic spread of cancer cells. It has been widely reported that
the expression of miR-200b can influence invasion and metastasis of
various types of cancer, including prostate (16), colon (17), hepatic (18), gastric (19) and bladder (20) cancer. In addition, miR-200b has
been reported to be significantly differentially expressed between
primary tumors and corresponding distant metastases in breast
cancer (14); however, the
association between miR-200b and metastatic processes in cervical
cancer remain to be elucidated. The present study aimed to
elucidate the potential molecular mechanism underlying the role of
miR-200b in cervical cancer metastasis.
Materials and methods
Cell culture
The HeLa human cervical cancer cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 µg/ml streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2.
Cell transfection
Small interfering (si)RNA-RhoE, miRNA-200b mimics
and inhibitors, and the negative control for siRNA-NC, were
designed by and purchased from Guangzhou RiBoBio Co., Ltd.
(Guangzhou, China). Cells were seeded in 6-well plates at a density
of 40%, and after an overnight incubation were transfected with the
miRNAs, siRNAs, miRNA inhibitors and NC using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After 6 h, the culture medium was replaced
with fresh DMEM. Total RNA and protein were subsequently extracted
after 48 h, for analysis using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) or western blotting,
respectively.
Cell migration assay
Transwell insert chambers containing an 8-µm
porous membrane (Corning Inc., Corning, NY, USA) were used to
performed the migration assay. A total of 1×105 cells
suspended in serum-free medium were placed on each upper chamber,
and 500 µl medium containing 10% FBS was added to the bottom
chamber. The cells were incubated for 24 h at 37°C in a humidified
incubator containing 5% CO2. To quantify the number of
migrated cells, cells on the upper surface of the Transwell
membrane were removed using a cotton swab. The migrated cells on
the reverse of the membrane were fixed in methanol, stained with
crystal violet (Sigma-Aldrich, St. Louis, MO, USA) and images were
captured under a microscope (BX31, Olympus Corporation, Tokyo,
Japan) at ×100 magnification. Six random fields from the triplicate
membranes of each experimental group were observed.
Cell lysis and western blotting
Whole proteins were extracted from the cell lysates
using radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Hangzhou, China). The protein concentration was
determined using the BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). Equal quantity of proteins (25 µg/lane)
were separated by 10–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (Goodbio Technology, Wuhan, China) and were
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then blocked
with 5% non-fat dried milk at room temperature and were incubated
with monoclonal mouse anti-human RhoE (1:2,000; cat. no. MAB6618;
R&D Systems, Minneapolis, MN, USA), monoclonal mouse anti-human
E-cadherin (1:1,000; cat. no. 3195), monoclonal rabbit anti-human
vimentin (1:1,000; cat. no. 5741), polyclonal rabbit anti-human
matrix metalloproteinase (MMP)-9 (1:1,000; cat. no. 4022) and
monoclonal rabbit anti-human GAPDH (1:1,000; cat. no. 2118)
antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA).
After being washed three times with Tris-buffered saline containing
Tween (0.02%), the membranes were incubated with goat anti-mouse
IgG2b (1:8,000; cat. no. sc-2062) and goat anti-rabbit IgG2b
(1:8,000; cat. no. sc-2004) horseradish peroxidase-conjugated
secondary antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) for 60 min at room temperature. The blots were visualized
using an enhanced chemiluminescence system (EMD Millipore). GAPDH
was used as an endogenous internal control. The immune complexes
were quantified using Image J (version 1.46; National Institutes of
Health, Bethesda, MA, USA)
RNA preparation and RT-qPCR
Total RNA, including mRNA, was isolated from the
cells post-transfection using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol
and treated with 2 µl RQ1 RNase-Free DNase (Promega
Corporation, Madison, WI, USA). For mRNA analysis, cDNA was
amplified from 2.0 µg total RNA in a final volume of 20
µl using the Revert Aid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.). The primers used for the RT
reaction were miR200b, 5′-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC
GCA CT GGAT ACG ACT CAT CAT-3′ and U6, 5′-CGC TTC ACG AAT TTG CGT
GTCA-3′. The reaction was incubated at 42°C for 15 min, then heated
to 95°C for 5 min and finally incubated at 5°C for 5 min. Human
GAPDH was used as an internal control. The PCR primer sequences
were as follows: RhoE, F 5′-ATA GAG TTG AGC CTG TGG GACAC-3′ and R
5′-AGG GTC TCT GGT CTA CTG ATGTC-3′; and GAPDH, F 5′-TGC ACCA CCA
ACT GCT TAGC-3′ and R 5′-GGC ATG GAC TGT GGT CAT GAG-3′, miR-200b,
F 5′-GCG GCT AAT ACT GCC TGG TAA-3′ and R 5′-GTG CAG GGT CCG
AGGT-3′, U6, F 5′-CGC TTC GGC AGC ACA TAT ACTA-3′ and R 5′-CGC TTC
ACG AAT TTG CGT GTCA-3′ obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The RT-qPCR reaction was performed using SYBR
Green Master mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.), with the following cycling conditions: 95°C for 10 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min.
