Introduction
Mesenchymal stem cells (MSCs) are progenitor cells
that have been described to localize within breast carcinomas,
where stem cells integrate into tumor-associated stromal tissues
and promote breast cancer invasion and metastasis (1,2). The
MSCs isolated from bone marrow have been demonstrated to greatly
increase the metastatic potency of weakly metastatic human breast
carcinoma cells (3). This
phenomenon was observed in MCF-7 cells, where an increase in cancer
cell proliferation was observed when the cancer cells were
co-cultured on bone marrow-derived MSC (BMSC) feeder layers. Thus,
this co-culture of BMSCs and cancer cells may be used as a model to
identify a potential method for reducing the propagation
characteristics (growth and proliferation rate) of cancer
cells.
The present study aimed to investigate the growth
reduction effects of BMSCs that had been pretreated with
pioglitazone and/or rosiglitazone on the growth and proliferation
rate of breast cancer cells. Pioglitazone and rosiglitazone are
prescription drugs of the thiazolidinedione (TZD) class that are
commonly used for the treatment of type II diabetes mellitus.
Although pioglitazone and rosiglitazone are currently widely used
clinically, neither pioglitazone nor rosiglitazone have any role in
the treatment of human breast cancer. Indeed, the use of these
drugs to treat cancer cells is not necessarily a promising strategy
in breast cancer therapy. For example, although pioglitazone and
rosiglitazone have been demonstrated to reduce breast cancer cell
proliferation and invasion (4,5),
these drugs, however, also demonstrate certain disadvantages,
including cellular lipid accumulation, as determined from an in
vitro assay, heart failure and bone destruction in female
patients. Therefore, it would be beneficial to administer
pioglitazone and rosiglitazone indirectly to breast cancer
patients, for example, via the interaction of stem and cancer
cells. Through this process, the modified and viable pretreated
stem cells would be subsequently administered to patients, and the
cells would allowed to interact with cancer cells in the body of
the patients.
In the present study, the effect of soluble growth
factors in the conditioned medium of the pretreated BMSCs on the
proliferation rate of MCF-7 cells was investigated using a
fibroblast growth factor 4 (FGF4) neutralizing antibody. It was
hypothesized that the pretreated stem cells would reduce cancer
cell growth (colony size) and the proliferation rate (colony
number) in vitro (Fig. 1).
This phenomenon may be attributed to the reduction of specific
soluble growth factors in the pretreated BMSCs; therefore, studying
the expression pattern of growth and inflammatory
response-associated molecules, including FGF4, chemokine (C-C
motif) ligand-5 (CCL5; also termed RANTES) and interleukin-6
(IL-6), may provide insights into the regulation of stem cells in
carcinogenesis. The results of the present study may also provide
valuable insights into the usefulness of pioglitazone- and/or
rosiglitazone-pretreated BMSCs, which may expand the benefits of
using pretreated BMSCs in future medical studies. The pioglitazone-
and/or rosiglitazone-pretreated BMSCs may also have a potential
application in stem cell-mediated therapy for human breast cancer,
as well as for other malignancies.
Materials and methods
Culture of the BMSCs and MCF-7 cell
lines
The BMSC cell line was purchased from AseaCyte Sdn
Bhd (Precision Cell Technology, Subang Jaya, Malaysia) and was
routinely cultured with growth medium for non-tumorigenic human
cells [low-glucose Dulbecco's Modified Eagle's Medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 mg/ml
streptomycin with stable glutamine and sodium pyruvate], whereas
the MCF-7 cell line was cultured using the growth medium for
tumorigenic human cells [high-glucose DMEM supplemented with 10%
FBS, 100 units/ml penicillin and 100 mg/ml streptomycin].
Occasionally, an optional supplement of 1X MycoKill (PAA
Laboratories; GE Healthcare Life Sciences, Chalfont, UK) and an
antibiotic cocktail were added to the two growth media to prevent
mycoplasma and fungal contaminations, respectively. The cell lines
were maintained at 37°C in a humidified atmosphere of 5% (v/v)
CO2. The growth media for the BMSCs and MCF-7 cells were
changed every three to four days. Cell lines were subsequently
subcultured and maintained for adhesive and non-adhesive
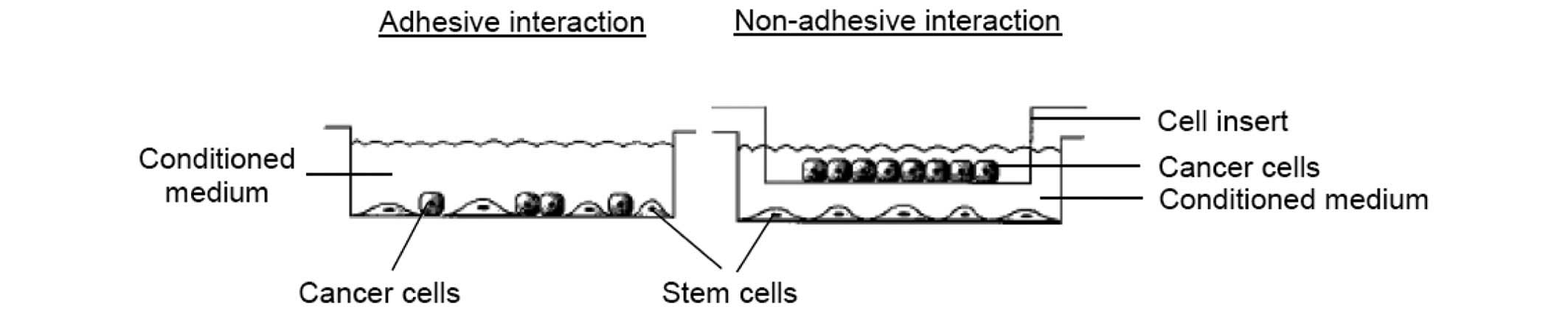
stem-and-cancer cell interaction, as described below (Fig. 2).
Analysis of the adhesive interaction of
MCF-7 cells with pioglitazone- and/or rosiglitazone-pretreated BMSC
feeder layers
The adhesive interaction or direct co-culture of
MCF-7 cells with BMSCs pretreated with growth media supplemented
with pioglitazone and/or rosiglitazone (both Sigma-Aldrich, St.
