Introduction
Endometrial carcinoma (EC) is the most common
malignancy of the female reproductive tract. One of its symptoms is
abnormal vaginal bleeding, which is similar to menstruation
(1). The main treatment method is
surgical resection. Several surgical pathological features of
endometrial cancer have been demonstrated to correlate with
prognosis. These include histological grade, histological type,
depth of myometrial invasion, cervical extension and the presence
of metastatic disease (2). The
majority of patients are already in the advanced stage when they
exhibit symptoms, leading to post-surgical relapse and a low
survival rate. Therefore, methods for early diagnosis and improving
prognosis are required. Non-estrogen dependent endometrial cancer
has a poor prognosis, however, its pathogenesis is not clear,
despite being associated with the abnormal expression of three
types of genes, including oncogenes, tumor suppressor genes and DNA
repair genes (3).
c-erbB-2, also termed HER-2/neu, is an oncogene in
neuroblastoma cells, and is predominantly expressed in embryonic
tissue and in certain normal adult tissues (4). Its proto-oncogene is located on the
long arm of chromosome 17 and encodes a transmembrane tyrosine
kinase receptor that has similarities to the epidermal growth
factor receptor. HER-2/neu overexpression may cause abnormal cell
proliferation, thus resulting in the malignant transformation of
cells. The development of tumor mechanisms of c-erbB-2 include the
inhibition of apoptosis, the formation of tumor blood vessels via
upregulating vascular endothelial growth factor and vascular
permeability factor, and increasing tumor invasiveness by
eradicating the anti-invasion barrier of body tissues. However, the
exact mechanism remains to be elucidated. c-erbB-2 is overexpressed
in various types of tumor, including breast cancer (20–30%), lung
cancer (5), gastric cancer
(6), tumors of the nervous system
(7), kidney neoplasms (8), oral squamous cell carcinoma (9) and ovarian cancer (10). It has been demonstrated that
c-erbB-2 can decrease tumor volume (11) and increase the survival rate in
primary and secondary breast cancer (12).
Macrophage migration inhibitory factor (MIF) is a
soluble factor identified during the activation of T lymphocytes
(13). MIF is a unique protein,
involved in inflammation, immune responses, cell growth and
angiogenesis. MIF has been implicated in natural killer cell
function, where it acts as an immunosuppressive cytokine through
the inhibition of natural killer cell activity (13). MIF is suggested to be important in
the occurrence and development of tumors, by increasing cell
migration (14), proliferation
(15) and angiogenesis (16), and is also able to inhibit
p53-mediated or mitochondrial apoptosis (17). In addition, the pro-tumorigenic
potential of MIF has been reported in glioblastoma multiforme
(18,19), ovarian cancer (20), gastric cancer (21) and non-small cell lung cancer
(22).
Previous studies have reported that c-erbB-2 and MIF
are associated with the occurrence and development of tumors
(23,24). In order to correctly identify that
the targeted therapy of c-erbB-2 and MIF can benefit patients with
endometrial cancer, the first and key step is to accurately detect
the expression of c-erbB-2 and MIF in endometrial cancer. To the
best of our knowledge, there are currently no studies on the
correlation between MIF and c-erbB-2 expression in endometrial
cancer. Consequently, in the present study, the expression of
c-erbB-2 and MIF was detected in normal endometrial (NE),
endometrial hyperplasia and endometrial cancer tissues by
immunohistochemistry and reverse transcription quantitative
polymerase chain reaction (RT-qPCR), and their role in the
occurrence and development of endometrial cancer was discussed.
Patients and methods
Patients
A total of 80 consecutive patients between October
2012 and May 2014, including 40 with EC, 20 with endometrial
hyperplasia and 15 with NE were recruited from The Fourth Hospital
of Harbin Medical University (Harbin, China). Patients were aged
between 40 and 82 years. The presenting symptom was postmenopausal
or intermenstrual bleeding; endometrial adenocarcinoma was the most
common type of cancer and was therefore selected as the cancer that
would be studied. The clinicopathological parameters evaluated were
age, International Federation of Gynecology and Obstetrics (FIGO)
stage, type of carcinoma and depth of myometrial invasion. Among
the 50 patients with EC, according to the 2009 FIGO formulation of
the clinical staging criteria, there were 8 patients with stage I,
30 cases with stage II; 12 cases with stage III–IV; 37 cases with
high differentiation (G1) and 13 cases with moderate/low
differentiation (G2–G3); 30 cases with metastasis and 20 cases with
lymph node metastasis; 29 with a uterine muscular layer of <0.4
cm; and 21 cases with a uterine muscular layer of >0.4 cm. The
present study was approved by the regional ethics committee of The
Fourth Affiliated Hospital of Harbin Medical University. Written
informed consent was obtained from all patients.
Immunohistochemistry
Fresh tissue samples obtained by surgical excision
were fixed in formalin and embedded in paraffin (Sigma-Aldrich, St.