RT-qPCR was performed using a StepOnePlus system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). For quantification of
miR-200b, similar to the mRNA RT-qPCR, miRNA expression levels were
analyzed using specific primers with U6 as an internal control. The
primers were purchased from Guangzhou RiBoBio Co., Ltd. The results
were analyzed using the 2−ΔΔCq method relative to U6 or
GAPDH expression (21).
Luciferase assay
The 3′-UTR of RhoE was amplified by PCR using human
genomic DNA and cloned into the XhoI site downstream of
luciferase in the pLUC vector (Promega Corporation). A pLUC
construct containing a mutated 3′-UTR of RhoE that lacked the seed
sequence of miR-200b was also synthesized. For the luciferase
assay, HEK293T cells (American Type Culture Collection) were seeded
into 24-well plates and transfected with 200 ng pLMP or
pLMP-has-miR-200b vector (Guangzhou RiBoBio Co., Ltd.) alongside 20
ng wild type or mutant RhoE 3′-UTR using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.) at room temperature. Cells were collected
after 48 h and were analyzed using the Dual-Luciferase Reporter
Assay system (Promega Corporation). All experiments were performed
four times and normalized to Renilla luciferase
activity.
Statistical analysis
All experiments were performed independently in
triplicate. Student's t-test (two-tailed) and one-way analysis of
variance were performed in order to analyze the data. SPSS 19.0
software (IBM SPSS, Armonk, NY, USA) was used to analyze the data.
Data are expressed as mean ± standard error. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-200b expression regulates the EMT of
HeLa cells
Loss of E-cadherin expression and increased
invasiveness are markers of EMT. To determine whether miR-200b was
able to regulate the EMT of cervical cancer cells, the expression
levels of E-cadherin and vimentin were detected in HeLa cells
