Introduction
Rheumatoid arthritis (RA) is an inflammatory joint
disease that features hyperplasia of the synovial tissue and
formation of pannus, and their invasive growth into the cartilage,
which results in the destruction of cartilage and bone (1). Inflammatory cytokines, including
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF) α, are
expressed and functionally active in synovial tissues. Within a
complex regulatory network, cytokines are implicated in specific
immunological processes that promote chronic inflammation,
autoimmunity and tissue destruction (2).
Decoy receptor 3 (DcR3)/TR6/M68/TNFRSF6b is a member
of the TNF receptor (TNFR) superfamily, but is a secreted protein,
as it lacks the transmembrane domain of conventional TNFRs
(3). The three ligands of DcR3 are
the TNF superfamily members Fas ligand, lymphotoxin-related
inducible ligand that competes for glycoprotein D binding to
herpesvirus entry mediator on T cells (LIGHT) and TNF-like ligand
1A (TL1A) (4). Death receptor 3
(DR3) is the receptor for TL1A that induces apoptosis and the
activation of nuclear factor κ-light-chain-enhancer in activated B
cells. DcR3 antagonizes TL1A/DR3 signaling event (5). DcR3 is expressed in certain types of
normal tissues, including the colon, stomach, spleen, lymph nodes,
spinal cord, pancreas and lungs (3,6), but
not in NIH3T3 human fibroblast cells (7); furthermore, DcR3 is frequently
overexpressed in various tumor cell types (3,6,8). In
tumors, overexpression of DcR3 may facilitate the evasion of the
cytotoxic and regulatory effects of Fas ligand (3,9),
LIGHT (10) and TL1A (5). A previous study by our group reported
that DcR3 is expressed in fibroblast-like synoviocytes from
patients with rheumatoid arthritis (RA-FLS) and that DcR3
expression induced in RA-FLS by TNFα protected the cells from
Fas-induced apoptosis (11). These
results led to the hypothesis that DcR3 is a key regulatory
molecule for the proliferation of RA-FLS.
Studies have suggested that DcR3 directly induces
monocytes to form osteoclasts (12) and that reverse signaling of DcR3
triggers enhanced adhesion of monocytes (13). A previous study by our group also
reported that DcR3 induces very late antigen-4 expression in THP-1
macrophages to inhibit cycloheximide-induced apoptosis (14). Another study by our group found
that DcR3 binds to TL1A expressed on RA-FLS, resulting in negative
regulation of inflammatory cytokine-induced cell proliferation
(15). Furthermore, a
comprehensive genetic analysis using microarrays by our group
demonstrated that DcR3 regulates gene expression in RA-FLS
(16).
From these gene expression profiles, IL-12B was
identified by our group as a gene which is induced by DcR3 in
RA-FLS (16). IL-12B encodes the
IL-12B p40 subunit, which is common to IL-12 and IL-23 (17). IL-12 consists of IL-12A p35 and
IL-12B p40 and induces T-helper cell (Th)1 immune responses, which
are linked to autoimmune diseases, including inflammatory bowel
disease and psoriasis (18). IL-23
is comprised of IL-23A p19 and IL-12B p40 and is linked to
autoimmune diseases, including multiple sclerosis and inflammatory
bowel disease, via Th17 immune responses (19). IL-12 (20) and IL-23 (21,22)
have also been reported to be associated with the pathogenesis of
RA.
The present study demonstrated that DcR3 induces
IL-12B p40 expression in RA-FLS by binding to membrane-bound TL1A.
In turn, IL-23 upregulates DcR3 expression in RA-FLS. These results
suggested that DcR3 and IL-23 may interact in a feedback loop that
aggravates local inflammation in patients with RA.
Materials and methods
Isolation and culture of synovial
fibroblasts
Synovial samples were obtained from patients with RA
who fulfilled the criteria of the American College of Rheumatology
(formerly, the American Rheumatism Association) (23) and who had never been treated with
biologics during total hip or knee replacement. Patients included 3
males and 27 females aged 69.0±10.3 years old. Written informed
consent to participate in this study was obtained from all patients
in accordance with the World Medical Association Declaration of
Helsinki Ethical Principles for Medical Research Involving Human
Subjects. The Medicine Ethics Committee of Kobe University Graduate
School of Health Sciences (Kobe, Japan) approved the protocol
including consent procedures. Synovial samples from patients with
osteoarthritis (OA) were obtained during total knee replacement in
a similar manner (8 females; aged 71.1±10.6 years old). To isolate
FLS, synovial tissue specimens were minced and digested in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis,
MO, USA) containing 0.2% collagenase (Sigma-Aldrich) for 2 h at
37°C. Dissociated cells were cultured in DMEM supplemented with 10%
fetal bovine serum (Sigma-Aldrich) and 100 units/ml
penicillin/streptomycin (Meiji Seika Pharma Co., Ltd., Tokyo,
Japan). Following incubation overnight and removal of non-adherent
cells, adherent cells were further incubated in fresh medium. Cells
from passages 3–7 were used in all further experiments (11).