Louis, MO, USA) according to standard procedures. Sections (4
µm) of representative blocks from each case were
deparaffinized, rehydrated and immunostained by the peroxidase
method. Slides were then incubated for 30 min with primary
monoclonal antibodies: Rabbit anti-human c-erbB-2 (dilution, 1:250;
catalogue number, BS8245-006) and rabbit anti-human MIF (dilution,
1:250; catalogue number, BS6432-100) purchased from Beijing Boosen
Biological Technology Co., Ltd. (Beijing, China). Control slides
were incubated for the same period with non-immunized rabbit serum
(negative control). Bound antibody complexes were stained for 10
min with 0.05% diaminobenzidine (Sigma-Aldrich). Sections were then
briefly counterstained with hematoxylin (Sigma-Aldrich), mounted
and examined under a Nikon Eclipse ×400 microscope (Nikon, Tokyo,
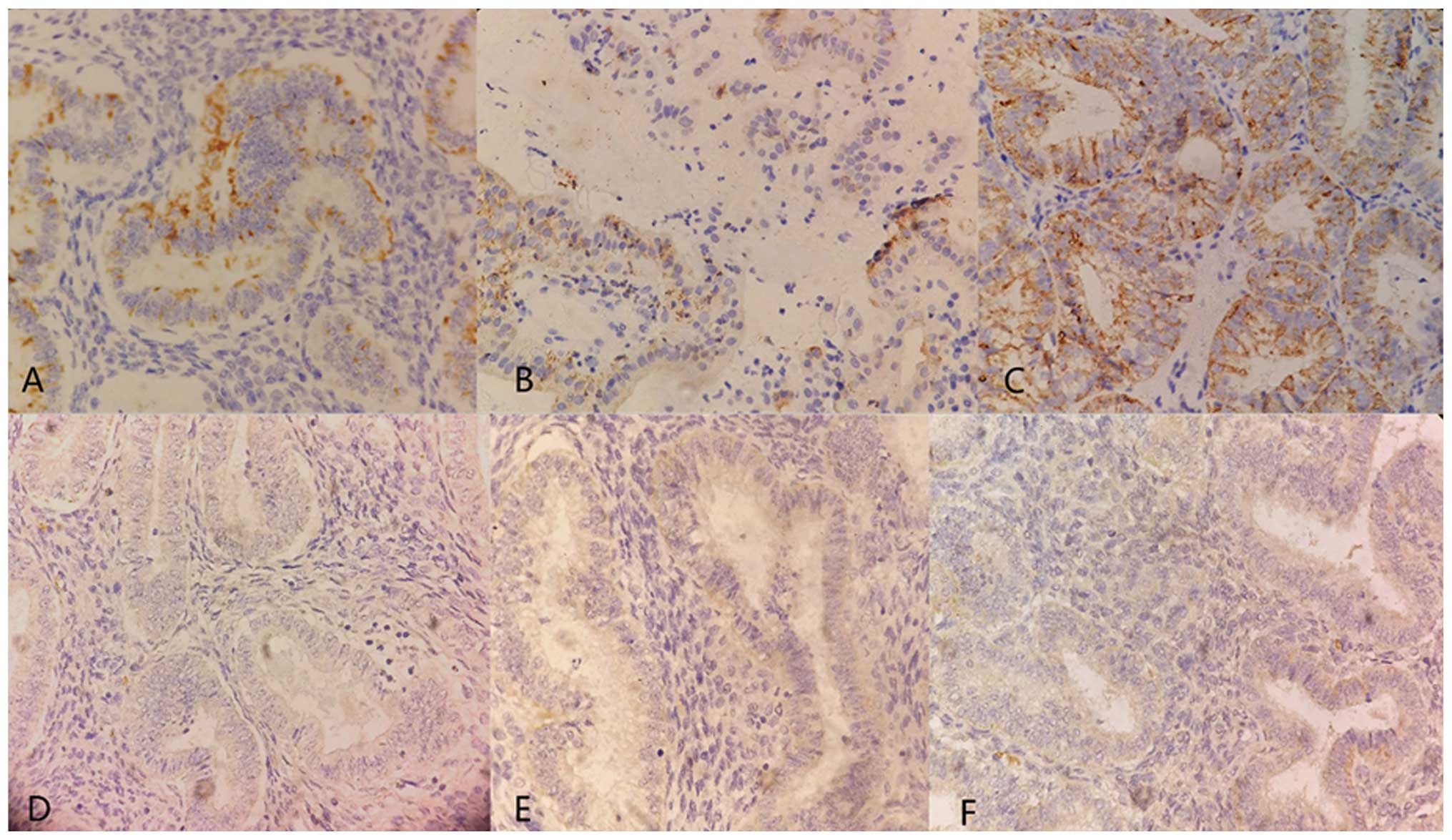
Japan). Positive MIF immunohistochemical staining was indicated by
brown yellow granules in the cytoplasm or cell nucleus. Positive
c-erbB-2 immunohistochemical staining was indicated by brown
granules located in the cell membrane or cytoplasm. The expression
intensity was determined by the degree of staining: No color (−);
light color and positive cells <5% (+); moderate staining, with
positive cells between 5 and 50% (++); dark staining, with >50%
positive cells (+++). The expression of HER-2 and MIF was
classified into two levels, namely, low (− or +) or high (++ or
+++).