transfected with miR-200b mimics, inhibitors, or a negative
control. The expression levels of miR-200b were analyzed by RT-qPCR
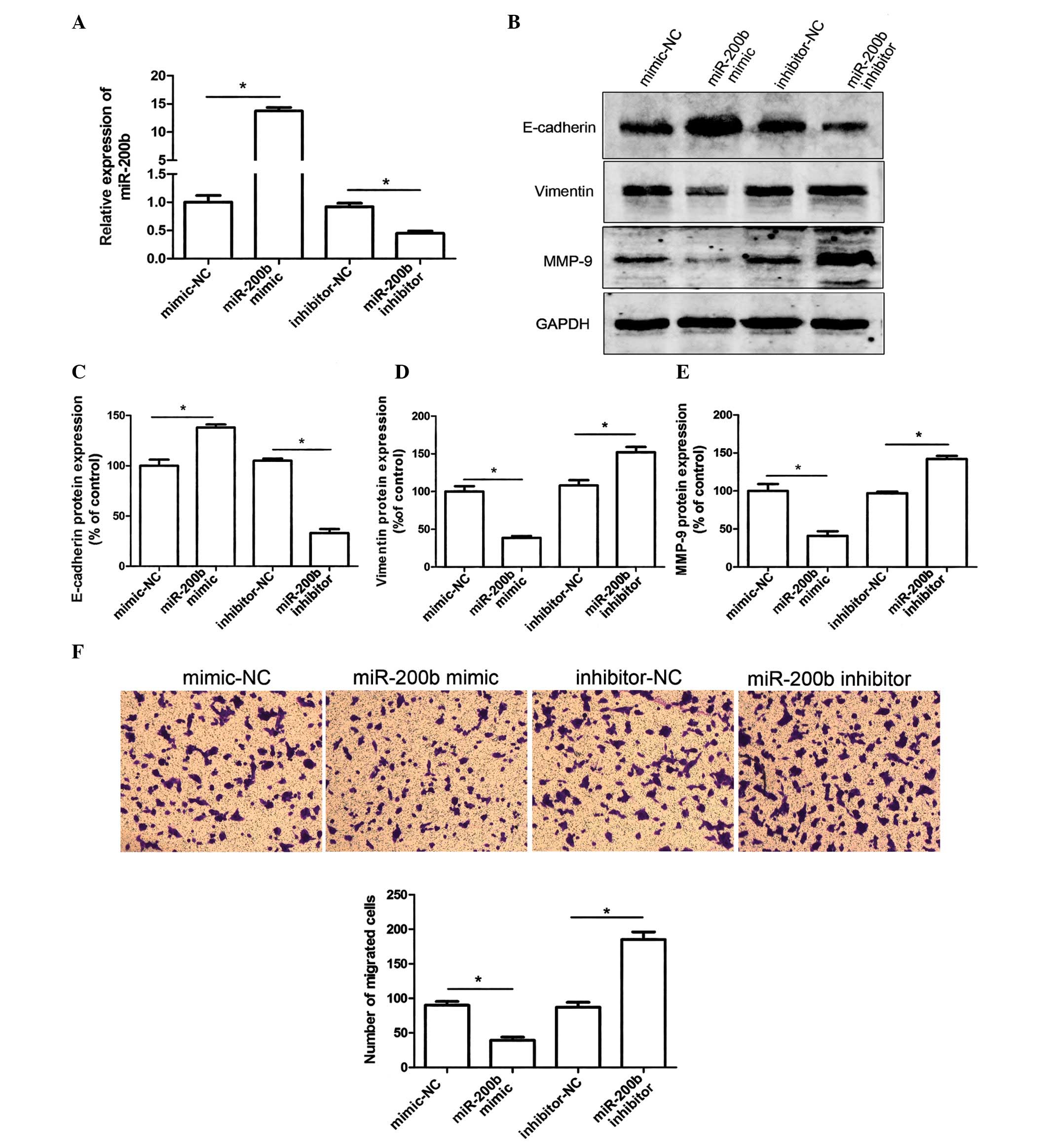
with U6 RNA as an internal control. As presented in Fig. 1A, the relative expression levels of
miR-200b were 14-fold higher in the cells transfected with the
miR-200b mimic, as compared with in the negative control cells
(mimic-NC). In addition, the cells transfected with the miR-200b
inhibitor exhibited a ~60% downregulation in miR-200b
expression.
Overexpression of miR-200b in the HeLa cells
resulted in upregulated E-cadherin and downregulated vimentin
protein expression levels, as compared with in the negative
control, as determined by western blotting (Fig. 1B–D). Furthermore, post-transfection
with the miR-200b inhibitor E-cadherin expression was reduced and
vimentin expression was increased (Fig. 1B–D). The expression levels of MMP-9
were also markedly decreased in the HeLa cells transfected with
miR-200b mimics. Conversely, transfection with the miR-200b
inhibitor significantly suppressed MMP-9 protein expression
(Fig. 1B and E). These results
suggest that metastasis of HeLa cells may, at least partially, be
attributed to MMP-9 upregulation. In addition, miR-200b may be
considered a powerful regulator of EMT in HeLa cells, due to the
regulation of E-cadherin and vimentin expression. Loss of miR-200b
expression may therefore have the potential to promote cervical
cancer cell migration by initiating EMT.
miR-200b levels regulate HeLa cell
migration
The present study investigated the effects of
miR-200b on the migration of HeLa cells using a Transwell migration
assay. Transfection of HeLa cells with miR-200b mimics suppressed
cell migratory ability. Conversely, transfection of the cells with
a miR-200b inhibitor resulted in a marked decrease in migratory
ability (Fig. 1F). These results
indicate that miR-200b levels may regulate HeLa cell migration.