Cell treatments
For quantification of IL-12B mRNA expression in
RA-FLS by reverse transcription quantitative polymerase chain
reaction (RT-qPCR), cells (1×106/well) were stimulated
with 10, 100 or 1,000 ng/ml recombinant human DcR3-Fc chimera
protein (DcR3-Fc; R&D Systems, Minneapolis, MN, USA), 1,000
ng/ml immunoglobulin (Ig)G1 (R&D Systems) as a control, or left
untreated by incubation in serum-free Opti-MEM medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 12 h.
Furthermore, RA-FLS (1×106 cells/well) were stimulated
with 1,000 ng/ml DcR3-Fc for 0, 6, 12 and 24 h.
In another experiment, RA-FLS (1×106
cells/well) were pre-incubated with 5.0 µg/ml monoclonal
mouse anti-human TL1A antibody (clone 6E6; 322204; Biolegend, San
Diego, CA, USA), 5.0 µg/ml mouse IgG1 (BA343; Acris, San
Diego, CA, USA) or serum-free Opti-MEM overnight at 37°C prior to
stimulation with 1,000 ng/ml DcR3-Fc for 12 h and analysis of
IL-12B mRNA levels by RT-qPCR.
Further batches of RA-FLS (1×106
cells/well) were stimulated with 10 or 100 ng/ml recombinant human
IL-12 (R&D Systems), 10 or 100 ng/ml recombinant human IL-23
(R&D Systems) or serum-free Opti-MEM for 12 h for subsequent
assessment of DcR3, TL1A and DR3 mRNA by RT-qPCR.
For quantification of IL-12B mRNA expression in
OA-FLS stimulated with DcR3-Fc, cells (1×106/well) were
incubated with 1,000 ng/ml DcR3-Fc or IgG1 in serum-free
Opti-MEM for 12 h.
For assessment of the expression of IL-12B p40
protein in RA-FLS by western blot analysis, cells
(1×106/well) were stimulated with 1,000 ng/ml DcR3-Fc,
1,000 ng/ml IgG1 or left untreated in serum-free Opti-MEM for 24
h.
RT-qPCR analysis
RA-FLS and OA-FLS were cultured in six-well plates
at 1×106 cells/well with various stimulants as described
above. RNA was extracted using the QIAshredder and RNeasy mini kits
(Qiagen, Hilden, Germany) according to the manufacturer's
protocols. Oligo (dT)-primed first-strand complementary DNA (cDNA)
was synthesized from 2 µg total RNA using a High Capacity
cDNA Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Relative expression levels of mRNA encoding
IL-12B p40, DcR3, TL1A and DR3, which also binds to TL1A, were
compared using TaqMan® real-time PCR on a StepOne™
real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) as follows: 50°C for 2 min and 95°C for 10 min, followed by
45 cycles of 95°C for 15 sec and 60°C for 1 min. Pre-designed
primers and probes for IL-12B (Hs01011518_m1), DcR3
(Hs00187070_m1), TL1A (Hs00270802_s1), DR3 (Hs00600930_g1), and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs99999905_m1) as
the control were obtained from Applied Biosystems (Thermo Fisher
Scientific, Inc.). Comparative analyses of each of these genes in
individual patients were performed using StepOne™ 2.1 software
(Applied Biosystems; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. All amplifications were conducted in
duplicate. mRNA expression levels of each gene were calculated
using the comparative threshold cycle (δδCq) method, as previously
described (24).
Western blot analysis
Following stimulation, cells were washed on ice and
lysed using a solution of protease inhibitor cocktail (Nacalai
Tesque Inc., Kyoto, Japan), phosphatase inhibitor cocktail 2/3
(Sigma-Aldrich) and hypotonic lysis buffer, which contained 25 mM
Tris (Nacalai Tesque Inc.), 150 mM NaCl (Sigma-Aldrich), 1% NP-40
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 1.5 mM
ethylene glycol tetraacetic acid (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). The lysate was incubated for 40 min at 4°C
and was subsequently centrifuged at 20,400 × g for 12 min at 4°C in
order to isolate the supernatant containing the cytoplasmic
proteins. Cytoplasmic proteins were quantified via the Bradford
method using the Bio-Rad Protein Assay Dye Reagent Concentrate
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following dilution
to an equal concentration with hypotonic lysis buffer (25 mM Tris,
150 mM NaCl, 1% NP-40 and 1.5 mM ethylene glycol tetraacetic acid),
each sample was loaded (80 ng/lane) and electrophoresed on a
7.5–15% polyacrylamide gradient gel (Biocraft, Tokyo, Japan) and
electrotransferred onto a blotting membrane (GE Healthcare, Little
Chalfont, UK). The membrane was blocked with 0.05 g/ml skimmed milk
(Megmilk Snow Brand Co., Ltd., Tokyo, Japan) diluted with
Tris-buffered saline with Tween® 20 [TBST; 20 mM Tris
(Nakalai Tesque, Inc.), 150 nM NaCl (Sigma-Aldrich) and 5%
Tween® 20 (Bio-Rad Laboratories, Inc.)] for 1 h at room
temperature. Following washing three times with TBST, the membrane
was incubated with primary antibody diluted with Can Get
Signal® Immunoreaction Enhancer Solution 1 (Toyobo Co.,
Ltd., Osaka, Japan) overnight at 4°C. Following incubation, the
membrane was washed three times with TBST and incubated with
secondary antibody diluted with Can Get Signal® Immunoreaction
Enhancer Solution 2 (Toyobo Co., Ltd.) for 1 h at room
temperature.
