Introduction
Bronchial asthma is a complex chronic inflammatory
disease of the airways characterized by repeated episodes of airway
hyperresponsiveness (AHR) (1) and
an allergic inflammatory response. This process involves the
infiltration of inflammatory cells, including mast cells,
eosinophils, neutrophils and lymphocytes, into the pulmonary tissue
(2,3).
A number of epidemiological studies have
investigated the incidence of asthma in adults, estimated incidence
rates are varied, ranging between 1/1000 and 7/1000 person-years
(4,5). An overall incidence rate of 2/1000
person-years in the 20–50 years age group has been determined in
previous studies with a prospective design (6,7).
Therapy with medium to high doses of inhaled corticosteroids are
currently generally effective in achieving symptom relief (8).
Mitogen-activated protein kinases (MAPKs) are
intracellular signal transduction molecules implicated in airway
inflammation and AHR (9). There
are three major groups of MAPKs in mammalian cells, including
extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK and
c-JUN N-terminal kinase (JNK) (10). ERK1/2 is significantly increased in
lung tissue in response to allergens (11), and promotes the production of
inflammatory cytokines and chemokines (12). In addition, ERK1/2 contributes to
goblet cell metaplasia (13),
T-helper 2 (Th2) cell differentiation, proliferation of mature B
cells and the migration of eosinophils (14).
Zanthoxylum bungeanum Maxim, known in China
as Hua Jiao, is a member of the Rutaceae family. The fruits of
Zanthoxylum are used as a spice in Chinese cuisine, and as a
traditional medicine for the treatment of stomachache, toothache,
abdominal pain, ascariasis and diarrhea (15). Previous studies have supported the
therapeutic properties of Zanthoxylum, and it was indicated
that the chloroform-soluble extract of Zanthoxylum is able
to inhibit hepatitis B virus DNA replication (16–19).
Furthermore, Z. bungeanum seed oil (Z. seed oil)
demonstrated a significant antioxidant activity (20). However, the therapeutic potential
of Z. seed oil remains undefined, particularly in inflammatory
diseases, such as asthma. In the present study, the effect of Z.
seed oil was investigated in an ovalbumin (OVA)-induced asthmatic
murine model, and the data suggested that Z. seed oil may serve as
a potential anti-inflammatory agent for asthmatic patients.
Materials and methods
Preparation of Z. seed oil
Whole Z. bungeanum seeds were collected from
Hangcheng (Shaanxi, China) in September 2008 and dried; the oil was
then extracted by squeezing 100 kg of broken seeds using a
multifunctional oil press (Gongyi Xiaoyi Jinwang Machinery Factory,
Gongyi, China). Voucher specimens of the seeds and oil extract were
deposited at the Xin Run Pharmaceutical Company (Xi'an, China). The
crude oil was purified using a silica gel column (Qingdao Yonghai
Silica Gel Co., Ltd., Qingdao, China) and activated carbon
chromatography. The ratio of crude oil, silica gel and activated
carbon (Lv Bao Shi Industry and Trade Co., Ltd., Xi'an, China) in
the glass column was 1:5:0.5. The purified oil (one of 16
fragments) was eluted from the column with petroleum ether
(Li'an-Long Bohua Medical Chemical Co., Ltd., Shaanxi, China) and
ethanol (Li'an-Long Bohua Medical Chemical Co., Ltd.) at a ratio of
8:2 at room temperature. Eluates were then concentrated under
reduced pressure (0.6 kPa).
Animals and treatments
All experimental protocols were approved by the
Institutional Ethical Committee for Animal Experimentation of The
Fourth Military Medical University (Xi'an, China). Male BALB/c mice
(Experimental Animal Center, The Fourth Military Medical
University; age, 6–8 weeks; weight, 22±2 g, n=240) were fed with a
standard diet and a constant supply of tap water. In addition, mice
were housed in a 12-h light/dark cycle at room temperature (23±3°C)
with 40–60% relative humidity levels. The mice were divided into
three groups of 80 (which was further divided into 5 time points,
n=16): Vehicle control (non-asthmatic), OVA-treated and OVA + Z.
seed oil-treated. The vehicle control group was administered with
200 µl saline (Shandong Jiejing Pharmaceutical Co., Ltd.,
Rizhou, China) only. To induce allergic asthma in the OVA-treated
and OVA + Z. seed-treated groups, mice were administered with 20 mg
OVA (Sigma-Aldrich, St. Louis MO, USA) emulsified with 40 mg
aluminum hydroxide (Shanghai Huashi Pharmaceutical Co., Ltd.,
Shanghai, China) in 200 µl 0.9% saline intraperitoneally
(i.p.). Eight days after the initial sensitization, mice were
administered 5 mg OVA emulsified with 10 mg aluminum hydroxide in
200 µl saline (i.p.). On day 14, the OVA-treated mice were
administered 50 µl saline (containing 20 µg/50
µl OVA) via intranasal inhalation for 24 and 48 h, and 3, 7
and 14 days. Mice in the Z. seed oil-treated group were
administered 50 µl saline (containing 20 µg/50
µl OVA) via intranasal inhalation, after 30 min 2 g/kg Z.
seed oil was administered orally, once per day for 24 and 48 h and
3, 7 and 14 days, while mice in the vehicle and OVA-treated groups
were administered placebo edible oil (Yihai Kerry Foodstuffs
Marketing Co., Ltd., Shenzhen, China) once per day.
Following treatment, six mice from each group were
selected for bronchoalveolar lavage fluid (BALF) collection.
Briefly, 60 mg/kg sodium pentobarbital (Guangzhou Chemical Reagent
Factory, Guangzhou, China) was administered i.p. and a tracheal
intubation tube was inserted. Lungs were flushed three times with
0.5 ml sterilized saline to recover ~1.2 ml BALF. These mice were
then sacrificed by cervical dislocation. In addition, for each time
point, eight mice per group were sacrificed by cervical dislocation
and their lungs were collected. Four pairs of lungs per group were
fixed in 4% buffered paraformaldehyde solution (Tianjin Baishi
Chemical Co., Ltd., Tianjin, China) and embedded in paraffin (Leica
Microsystems GmbH, Wetzlar, Germany) for histological examination.
The remaining four pairs of lungs were washed with ice-cold saline,
dehydrated with filter paper and stored at −80°C for further
analysis.
Measurement of interleukin-4 (IL-4),
IL-5, interferon γ (IFNγ), leukocytes and eosinophils levels in
BALF
The collected BALF was centrifuged for 5 min at 380
× g and 4°C to separate the cells from the supernatant. The
supernatant was stored at −80°C to be used for the measurement of
cytokines. As indicators of inflammation, the concentrations of
IL-4, IL-5 and IFN-γ were measured in the BALF supernatants using
enzyme linked immunosorbent assays, according to the manufacturer's
protocol (Shanghai Bio-Tech Co., Ltd., Shanghai, China). The BALF
cell pellet was resuspended in 100 µl Wright staining
solution (Zhuhai Beisuo Biotechnology Co., Ltd., Zhuhai, China) and
total leukocytes were measured with a Neubauer hemocytometer
(Jiangyin Medical Devices Co., Ltd., Jiangyin, China). To quantify
the eosinophil and neutrophil cell populations, 50 µl of the
resuspended cells were adhered onto a glass slide using an
Invitrogen Cytospin centrifuge (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 400 × g for 4 min. Slides were stained with
Giemsa stain (Zhuhai Beisuo Biotechnology Co., Ltd.) following
standard protocols. Eosinophils were identified by a rose-red
cytoplasm and neutrophils by a light pink cytoplasm. A total of 500
cells were counted under a microscope (DM LB; Leica Microsystems
GmbH; ×1,000 magnification) and the percentage of eosinophils was
calculated.
Histological analysis of lung sections
and lung inflammation grading
The paraffin-embedded fixed lung samples were cut
into 4-µm tissue sections. For the examination of bronchial
inflammation, lung tissue sections were stained with hematoxylin
and eosin (H&E; Zhuhai DL Biotech Co., Ltd., Zhuhai, Guangdong,
China) visualized with a DM LB microscope and scored using
established criteria (21) in a
blinded manner by two pathologists averaging their judgments for
the categorization. Inflammatory cell infiltration was graded into
four categories: Grade 0, normal lung structure; Grade I,
infiltration of a small number of diffuse inflammatory cells; Grade
II, a 1-cell thick ring of inflammatory cells; Grade III, a 2–4
cell thick ring of inflammatory cells; Grade IV, a ring of
inflammatory cells >4 cells deep.