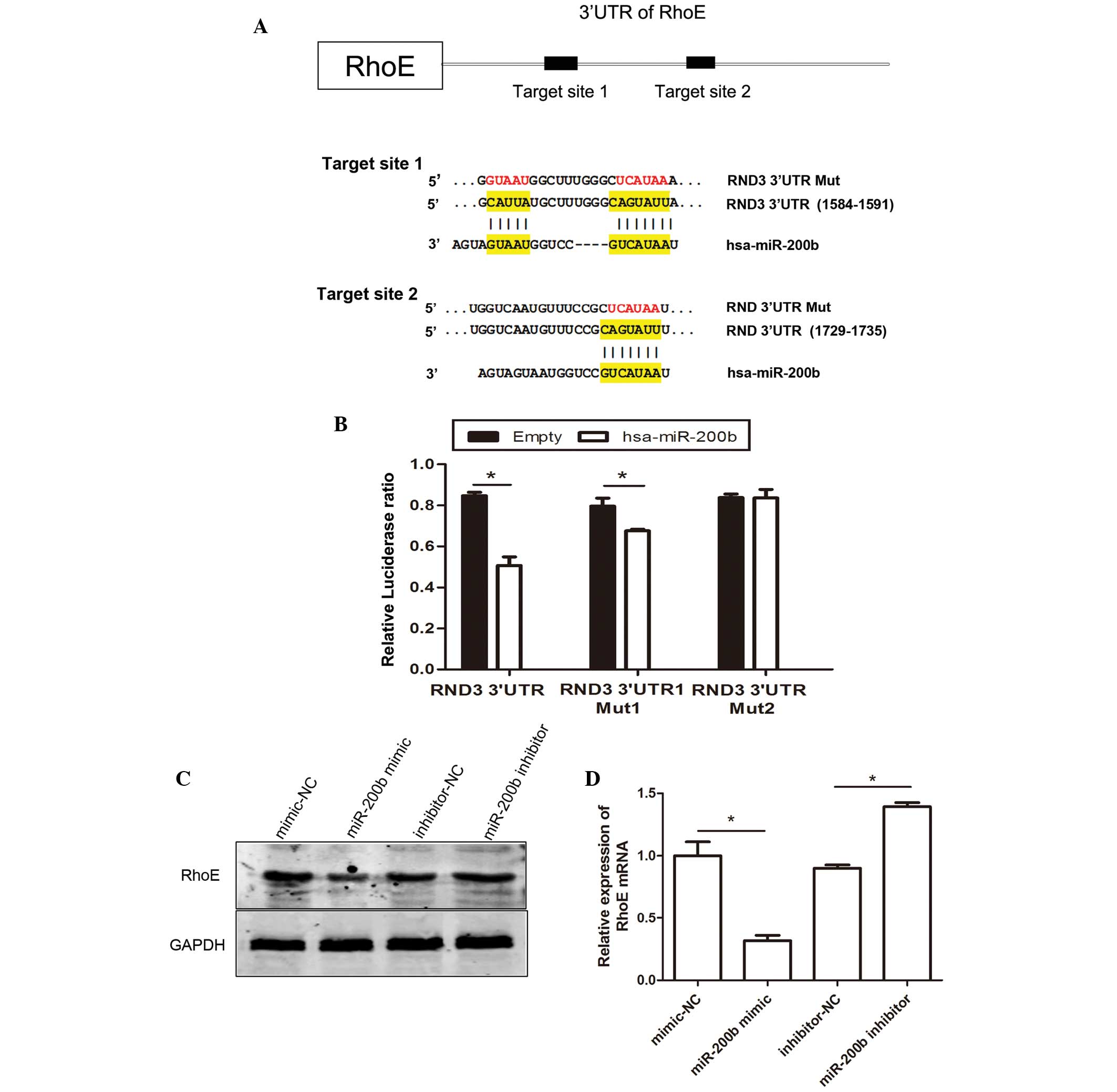
miR-200b directly targets RhoE
To explore the mechanisms underlying
miR-200b-mediated reductions in cell metastasis, potential miR-200b
target genes were investigated using online miRNA target prediction
databases (Targetscan, targetscan.org;
microrna.org/microrna/home.do and
genie.weizmann.ac.il/pubs/mir07/mir07_data.html). Among these
target genes, RhoE was identified, which is associated with
regulation of the actin cytoskeleton and migration via alterations
in cell motility (22). To
validate whether RhoE is a direct target gene of miR-200b, a
luciferase reporter vector containing the full length 3′-UTR of
RhoE was cloned. miR-200b significantly suppressed the relative
luciferase activity by targeting the 3′-UTR of RhoE. As shown in
Fig. 2A, two predicted target
sites of miR-200b have been identified, predictive target site 1
(from 1584 to 1591) and predictive target site 2 (from 1729 to
1735) in 3′UTR of RhoE. To analyze which predictive target site is
regulated by miR-200b, a wild type RhoE 3′-UTR (containing the two
prediction target sites) reporter construct and a mutated RhoE
3′-UTR reporter construct were generated. The results demonstrated
that the first mutant reporter construct had less of an effect on
luciferase activity, and the second mutant reporter construct had
no effect on luciferase activity, as compared with the report
vector containing the full 3′-UTR of RhoE. The luciferase assays
revealed that miR-200b may directly target the 3′-UTR of RhoE by
targeting predicted target sites 1 and 2 with both sites having the
same effect. The effects of miR-200b on the endogenous expression
of RhoE were subsequently examined by western blotting.