The expression of IL-12B p40 and α-tubulin was
detected using mouse anti-human IL-12B p40 antibody (clone 169516;
MAB6091; R&D Systems) and mouse anti-human α-tubulin antibody
(clone DM1A; T9026; Sigma-Aldrich) as primary antibodies,
respectively. Polyclonal sheep horseradish peroxidase-conjugated
anti-mouse IgG antibody (NA931; GE Healthcare) was used as the
secondary antibody, and antibodies were visualized using the ECL™
plus reagent (GE Healthcare) according to the manufacturer's
protocols using the Chemilumino analyzer LAS-3000 mini (FujiFilm,
Tokyo, Japan). Protein expression was evaluated by
semi-quantification of digitally captured images using the public
domain of the US National Institutes of Health Image program
(http://rsb.info.nih.gov/nih-image/)
with normalization to α-tubulin expression.
Statistical analysis
Values are expressed as the mean ± standard
deviation unless otherwise indicated. The Wilcoxon signed-rank test
was used to evaluate the differences between two groups. The
Kruskal-Wallis test was used to evaluate the differences among
three or more groups. If the Kruskal-Wallis test indicated
statistical significance, a post-hoc analysis was performed for
these groups. Statistical analyses conducted using Statcel (version
3; OMS Publishing, Inc., Tokyo, Japan). P<0.05 was considered to
indicate a statistically significant difference.
Results
DcR3-Fc increases IL-12B mRNA expression
in RA-FLS
RT-qPCR analysis revealed that the expression of
IL-12B mRNA in RA-FLS was significantly increased by DcR3-Fc at the
highest concentration of 1,000 ng/ml following incubation for 12 h
(Fig. 1A). Time-course experiments
showed that following 6 and 24 h of stimulation with DcR3-Fc (1,000
ng/ml), the expression of IL-12B mRNA in RA-FLS was significantly
increased to a similar extent and exhibited a peak at 12 h of
stimulation (Fig. 1B).
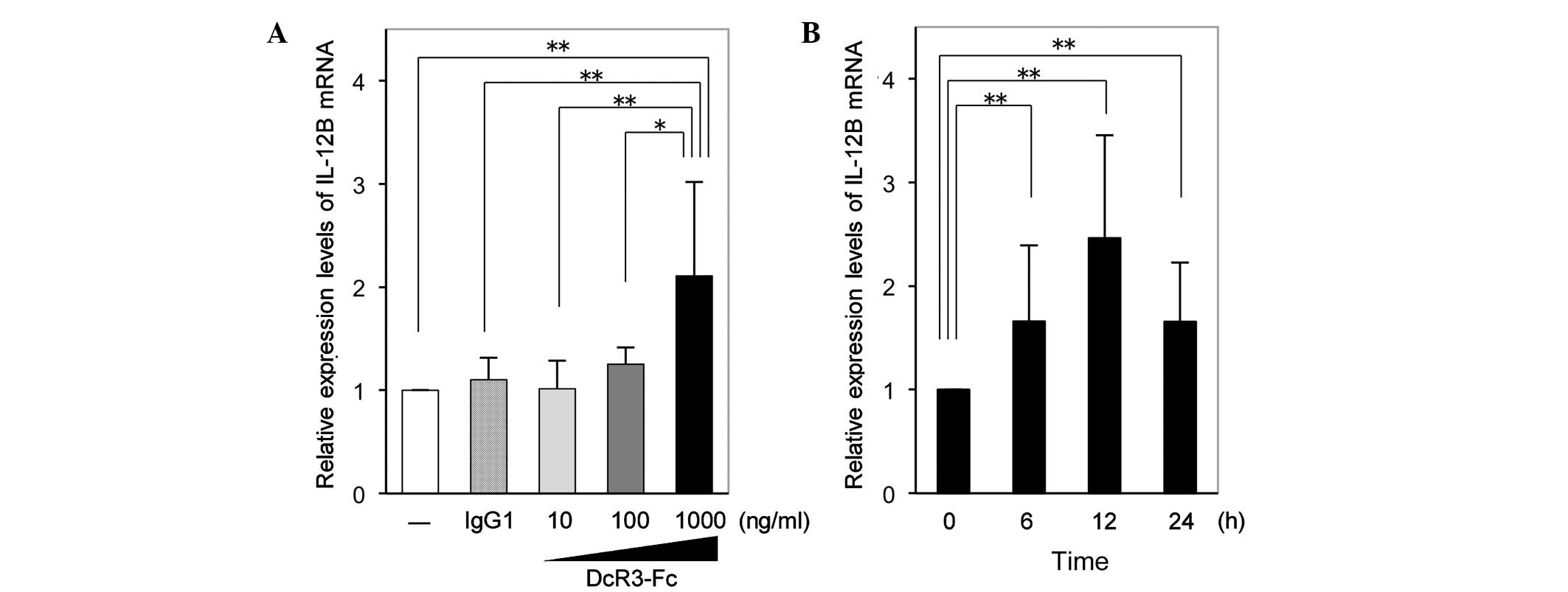 | Figure 1Expression of IL-12B mRNA in RA-FLS.