Immunohistochemistry
Lung tissue sections were deparaffinized with xylene
(Li'an-Long Bohua Medical Chemical Co., Ltd.) and washed in serial
dilutions of ethanol for immunohistochemical staining analysis.
Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide in methanol (Li'an-Long Bohua Medical Chemical Co., Ltd.)
for 5 min, and non-specific binding was blocked with 1% bovine
serum albumin (Chembase Bio; Beijing, China) in phosphate-buffered
saline (PBS; EUROIMMUN AG, Luebeck, Germany) for 1 h. Sections were
incubated with rabbit polyclonal proliferating cell nuclear antigen
(PCNA) primary antibody (1:600; BIOSS, Beijing, China; cat. no.
bS-0941R) overnight at 4°C. Following three PBS washes (2 ml),
slides were incubated with horseradish peroxidase-conjugated
AffiniPure goat anti-mouse IgG (H + L) for 20 min (cat. no.
ZB-2305) for 20 min. Immunoreactivity was visualized with a
3,3′-diaminobenzi-dine substrate (Zymed Laboratories; Thermo Fisher
Scientific, Inc.), the slides were counterstained with H&E, and
then mounted using resin adhesive (Leica Microsystems GmbH). All
stained tissue sections were analyzed with bright-field microscopy
using a DM LB microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For RT-qPCR analysis, frozen tissue samples were
homogenized in TRIzol (Takara Bio, Inc., Otsu, Japan) using 1 ml
TRIzol per 50 mg of lung tissue, and total RNA was extracted using
RNAiso Plus kit (Takara Bio, Inc.) according to the manufacturer's
protocol. RNA concentration was measured using a spectrophotometer
(DU-800; Beckman Coulter, Inc., Brea, CA, USA). RT-qPCR was
conducted using the PrimeScript™ RT Master mix kit (Takara Bio,
Inc.) according to the manufacturer's protocols. Probes were
synthesized by Takara Bio, Inc.
Following RT, the cDNA products were amplified in a
Bio-Rad iQ™ 5 Real Time PCR detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) with reaction conditions, as follows: 95°C
for 30 sec, followed by 40 cycles at 95°C for 10 sec and 60°C for
20 sec). Each sample had a final volume of 20 µl containing
80 ng cDNA, 0.8 µl forward and reverse primers, 10 µl
2X SYBR® Premix Ex Taq (Takara Bio, Inc.) and 6.4
µl dH2O. mRNA expression levels were determined
using a Bio-Rad iQ™ 5 Real Time PCR detection system (Bio-Rad
Laboratories, Inc.) using the specific primers indicated in
Table I. Following amplification,
melting curve analysis was performed to assess the specificity of
the amplified PCR products. qPCR products were quantified by the
quantification cycle at which specific fluorescence became
detectable (22). The mRNA levels
of each gene were normalized to those of the housekeeping gene
GAPDH.
 | Table INucleotide sequences of reverse
transcription-quantitative polymerase chain reaction. |
Table I
Nucleotide sequences of reverse
transcription-quantitative polymerase chain reaction.
| Target mRNA | Accession no. | Forward primer | Reverse primer |
|---|
| LTC4S | NM 008521.1
(mouse) |
5′-CTGTGGCTGGCAACATGAAG-3′ |
5′-CCTTCGTGCAGAGATCACCTGTAG-3′ |
| IL-8 | NM 009909.3
(mouse) |
5′-GGGCCCTGATCCCTGTGTAGTA-3′ |
5′-GAGGTGCTAGGATTTGAGCCTGAG-3′ |
| MCP-1 | NM 011333.1
(mouse) |
5′-GCATCCACGTGTTGGCTCA-3′ |
5′-CTCCAGCCTACTCATTGGGATCA-3′ |
| CCR-3 | NM 009914.4
(mouse) |
5′-ATGGAGTGGCTATGGCTCAGG-3′ |
5′-TGTTCTGCTCAAATAGTTGTCATGG-3′ |
| ERK | NM 001038663.1
(mouse) |
5′-AACGTTCTGCACCGTGACCTC-3′ |
5′-ACCAACGTGTGGCTACGTACTCTG-3′ |
| GAPDH | NM 008084.2
(mouse) |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
| TNF-α | NM 013693.2
(mouse) |
5′-AAGCCTGTAGCCCACGTCGTA-3′ |
5′-GGCACCACTAGTTGGTTGTCTTTG-3′ |
| ICAM-1 | NM 010493.2
(mouse) |
5′-AACTGTGGCACCGTGCAGTC-3′ |
5′-AGGGTGAGGTCCTTGCCTACTTG-3′ |
Protein extraction and western
blotting
Pulmonary tissue was washed with ice-cold PBS, lysed
with radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China; 50 mM Tris-HCl, pH 7.2; 150 mM NaCl;
1% NP 40; 0.1% SDS; 1 mM EDTA; 1 mM phenylmethanesulfonyl fluoride)
and then homogenized in ice-cold water for 2–3 min. The lung
homogenate samples were incubated for 30 min at 4°C and shaken by
hand every 10 min. Samples were then centrifuged at 24,000 × g for
10 min at 4°C and the supernatants were collected. Total protein
concentration was determined using the BCA protein assay (Beyotime
Institute of Biotechnology) and the samples were denatured in 5X
loading buffer by incubation at 100°C for 10 min. Equal quantities
of protein from each treatment group were separated on 10%
SDS-polyacrylamide gels run at 120 V for 60 min and then
electro-transferred to a polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were incubated with
primary antibodies, as follows rabbit polyclonal anti-Ras (1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA; cat. no. 3965),
rabbit polyclonal anti-ERK1/2 (1:1,000; Santa Cruz Biotechnology,
Inc.; cat. no. sc-94), rabbit polyclonal anti-phosphorylated
(p)-ERK1/2 (1:600; Santa Cruz Biotechnology, Inc.; sc-7383), rabbit
polyclonal anti-p38 MAPK (1:800; Santa Cruz Biotechnology, Inc.;
sc-7149), rabbit polyclonal anti-p-p38 MAPK (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 9211), rabbit polyclonal
anti-JNK (1:800; Santa Cruz Biotechnology, Inc.; sc-572), rabbit
polyclonal anti-nuclear factor-κB-p65 (NF-κB-p65; 1:1,000; Santa
Cruz Biotechnology, Inc.; cat. no. sc-372), rabbit polyclonal
anti-c-fos (1:1,000; Santa Cruz Biotechnology, Inc.; cat. no.
sc-52), rabbit polyclonal anti-c-JUN (1:600; Santa Cruz
Biotechnology, Inc.; cat. no. sc-1694), rabbit polyclonal β-actin
(1:600; Wuhan Boster Biological Technology, Ltd., Wuhan, China;
cat. no. MK1656), mouse monoclonal anti-p-JNK (1:600; Cell
Signaling Technology, Inc.; cat. no. sc-6254), mouse monoclonal
anti-p-activating transcription factor-2 (ATF-2; 1:600; Santa Cruz
Biotechnology, Inc.; cat. no. sc-8398), rabbit monoclonal
anti-p-NF-κB-p65 (1:1,000; Cell Signaling Technology, Inc.; cat.
no. 3033), rabbit polyclonal intercellular adhesion molecule 1
(ICAM-1; 1:600; BIOSS; cat. no. bS-0608R), rabbit polyclonal
anti-tumor necrosis factor-α (TNF-α; 1:600; BIOSS; cat. no.
bS-00789), rabbit polyclonal anti-CD14 (1:600; Wuhan Boster
Biological Technology, Ltd.; cat. no. BA0719-2), rabbit polyclonal
anti-TNF-receptor (TNF-R; 1:600; BIOSS; cat. no. bS-2941R), rabbit
polyclonal anti-toll-like receptor (TLR)2 (1:600; BIOSS; cat. no.
bS-1019R) and rabbit polyclonal anti-TLR4 (1:600; BIOSS; cat. no.
bS-1021R). The membranes were then incubated with AffiniPure
anti-mouse (ZB-2301) or anti-rabbit (ZB-2305) horseradish
peroxidase-conjugated AffiniPure IgG (H + L) secondary antibodies
(ZSGB-BIO, Beijing, China). After washing with Tris-buffered saline
and Tween 20, membranes were incubated with enhanced
chemiluminescence substrate (Pierce Chemical Co., Dallas, TX, USA)
and signals were visualized by exposure to X-ray films (Tianjin
Media Imaging Materials Co., Ltd., Tianjin, China). Proteins were
quantified by densitometry using Gel-Pro Analyzer Image software
version 4.0 (Media Cybernetics Inc., Rockville, MD, USA). All
protein levels were normalized relative to the β-actin
expression.