Trans-fection of the HeLa cells with miR-200b mimics resulted in a
marked decrease in the protein expression levels of RhoE (Fig. 2C). Furthermore, transfection with a
miR-200b inhibitor upregulated the expression levels of RhoE in the
cells. miRNA is known to post-transcriptionally regulate gene
expression either via translational repression or mRNA degradation
(9). In the present study RhoE
mRNA expression levels were decreased, as determined using RT-qPCR
analysis (Fig. 2D). These results
suggest that miR-200b could inhibit RhoE expression at the
transcriptional level.
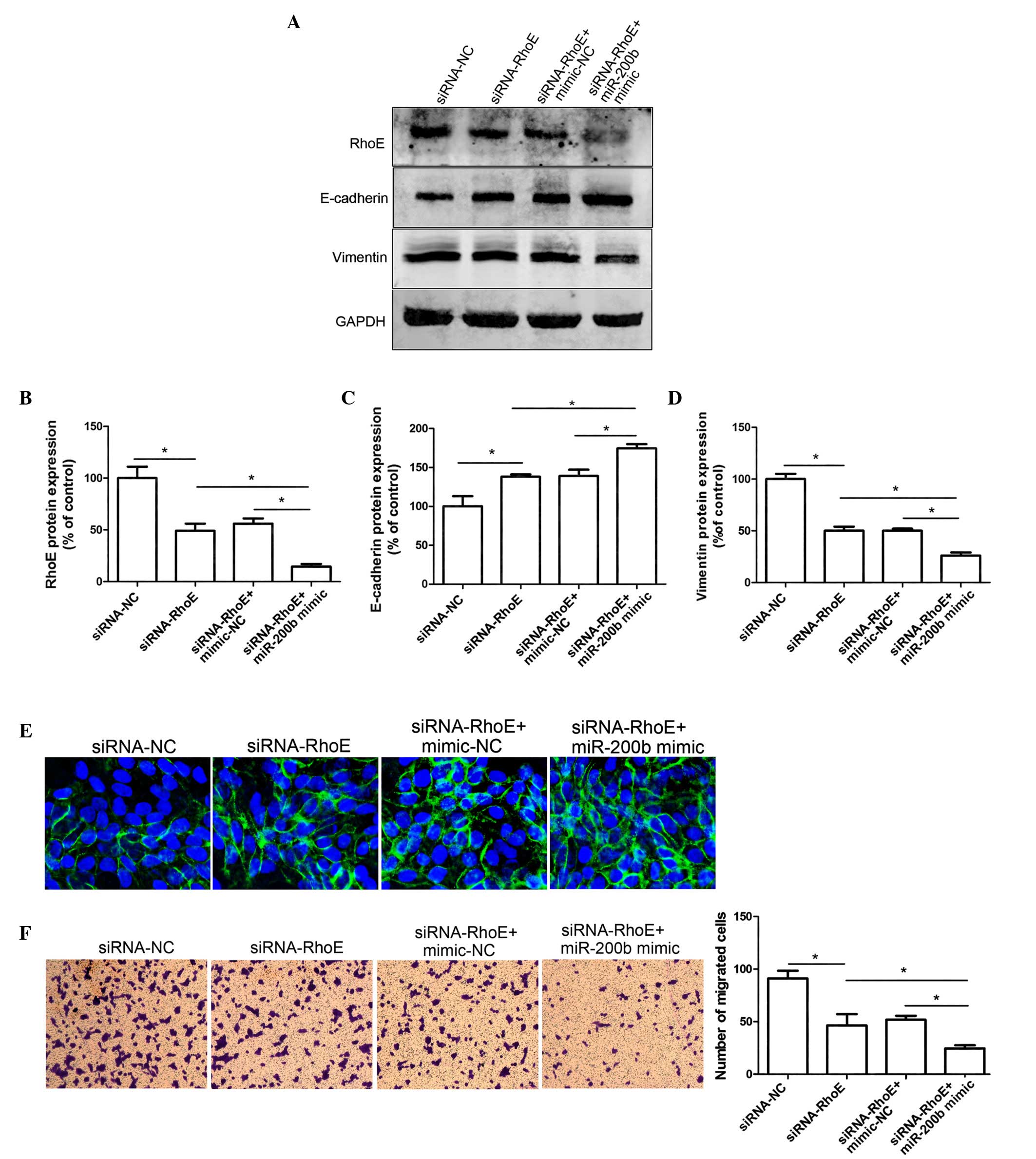
Silencing of RhoE inhibits EMT in HeLa
cells
The present study aimed to investigate whether RhoE
is the main factor affecting miR-200b-regulated cell migration in
HeLa cells; therefore HeLa cells were transfected with siRNA-RhoE.