(A) Relative expression levels of IL-12B mRNA in RA-FLS after 12 h
of incubation with 10, 100 or 1,000 ng/ml DcR3-Fc or 1,000 ng/ml
IgG1, or serum-free medium only. Unstimulated cells were assigned a
value of 1. Values are expressed as the mean ± standard deviation
(n=7). (B) Relative expression levels of IL-12B mRNA in RA-FLS
after 0, 6, 12 or 24 h of incubation with 1,000 ng/ml DcR3-Fc.
Expression at time-point zero was assigned a value of 1. Values are
expressed as the mean ± standard deviation (n=13).
*P<0.05; **P<0.01. IL, interleukin;
IgG, immunoglobulin G; FLS, fibroblast-like synoviocytes; RA,
rheumatoid arthritis; DcR3, decoy receptor 3. |
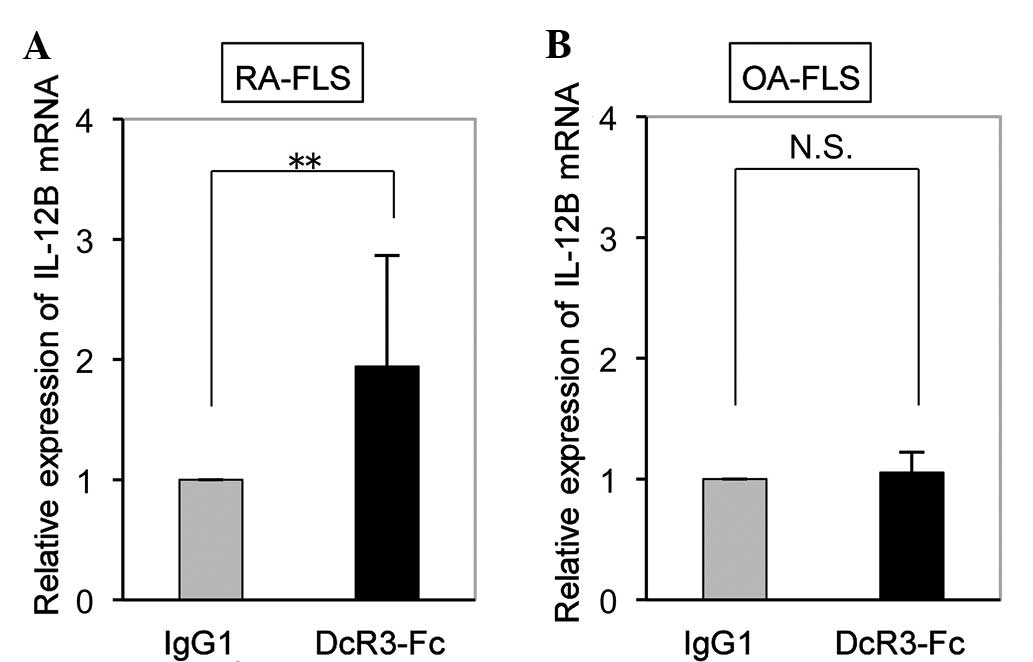
Upregulation of IL-12B mRNA by DcR3-Fc in
FLS is RA-specific
While the expression of IL-12B mRNA in RA-FLS was
significantly increased by DcR3-Fc (Fig. 2A), it was not affected in OA-FLS
(Fig. 2B). This finding suggested
that IL-12B mRNA expression was upregulated by DcR3-Fc in FLS an
RA-specific manner.