Nuclear protein extraction
Nuclear extracts were prepared using the Nuclear and
Cytoplasmic Protein Extraction kit, according to the manufacturer's
protocol (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Briefly, 100 mg lung tissue from each group were homogenized in
ice-cold PBS and incubated for 5 min on ice to allow large
particles to settle. The supernatant was then transferred and
centrifuged at 500 × g for 3 min. The supernatant was discarded and
100 µl cold Buffer A (supplemented with protease inhibitors)
was added for every 10 µl of packed cells. Samples were
vortexed for 15 sec and incubated on ice for 10–15 min prior to
addition of cold Buffer B. Samples were then vortexed and
centrifuged at 16,000 × g at 4°C for 5 min, and the supernatant
containing cytoplasmic proteins was collected. Nuclear proteins
were extracted by incubating the remaining pellet with cold Buffer
C. Samples were incubated on ice for 40 min, shaken by hand every
10 min and centrifuged at 16,000 × g at 4°C for 10 min. The
supernatant containing nuclear proteins was collected, flash-frozen
in liquid nitrogen and stored at −80°C.
Statistical analysis
The experimental results are presented as the mean ±
standard deviation. Statistical analysis was performed using the
paired, nonparametric Student's t-test or one-way analysis of
variance, followed by the Bonferroni correction or the Games-Howell
test. SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA)
was used perform all tests and a value of P<0.05 was considered
to indicate a statistically significant difference.
Results
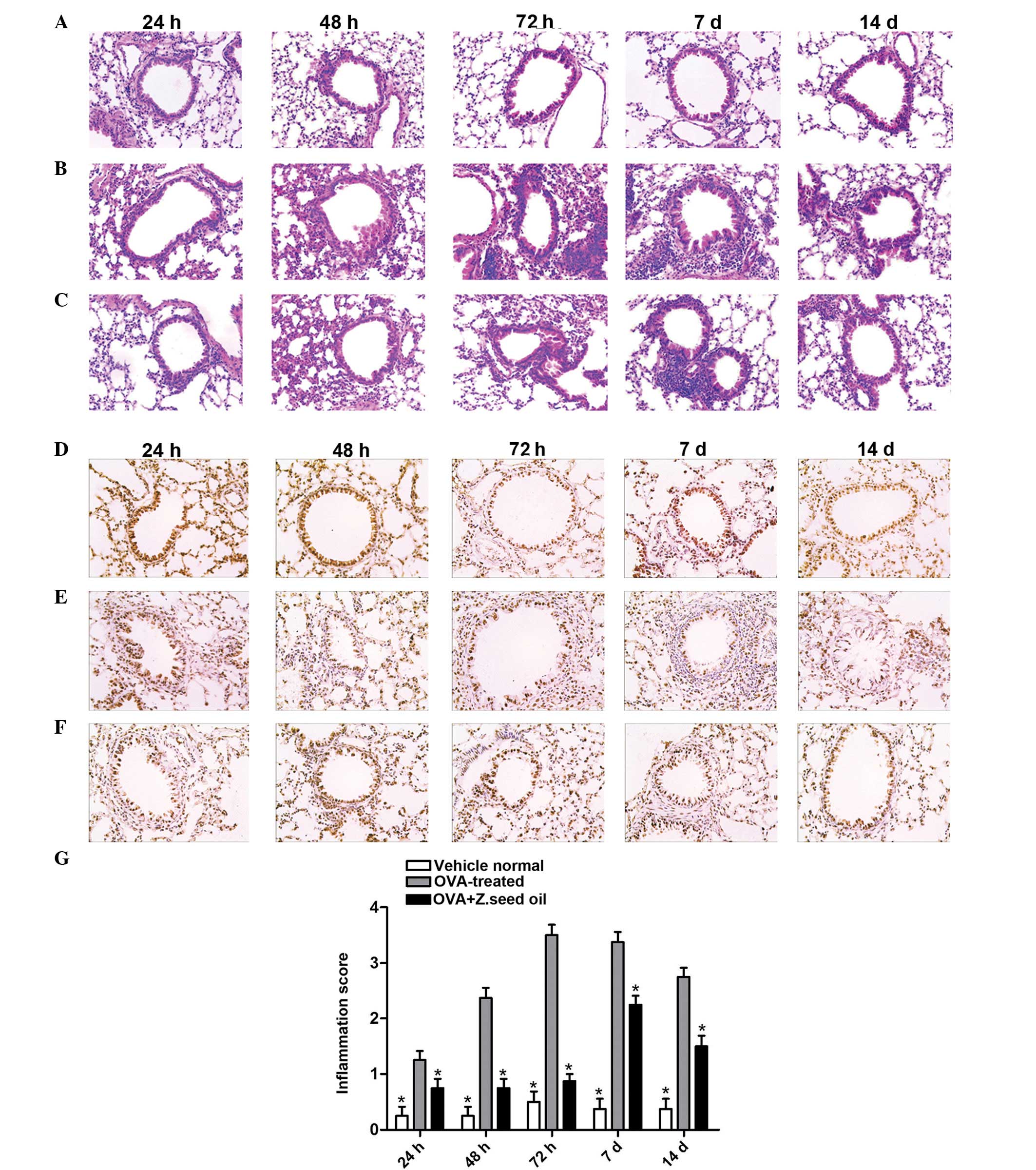
Histological analyses of the effect of Z.
seed oil in OVA-induced asthmatic mice
To investigate the therapeutic efficacy of Z. seed
oil in the lung tissue of asthmatic mice, a well-established murine
model of asthma was employed to induce asthma by OVA exposure for
time periods ranging between 24 h and 14 days. In addition to the
OVA treatment, mice were administered either placebo edible oil or
Z. seed oil. Following treatment, mice were sacrificed and the lung
tissue was collected for analysis by H&E and
immunohistochemical staining (Fig.
1). Lung tissue sections from vehicle-treated mice demonstrated
an inflammatory infiltration score of 0.30±0.10 (24 h), 0.30±0.10
(48 h), 0.43±0.11 (72 h), 0.36±0.05 (7 days) and 0.50±0.26 (14
days) (Fig. 1A, D and G),
indicating healthy and intact lung structure without significant
inflammation. By contrast, lung tissue from OVA-treated mice
revealed marked infiltration of inflammatory cells (primarily
eosinophils and neutrophils) into the peribronchial and
perivascular connective tissues as early as 24 h after OVA
treatment (Fig. 1B and E). The
OVA-treated group had a pulmonary injury score of 2.36±0.48 at 48 h
(Fig. 1G), and grade II
inflammation was observed, as indicated by the one-cell thick ring
of infiltrated inflammatory cells in Fig. 1B. This demonstrated significant
pulmonary inflammation at an early stage of asthma sensitization.
Furthermore, the inflammation score was significantly higher in the
OVA-treated group compared with the vehicle at all time points
(P<0.05; Fig. 1G).
Lung inflammation and tissue damage substantially
increased following 48 h of sustained OVA challenge. Infiltration
of inflammatory markers markedly increased, and swelling on the
alveolar walls, alveolar epithelial cells and a massive influx of
polymorphonuclear leukocytes were observed (Fig. 1B and E). Analysis of the lung
tissue at 48 h revealed an inflammatory cell infiltration score of
2.4±0.45 (Fig. 1G), with class III
inflammation, as indicated by the 2–4 cell thick ring of
inflammatory cells (Fig. 1B).
After 72 h of OVA challenge, inflammation was even more pronounced,
with an infiltration score of 3.56±0.72 (Fig. 1G), and grade IV inflammation, as
indicated by the >4-cell thick ring of inflammatory cells
(Fig. 1B). In addition to the
phenotypes observed at 48 h, swollen bronchial epithelial cells,
detachment of cells and hypertrophic sub-mucosal mucous glands with
mucosal and sub-mucosal edema were observed. In Fig. 1B, a significant hyperplasia of
bronchial smooth muscle cells, formation of lymph follicles and
constricting of the bronchial airway due to increased cell mass
were also demonstrated after 72 h.