Louis, MO, USA) was performed by seeding 1.0×103
BMSCs/ml per well in a four-well chamber slide. The cells were
allowed to adhere overnight. The low-glucose DMEM growth medium for
the BMSCs was changed every three to four days, when the cells
reached 90% confluence. The BMSCs were subsequently incubated in
growth medium supplemented with pioglitazone and/or rosiglitazone
for one week. Pioglitazone (40 µM) or rosiglitazone (40
µM), or pioglitazone (20 µM) + rosiglitazone (20
µM), which modified the BMSCs but did not significantly
decrease their viability, was added to the growth medium to treat
the BMSCs. Dimethylsulfoxide (DMSO; Bio Basic Canada, Inc.,
Markham, ON, Canada) was used as a control in the present study,
since it has been demonstrated to exert no growth-reducing effect
on MCF-7 cells. The incubated cells were observed on a daily basis,
and the growth medium supplemented with pioglitazone and/or
rosiglitazone was changed every three to four days. After one week,
the growth medium supplemented with pioglitazone and/or
rosiglitazone was removed. The BMSC feeder layer that had been
incubated with the growth media supplemented with pioglitazone
and/or rosiglitazone was carefully washed several times with
pre-warmed phosphate-buffered saline (PBS). Once the growth medium
supplemented with pioglitazone and/or rosiglitazone had been
removed, the BMSC feeder layer was referred to as the pioglitazone-
and/or rosiglitazone-pretreated BMSC feeder layer. A suspension of
MCF-7 cells (10 cells/well) was added to the pretreated BMSC feeder
layer. Cultured MCF-7 cells alone were used as a control in the
present experiment. Following one week of co-culture, the
morphology, number and size of the MCF-7 colonies that were formed
on the pretreated BMSC feeder layer were monitored and quantified
using Oil Red O staining under a Motic AE31 inverted microscope
(Motic Intruments, Inc., Richmond, BC, Canada). The size (diameter)
of each MCF-7 colony on the pretreated BMSC feeder layer was
measured at a magnification of ×40 using the inverted microscope.
The co-culture was repeated in at least two independent experiments
to ensure the reproducibility of the results.
Analysis of the non-adhesive interaction
of MCF-7 cells with pioglitazone- and/or rosiglitazone-pretreated
BMSC conditioned medium
The non-adhesive interaction or indirect co-culture
of MCF-7 cells with the conditioned medium of BMSCs pretreated with
pioglitazone and/or rosiglitazone was performed by seeding
1.0×104 BMSCs/ml per well in a six-well plate. The cells
were allowed to adhere overnight. The low-glucose DMEM growth
medium for the BMSCs was changed every three to four days. Once the
cells had reached 90% confluence, the growth medium used to
maintain the BMSCs was removed, and the BMSC feeder was
subsequently added with the growth media supplemented with
pioglitazone and/or rosiglitazone. The pioglitazone- and/or
rosiglitazone-containing growth medium used to pretreat the BMSCs
was changed every three to four days. After one week, the growth
medium supplemented with pioglitazone and/or rosiglitazone was
removed. Subsequently, the pioglitazone- and/or
rosiglitazone-pretreated BMSC feeder layer was carefully washed
several times with pre-warmed PBS. Fresh growth medium was
subsequently added to the pretreated BMSCs, and the pretreated BMSC
feeder layer was incubated in fresh growth medium for one week.
Following one week of incubation, the growth medium, which is now
referred to as conditioned medium for all culture conditions, was
collected in 15 ml falcon tubes (BD Biosciences, San Jose, CA,
USA). The conditioned medium was subsequently centrifuged using an
Eppendorf 5804R (Eppendorf, Germany) at maximum speed (15,000 × g)
for 20 min at 4°C to precipitate any particles or cell debris in
the medium, and the supernatants were collected and used for the
MCF-7 cell incubation in the analysis of non-adhesive interaction,
as described below. The conditioned medium was also used for
immunoassays, as described below. For the non-adhesive interaction,
the MCF-7 cells were seeded (1.0×103 cells/ml per well)
and maintained in high-glucose DMEM growth medium in a 12-well
plate. Following 24 h of incubation, the old growth medium was
removed, and the collected conditioned medium was added to the
MCF-7 cells. The MCF-7 cells were incubated in the conditioned
medium for one week, and the incubation of the MCF-7 cells with
each conditioned medium was performed in triplicate. Following one
week of incubation, the MCF-7 cells were trypsinized using 0.25%
trypsin-EDTA containing phenol red, and the proliferation rate of
the cells was quantified using a Trypan Blue Exclusion assay using
0.4% (w/v) Trypan Blue solution (Gibco). The co-culture was
repeated in at least two independent experiments to ensure the
reproducibility of the results.
Immunoassay of soluble growth factors in
conditioned medium of BMSCs pretreated with pioglitazone and/or
rosiglitazone
The levels of FGF4, CCL2, CCL5, IL-6, vascular
endothelial growth factor (VEGF) and transforming growth factor β
(TGFβ) in the conditioned medium of BMSCs pretreated with
pioglitazone and/or rosiglitazone were determined using
RayBio® enzyme-linked immunosorbent assay (ELISA) kits
(RayBiotech, Inc., Norcross, GA, USA). In these kits, antibodies
specific for the proteins were pre-coated onto microtiter plates.
The samples (conditioned medium) were subsequently added to the
wells and allowed to react with the bound antibody for 2.5 h at
room temperature. The unbound substances were washed away with wash
solution, according to the manufacturer's protocol. Subsequently,
an enzyme-linked antibody specific to the protein in the
conditioned medium was added to the wells. The antibody was
incubated with the target protein for 1 h. Following a further
washing step, substrate solution was added to the wells for color
development. Development of the color was proportional to the
quantity of protein present in the samples. The color intensity was
subsequently measured using an ELISA reader at a wavelength of 450
nm, and the level of each specific protein in the conditioned
medium was calculated. The ELISA was performed in triplicate and
repeated in at least two independent experiments.
Analysis of the levels of FGF4 transcript
in BMSCs pretreated with pioglitazone and/or rosiglitazone
The total RNA of the pretreated BMSCs was extracted
using the RNeasy™ Total RNA kit (Qiagen, Inc., Valencia,
CA, USA). The integrity of the extracted total RNA was confirmed by
1% agarose gel electrophoresis at 90 V for 35 min, and the purity,
as well as the concentration of extracted total RNA, was measured
using a Nanodrop 2000c spectrophotometer (Nanodrop; Thermo Fisher
Scientific, Inc.) at 260/280 nm. Subsequently, 1.0 µg RNA
was reverse-transcribed into cDNA using a commercially available
Revert-Aid First-Strand cDNA Synthesis kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.), and this cDNA was used to analyze
the level of FGF4 transcript in the pretreated BMSCs using
real-time (RT)-quantitative (q)PCR. During this process, the cDNA
that was reverse-transcribed from the extracted total RNA of
pioglitazone- and/or rosiglitazone-pretreated adipose
tissue-derived mesenchymal stem cells (ATSCs) was also used to
analyze the level of the FGF4 transcript by RT-qPCR. Primers
specific to the genes were designed using Primer Express software
version 2.0 (Applied Biosystems) (Table I), and RT-qPCR was performed using
a Rotor-Gene 600 PCR system (Qiagen, Inc.), according to the
manufacturer's protocol. The reactions were performed in a total
volume of 25 µl in optical reaction tubes that included
Power SYBR® Green Master mix (Applied Biosystems), 900
nM each primer and cDNA, as prepared above. The reaction program
was subsequently initiated at 95°C for 10 min to activate the
enzyme, and this step was followed by 40 cycles of denaturation at
95°C for 15 sec and primer annealing combined with extension at
60°C for 1 min. The Ct value of the gene in each unknown sample was
normalized to that of β-actin, and the associated expression level
of the gene and the fold change in gene expression were calculated,
according to the manufacturer's protocol. RT-qPCR was performed in
triplicate, and repeated in at least two independent
experiments.