As shown in Fig. 3A, the
expression levels of RhoE decreased by >60% following
transfection with siRNA-RhoE, as compared with the negative
control-cells (Fig. 3A and B). In
addition, siRNA-mediated RhoE downregulation resulted in increased
E-cadherin expression and reduced vimentin protein expression in
HeLa cells (Fig. 3C and D).
Immunofluorescent labeling with an anti-E-cadherin antibody
exhibited similar results; E-cadherin was significantly increased
following transfection with siRNA-RhoE, as compared with the
negative control HeLa cells (Fig.
3E). A Transwell assay was conducted to determine whether RhoE
was able to influence the migratory ability of cervical cancer
cells. The migratory ability of the Hela cells was markedly
decreased when RhoE expression was silenced, as compared with the
negative control group, thus indicating that downregulation of RhoE
expression may suppress the cell migratory ability. These results
suggest that downregulation of RhoE may inhibit EMT and cell
migration in vitro.
miR-200b regulates EMT in HeLa cell by
reducing the expression of RhoE
The present study aimed to investigate whether
miR-200b influences the metastatic potential of HeLa cells via
regulating the expression of RhoE. As presented in Fig. 3F, overexpression of miR-200b in
siRNA-RhoE-transfected cells significantly impaired their migratory
ability, as compared with the siRNA-RhoE cells transfected with
mimic-NC. In addition, overexpression of miR-200b in the
siRNA-RhoE-transfected cells resulted in an upregulation of
E-cadherin expression, as compared with the mimic-NC group
(Fig. 3A and F). These results
indicate that miR-200b may function as a potent suppressor of
migration in HeLa cells via regulation of RhoE expression.
Discussion
Metastasis is defined as a multistep biological
process during which primary cancer cells form distant secondary
tumors. Approximately 90% of patients with cancer succumb to
metastatic disease (23);
therefore, targeting metastasis is a key anticancer strategy. EMT,
which is recognized as the initiation of cancer metastasis
(24). Numerous studies have
reported the role of miRNAs as promoters (25-27)
and suppressors of metastasis (28-30);
therefore, molecular regulation of miRNA expression may serve as a
potential strategy for therapeutic intervention. High levels of
miR-196a have been demonstrated to promote the migration and
invasion of colorectal cancer cells via the repression of Hox genes
(31). In addition, tumor
suppressor miRNAs, including miR-29a, have also been reported,
which inhibit cervical cell metastasis by targeting heat shock
protein 47 (32). As a member of
the miR-200 family, miR-200b has been identified as a powerful
regulator of EMT in various types of cancer via the regulation of
numerous genes; however, the function of miR-200b in cervical
cancer remains to be determined. A significant correlation has
previously been reported between the expression of miR-200 and the
E-cadherin/vimentin ratio in NCI60 cells, and inhibition of
endogenous miR-200 expression was sufficient to induce EMT
(14,15). Based on these findings, the present
study hypothesized that miR-200b may be associated with the
metastatic processes of cervical cancer.
To explore the mechanism underlying the effects of
miR-200b on metastasis, potential miR-200b target genes were
searched for using online miRNA target prediction databases, and
numerous genes were identified. The present study selected one
target gene of miR-200b, RhoE, which has a clear role in the
regulation of the actin cytoskeleton and influences migration via
alterations in cell motility, in order to determine the mechanisms
underlying the effects of miR-200b on the regulation of EMT in
cervical cancer cells. A previous study reported that RhoE may act
as a target gene of miR-200b in regulating cell cycle progression
of HeLa cells (33). In the
present study, luciferase assays, RT-qPCR and western blotting
provided direct evidence suggesting that miR-200b may bind to the
3′-UTR of RhoE, suppressing the expression of RhoE at the mRNA and
protein levels. EMT is a key process that contributes to cancer
metastasis, which is characterized by a loss of epithelial markers,
including E-cadherin; an increase in mesenchymal markers, such as
vimentin (5,6); and an increase in migratory and
invasive behavior. In the present study, overexpression of miR-200b
in HeLa cells led to upregulated E-cadherin expression and
downregulated vimentin expression. Conversely, transfection with an
miR-200b inhibitor had the opposite effect on the HeLa cells. These
findings suggested that miR-200b may be associated with the EMT and
tumor metastasis of cervical cancer cells, and may serve as a
potential therapeutic target for the treatment of invasive
carcinoma.