Following 7 days of sustained OVA challenge, the
inflammatory infiltration score was 3.56±0.64, suggesting that the
inflammatory infiltration had not progressed past 72 h (Fig. 1G). The inflammatory response at day
7 appeared to induce airway remodeling, as bronchial smooth muscle
hypertrophy and an increase in goblet cells were observed among
changes in the ciliated epithelial cells and basement membrane
thickening (Fig. 1B) compared with
the vehicle-treated group (Fig.
1A). Following 14 days of sustained OVA challenge, inflammatory
cell infiltration significantly decreased, with an infiltration
score of 2.70±0.26 compared with the vehicle-treated group
(0.50±0.26; P<0.05; Fig. 1G)
and decreased compared with infiltration score at 72 h (3.56±0.64)
and 7 days (3.36±0.64). Bronchial smooth muscle hyperplasia
markedly increased, with lymph follicles surrounding the airway.
Furthermore, metaplasia of goblet cells in the tracheal and
bronchial mucosa and increased bronchial epithelial cell
proliferation in the OVA-challenged mice was observed (Fig. 1B) compared with the vehicle-treated
group at 14 days (Fig. 1A).
Administration of Z. seed oil (2 g/kg) to asthmatic
mice significantly alleviated damage to the lung tissue. Leukocyte
infiltration was markedly decreased in the Z. seed oil-treated mice
(Fig. 1C), with significantly
reduced infiltration scores at each time point compared with the
OVA-treated group (0.80±0.17 at 24 h, 0.73±0.40 at 48 h, 0.93±0.30
at 72 h, 2.20±0.52 at 7 days and 1.5±0.30 at 14 days; P<0.05;
Fig. 1G). Pulmonary tissue injury
was markedly alleviated, and airway remodeling, lymph follicles
formation, goblet cells and bronchial epithelial cell hyperplasia
were all reduced during the full time period compared with the
vehicle-treated group (Fig. 1C). A
reduction in PCNA staining in the bronchial and peribronchial
epithelia of OVA-treated mice was detected (Fig. 1E) compared with the vehicle-treated
mice (Fig. 1D). However,
administration of Z. seed oil resulted in an increase in PCNA
expression in the peribronchial epithelium and perivascular
connective tissue at each time point (Fig. 1F) compared with the OVA-treated
mice (Fig. 1E).
Z. seed oil inhibits the infiltration of
total leukocytes and eosinophils into the airways
To examine the effect of Z. seed oil on disease
progression, leukocyte and eosinophil populations were analyzed in
the BALF of Z. seed oil- or placebo oil-treated mice.
Administration of Z. seed oil significantly reduced the total
leukocyte population by 21–33% (P<0.05; Fig. 2A) and the eosinophil population by
5–51% (P<0.05; Fig. 2B)
compared with the OVA-treated group. Additionally, the levels of
IL-4, IL-5 and IFNγ in the BALF were measured. Compared with the
OVA-treated group, Z. seed oil reduced the levels of IL-5 levels by
13–53% (P<0.05; Fig. 2C) and
IL-4 by 22–49% (P<0.05; Fig.
2D) over the course of the experiment. The reduction of BALF
IL-4 and IL-5 levels due to Z. seed oil administration was most
pronounced after 7 days of sustained OVA challenge compared with
the OVA-treated mice (P<0.05; Fig.
2C and D). Furthermore, administration of Z. seed oil increased
BALF IFNγ levels by 6–8% over the course of the experiment
(Fig. 2E). These results indicate
that Z. seed oil attenuates the inflammatory response in asthmatic
mice, particularly the infiltration of inflammatory cells and the
production of inflammatory cytokines in the bronchial airway.
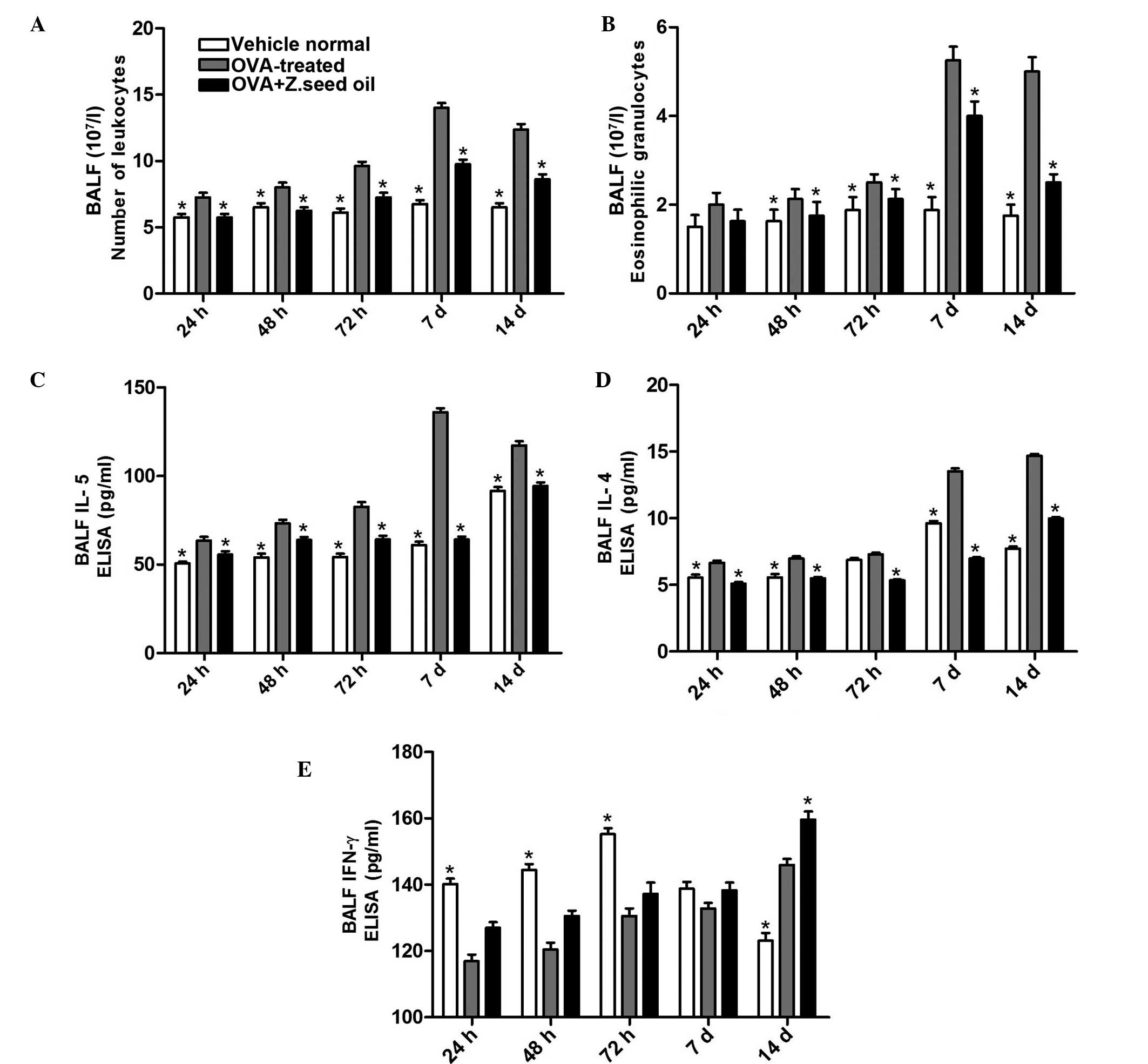 | Figure 2Effect of Z. seed oil on the
recruitment of inflammatory cells, and IL-4, IL-5 and IFNγ
expression levels in the BALF of OVA-induced asthmatic mice. (A)
Analysis of total leukocyte and (B) Eosinophil counts in the BALF
of vehicle-, OVA- and OVA + Z. seed oil-treated mice. (C–E)
Cytokine production in BALF collected after 24, 48, 72 h, and 7 and
14 d treatment. Data represent the mean ± standard deviation (n=8).