RT-qPCR
Following removing and cutting the uterus, a portion
of the tissue (0.5×0.5 cm) was immediately scraped into a small EP
tube with RNA preservation solution and stored in a deep-freezer
(−80°C) until assayed. Total RNA from cells was isolated using
TRIzol reagent (Fuzhou Maixin Biotechnology Development Co., Ltd.,
Fuzhou, China) according to the manufacturer's protocol. Equal
quantities of RNA were reverse-transcribed and qPCR analysis was
performed using qPCR Master-mix [2 µl templated cDNA reverse
transcriptase, 0.5 µl gene sense primers, 0.5 µl gene
antisense primer, 10 µl Taq DNA polymerase (Shanghai Sangon
Biological Engineering Technology & Services Co., Ltd.,
Shanghai, China), 7 µl deionized water 10X PCR buffer and 1
µl SYBR Green (Thermo Fisher Scientific, Inc.)]. All primers
were synthesized by Shanghai Invitrogen Biotechnology Co., Ltd.
(Shanghai, China). The gene sequences were as follows: MIF, forward
5′-GCACAGCATCGGCAAGAT-3′ and reverse 3′-GAGTTGTTCCAGCCCACATT-5′;
c-erbB-2, forward 5′-CTGAACAATACCACCCCTGTC-3′ and reverse
3′-AGATGTCCTTCCACAAAATCGT-5′; GAPDH forward
5′-AGGTGAAGGTCGGAGTCAAC-3′ and reverse 3′-CGCTCCTGGAAGATGGTGAT-5′.
PCR was performed in a PX2 thermacycler (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) The reaction conditions were as follows:
Denaturation at 94°C for 1 min; amplification and quantification:
30 cycles of 94°C for 30 sec, 56°C for 30 sec and 70°C for 40 sec;
melting curve: 70°C for 7 min. The conditions for c-erbB-2 were the
same as MIF and GAPDH except the annealing temperature was 55°C.
The relative quantity of each target gene mRNA to the housekeeping
gene (GAPDH) was calculated as ΔCq, where ΔCq=Cq gene − GAPDHCq.
The fold change of each target gene mRNA to the corresponding
normal tissue was calculated as 2(−ΔΔCq), where ΔΔCq=ΔCq
target gene of the experimental group − ΔCq target gene in the
control group.
Statistical analysis
Data were analyzed using SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA). MIF and c-erbB-2 mRNA expression
results of the three groups of tissues are presented as the mean ±
standard deviation. Differences were analyzed using χ2
test and the two genes in each group were compared using
independent samples t-test. The correlation between the expression
of MIF protein and c-erbB-2 protein was analyzed using McNemar's
test. The confidence interval was 95% and P≤0.05 was considered to
indicate a statistically significant difference.
Results
Protein expression of c-erbB-2 and
MIF
Immunoreactivities were scored under a light
microscope at a magnification of ×400 and the mean percentage of
tumor cells that demonstrated positive staining was assessed.
c-erbB-2 and MIF protein were detected in NE, atypical hyperplasia
and EC (Fig. 1; Table I). The positive rates of MIF
protein in NE, atypical hyperplasia and EC were 20, 45 and 70%,
respectively (P=0.03). c-erbB-2 protein was positive in four normal
cases (26.7%), seven atypical hyperplasia cases (35%) and 32 EC
cases (64%).
 | Table INumber of cases with a positive
expression of MIF and c-erbB-2 in normal endometrium, atypical
hyperplasia and endometrial carcinoma. |
Table I
Number of cases with a positive
expression of MIF and c-erbB-2 in normal endometrium, atypical
hyperplasia and endometrial carcinoma.
| Group | No. of cases | MIF (+) | Positive (%) | c-erbB-2 (+) | Positive (%) |
|---|
| Normal
endometrium | 15 | 3 | 20 | 4 | 26.7 |
| Atypical
hyperplasia | 20 | 9 | 45 | 7 | 35 |
| Endometrial
cancer | 50 | 35 | 70 | 32 | 64 |
MIF protein expression was significantly lower in
endometrial carcinoma with early FIGO stages (P=0.036), low grading
G1 (P=0.013) and no lymphovascular invasion (P=0.012). c-erbB-2
expression was significantly lower in endometrial carcinoma with
early FIGO stages (P= 0.036), depth of myometrial invasion <0.4
cm (P=0.007) and no lymphovascular invasion (P=0.012; Table II). However, no statistically
significant difference was identified between the expression of MIF
and c-erbB-2 in different age groups. Subsequently, the protein
expression of MIF and c-erbB-2 was analyzed (Table III). When comparing MIF and
c-erbB-2 protein expression with clinical stage, histological
grade, depth of myometrial invasion and lymph node metastasis, it
was found that MIF protein had a higher expression in tumors at
stages I–II (χ2=6.632; P=0.01), grade G1
(χ2=11.064; P=0.001) and with no lymph node metastasis
(χ2=6.556; P=0.01). By contrast, c-erbB-2 protein
expression was higher in tumors at stages III–IV
(χ2=4.800; P=0.024), grade G2–G3 (χ2=6.788;
P=0.009) and with lymph node metastasis (χ2=6.149;
P=0.013). In addition, no consistency was observed between MIF and
c-erbB-2 (χ2=3.35; P<0.05; Table IV).
 | Table IIAssociation between the protein
expression of MIF and c-erbB-2 and the pathological characteristics
of endometrial carcinoma. |
Table II
Association between the protein
expression of MIF and c-erbB-2 and the pathological characteristics
of endometrial carcinoma.