A previous study has suggested that RhoE is
implicated in cancer motility; however, the role of RhoE in cancer
cell invasion remains at a preliminary stage (34). B-Raf-mediated upregulation of RhoE
has been shown to promote melanoma cell invasion by reducing
RhoA/RhoA kinase activity (35),
whereas p53-mediated induction of RhoE has been linked to
inhibition of cancer cell invasion (36), thus suggesting that RhoE may exert
a dual function in modulating cell mobility and invasion. In
colorectal cancer, RhoE expression has been shown to be
significantly correlated with depth of invasion, and lymph node and
distant metastasis (37),
suggesting the role of RhoE in migration and invasion. Furthermore,
RhoE expression was significantly reduced in colorectal cancer
tissues, as compared with in normal tissues (38). The results of the present study
demonstrated that siRNA-mediated knockdown of RhoE resulted in
suppressive effects on EMT, as determined by reduced vimentin
expression and increased E-cadherin expression. Furthermore, a
Transwell migration assay indicated that knockdown of RhoE
inhibited the cell migratory ability. These results indicated that
miR-200b has a suppressive role in cell migration thereby
influencing the EMT process, which may partly be due to direct
inhibition of RhoE expression. The available data suggested that
stress fibers act to maintain cell tension and mobility, and the
loss of these actin structures directly leads to cell spreading
(39). Previous studies have
reported that loss of stress fibers is accompanied by rapid cell
spreading (40,41). It is well known that RhoA, which is
a member of the Rho family, is involved in maintaining cell tension
and a round morphology. A previous study demonstrated that RhoE may
inhibit signaling downstream of RhoA to prevent RhoA from
stimulating stress fiber formation, and time-lapse videomicroscopy
has shown that following microinjection of RhoE, cells were much
flatter and spread out, as compared with control cells (34). These findings suggested that RhoE
may regulate cell spreading via the inhibition of RhoA signaling.
Notably, overexpression of dominant-negative N-terminally truncated
ROKα, a downstream target for RhoA, induced cell spreading in HeLa
and 3T3 cells (41). However,
further experiments are required.
In conclusion, the present study is the first, to
the best of our knowledge, to describe the miR-200b/RhoE link and
identify miR-200b as a regulator of EMT in cervical cancer cells by
influencing the expression of RhoE. As a result, these findings may
provide novel insights into the role of miR-200b in the development
of cervical cancer and suggest that miR-200b may be considered an
effective target for the treatment of patients with highly
metastatic cervical cancer.
Acknowledgments
The authors are grateful for the support provided by
the Natural Science Foundation of China (grant nos. 81302273 and
81201196), the Science and Technology Department of Hubei Province,
China (grant no. ZRY039), the Health and Family Planning Commission
of Hubei Province, China (grant no. 2012ZY02) and the Chinese
Postdoctoral Science Foundation (grant no. 2011M500857).
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2015. Atlanta: American Cancer Society; 2015
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Büchler MW: Colorectal cancer. Lancet. 365:153–165. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fidler IJ: The organ microenvironment and
cancer metastasis. Differentiation. 70:498–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirohashi S: Inactivation of the
E-cadherin-mediated cell adhesion system in human cancers. Am J
Pathol. 153:333–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dykxhoorn DM: MicroRNAs and metastasis:
Little RNAs go a long way. Cancer Res. 70:6401–6406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Korpal M and Kang Y: The emerging role of
miR-200 family of microRNAs in epithelial-mesenchymal transition
and cancer metastasis. RNA Biol. 5:115–119. 2008. View Article : Google Scholar
|
|
14
|
Gravgaard KH, Lyng MB, Laenkholm AV,
Søkilde R, Nielsen BS, Litman T and Ditzel HJ: The miRNA-200 family
and miRNA-9 exhibit differential expression in primary versus
corresponding metastatic tissue in breast cancer. Breast Cancer Res
Treat. 134:207–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong D, Li Y, Wang Z, Banerjee S, Ahmad A,
Kim HR and Sarkar FH: miR-200 regulates PDGF-D-mediated
epithelial-mesenchymal transition, adhesion, and invasion of
prostate cancer cells. Stem Cells. 27:1712–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tryndyak VP, Beland FA and Pogribny IP:
E-cadherin transcriptional down-regulation by epigenetic and
microRNA-200 family alterations is related to mesenchymal and
drug-resistant phenotypes in human breast cancer cells. Int J
Cancer. 126:2575–2583. 2010.