*P<0.05 vs. the OVA-treated group. OVA, ovalbumin; Z.
seed oil, Zanthoxylum bungeanum seed oil; BALF,
bronchoalveolar lavage fluid; d, days; IL, interleukin; IFNγ,
interferon γ. |
Z. seed oil treatment reduces mRNA
expression of chemo- tactic factor levels in the lungs of
OVA-challenged mice
Environmental stress can lead to the production of
chemotactic factors that promote the selective migration of
eosinophils and neutrophils to the sites of inflammation (23,24).
mRNA expression levels were examined to identify whether Z. seed
oil may affect the expression of the chemotactic factor leukotriene
C4 synthase (LTC4S), IL-8, monocyte chemoattractant protein-1
(MCP-1) and chemokine (C-C motif) receptor-3 (CCR-3) in the lung
tissue of asthmatic mice. LTC4S, IL-8, MCP-1 and CCR-3 mRNA
expression levels were significantly upregulated in the lung tissue
of the OVA-challenged mice compared with the vehicle-treated mice
at all time points (P<0.05; Fig.
3). Administration of Z. seed oil to the OVA-challenged mice
significantly downregulated the levels of LTC4S, IL-8, MCP-1 and
CCR-3 at 24, 48 and 72 h, and 7 and 14 days (P<0.05; Fig. 3), suggesting that Z. seed oil may
attenuate the induction of chemotactic factors in the lung tissue
of asthmatic mice.
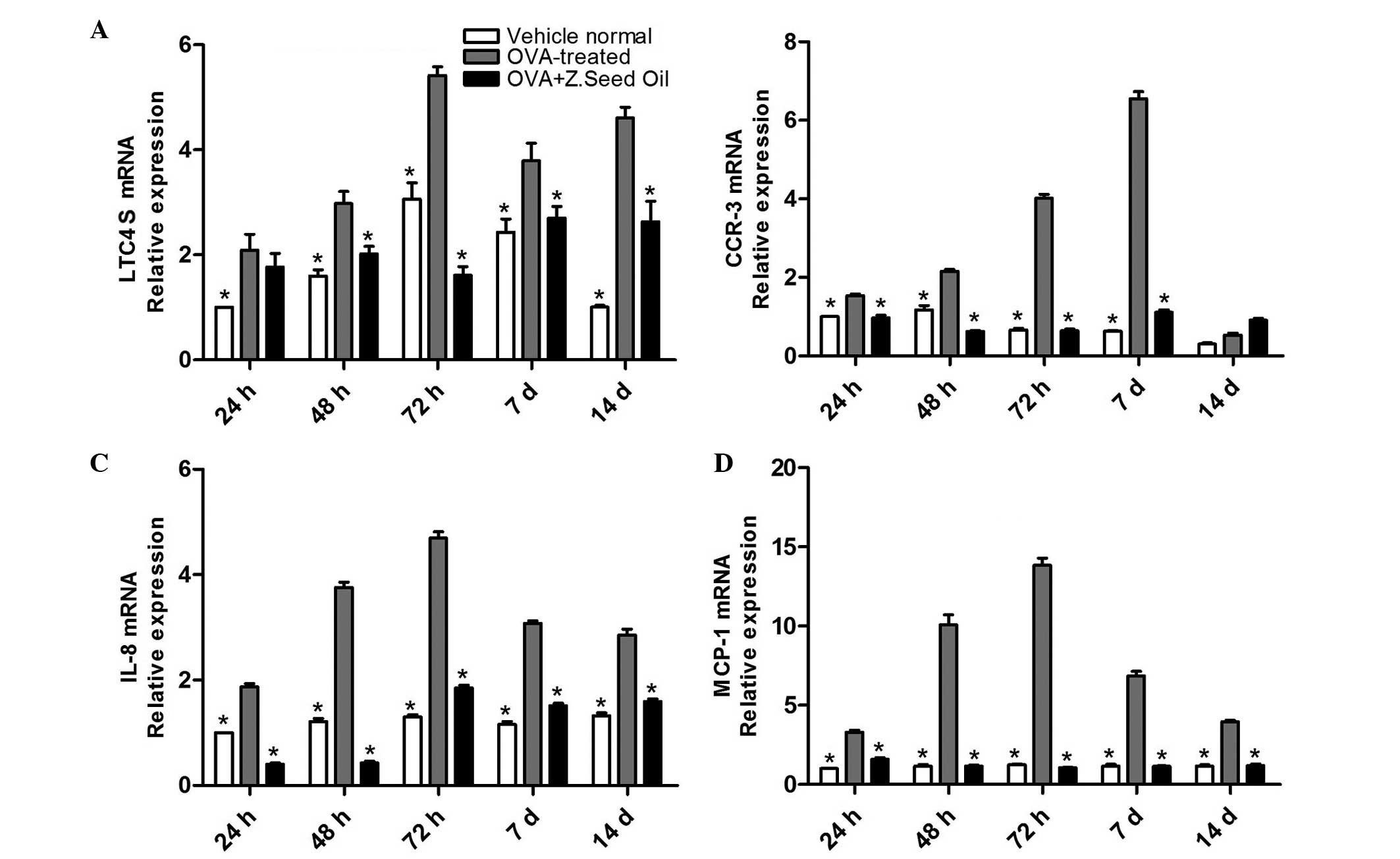 | Figure 3Effect of Z. seed oil on LTC4S,
CCR-3, IL-8 and MCP-1 mRNA expression levels in the lung tissue of
OVA-induced asthmatic mice. Lungs from vehicle-, OVA- and OVA + Z.
seed oil-treated mice were excised following 24, 48 and 72 h, and 7
and 14 d treatment. mRNA expression levels of (A) LTC4S, (B) CCR-3,
(C) IL-8 and (D) MCP-1 were measured by RT-qPCR. Data are presented
as the mean ± standard deviation (n=3). *P<0.05 vs.
the OVA-treated group. OVA, ovalbumin; Z. seed oil, Zanthoxylum
bungeanum seed oil; LTC4S, leukotriene C4 synthase; d, days;
CCR-3, chemokine (C-C motif) receptor 3; IL-8, interleukin-8;
MCP-1, monocyte chemoattractant protein-1. |
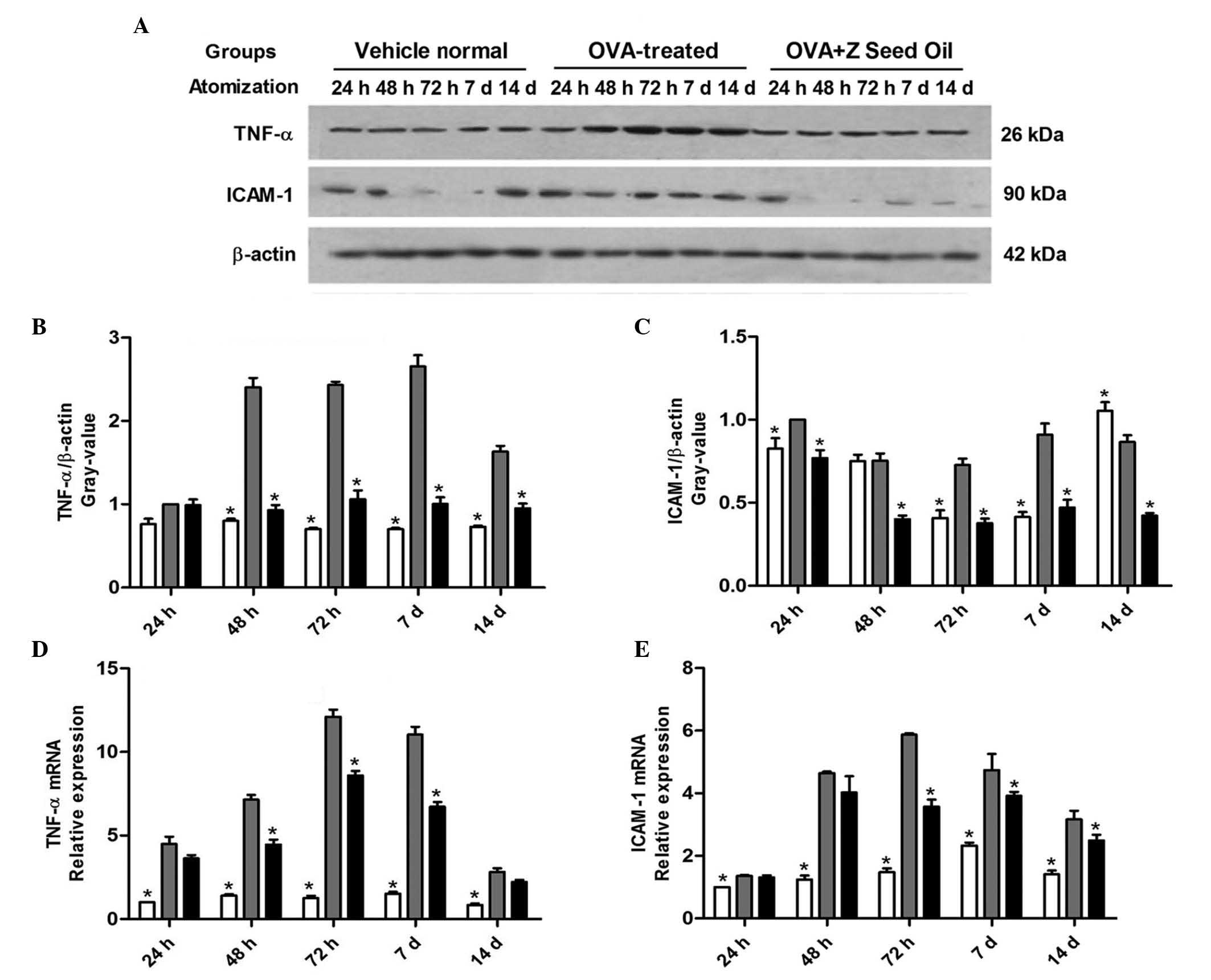
Z. seed oil inhibits the induction of
TNF-α and ICAM-1 protein and mRNA expression in the lungs of
asthmatic mice
Leukocyte recruitment and adhesion are required for
sufficient immune surveillance and inflammatory response. The
adhesion of leukocytes to the vascular endothelium is mediated by
specific endothelial cell adhesion molecules, including ICAM-1,
VCAM-1 and TNF-α (25–27). The expression levels of these
adhesion molecules were analyzed in the lung tissue of asthmatic
mice by western blotting (Fig.