| Clinical feature | Cases | c-erbB-2
| MIF
|
|---|
| + | − | Positive (%) | P-valuea | + | − | Positive (%) | P-valuea |
|---|
| Age (years) | | | | | | | | | |
| ≤60 | 28 | 18 | 10 | 64.3 | 0.962 | 19 | 9 | 78.6 | 0.709 |
| >60 | 22 | 14 | 8 | 63.6 | | 16 | 6 | 63.6 | |
| Stage (2009
FIGO) | | | | | | | | | |
| I | 8 | 3 | 5 | 37.5 | 0.036 | 2 | 6 | 25.0 | 0.009 |
| II | 30 | 18 | 12 | 60.0 | | 23 | 7 | 76.7 | |
| III–IV | 12 | 11 | 1 | 91.7 | | 10 | 2 | 83.3 | |
| Grade | | | | | | | | | |
| G1 | 37 | 20 | 17 | 54.1 | 0.013 | 25 | 12 | 78.4 | 0.527 |
| G2–G3 | 13 | 12 | 1 | 92.3 | | 10 | 3 | 76.9 | |
| Myometrial
invasion | | | | | | | | | |
| <0.4 cm | 29 | 17 | 12 | 58.6 | 0.352 | 16 | 13 | 55.2 | |
| >0.4 cm | 21 | 15 | 6 | 71.4 | | 19 | 2 | 90.5 | 0.007 |
| Lymph node
metastasis | | | | | | | | | |
| No | 30 | 15 | 15 | 50.0 | 0.012 | 17 | 13 | 56.7 | |
| Yes | 20 | 17 | 3 | 85.0 | | 18 | 2 | 90.0 | 0.012 |
 | Table IIIComparison between the protein
expression of MIF and c-erbB-2 and the pathological characteristics
of EC. |
Table III
Comparison between the protein
expression of MIF and c-erbB-2 and the pathological characteristics
of EC.
| Clinical
feature | c-erbB-2
| P-valuea | MIF
| P-valuea |
|---|
| Cases (+)b | Low | High | χ2 | Cases (+)b | Low | High | χ2 |
|---|
| Age (years) | | | | | | | | | | |
| ≤60 | 18 | 10 | 8 | 0.508 | 0.722 | 19 | 11 | 8 | 2.485 | 0.115 |
| >60 | 14 | 6 | 8 | | | 16 | 5 | 11 | | |
| Stage (2009
FIGO) | | | | | | | | | | |
| I–II | 21 | 14 | 7 | 6.788 | 0.009 | 25 | 8 | 17 | 6.632 | 0.010 |
| III–IV | 11 | 2 | 9 | | | 10 | 8 | 2 | | |
| Grade | | | | | | | | | | |
| G1 | 20 | 13 | 7 | 4.800 | 0.028 | 25 | 7 | 18 | 11.064 | 0.001 |
| G2–G3 | 12 | 3 | 9 | | | 10 | 9 | 1 | | |
| Myometrial
invasion | | | | | | | | | | |
| <0.4 cm | 17 | 7 | 10 | 1.129 | 0.479 | 16 | 4 | 12 | 5.096 | 0.024 |
| >0.4 cm | 15 | 9 | 6 | | | 19 | 12 | 7 | | |
| Lymph node
metastasis | | | | | | | | | | |
| No | 15 | 11 | 4 | 6.149 | 0.013 | 17 | 4 | 13 | 6.556 | 0.010 |
| Yes | 17 | 5 | 12 | | | 18 | 12 | 6 | | |
 | Table IVCorrelation between MIF and
c-erbB-2. |
Table IV
Correlation between MIF and
c-erbB-2.
| c-erbB-2 | MIF
|
|---|
| + | − | Total |
|---|
| + | 25 | 7 | 15 |
| − | 10 | 8 | 18 |
| Total | 35 | 15 | 18 |
mRNA expression of c-erbB-2 and MIF
DNA sequencing confirmed the amplification of the
intended target sequence for MIF, c-erbB-2 and GAPDH. MIF mRNA and
c-erbB-2 mRNA could be detected in all samples analyzed. GAPDH was
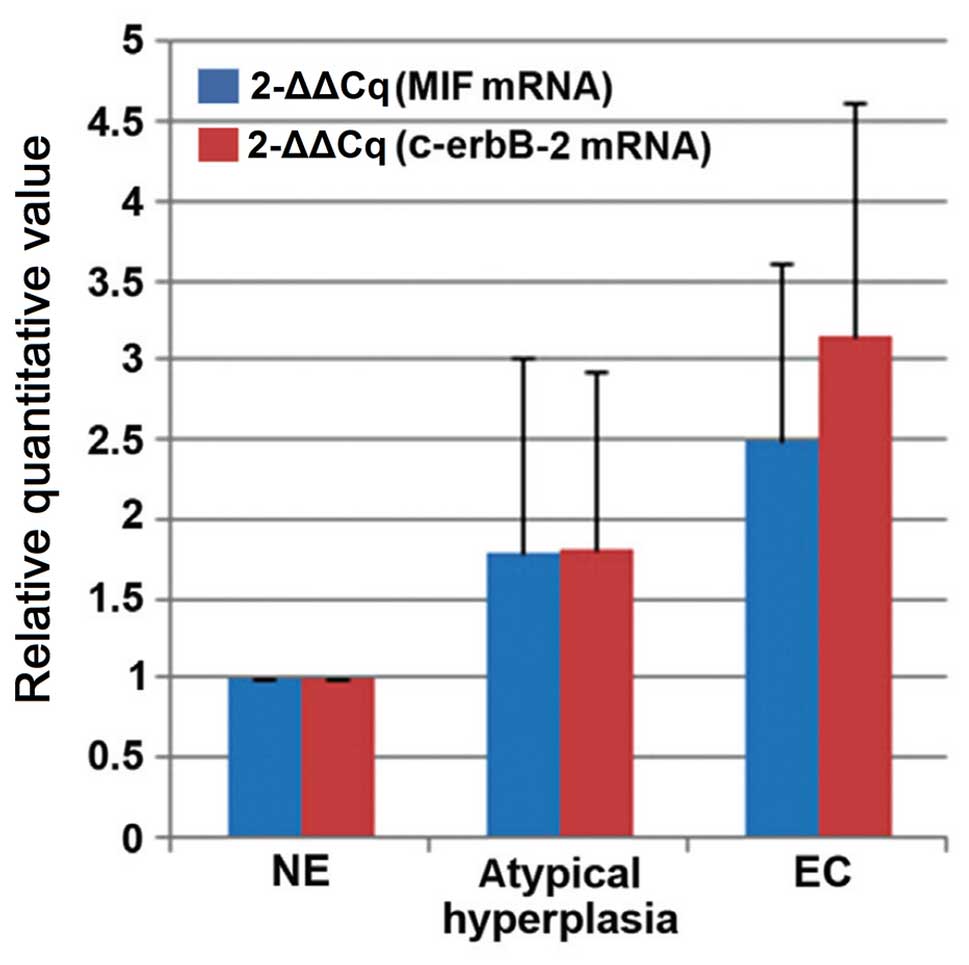
used as the internal control gene for endometrial samples (Fig. 2). Normalized expression levels of
MIF differed among the samples: The normal endometrial cancer
tissue was used as a control and atypical hyperplasia samples
demonstrated an average expression value of 1.798±1.216, and EC
samples had an expression value of 2.494±1.108. The c-erbB-2 mRNA
expression value in the atypical hyperplasia and EC samples was
1.808±1.127 and 3.147±1.471, respectively. The MIF and c-erbB-2
mRNA expression levels were upregulated in EC samples, in
comparison with in atypical hyperplasia and normal endometrium