 | Table IPrimers used for real
time-quantitative PCR. |
Table I
Primers used for real
time-quantitative PCR.
| Gene(s) | Sequence(s) | Amplicon (bp) |
|---|
| FGF4 | Forward:
5′-CAACTACAACGCCTACGAGTCCTA-3′ | 77 |
| Reverse:
5′-CCTTCTTGGTCTTCCCATTCTTG-3′ | |
| Ki-67a | Forward:
5′-AACTATCCTCGTCTGTCCCAACAC-3′ | 106 |
| Reverse:
5′-CGGCCATTGGAAAGACAGAT-3′ | |
| PCNAb | Forward:
5′-AGAAGGTGTTGGAGGCACTCA-3′ | 72 |
| Reverse:
5′-GGTTTACACCGCTGGAGCTAA-3′ | |
| β-actin | Forward:
5′-CATTGCCGACAGGATGCA-3′ | 102 |
| Reverse:
5′-CCGATCCACACGGAGTACTTG-3′ | |
Analysis of the effect of an
FGF4-neutralizing antibody on the non-adhesive interaction of MCF-7
cells with BMSCs
The effect of a FGF4-neutralizing antibody on the
non-adhesive interaction of MCF-7 cells with BMSCs was analyzed by
seeding 5.0×103 BMSCs/ml per well in a 24-well plate.
The cells were allowed to adhere until 70% confluence had been
reached. At this point, the old growth medium used to maintain the
BMSCs was subsequently removed, and the BMSC feeder layer was added
with fresh growth medium. The fresh growth medium contained 6, 10
or 14 µg/ml anti-human FGF4-neutralizing antibody (R&D
Systems, Inc., Minneapolis, MN, USA). The incubation of the stem
cells with fresh growth medium without FGF4-neutralizing antibody
was used as a control in the present study. A polycarbonate
Transwell cell insert with a membrane pore size of 8.0 µm
was subsequently attached to each well of the plate as a transwell
configuration. A 100 µl suspension of MCF-7 cells (~100
cells/well) was subsequently added onto the cell insert. The cancer
cells were allowed to interact with the stem cells in the
respective growth medium with or without the neutralizing antibody
for one week. The growth media was changed once during the
incubation period for the stem-and-cancer cell interaction. After
one week of incubation, to quantify the exact proliferation rate of
incubated MCF-7 cells, the MCF-7 cells were trypsinized, as
previously described, and subjected to RNA extraction and cDNA
synthesis, and the resulting cDNA was subsequently used to analyze
the levels of Ki-67 and proliferating cell nuclear antigen (PCNA)
transcripts by RT-qPCR, as described above. The co-culture was
repeated in at least two independent experiments to ensure the
reproducibility of the results.
Statistical analysis
All the graphs and statistical calculations were
generated and performed using GraphPad 6.01 software (GraphPad
Software, Inc., La Jolla, CA, USA). Despite the adhesive nature of
the co-culture, the majority of the experiments were performed in
triplicate, and repeated several times independently to confirm the
reproducibility of the results. Data are expressed as the mean ±
standard deviation. P<0.05 was taken to indicate a statistically
significant difference.
Results
Adhesive interaction of MCF-7 cells grown
on pioglitazone- and/or rosiglitazone-pretreated BMSC feeder
layers
For the adhesive interaction, the growth and
proliferation rate of MCF-7 cells on the pioglitazone- and/or