4A–D) and RT-qPCR analyses (Fig.
4E and F). ICAM-1 and TNF-α protein and mRNA expression levels
were significantly elevated in OVA-induced asthmatic mice compared
with the vehicle-treated mice at the majority of time points
(P<0.05; Fig. 4), consistent
with the increased inflammatory response observed. Administration
of Z. seed oil attenuated the induction of ICAM-1 and TNF-α mRNA
and protein expression levels compared with the placebo-treated
mice at 24, 48 and 72 h, and 7 and 14 days (P<0.05; Fig. 4C–F). These results suggest that Z.
seed oil inhibits the expression of adhesion molecules required for
leukocyte recruitment, which may contribute to its
anti-inflammatory effect.
Effect of Z. seed oil on the protein
expression levels of TNF-R, CD14, and TLR2 and 4
The production of pro-inflammatory cytokines is
controlled by the TNF-R, CD14, and TLR2 and 4 signal transduction
pathways in human monocytes (28).
Due to the marked inflammatory response in OVA-challenged mice, the
expression levels of these signaling proteins were investigated in
the presence and absence of Z. seed oil (Fig. 5A). Western blot analysis indicated
a significant change in CD14 and TLR4 protein expression levels in
only two of the five time points in the OVA-treated group compared
with the vehicle-treated group (Fig.
5B and C). In addition, a significant change was observed in
only three of the five time points following Z. seed oil
administration compared with the OVA-treated group (Fig. 5B and C). By contrast, the protein
expression levels of TNF-R and TLR2 were significantly upregulated
in the majority of time points in OVA-treated mice compared with
the vehicle-treated mice (P<0.05; Fig. 5D and E). However, only the
induction of TLR2 expression was significantly attenuated by Z.
seed oil administration (P<0.05; Fig. 5D), whereas TNF-R, CD14 and TLR4
expression levels were similar compared with the Z. seed oil- or
placebo oil-treated mice (Fig. 5B, C
and E).
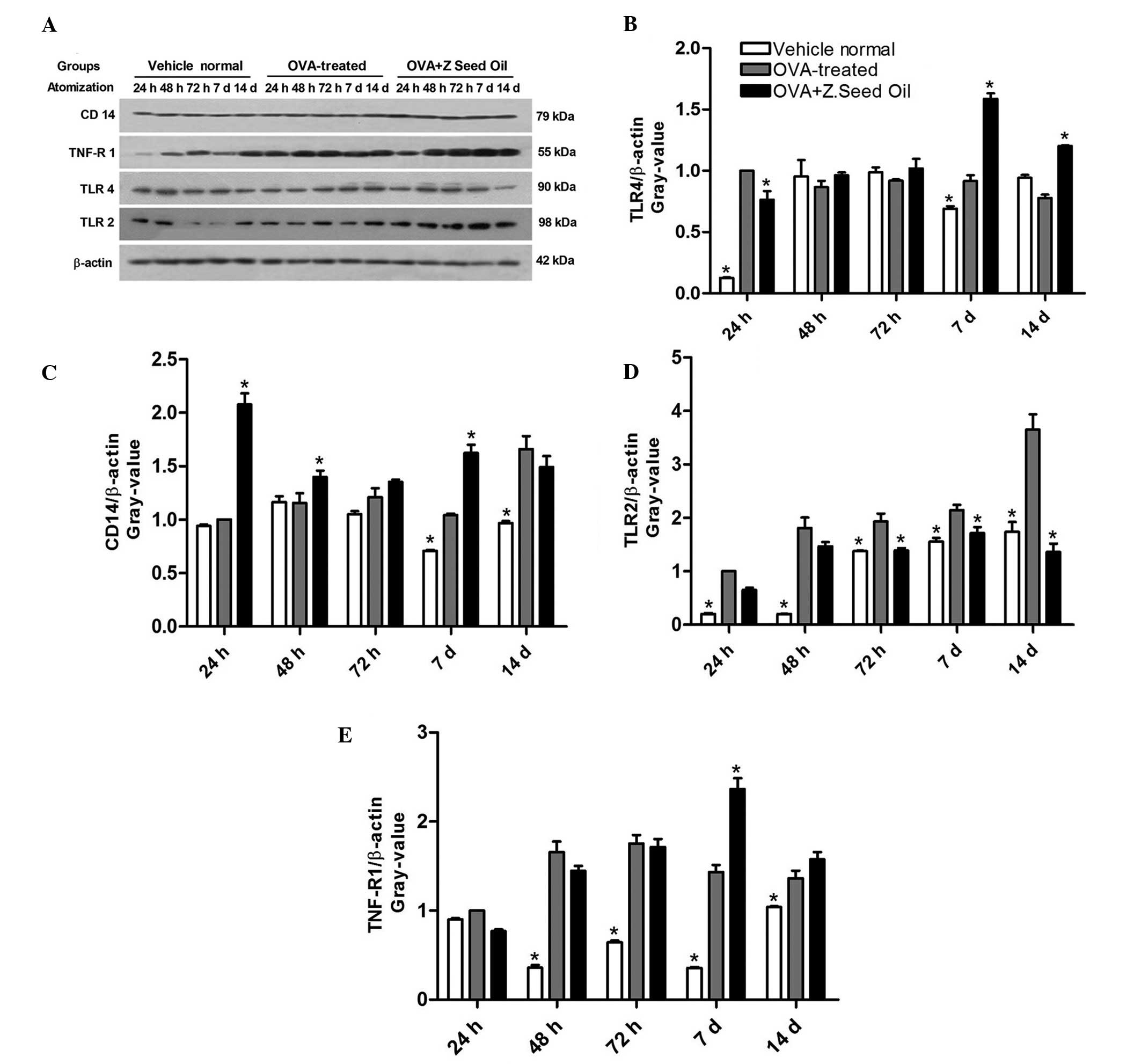 | Figure 5Effect of Z. seed oil on TLR4, CD14,
TLR2 and TNF-R1 expression levels in OVA-induced asthmatic mice.
(A) Western blot analysis of CD14, TNF-R1, TLR4, TLR2 and β-actin
in vehicle-, OVA- and OVA + Z. seed oil-treated mice.
Quantification of (B) TLR4, (C) CD14, (D) TLR2 and (E) TNF-R1.
Expression levels were normalized to β-actin. Data are expressed as
the mean ± standard deviation (n=3). *P<0.05 vs. the
OVA-treated asthmatic mice. OVA, ovalbumin; Z. seed oil,
Zanthoxylum bungeanum seed oil; d, days; TNF-R, tumor
necrosis factor-receptor; TLR, toll-like receptor. |
Effect of Z. seed oil on Ras protein
expression levels and MAPK signaling in the lungs of asthmatic
mice
The MAPK pathway contributes to airway inflammation
and AHR (29). The role of
intracellular ERK1/2 signaling in the bronchial inflammatory
response was observed in OVA-induced asthma and Z. seed oil-treated
mice. Western blot analysis (Fig.