|
|
18
|
Hung CS, Liu HH, Liu JJ, Yeh CT, Chang TC,
Wu CH, Ho YS, Wei PL and Chang YJ: MicroRNA-200a and -200b mediated
hepatocellular carcinoma cell migration through the epithelial to
mesenchymal transition markers. Ann Surg Oncol. 20(Suppl 3):
S360–S368. 2013. View Article : Google Scholar
|
|
19
|
Kurashige J, Kamohara H, Watanabe M,
Hiyoshi Y, Iwatsuki M, Tanaka Y, Kinoshita K, Saito S, Baba Y and
Baba H: MicroRNA-200b regulates cell proliferation, invasion, and
migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg
Oncol. 19(Suppl 3): S656–S664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adam L, Zhong M, Choi W, Qi W, Nicoloso M,
Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, et al:
miR-200 expression regulates epithelial-to-mesenchymal transition
in bladder cancer cells and reverses resistance to epidermal growth
factor receptor therapy. Clin Cancer Res. 15:5060–5072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Chardin P: Function and regulation of Rnd
proteins. Nat Rev Mol Cell Biol. 7:54–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arias AM: Epithelial mesenchymal
interactions in cancer and development. Cell. 105:425–431. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Q, Gumireddy K, Schrier M, Ie Sage
C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, et al:
The microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gaziel-Sovran A, Segura MF, Di Micco R,
Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert
JF, Shang S, Kerbel RS, et al: miR-30b/30d regulation of GalNAc
transferases enhances invasion and immunosuppression during
metastasis. Cancer Cell. 20:104–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar
|
|
29
|
Fang JH, Zhou HC, Zeng C, Yang J, Liu Y,
Huang X, Zhang JP, Guan XY and Zhuang SM: MicroRNA-29b suppresses
tumor angiogenesis, invasion, and metastasis by regulating matrix
metalloproteinase 2 expression. Hepatology. 54:1729–1740. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schimanski CC, Frerichs K, Rahman F,
Berger M, Lang H, Galle PR, Moehler M and Gockel I: High miR-196a
levels promote the oncogenic phenotype of colorectal cancer cells.
World J Gastroenterol. 15:2089–2096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamamoto N, Kinoshita T, Nohata N, Yoshino
H, Itesako T, Fujimura L, Mitsuhashi A, Usui H, Enokida H, Nakagawa
M, et al: Tumor-suppressive microRNA-29a inhibits cancer cell
migration and invasion via targeting HSP47 in cervical squamous
cell carcinoma. Int J Oncol. 43:1855–1863. 2013.PubMed/NCBI
|
|
33
|
Xia W, Li J, Chen L, Huang B, Li S, Yang
G, Ding H, Wang F, Liu N, Zhao Q, et al: MicroRNA-200b regulates
cyclin D1 expression and promotes S-phase entry by targeting RND3
in HeLa cells. Mol Cell Biochem. 344:261–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guasch RM, Scambler P, Jones GE and Ridley
AJ: RhoE regulates actin cytoskeleton organization and cell
migration. Mol Cell Biol. 18:4761–4771. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klein RM and Aplin AE: Rnd3 regulation of
the actin cyto-skeleton promotes melanoma migration and invasive
outgrowth in three dimensions. Cancer Res. 69:2224–2233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gadea G, de Toledo M, Anguille C and Roux
P: Loss of p53 promotes RhoA-ROCK-dependent cell migration and
invasion in 3D matrices. J Cell Biol. 178:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou J, Yang J, Li K, Mo P, Feng B, Wang
X, Nie Y and Fan D: RhoE is associated with relapse and prognosis
of patients with colorectal cancer. Ann Surg Oncol. 20:175–182.
2013. View Article : Google Scholar
|
|
38
|
Luo H, Zou J, Dong Z, Zeng Q, Wu D and Liu
L: Up-regulated miR-17 promotes cell proliferation, tumour growth
and cell cycle progression by targeting the RND3 tumour suppressor
gene in colorectal carcinoma. Biochem J. 442:311–321. 2012.
View Article : Google Scholar
|
|
39
|
Katoh H, Harada A, Mori K and Negishi M:
Socius is a novel Rnd GTPase-interacting protein involved in
disassembly of actin stress fibers. Mol Cell Biol. 22:2952–2964.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leung T, Chen XQ, Manser E and Lim L: The
p160 RhoA-binding kinase ROK alpha is a member of a kinase family
and is involved in the reorganization of the cyto-skeleton. Mol
Cell Biol. 16:5313–5327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ridley AJ and Hall A: The small
GTP-binding protein rho regulates the assembly of focal adhesions
and actin stress fibers in response to growth factors. Cell.
70:389–399. 1992. View Article : Google Scholar : PubMed/NCBI
|