rosiglitazone-pretreated BMSC feeder layers were measured by the
colony size (growth) and the colony number (proliferation rate) of
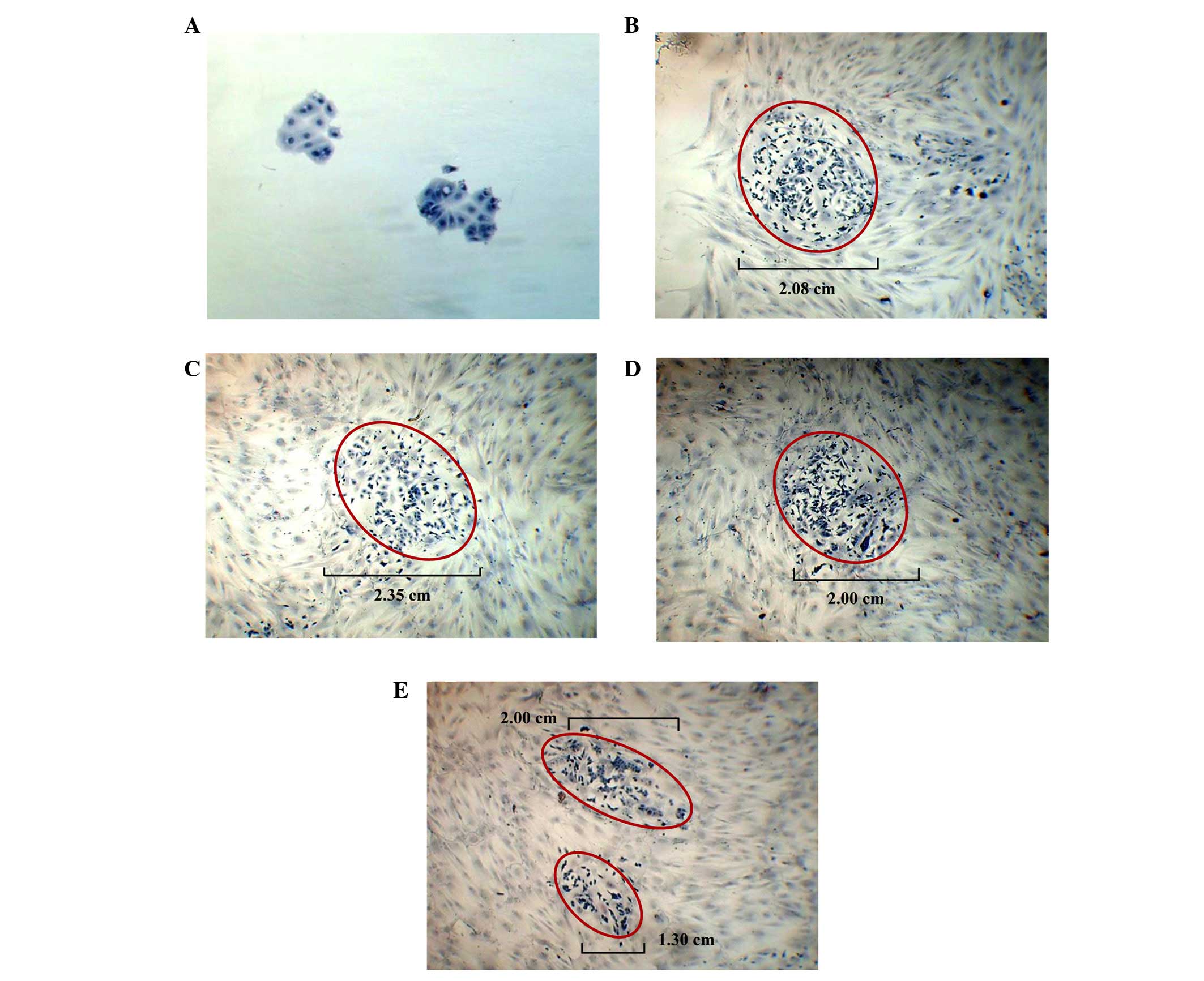
the cancer cells, respectively. In general, compared with the MCF-7
cells grown on plastic only for one week (Fig. 3A), the growth of the MCF-7 cells
(colony size) was observed to be greater and more diffuse when the
cancer cells were co-cultured on the feeder layer of BMSCs
pretreated with DMSO alone (control; Fig. 3B). A similar phenomenon was
observed when the MCF-7 cells were grown on the feeder layers of
BMSCs pretreated with 40 µM pioglitazone (Fig. 3C) and 40 µM rosiglitazone
(Fig. 3D). The overall size of the
MCF-7 colonies formed on the feeder layer of BMSCs pretreated with
DMSO alone was assigned a value of 100% in the present study
(control feeder layer). The MCF-7 colonies formed on the feeder
layer of BMSCs pretreated with 40 µM pioglitazone and 40
µM rosiglitazone were ~113.0 to 115.3%, and ~96.2 to 104.1%,
respectively, compared with the MCF-7 colonies formed on the
control feeder layer. The appearance of these MCF-7 colonies was
also revealed to be single cells, without evidence of direct
cell-to-cell contact. However, when MCF-7 cells were co-cultured on
feeder layers of BMSCs that were pretreated with 20 µM
pioglitazone + 20 µM rosiglitazone, the overall size of the
MCF-7 colonies that were formed on the feeder layer was markedly
decreased to ~62.5 to 77.6% (Fig.
3E) compared with the control feeder layer. The two small
colonies in the field of view are not comparable to a large colony,
since there is an appreciable distance between the areas of the two
colonies. Thus, BMSCs pretreated with 20 µM pioglitazone +
20 µM rosiglitazone may have the potential to reduce the
growth of MCF-7 cells.
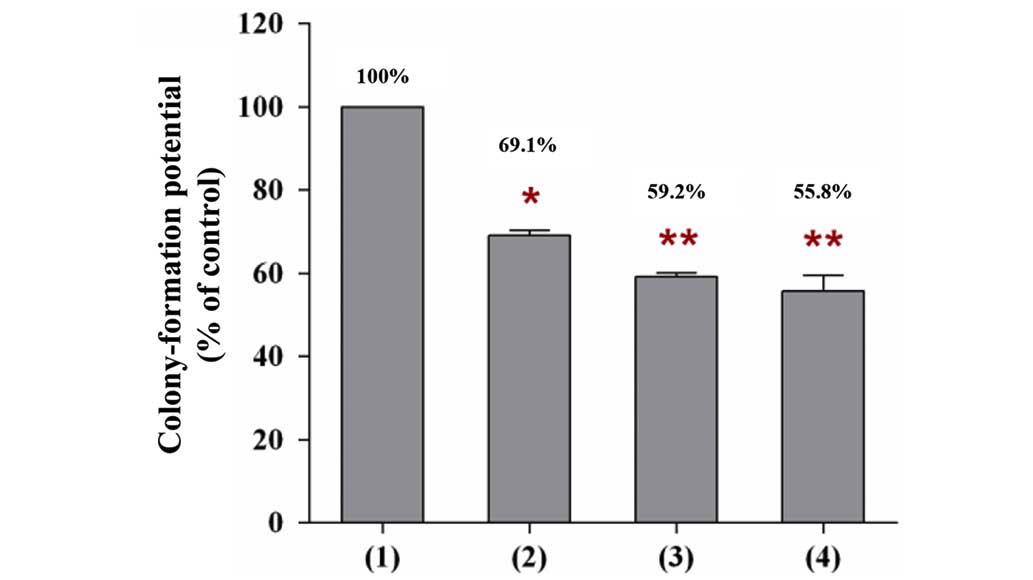
In addition to the growth of MCF-7 cells, a similar
phenomenon was observed on the proliferation rate of MCF-7 cells
(colony number). When MCF-7 cells were co-cultured on the feeder
layer of BMSCs that were pretreated with a single type of TZD drug,
or with the two of them, the number of MCF-7 colonies that were
formed on the BMSC feeder layer was markedly decreased compared
with the number of colonies that were formed on the control feeder
layer (Fig. 4). The number of
MCF-7 colonies formed was decreased to 69.1% by co-culturing with
the feeder layer of BMSCs pretreated with 40 µM pioglitazone
(P<0.05), whereas the number decreased to 55.8% by co-culturing
with the feeder layer of BMSCs pretreated with 40 µM
rosiglitazone (P<0.01), and to 59.2% by co-culturing with the
feeder layer of BMSCs pretreated with 20 µM pioglitazone +
20 µM rosiglitazone (P<0.01). The calculation was based
on the number of MCF-7 colonies that were formed on the feeder
layer of BMSCs pretreated with DMSO, which was set to 100% in the
present study (control feeder layer). Thus, BMSCs pretreated with
one, or a combination, of the TZD drugs may have the potential to
reduce the proliferation rate of cancer cells.