6A) indicated that ERK1/2 phosphorylation was significantly
increased in the lung tissue of asthmatic mice compared with
control non-asthmatic mice at each time point (P<0.05; Fig. 6B), indicating elevated ERK
activity. Simultaneously, significantly elevated JNK
phosphorylation and reduced p38 MAPK phosphorylation were detected
in the lung tissue of asthmatic mice relative to the non-asthmatic
control lungs at each time point analyzed (P<0.05; Fig. 6C and D). Elevated ERK1/2 and JNK
phosphorylation were sustained over the total of 14 days of OVA
challenge, suggesting that these signaling pathways may contribute
to the infiltration of inflammatory cells into the bronchial airway
during asthma attacks. Administration of Z. seed oil significantly
attenuated ERK and JNK phosphorylation in the lung tissue of
asthmatic mice at each time point (P<0.05), but did not have an
effect on Ras or p38 MAPK phosphorylation expression levels
compared with the placebo oil-treated mice (Fig. 6C and E).
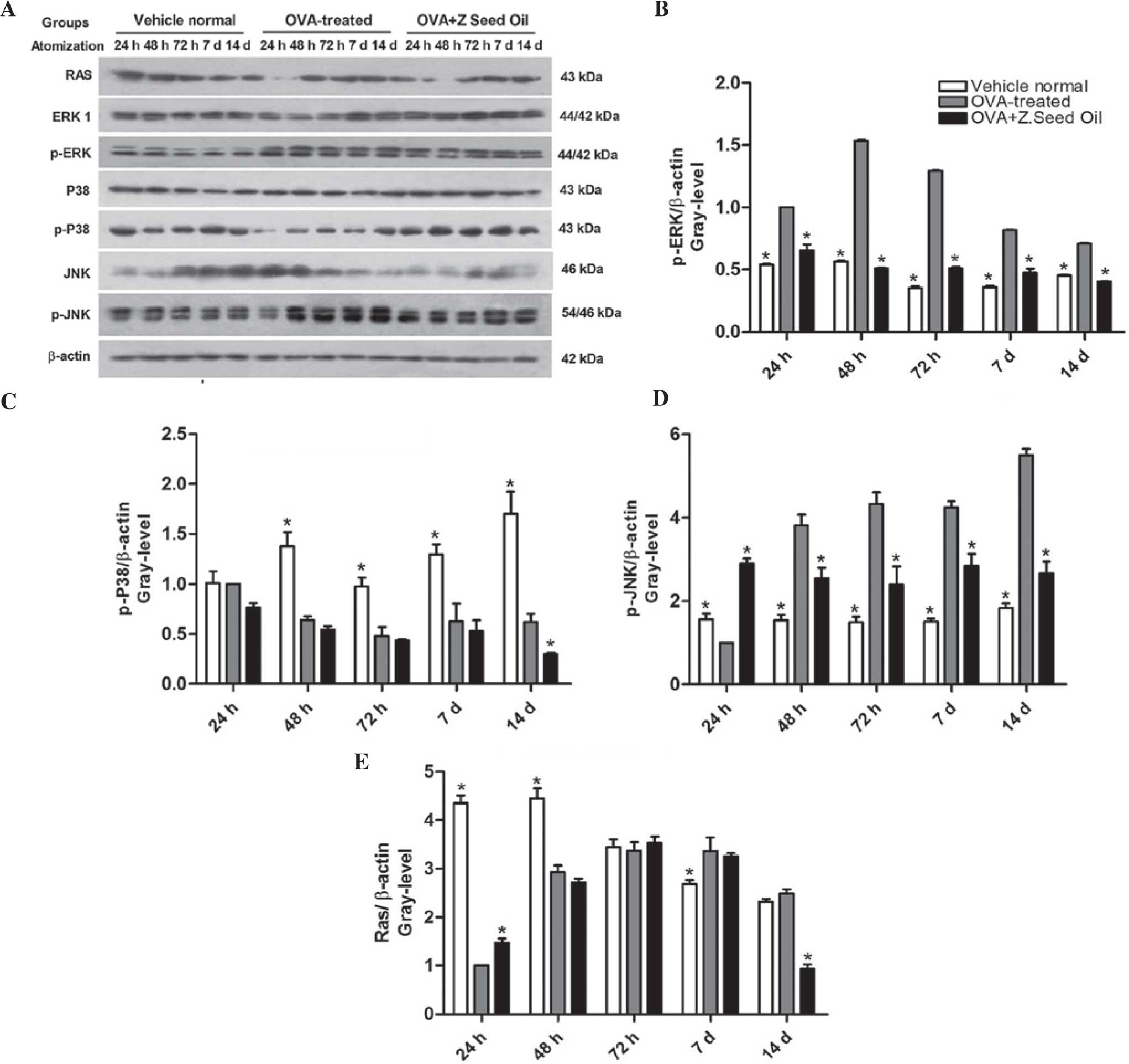 | Figure 6Effect of Z. seed oil on MAPK
signaling in the lung tissue of OVA-induced asthmatic mice. (A)
Western blot analysis of phosphorylated and unphosphorylated
ERK1/2, p38 MAPK, JNK, Ras and β-actin expression in vehicle-, OVA-
and OVA + Z. seed oil-treated mice. Quantitative analysis of the
expression levels of (B) p-ERK1/2, (C) p-p38 MAPK, (D) p-JNK and
(E) Ras. Protein expression levels were normalized to β-actin. Data
are expressed as the mean ± standard deviation (n=3).
*P<0.05 vs. the OVA-treated asthmatic mice. OVA,
ovalbumin; Z. seed oil, Zanthoxylum bungeanum seed oil; d,
days; p-ERK, phosphorylated-extracellular signal regulated kinase;
JNK, c-jun N-terminal kinase. |
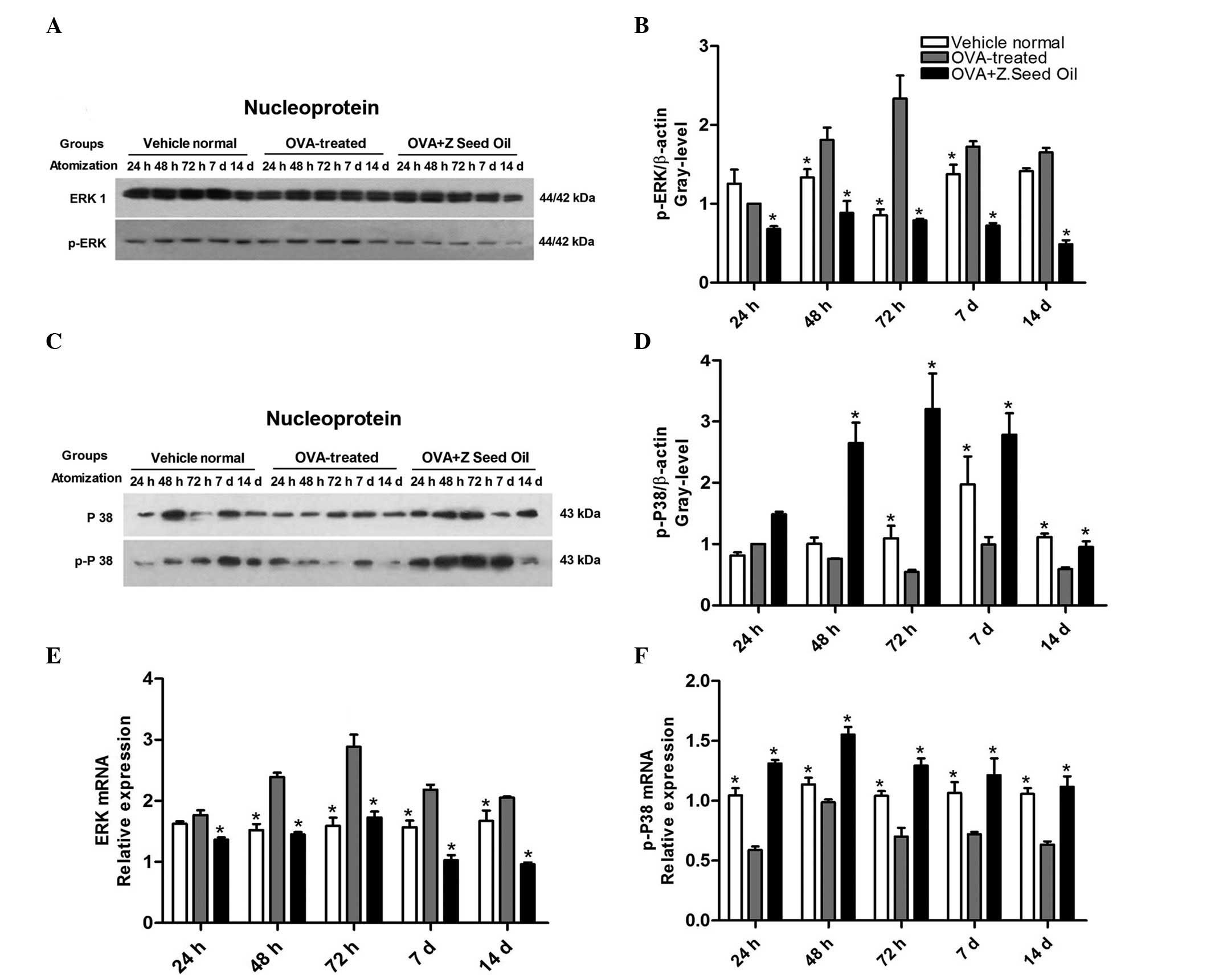
Z. seed oil attenuates the induction of
nuclear ERK1/2 protein and mRNA expression
In order to further evaluate the effect of Z. seed
oil on ERK signaling in the lung tissue of the OVA-treated
asthmatic mice, the levels of nuclear p-ERK and p-p38 MAPK were
analyzed. Western blot analysis of lung nuclear protein extracts
demonstrated significantly elevated ERK phosphorylation in the
nuclei of lung cells from OVA-induced asthmatic mice compared with
the non-asthmatic control mice (P<0.05; Fig. 7A and B). Compared with the
non-asthmatic mice, phosphorylation of p38 MAPK was significantly
reduced at 72 h, 7 and 14 days of sustained OVA challenge
(P<0.05; Fig. 7C and D). These
changes in ERK1/2 phosphorylation were attenuated and p38 MAPK
phosphorylation was upregulated by Z. seed oil treatment. The
results suggest that nuclear translocation of ERK1/2 is enhanced in
the lung tissue of asthmatic mice, consistent with elevated MAPK
activity following asthma induction.