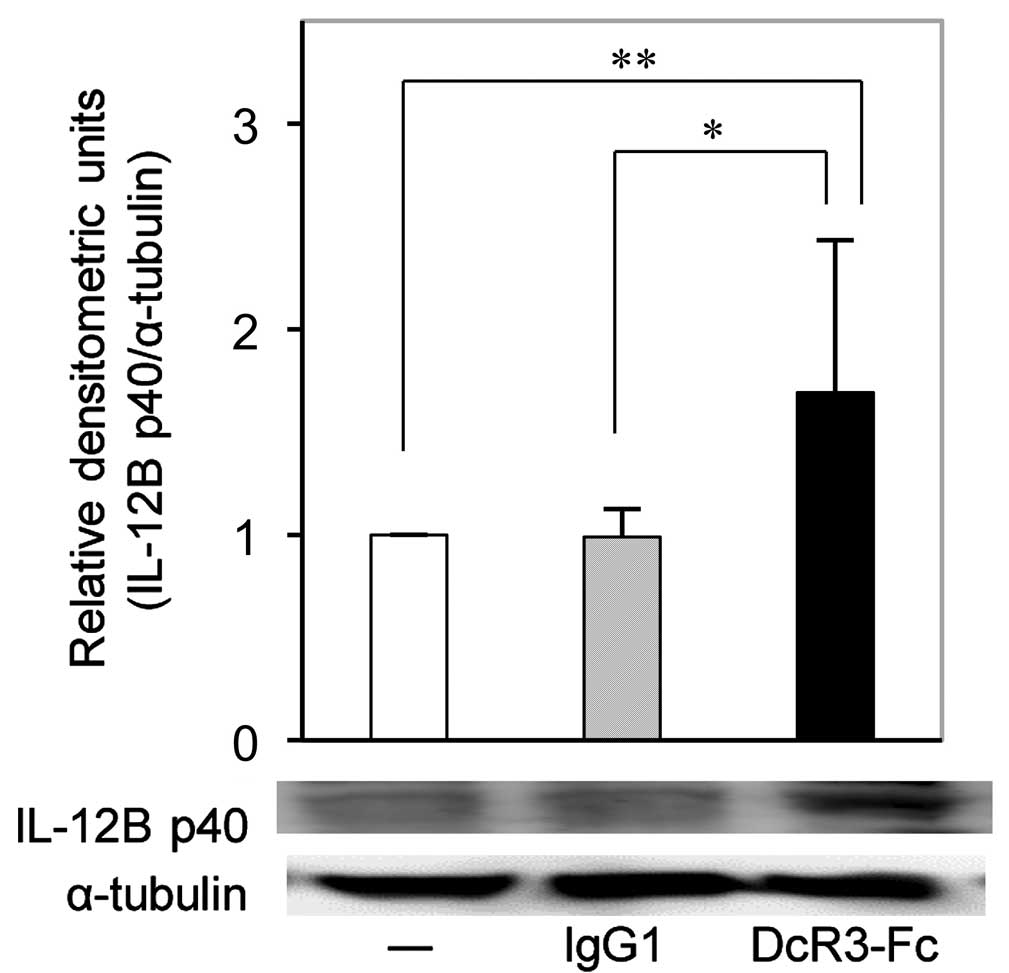
DcR3-Fc increases IL-12B p40 protein
expression in RA-FLS
In accordance with the RT-qPCR results, western blot
analysis confirmed that the expression of IL-12B p40 protein in
RA-FLS was also significantly increased by DcR3-Fc (Fig. 3).
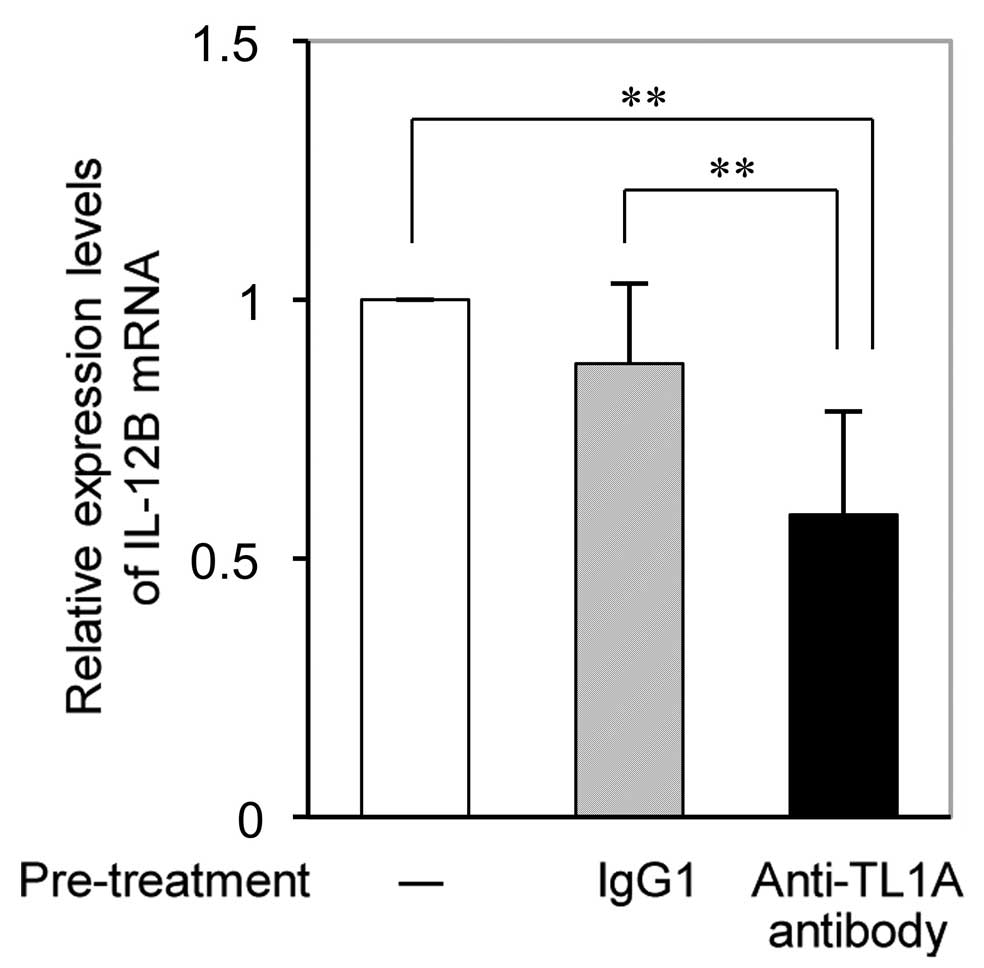
TL1A antibody suppresses DcR3-induced
IL-12B expression in RA-FLS
RT-qPCR analysis revealed that the DcR3-Fc-induced
increases in IL-12B mRNA expression in RA-FLS were significantly
reduced by pre-treatment with anti-TL1A antibody (Fig. 4). While pre-treatment with IgG as a
control slightly but not significantly inhibited DcR3-Fc-induced
IL-12B expression, and the effect of anti-TL1A on IL-12B expression
was significantly higher.
IL-23, but not IL-12 induces DcR3 mRNA
expression in RA-FLS
RT-qPCR revealed that IL-23 induced the expression
of DcR3 mRNA, but not that of TL1A or DR3 mRNA, in RA-FLS. However,
IL-12 did not induce the expression of DcR3, TL1A or DR3 mRNA
(Fig. 5).
Discussion
Previous studies by our group have demonstrated that
DcR3 has a substantial role in local inflammation in RA as a decoy
receptor (11) and as a ligand for
membrane-bound TL1A (15,16). A recent study by our group used a
cDNA microarray assay to reveal the expression profiles of genes
regulated by DcR3 in RA-FLS (16).
The profile revealed upregulation of IL-12B (fold change, 1.65;
P=0.008), which was assigned to the major functional clustering
categories of cell motility and glycosylation (16). IL-12B mRNA encodes the IL-12B p40
subunit of IL-23 and IL-12. IL-23 regulates Th17 and is involved in