Non-adhesive interaction of MCF-7 cells
grown in pioglitazone- and/or rosiglitazone-pretreated BMSC
conditioned medium
Unlike the experiment investigating adhesive
interaction, only the proliferation rate was determined in the case
of non-adhesive interaction. The non-adhesive interaction of MCF-7
cells with the conditioned medium of BMSCs increased the
proliferation rate of the cancer cells by ~16.6% compared with the
proliferation rate of the cancer cells that were incubated in the
growth medium only (Fig. 5). This
phenomenon indicated that the increase in the proliferation rate of
cancer cells cannot be correlated with a direct physical
stem-and-cancer cell interaction, since similar findings were
observed for the adhesive and the non-adhesive interaction
conditions. Furthermore, the incubation of MCF-7 cells with BMSC
conditioned medium caused the cells to convert from growing in
clusters into appearing as single cells, without evidence of direct
cell-to-cell contact. However, the incubation of MCF-7 cells with
the conditioned medium of BMSCs pretreated with pioglitazone and/or
rosiglitazone reduced the proliferation rate of the cancer
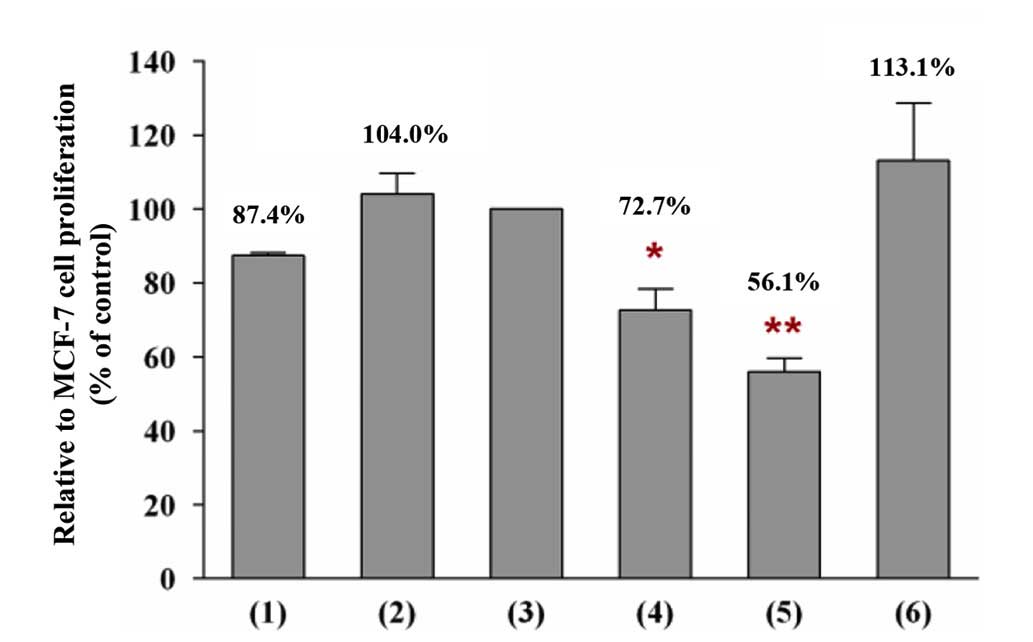
cells.
Incubation of MCF-7 with the conditioned medium of
BMSCs pretreated with pioglitazone and/or rosiglitazone decreased
the proliferation rate of the cancer cells compared with the
incubation of MCF-7 cells in the conditioned medium of BMSCs
pretreated with DMSO only (100%; control). The present study
determined that the proliferation rate of MCF-7 cells was decreased
to 72.7% (P<0.05) when the cancer cells were incubated in the
conditioned medium of BMSCs pretreated with 40 µM
pioglitazone (Fig. 5). The growth
reduction effect on the proliferation rate was observed to be more
potent when the MCF-7 cells were incubated in the conditioned
medium of BMSCs pretreated with 20 µM pioglitazone + 20
µM rosiglitazone, which decreased the proliferation rate of
MCF-7 cells to 56.1% (P<0.01). However, the incubation of MCF-7
cells with the conditioned medium of BMSCs pretreated with 40
µM rosiglitazone did not reveal any growth reduction effect
on the proliferation rate of cancer cells. This finding indicates
that the growth reduction effect on cancer cells may be caused by a
reduced secretion of specific soluble growth factors by pretreated
BMSCs into the conditioned medium.
Levels of soluble growth factors in the
conditioned medium of BMSCs pretreated with pioglitazone and/or
rosiglitazone
The present study identified that the soluble growth
factors secreted by pretreated BMSCs in the conditioned medium
differed, depending on the TZD treatment (Fig. 6). Decreased levels of FGF4 were
first detected in the conditioned medium of BMSCs that were
pretreated with pioglitazone and/or rosiglitazone, as determined by
ELISA. The present study revealed that the levels of FGF4 in the
conditioned medium of BMSCs pretreated with 40 µM
pioglitazone and 40 µM rosiglitazone were 34.0% (P<0.001)
and 18.8% (P<0.001), respectively, compared with the level of
FGF4 in the conditioned medium of BMSCs pretreated with DMSO (100%;
control). When the BMSCs were pretreated with a combination of
pioglitazone and rosiglitazone, a more marked reduction in the
level of FGF4 in the conditioned medium of pretreated BMSCs was
observed. The level of FGF4 in the conditioned medium of BMSCs
pretreated with 20 µM pioglitazone + 20 µM
rosiglitazone was only 14.8% (P<0.001) compared with the
control. The marked reduction in the level of FGF4 may contribute
to the potent decrease in the proliferation rate of MCF-7 cells.
Indeed, the levels of CCL5 and IL-6 were also identified to be
significantly higher in the conditioned medium of BMSCs pretreated
with 40 µM rosiglitazone (P<0.05), and the level of VEGF
was significantly higher in the conditioned medium of BMSCs
pretreated with 40 µM pioglitazone (P<0.05), indicating
the negative implications of pioglitazone use. The high levels of
CCL5 and IL-6 in the conditioned medium of BMSCs pretreated with 40
µM rosiglitazone may explain why the proliferation rate of
MCF-7 cells did not decrease when the cancer cells were incubated
in the conditioned medium.