(P<0.05).
MIF mRNA and c-erbB-2 mRNA levels were also analyzed
according to the following parameters of EC samples: Age, clinical
stage, histological grade, depth of myometrial invasion and lymph
node metastasis (Fig. 3). The
analysis of MIF mRNA expression demonstrated no statistically
significant difference in the age and depth of myometrial invasion
(P>0.05). MIF mRNA overexpression appeared to correlate with
lower aggressiveness: It was significantly associated with early
FIGO stages, (P=0.001), low grading G1 (P=0.004) and no
lymphovascular invasion (P=0.012). It was observed that c-erbB-2
mRNA levels had no difference in age (P>0.05). The analysis
demonstrated that the levels of c-erbB-2 mRNA were higher in FIGO
stages III–IV, grading G2-3, deeper invasion and lymphovascular
invasion, compared with early FIGO stages, low grading G1, a depth
of myometrial invasion <0.4 cm and no lymphovascular invasion,
respectively (3.354±1.254 vs. 2.953±1.686, P=0.630; 2.291±1.231 vs.
4.323±1.221, P=0.001; 2.323±1.264 vs. 4.263±1.250, P=0.003;
2.459±1.307 vs. 4.028±1.397, P=0.033; 2.352±1.389 vs. 4.067±1.284,
P=0.024).
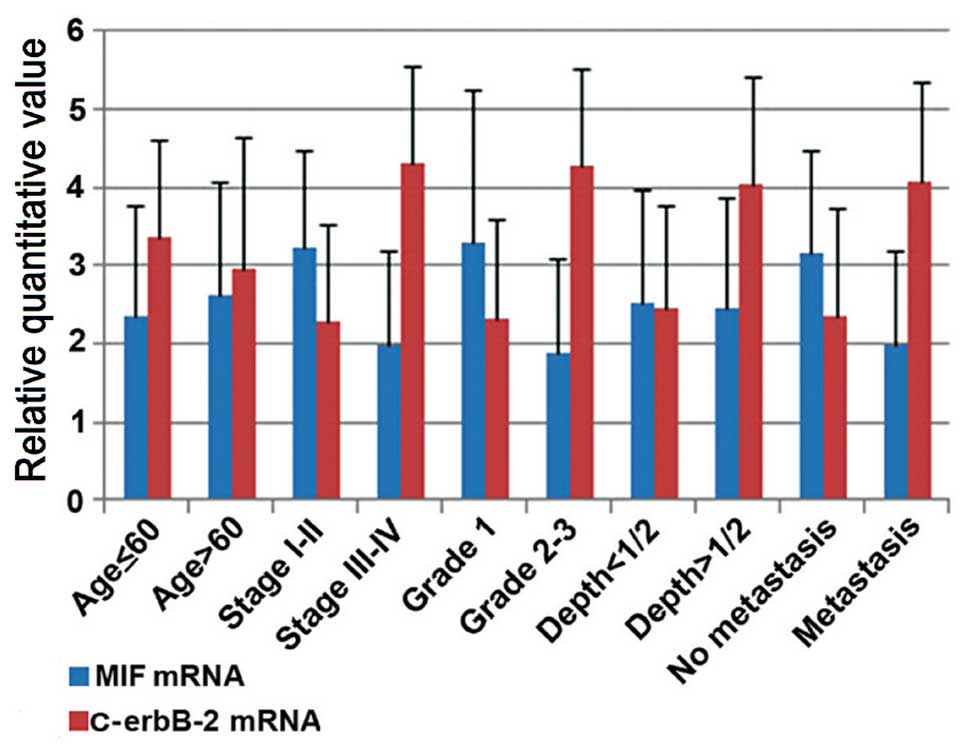 | Figure 3Abscissa indicates pathological
characteristics, the vertical axis represents gene content. The
expression of MIF (blue) and c-erbB-2 (red) in endometrial
carcinoma was compared with pathological characteristics, including
age, FIGO stage, histological grade, depth of myometrial invasion
and lymph node metastasis. The two genes in each group were
compared using an independent samples t-test. MIF overexpression is
significantly associated with characteristics of low invasiveness,
including early stage, low grading and no lymphovascular invasion
(P<0.05), whereas c-erbB-2 mRNA levels were higher in tumors
with FIGO stages III–IV, grading G2-3, deeper invasion and
lymphovascular invasion, compared with tumors with early FIGO
stages, low grading G1, depth of myometrial invasion <0.4 cm and
no lymphovascular invasion. MIF, macrophage migration inhibitory
factor; FIGO, International Federation of Gynecology and
Obstetrics. |
Discussion
Several studies have demonstrated that the c-erbB-2
gene is associated with the metastasis and invasion of breast
cancer, which reflects the potential ability of local growth,
invasion and lymphatic metastasis of this tumor (25,26).
It may therefore be a detection index of early recurrence, shorter
survival rate and prognosis. Another study demonstrated that
c-erbB-2 had a significantly higher expression in breast cancer
with bone metastasis (P=0.029) (27). The study also demonstrated that
c-erbB-2 contributed to tumor invasion and metastasis, and thus can
be used as a prognostic indicator. A previous study confirmed that
the angiogenic microvessel density was significantly higher in
breast cancer patients with overexpression of c-erbB-2 protein,
compared with the c-erbB-2 negative groups (P<0.05) (28). Therefore, c-erbB-2 is an
independent prognostic indicator in breast cancer.
The present study demonstrated that c-erbB-2 is
associated with clinical stage, histological grade, lymph node