the pathogenesis of inflammatory diseases (19,25,26).
By contrast, IL-12 shifts the balance of the Th1 vs. the Th2
response towards the Th1 phenotype (18) and induces interferon gamma
production via Th1 to be involved in the pathogenesis of
inflammatory diseases (27). IL-12
and IL-23 are also key mediators of psoriasis including psoriatic
arthritis and are targeted by Ustekinumab, a human anti-p40
monoclonal antibody therapeutic (28). Mannon et al (29) reported that patients with Crohn's
disease responded well to treatment with monoclonal antibodies
against the p40 subunit of IL-12/IL-23.
The present study confirmed the constitutive
expression of IL-12B mRNA and p40 protein in RA-FLS and
demonstrated that mRNA and protein expression levels were increased
following stimulation with DcR3. Furthermore, while IL-12B mRNA
expression was detected in RA-FLS as well as OA-FLS, DcR3 induced
overexpression of IL-12B mRNA only in RA-FLS, while not affecting
IL-12B expression in OA-FLS. Although the effect of DcR3 on p40
protein expression levels in OA-FLS was not assessed in the present
study, it is unlikely to be affected without detectable effects on
IL-12B mRNA expression. The results of the present study
demonstrated that upregulation of IL-12B mRNA by DcR3-Fc in FLS is
disease-specific for RA, as compared with OA which also causes
severe destructive arthritis that may necessitate joint replacement
but does not accompany autoimmune abnormality. However, other
arthritic conditions are less likely to cause joint destruction,
and these should be investigated in future studies.