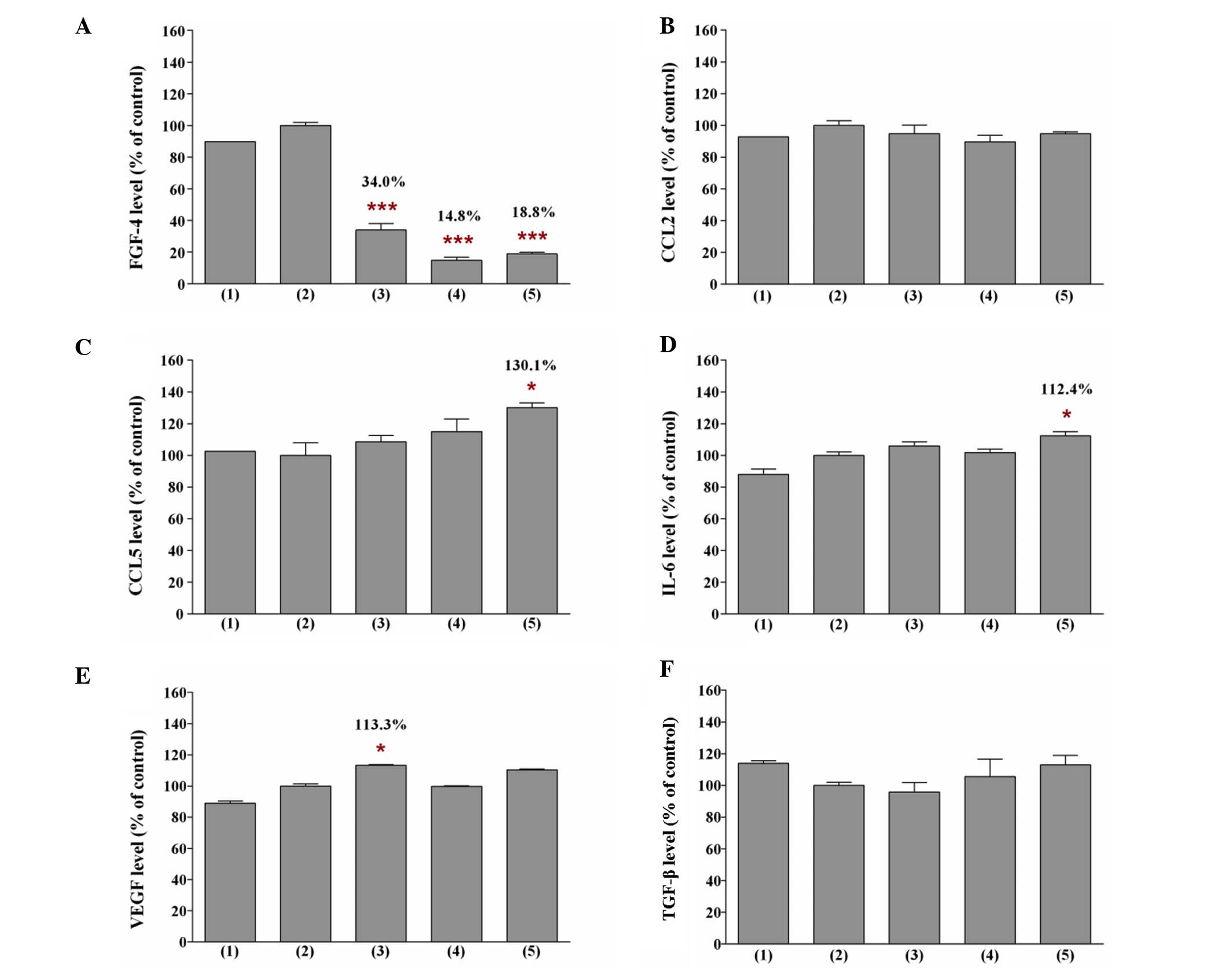 | Figure 6Levels of (A) FGF4, (B) CCL2, (C)
CCL5, (D) IL-6, (E) VEGF and (F) TGF-β in the conditioned medium of
BMSCs pretreated with pioglitazone and/or rosiglitazone. The
experimental conditions were: (1)
conditioned medium of BMSCs, (2)
conditioned medium of BMSCs pretreated with DMSO, (3) conditioned medium of BMSCs pretreated
with 40 µM pioglitazone, (4) conditioned medium of BMSCs pretreated
with 20 µM pioglitazone + 20 µM rosiglitazone, and
(5) conditioned medium of BMSCs
pretreated with 40 µM rosiglitazone. The levels of these
soluble growth factors in the conditioned medium were assayed by
enzyme-linked immunosorbent assay. The levels of soluble growth
factors in the conditioned medium of BMSCs pretreated with DMSO
were defined as 100%. Data are expressed as the means ± standard
deviation from three separate experiments. One-way analysis of
variance was used to compare the levels of soluble growth factor in
BMSCs pretreated with DMSO only (control) with the BMSCs pretreated
with the thiazolidinedione(s). *P<0.05,
***P<0.001 compared with the control. FGF4,
fibroblast growth factor 4; CCL2/5, chemokine (C-C motif)
ligand-2/5; IL-6, interleukin-6; VEGF, vascular endothelial growth
factor; TGF-β, transforming growth factor-β; BMSCs, bone
marrow-derived mesenchymal stem cells; DMSO, dimethylsulfoxide. |
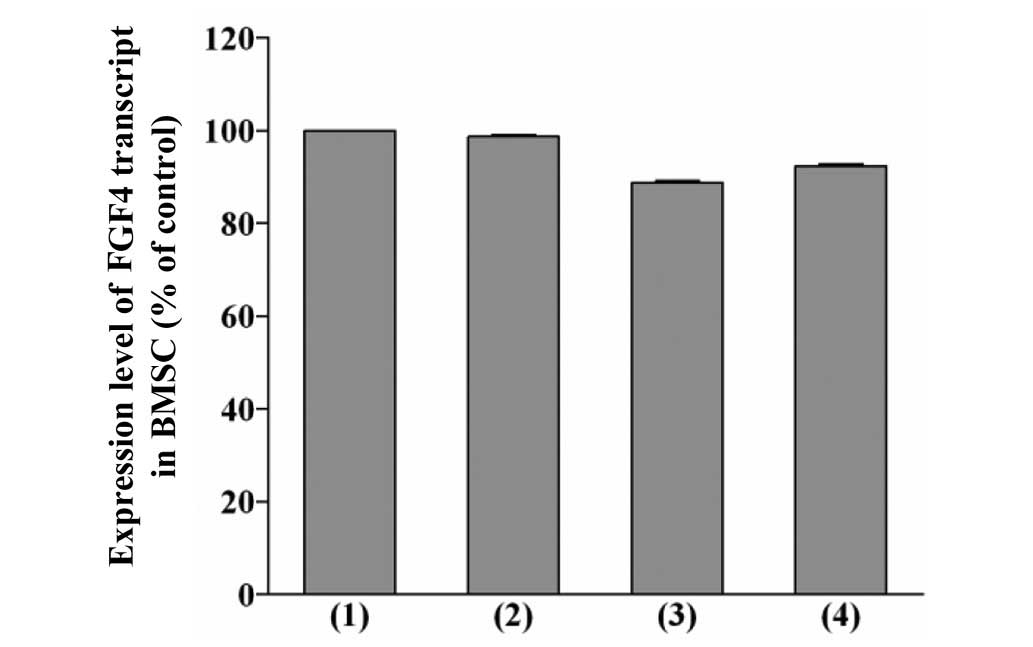
Levels of FGF4 transcript in BMSCs
pretreated with pioglitazone and/or rosiglitazone
A reduction in the levels of FGF4 transcript in
BMSCs pretreated with pioglitazone and/or rosiglitazone by RT-qPCR
was observed (Fig. 7). However,
the reduction in the levels of FGF4 transcript in BMSCs pretreated
with pioglitazone and/or rosiglitazone was not statistically
significant compared with the levels observed in the BMSCs
pretreated with DMSO only. The level of FGF4 transcript in the
BMSCs pretreated with 20 µM pioglitazone + 20 µM
rosiglitazone was only 88.78% compared with the control (100%).
This phenomenon indicated that a reduction in the expression level
of the FGF4 protein only contributed to the growth reduction effect
on MCF-7 cells when the cancer cells interacted with the pretreated
stem cells.