metastasis (P<0.05), but not with age and depth of myometrial
invasion (P>0.05). In addition, c-erbB-2 had a higher expression
in tumors with FIGO stages III–IV, grade G2-3, deeper invasion and
lymphovascular invasion. This was confirmed in a previous study
(29). Morrison et al
analyzed 110 cases of endometrial cancer and the experimental
results demonstrated that c-erbB-2 was correlated with histological
grade (the positive rate of G1 tumor was significantly higher than
G2 and G3 tumor), but not with age, clinical stage, histological
type or the depth of myometrial invasion (30). In regards to the high expression
level in G1, this study contrasts with the results of the present
study.
To the best of our knowledge, there is only one
study focusing on MIF in endometrial cancer: Bondza et al
found that MIF treatment significantly stimulated vascular
endothelial growth factor expression in a dose- and time-dependent
manner in EC (31). A previous
study associated MIF with tumor growth and progression by
stimulating tumor-associated angiogenesis, but not in endometrial
cancer. Hagemann et al observed that MIF was strongly
expressed in malignant ascites, and that MIF generated by ovarian
cancer cells could stimulate the expression of cytokines,
chemokines and tumor angiogenesis factors, and contribute towards
the vascularization and angiogenesis of tumors (32). The authors found that MIF was
strongly expressed in malignant ascites, which suggests that MIF
autocrine generated by ovarian cancer cells stimulated other
cytokines, chemokines, angiogenesis factor, and contributed to the
vascularization and angiogenesis of the tumor (32). Nishihira et al concluded
that MIF is closely associated with tumor growth and angiogenesis
through the treatment of mice colon cancer cells with the antisense
MIF gene (33). This study
demonstrated that MIF can promote tumor angiogenesis, growth,
invasion and metastasis.
The presence of MIF mRNA and protein could be
observed in all endometrial samples. The overexpression of MIF mRNA
and protein is associated with low histological grade, early FIGO
stages and no lymphovascular invasion (P<0.05). Similarly,
previous studies found that MIF overexpression correlates with
lower aggressiveness and was significantly associated with early
FIGO stage, low grading G1-2, no lymphovascular invasion and
confirms the data reported by other authors on other tumor types
(21,34,35).
This suggests that, in patients with endometrial cancer, the
upregulation of MIF may be associated with the inhibition of
metastatic spread.
Finally, the correlation between MIF and c-erbB-2
was analyzed and no significant association was found between them
(χ2=3.35; P>0.05). In the mouse model of HER2-driven
breast cancer, Schulz et al concluded that HER2
overexpression can inhibit MIF activity (36). The present study failed to come to
this conclusion. Since the amount of endometrial hyperplasia is not
sufficient, the samples can not be divided into groups of more
detail, and thus the association between the expression of MIF and
c-erbB-2 in endometrial hyperplasia cannot be confirmed.