Sakkas et al (30) showed that the levels of IL-12B mRNA
are higher in the synovial membrane of patients with RA than those
in patients with OA, although this difference was not statistically
significant. Kitagawa et al (31) reported that the level of
constitutive IL-12 p40 production by synovial cells (SC) from
patients with RA was greater than that by SC from non-RA patients,
including those with OA and ankylosing spondylitis, and that IL-12
p40 induction by lipopolysaccharide, a potent inducer of IL-12
production in macrophages and dendritic cells, in SC from patients
with RA was significantly higher than that in SC from non-RA
patients. Combined with the results of the present study, it is
therefore suggested that DcR3/IL-12B mediates functions that
comprise a disease-specific pathway in RA.
Regarding the mechanisms of the DcR3 - IL-12B
interaction, the present study revealed that binding of DcR3 to
TL1A, which is expressed on RA-FLS, leads to enhanced expression of
IL-12B. In addition, it was demonstrated that IL-23, but not IL-12,
induced the expression of DcR3 in RA-FLS. However, the expression
of TL1A and DR3 was not affected by either IL-23 or IL-12. These
results indicated that DcR3 as a key component of the TL1A/DR3/DcR3
signaling pathway interacts with IL-23 in a feedback loop.
In the present study, the effects of DcR3 on IL-12B
mRNA and p40 protein expression levels were investigated. Although
the effects of TL1A, Fas ligand and LIGHT on IL-12B and p40
expression levels were were not investigated in the present study,
further studies are required in order to assess these effects.
In conclusion, the present study suggested that DcR3
enhances the expression of IL-12B p40 in RA-FLS by binding to
membrane-bound TL1A and may increase local IL-23 and IL-12
expression in the rheumatoid synovium. In addition, IL-23, but not
IL-12, may induce the expression of DcR3 in RA-FLS. DcR3 and IL-23
may be interact in a feedback loop that aggravates local
inflammation in patients with RA. Controlling the expression of
local DcR3 or IL-12B may reduce inflammation in the rheumatoid
synovium and may represent an approach for developing strategies to
treat RA.
Acknowledgments
The authors would like to thank Mr. Kyoko Tanaka,
Ms. Minako Nagata and Ms. Maya Yasuda (Department of Orthopaedic
Surgery, Kobe University Graduate School of Medicine) for technical
assistance. The present study was supported by a grant-in-aid from
the Ministry of Health and Welfare of Japan (grant nos. 24592261
and 15k10473).
References
|
1
|
Goldring SR: Pathogenesis of bone and
cartilage destruction in rheumatoid arthritis. Rheumatology
(Oxford). 42(Suppl 2): ii11–ii16. 2003. View Article : Google Scholar
|
|
2
|
McInnes IB and Schett G: Cytokines in the
pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7:429–442.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pitti RM, Marsters SA, Lawrence DA, Roy M,
Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT,
et al: Genomic amplification of a decoy receptor for Fas ligand in
lung and colon cancer. Nature. 396:699–703. 1998. View Article : Google Scholar
|
|
4
|
Shi G, Wu Y, Zhang J and Wu J: Death decoy
receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo.
J Immunol. 171:3407–3414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Migone TS, Zhang J, Luo X, Zhuang L, Chen
C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al: TL1A is a
TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell
costimulator. Immunity. 16:479–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bai C, Connolly B, Metzker ML, Hilliard
CA, Liu X, Sandig V, Soderman A, Galloway SM, Liu Q, Austin CP and
Caskey CT: Overexpression of M68/DcR3 in human gastrointestinal
tract tumors independent of gene amplification and its location in
a four-gene cluster. Proc Natl Acad Sci USA. 97:1230–1235. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Zhang L and Kim S: Quantification
and detection of DcR3, a decoy receptor in TNFR family. J Immunol
Methods. 285:63–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohshima K, Haraoka S, Sugihara M, Suzumiya
J, Kawasaki C, Kanda M and Kikuchi M: Amplification and expression
of a decoy receptor for fas ligand (DcR3) in virus (EBV or HTLV-I)
associated lymphomas. Cancer Lett. 160:89–97. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuji S, Hosotani R, Yonehara S, et al:
Endogenous decoy receptor 3 blocks the growth inhibition signals
mediated by Fas ligand in human pancreatic adenocarcinoma. Int J
Cancer. 106:17–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu KY, Kwon B, Ni J, Zhai Y, Ebner R and
Kwon BS: A newly identified member of tumor necrosis factor
receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J
Biol Chem. 274:13733–13736. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayashi S, Miura Y, Nishiyama T, Mitani M,
Tateishi K, Sakai Y, Hashiramoto A, Kurosaka M, Shiozawa S and
Doita M: Decoy receptor 3 expressed in rheumatoid synovial
fibroblasts protects the cells against Fas-induced apoptosis.
Arthritis Rheum. 56:1067–1075. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang CR, Wang JH, Hsieh SL, Wang SM, Hsu
TL and Lin WW: Decoy receptor 3 (DcR3) induces osteoclast formation
from monocyte/macrophage lineage precursor cells. Cell Death
Differ. 11(Suppl 1): S97–S107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu MJ, Lin WW, Tsao WC, Chang YC, Hsu TL,
Chiu AW, Chio CC and Hsieh SL: Enhanced adhesion of monocytes via
reverse signaling triggered by decoy receptor 3. Exp Cell Res.