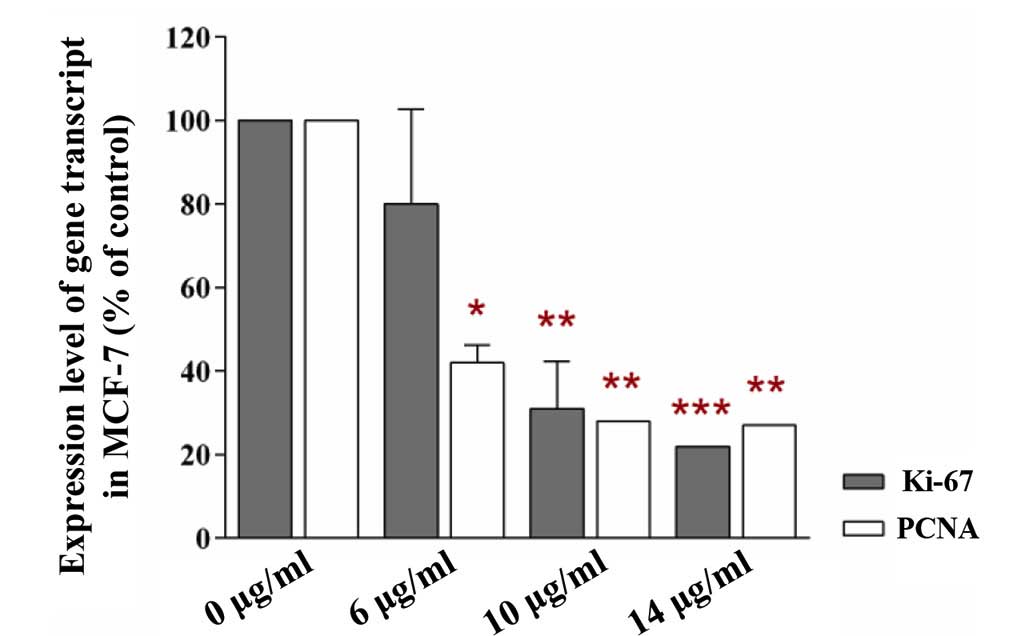
Effect of an FGF4-neutralizing antibody
on the non-adhesive interaction of MCF-7 cells with BMSCs
The present study revealed reduced levels of Ki-67
and PCNA transcripts in MCF-7 cells that interacted non-adhesively
with BMSCs in growth medium containing the FGF4-neutralising
antibody (Fig. 8). The levels of
the transcripts in MCF-7 cells that underwent the identical
interaction with BMSCs in growth medium without the antibody was
set to 100% (control). The reduction in the levels of Ki-67 and
PCNA transcripts in the MCF-7 cells that had interacted with the
BSMCs was statistically significant. In fact, the levels of the two
transcripts in MCF-7 cells that interacted with BMSCs in growth
medium containing 6 µg/ml FGF4-neutralizing antibody were
80% and 42% (P<0.01), respectively, compared with the control
(100%). When the cancer cells that had interacted with BMSCs were
incubated with medium containing 10 and 14 µg/ml
FGF4-neutralizing antibody, the levels of the Ki-67 and PCNA
transcripts were reduced to 31% (P<0.001) and 28% (P<0.001),
and to 22% (P<0.001) and 27% (P<0.001), respectively,
compared with the identical control. Since Ki-67 and PCNA are
widely used as proliferation-associated markers, this phenomenon
indicated that the neutralization of FGF4 secreted from the BMSCs
in growth medium effected by the FGF4-neutralizing antibody reduced
the proliferation rate of the MCF-7 cells. Thus, the decrease in
the proliferation rate of MCF-7 cells was likely to have been
caused by the reduction in the level of FGF4.
Discussion
BMSCs have been previously shown to secrete soluble
growth factors into conditioned medium (6–14).
Thus, pretreated BMSCs may be used to alter the growth and
proliferation rate of MCF-7 cells via changes in the levels of
soluble growth factors secreted by the pretreated BMSCs into the
conditioned medium. In the present study, it is proposed that the
inhibition of the MCF-7 growth and proliferation rate was likely to
have been due to a reduction in the secretion of specific soluble
growth factors by the pretreated BMSCs, regardless of the presence
or absence of a direct physical stem-and-cancer cell interaction
between the two cell lines. The level of soluble growth factors in
the conditioned medium secreted by pretreated BMSCs were likely to
exert a growth reduction effect, which prohibited the growth and
proliferation rate of the MCF-7 cells. The soluble growth factors
assessed in the present study, including FGF4, CCL2, CCL5 (also
termed RANTES), IL-6, VEGF and TGF-β, have been previously reported
to exhibit proliferation-associated effects on cancer cell growth
(6–14). However, a reduction in FGF4 was
only identified in the conditioned medium of BMSCs pretreated with
pioglitazone and/or rosiglitazone.
Pioglitazone and rosiglitazone exhibit a similar
safety profile and effect on cancer cells. Consistently with these
findings, the present study identified high levels of soluble
growth factors, which are usually increased in the inflammatory
response and during cancer cell invasion, in the conditioned medium
of BMSCs pretreated with pioglitazone and rosiglitazone. VEGF,
which is known to exert an important role in angiogenesis, was also
present at markedly higher levels in the conditioned medium of
BMSCs pretreated with 40 µM pioglitazone, and therefore this
could explain the unsuitability of pioglitazone for pretreatment of
the BMSCs, even though the conditioned medium reduced the
proliferation rate of the MCF-7 cells. Furthermore, high levels of
CCL5 and IL-6 were identified in the conditioned medium of BMSCs
pretreated with 40 µM rosiglitazone, which may explain the
absence of a growth reduction effect on the proliferation rate of
MCF-7 cells when the cancer cells were incubated in the conditioned
medium. This phenomenon confirmed the negative implications of the
use of pioglitazone or rosiglitazone alone, such as the induction
of an inflammatory response in treated cells. Notably, this
phenomenon was not observed in the BMSCs pretreated with 20
µM rosiglitazone + 20 µM pioglitazone. The reduced
protein levels of FGF4 were only identified in the conditioned
medium of BMSCs pretreated with pioglitazone and/or rosiglitazone.
Additionally, the pretreatment of BMSCs with 20 µM
rosiglitazone + 20 µM pioglitazone did not increase the
levels of CCL5, IL-6 or VEGF in the conditioned medium. These
findings indicate that the application of BMSCs pretreated with 20
µM pioglitazone + 20 µM rosiglitazone to cancer
patients via cell-cell interactions may be an effective strategy
for the treatment of human breast cancers.
FGF4 is a protein encoded by the FGF4 gene that
demonstrates multiple oncogenic activities, including tumor growth
and invasion (15). FGF4 is
expressed in human breast cancer cells (16). Previous studies demonstrated that
the binding site of Oct-4, a POU-domain transcription factor that
is markedly expressed in pluripotent embryonic stem cells and
exerts an important role in stem cell regulation, was identified in
FGF4 (17,18). By studying the expression pattern
of FGF4, the present study may lend insights into the regulation of
stem cells in carcinogenesis. Consistently with this aim, a
reduction in FGF4 was identified in BMSCs pretreated with 20
µM pioglitazone + 20 µM rosiglitazone, which may have
potential therapeutic value for breast cancer, compared with BMSCs
pretreated with a single TZD. Furthermore, the modification of
BMSCs through pretreatment with pioglitazone and rosiglitazone did
not change the morphology of the stem cells. This finding indicates
that the pretreated stem cells may be injected into the mammary fat
pads or bloodstream to reduce cancer cell growth in patients, or
may be used in various cell-mediated therapies. Notably,
unpublished data in our laboratory revealed that this strategy was
more effective when BMSCs were used: A 1.13-fold change in the
transcript levels of FGF4 was identified in BMSCs pretreated with
pioglitazone and rosiglitazone compared with the control. A
decreased effect was demonstrated when the identical treatment was
applied to ATSCs (a 1.04-fold change in the FGF4 transcript level).