To the best of our knowledge, the present study is
the first to focus on the conjoint analysis of MIF and c-erbB-2 by
RT-qPCR and immunohistochemistry. In our population study, the
results are consistent between these two types of test. MIF and
c-erbB-2 were overexpressed in endometrial cancer samples
suggesting that MIF and c-erbB-2 are involved in the occurrence and
development of tumors. It is hypothesized that the imbalance in the
expression of MIF and c-erbB-2 could be a possible critical step in
the progression of endometrial cancer. Although this hypothesis
needs to be confirmed in a larger number of cases, it may be
clinically relevant.
These data suggest that overexpression of MIF and
c-erbB-2 is associated with the occurrence and development of
endometrial cancer. The upregulation of MIF may be associated with
the inhibition of metastatic spread, however, upregulation of MIF
may promote tumor progression. In conclusion, MIF and c-erbB-2 are
correlated with the occurrence and the development of endometrial
cancer, and thus can be used for the early diagnosis and prognosis
of endometrial cancer. However, the detailed functional
significance of MIF and c-erbB-2 in endometrial cancer remains to
be determined. Taken together, the current aim is to identify new
prognostic factors to customize adjuvant therapies and new targets
for anticancer therapies.
Acknowledgments
This study was supported by the Science Foundation
of Heilongjiang Province of China (grant no. H201429).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mhawech-Fauceglia P, Wang D, Samrao D,
Godoy H, Pejovic T, Liu S and Lele S: Pair-Box (PAX8) protein
positive expression is associated with poor disease outcome in
women with endometrial cancer. Br J Cancer. 107:370–374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verit FF and Yucel O: Endometriosis,
leiomyoma and adenomyosis: The risk of gynecologic malignancy.
Asian Pac J Cancer Prev. 14:5589–5597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Z and Ford BD: Uperegulation of erbB
recepters in rat brain after middle cerebrial arterial occlusion.
Neurosci Lett. 375:181–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Araújo A, Ribeiro R, Azevedo I, Coelho A,
Soares M, Sousa B, Pinto D, Lopes C, Medeiros R and Scagliotti GV:
Genetic polymorphisms of the epidermal growth factor and related
receptor in non-small cell lung cancer-a review of the literature.
Oncologist. 12:201–210. 2007. View Article : Google Scholar
|
|
6
|
Oshima CT, Lanzoni VP, Iriya K and Forones
NM: C-erbB-2 oncoprotein expression in gastric carcinoma:
Correlation with clinical stage and prognosis. Int J Biol Markers.
16:250–254. 2001.
|
|
7
|
Potti A, Forseen SE, Koka VK, Pervez H,
Koch M, Fraiman G, Mehdi SA and Levitt R: Determination of
HER-2/neu overexpression and clinical predictors of survival in a
cohort of 347 patients with primary malignant brain tumors. Cancer
Invest. 22:537–544. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rotter M, Block T, Busch R, Thanner S and
Höfer H: Expression of Her-2/neu in renal-cell carcinoma.
Correlation with histologic subtypes and differentiation. Int J
Cancer. 52:213–217. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manavi M, Bauer M, Baghestanian M, Berger
A, Kucera E, Pischinger K, Battistutti W and Czerwenka K: Oncogenic
potential of c-erbB-2 and its association with c-K-ras in
premalignant and malignant lesions of the human uterine
endometrium. Tumour Biol. 22:299–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xi L, Satpathy M, Zhao Q, Qian W, Yang L
and Jiang H: HER-2/neu targeted delivery of a nanoprobe enables
dual photoacoustic and fluorescence tomography of ovarian cancer.
Nanomedicine. 10:669–677. 2014.
|
|
11
|
Carson WE, Parihar R, Lindemann MJ,
Personeni N, Dierksheide J, Meropol NJ, Baselga J and Caligiuri MA:
Interleukin-2 enhances the natural killer cell response to
Herceptin-coated Her2/neupositive breast cancer cells. Eur J
Immunol. 31:3016–3025. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Apte RS, Sinha D, Mayhew E, Wistow GJ and
Niederkorn JY: Cutting edge: Role of macrophage migration
inhibitory factor in inhibiting NK cell activity and preserving
immune privilege. J Immunol. 160:5693–5696. 1998.PubMed/NCBI
|
|
14
|
Rendon BE, Roger T, Teneng I, Zhao M,
Al-Abed Y, Calandra T and Mitchell RA: Regulation of human lung
adenocarcinoma cell migration and invasion by macrophage migration
inhibitory factor. J Biol Chem. 282:29910–29918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liao H, Bucala R and Mitchell RA:
Adhesion-dependent signaling by macrophage migration inhibitory
factor (MIF). J Biol Chem. 278:76–81. 2003. View Article : Google Scholar
|
|
16
|
Amin MA, Volpert OV, Woods JM, Kumar P,
Harlow LA and Koch AE: Migration inhibitory factor mediates
angiogenesis via mitogen-activated protein kinase and
phosphatidylinositol kinase. Circ Res. 93:321–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baumann R, Casaulta C, Simon D, Conus S,
Yousefi S and Simon HU: Macrophage migration inhibitory factor
delays apoptosis in neutrophils by inhibiting the
mitochondria-dependent death pathway. FASEB J. 17:2221–2230. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bacher M, Schrader J, Thompson N, Kuschela
K, Gemsa D, Waeber G and Schlegel J: Up-regulation of macrophage
migration inhibitory factor gene and protein expression in glial
tumour cells during hypoxic and hypoglycemic stress indicates a
critical role for angiogenesis in glioblastoma multiforme. Am J
Pathol. 162:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Repp AC, Mayhew ES, Apte S and Niederkorn
JY: Human uveal melanoma cells produce macrophage
migration-inhibitory factor to prevent lysis by NK cells. J
Immunol. 165:710–715. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krockenberger M, Dombrowski Y, Weidler C,
Ossadnik M, Hönig A, Häusler S, Voigt H, Becker JC, Leng L, Steinle
A, et al: Macrophage migration inhibitory factor contributes to the
immune escape of ovarian cancer by down-regulating NKG2D. J
Immunol. 180:7338–7348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia HH, Yang Y, Chu KM, Gu Q, Zhang YY, He
H, Wong WM, Leung SY, Yuen ST, Yuen MF, et al: Serum macrophage
migration-inhibitory factor as a diagnostic and prognostic
biomarker for gastric cancer. Cancer. 115:5441–5449. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White ES, Flaherty KR, Carskadon S, Brant
A, Iannettoni MD, Yee J, Orringer MB and Arenberg DA: Macrophage
migration inhibitory factor and CXC chemokine expression in
non-small cell lung cancer: Role in angiogenesis and prognosis.