292:241–251. 2004. View Article : Google Scholar
|
|
14
|
Tateishi K, Miura Y, Hayashi S, Takahashi
M and Kurosaka M: DcR3 protects THP-1 macrophages from apoptosis by
increasing integrin alpha4. Biochem Biophys Res Commun.
389:593–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahashi M, Miura Y, Hayashi S, Tateishi
K, Fukuda K and Kurosaka M: DcR3-TL1A signalling inhibits
cytokine-induced proliferation of rheumatoid synovial fibroblasts.
Int J Mol Med. 28:423–427. 2011.PubMed/NCBI
|
|
16
|
Fukuda K, Miura Y, Maeda T, Takahashi M,
Hayashi S and Kurosaka M: Decoy receptor 3 regulates the expression
of various genes in rheumatoid arthritis synovial fibroblasts. Int
J Mol Med. 32:910–916. 2013.PubMed/NCBI
|
|
17
|
Lupardus PJ and Garcia KC: The structure
of interleukin-23 reveals the molecular basis of p40 subunit
sharing with interleukin-12. J Mol Biol. 382:931–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haskó G and Szabó C: IL-12 as a
therapeutic target for pharmacological modulation in
immune-mediated and inflammatory diseases: Regulation of T helper
1/T helper 2 responses. Br J Pharmacol. 127:1295–1304. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paradowska-Gorycka A, Grzybowska-Kowalczyk
A, Wojtecka-Lukasik E and Maslinski S: IL-23 in the pathogenesis of
rheumatoid arthritis. Scand J Immunol. 71:134–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swaak AJ, van den Brink HG and Aarden LA:
Cytokine production in whole blood cell cultures of patients with
rheumatoid arthritis. Ann Rheum Dis. 56:693–695. 1997. View Article : Google Scholar
|
|
21
|
Liu FL, Chen CH, Chu SJ, Chen JH, Lai JH,
Sytwu HK and Chang DM: Interleukin (IL)-23 p19 expression induced
by IL-1beta in human fibroblast-like synoviocytes with rheumatoid
arthritis via active nuclear factor-kappaB and AP-1 dependent
pathway. Rheumatology (Oxford). 46:1266–1273. 2007. View Article : Google Scholar
|
|
22
|
Kim HR, Cho ML, Kim KW, Juhn JY, Hwang SY,
Yoon CH, Park SH, Lee SH and Kim HY: Up-regulation of IL-23p19
expression in rheumatoid arthritis synovial fibroblasts by IL-17
through PI3-kinase-, NF-kappaB- and p38 MAPK-dependent signalling
pathways. Rheumatology (Oxford). 46:57–64. 2007. View Article : Google Scholar
|
|
23
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH and Luthra
HS: The American Rheumatism Association 1987 revised criteria for
the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiel CT, Kraus C, Rauch A, Ekici AB,
Rautenstrauss B and Reis A: A new quantitative PCR multiplex assay
for rapid analysis of chromosome 17p11.2-12 duplications and
deletions leading to HMSN/HNPP. Eur J Hum Genet. 11:170–178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage distinct
from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwakura Y and Ishigame H: The IL-23/IL-17
axis in inflammation. J Clin Invest. 116:1218–1222. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manetti R, Parronchi P, Giudizi MG,
Piccinni MP, Maggi E, Trinchieri G and Romagnani S: Natural killer
cell stimulatory factor (interleukin 12 [IL-12]) induces T helper
type 1 (Th1)-specific immune responses and inhibits the development
of IL-4-producing Th cells. J Exp Med. 177:1199–1204. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gottlieb A and Narang K: Ustekinumab in
the treatment of psoriatic arthritis: Latest findings and clinical
potential. Ther Adv Musculoskelet Dis. 5:277–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mannon PJ, Fuss IJ, Mayer L, Elson CO,
Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL,
et al: Anti-interleukin-12 antibody for active Crohn's disease. N
Engl J Med. 351:2069–2079. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakkas LI, Johanson NA, Scanzello CR and
Platsoucas CD: Interleukin-12 is expressed by infiltrating
macrophages and synovial lining cells in rheumatoid arthritis and
osteoarthritis. Cell Immunol. 188:105–110. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kitagawa M, Mitsui H, Nakamura H, Yoshino
S, Miyakawa S, Ochiai N, Onobori M, Suzuki H and Sumida T:
Differential regulation of rheumatoid synovial cell interleukin-12
production by tumor necrosis factor alpha and CD40 signals.
Arthritis Rheum. 42:1917–1926. 1999. View Article : Google Scholar : PubMed/NCBI
|