The present study only investigated the expression level of FGF4;
other genes that are associated with FGF4, including stromal
cell-derived factor 1, platelet-derived growth factor homodimer,
epidermal growth factor and basic FGF, will be investigated in
future studies.
The approach taken in the present study did not
appreciably change the two stem and cancer cell morphologies.
Although pretreated stem cells have been shown to have a growth
reduction effect on breast cancer cells, there remains a risk that
these cells would advance, rather than suppress, cancer. As such,
further studies to precisely indicate the potential of pretreated
stem cells on breast cancer treatment, including the proliferative
and differentiation capacities of the pretreated stem cells, are
warranted. To translate these results into a clinical setting, it
must be determined how many systemically infused MSCs would
actually reach the tumor site, and how long the MSCs would survive
in the body post-infusion. Perhaps an in vivo model would be
the next optimal approach to prove that this study is clinically
relevant.
In conclusion, BMSCs pretreated with pioglitazone
and/or rosiglitazone reduced the growth and proliferation rate of
breast cancer, an effect that may be attributed to the reduction of
the protein level of FGF4 in the pretreated BMSCs. However, further
studies on the correlation of the protein levels of FGF4 in the
conditioned medium of pioglitazone- and/or rosiglitazone-pretreated
BMSCs is warranted.
Acknowledgments
This project was funded by the Research University
Grant Scheme for Individual (RUI) from Universiti Sains Malaysia
(no. 1001/CIPPM/811200) for the study of MSCs and the Fundamental
Research Grant Scheme (FRGS) Fasa 2/2013 (no. 203/CIPPM/6711336)
from the Ministry of Higher Education (MoHE), Malaysia for the
study of FGF4. The pioglitazone study was funded by an Exploratory
Research Grant Scheme (ERGS) Fasa 1/2013 (no. 203/CIPPM/6730098).
The first author is grateful for the support provided by the
Ernst-von-Leyden Scholarship from Berliner Krebsgesellschaft E.V.
(to BKG) during her postdoctoral training. The second author thanks
the Graduate Assistant Scheme offered by the Institute of
Postgraduate Studies (IPS), Universiti Sains Malaysia.
References
|
1
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Tran I, Seshareddy K, Weiss ML and
Detamore MS: A comparison of human bone marrow-derived mesenchymal
stem cells and human umbilical cord-derived mesenchymal stromal
cells for cartilage tissue engineering. Tissue Eng Part A.
15:2259–2266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hombauer H and Minguell JJ: Selective
interactions between epithelial tumour cells and bone marrow
mesenchymal stem cells. Br J Cancer. 82:1290–1296. 2000. View Article : Google Scholar
|
|
4
|
Liu H, Zang C, Fenner MH, Possinger K and
Elstner E: PPARgamma ligands and ATRA inhibit the invasion of human
breast cancer cells in vitro. Breast Cancer Res Treat. 79:63–74.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Magenta G, Borenstein X, Rolando R and
Jasnis MA: Rosiglitazone inhibits metastasis development of a
murine mammary tumor cell line LMM3. BMC Cancer. 8:472008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams J, Carder PJ, Downey S, Forbes MA,
MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ,
et al: Vascular endothelial growth factor (VEGF) in breast cancer:
Comparison of plasma, serum and tissue VEGF and microvessel density
and effects of tamoxifen. Cancer Res. 60:2898–2905. 2000.PubMed/NCBI
|
|
7
|
Robinson SC, Scott KA, Wilson JL, Thompson
RG, Proudfoot AE and Balkwill FR: A chemokine receptor antagonist
inhibits experimental breast tumor growth. Cancer Res.
63:8360–8365. 2003.PubMed/NCBI
|
|
8
|
Yaal-Hahoshen N, Shina S, Leider-Trejo L,
Barnea I, Shabtai EL, Azenshtein E, Greenberg I, Keydar I and
Ben-Baruch A: The chemokine CCL5 as a potential prognostic factor
predicting disease progression in stage II breast cancer patients.
Clin Cancer Res. 12:4474–4480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaday GG, Peehl DM, Kadam PA and Lawrence
DM: Expression of CCL5 (RANTES) and CCR5 in prostate cancer.
Prostate. 66:124–134. 2006. View Article : Google Scholar
|
|
10
|
Loberg RD, Ying C, Craig M, Yan L, Snyder
LA and Pienta KJ: CCL2 as an important mediator of prostate cancer
growth in vivo through the regulation of macrophage infiltration.
Neoplasia. 9:556–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghosh S, Sullivan CA, Zerkowski MP,
Molinaro AM, Rimm DL, Camp RL and Chung GG: High levels of vascular
endothelial growth factor and its receptors (VEGFR-1, VEGFR-2,
neuropilin-1) are associated with worse outcome in breast cancer.
Hum Pathol. 39:1835–1843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stacey DL, Gibala MJ, Martin-Ginis KA and
Timmons BW: Effects of recovery method after exercise on
performance, immune changes and psychological outcomes. J Orthop
Sports Phys Ther. 40:656–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartmann MC, Dwyer RM, Costello M, Potter
SM, Curran C, Hennessy E, Newell J, Griffin DG and Kerin MJ:
Relationship between CCL5 and transforming growth factor-β1 (TGFβ1)
in breast cancer. Eur J Cancer. 47:1669–1675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Ginestier C, Ou SJ, Clouthier SG,
Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, et
al: Breast cancer stem cells are regulated by mesenchymal stem
cells through cytokine networks. Cancer Res. 71:614–624. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galland F, Stefanova M, Lafage M and
Birnbaum D: Localization of the 5′ end of the MCF2 oncogene to
human chromosome 15q15-q23. Cytogenet Cell Genet. 60:114–116. 1992.
View Article : Google Scholar
|
|
16
|
Wang P, Branch DR, Bali M, Schultz GA,
Goss PE and Jin T: The POU homeodomain protein OCT3 as a potential
transcriptional activator for fibroblast growth factor-4 (FGF-4) in
human breast cancer cells. Biochem J. 375:199–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamb KA and Rizzino A: Effects of
differentiation on the transcriptional regulation of the FGF-4
gene: Critical roles played by a distal enhancer. Mol Reprod Dev.
51:218–224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boiani M and Schöler HR: Regulatory
networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell
Biol. 6:872–884. 2005. View
Article : Google Scholar : PubMed/NCBI
|