Clin Cancer Res. 9:853–860. 2003.PubMed/NCBI
|
|
23
|
Zheng J and Zhu YM: Expression of c-erbB-2
proto-oncogene in extrahepatic cholangiocarcinoma and its clinical
significance. Hepatobiliary Pancreat Dis Int. 6:412–415.
2007.PubMed/NCBI
|
|
24
|
Wu S, Lian J, Tao H, Shang H and Zhang L:
Correlation of macrophage migration inhibitory factor gene
polymorphism with the risk of early-stage cervical cancer and
lymphatic metastasis. Oncol Lett. 2:1261–1267. 2011.
|
|
25
|
Lambropoulou M, Stefanou D, Alexiadis G,
Tamiolakis D, Tripsianis G, Chatzaki E, Vandoros GP, Kiziridou A,
Papadopoulou E and Papadopoulos N: Cytoplasmic expression of
c-erbB-2 in endometrial carcinomas. Onkologie. 30:495–500. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhatavdekar JM, Patel DD, Shah NG, Vora
HH, Suthar TP, Chikhlikar PR, Ghosh N and Trivedi TI: Prognostic
significance of immunohistochemically localized biomarkers in stage
II and stage III breast cancer. A multivariate analysis. Ann Surg
Oncol. 7:305–311. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim R, Arihiro K, Emi M, Tanabe K and
Osaki A: Potential role of HER-2; in primary breast tumor with bone
metastasis. Oncol Rep. 15:1477–1484. 2006.PubMed/NCBI
|
|
28
|
Pérez-Regadera J, Sánchez-Muñoz A,
De-la-Cruz J, Ballestín C, Lora D, García-Martín R, Mendiola C,
Alonso L, Alba E and Lanzós E: Negative prognostic impact of the
coexpression of epidermal growth factor receptor and c-erbB-2 in
locally advanced cervical cancer. Oncology. 76:133–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toi M, Kashitani T and Tominaga T: Tumor
angiogenesis is an independent prognostic indicator in primary
breast carcinoma. Int J Cancer. 55:371–374. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morrison C, Zanagnolo V, Ramirez N, Cohn
DE, Kelbick N, Copeland L, Maxwell GL and Fowler JM: HER-2 is an
independent prognostic factor in endometrial cancer: Association
with outcome in a large cohort of surgically staged patients. J
Clin Oncol. 24:2376–2385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bondza PK, Metz CN and Akoum A: Macrophage
migration inhibitory factor upregulates alpha(v)beta (3) integrin
and vascular endothelial growth factor expression in endometrial
adenocarcinoma cell line Ishikawa. J Reprod Immunol. 77:142–151.
2008. View Article : Google Scholar
|
|
32
|
Hagemann T, Robinson SC, Thompson RG,
Charles K, Kulbe H and Balkwill FR: Ovarian cancer cell-derived
migration inhibitory factor enhances tumor growth, progression and
angiogenesis. Mol Cancer Ther. 6:1993–2002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishihira J, Ishibashi T, Fukushima T, Sun
B, Sato Y and Todo S: Macrophage migration inhibitory factor (MIF):
Its potential role in tumor growth and tumor-associated
angiogenesis. Ann NY Acad Sci. 995:171–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Denz A, Pilarsky C, Muth D, Rückert F,
Saeger HD and Grützmann R: Inhibition of MIF leads to cell cycle
arrest and apoptosis in pancreatic cancer cells. J Surg Res.
160:29–34. 2010. View Article : Google Scholar
|
|
35
|
Verjans E, Noetzel E, Bektas N, Schütz AK,
Lue H, Lennartz B, Hartmann A, Dahl E and Bernhagen J: Dual role of
macrophage migration inhibitory factor (MIF) in human breast
cancer. BMC Cancer. 9(230)2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schulz R, Streller F, Scheel AH, Rüschoff
J, Reinert MC, Dobbelstein M, Marchenko ND and Moll UM: HER2/ErbB2
activates HSF1 and thereby controls HSP90 clients including MIF in
HER2-overexpressing breast cancer. Cell Death Dis. 5:e9802014.
View Article : Google Scholar : PubMed/NCBI
|