To further support the western blot analysis data,
ERK and p38 MAPK mRNA expression levels were measured in the lung
tissue of asthmatic mice over the course of the experiment.
Consistent with the protein analysis, ERK mRNA expression levels
were significantly upregulated (P<0.05; Fig. 7E), and p38 MAPK mRNA levels were
significantly downregulated (P<0.05; Fig. 7F) in the lung tissue of OVA-induced
asthmatic mice compared with the non-asthmatic mice. Administration
of Z. seed oil reduced ERK and elevated p38 MAPK mRNA expression
levels in the lung tissue of asthmatic mice compared with the
untreated OVA-challenged mice at each time point (P<0.05;
Fig. 7E and F).
Z. seed oil regulates nuclear NF-κB
expression
To assess the effect of Z. seed oil on other
inflammatory pathways, c-fos, c-JUN, ATF-2 and NF-κB nuclear levels
were measured in the lung tissue of asthmatic mice by western
blotting (Fig. 8A). OVA-induced
asthma resulted in the significant upregulation of c-fos nuclear
levels at 24, 48 and 72 h time points (Fig. 8B), and c-JUN nuclear levels at 24,
72 h and 7 days (Fig. 8C) compared
with the non-asthmatic mice (P<0.05). Furthermore, a decrease in
the levels of p-NF-κB-p65 (Fig.
8D) and p-ATF-2 (Fig. 8E) were
observed in the nuclear extracts from the lung tissue of asthmatic
mice compared with the non-asthmatic mice (P<0.05).
Administration of Z. seed oil to asthmatic mice significantly
attenuated c-fos (Fig. 8B) and
c-JUN (Fig. 8C) induction
(P<0.05), suggesting that this treatment may inhibit the
transcription of pro-inflammatory factors in nuclear extracts from
the lung tissue of asthmatic mice compared with the placebo
oil-treated mice.
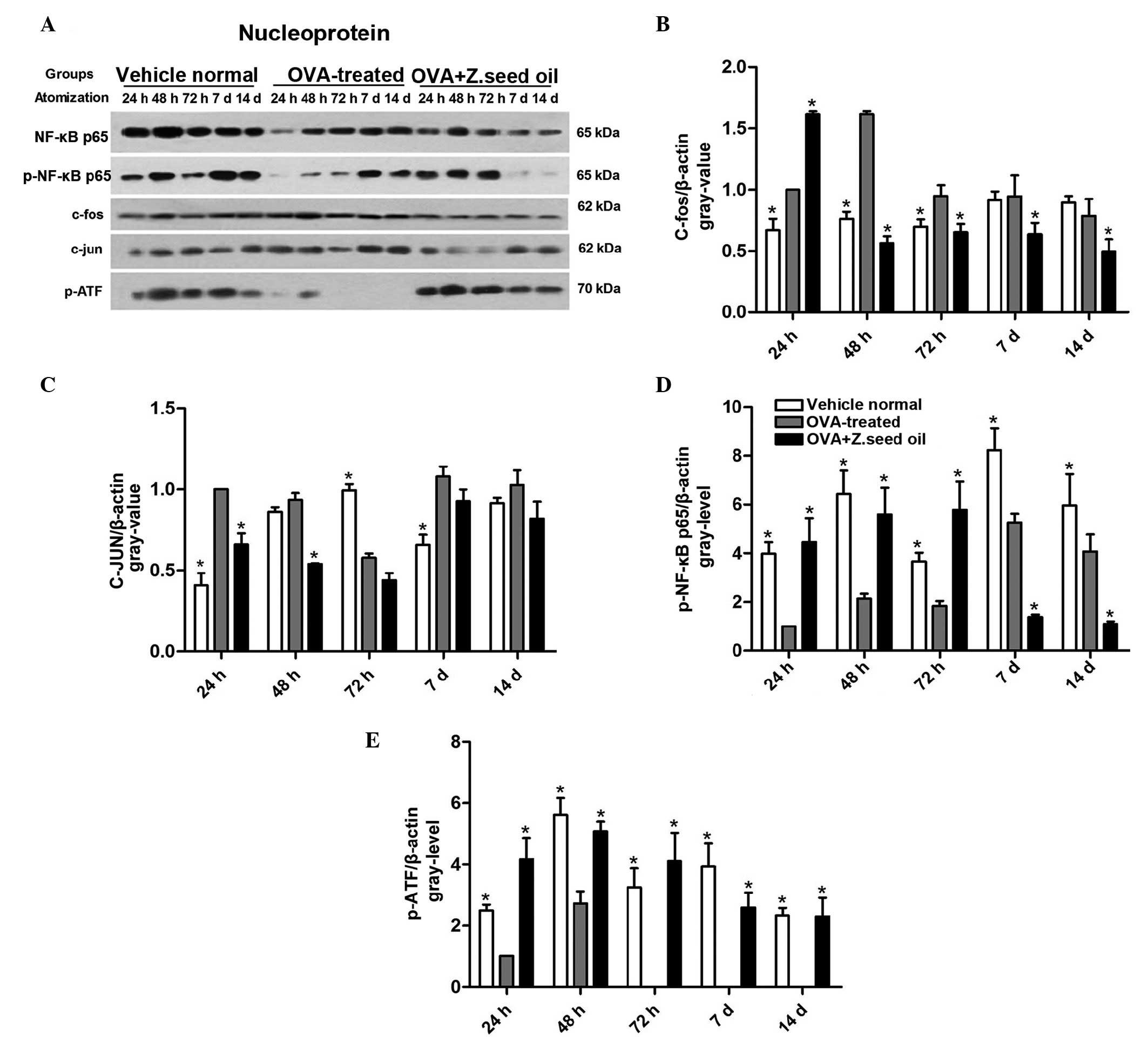 | Figure 8Effect of Z. seed oil on nuclear
NF-κB signaling in the lung tissue of asthmatic mice. (A) Western
blot analysis of NF-κB p65, p-NF-κB p65, c-fos, c-JUN, p-ATF-2 and
β-actin protein expression levels in vehicle-, OVA- and OVA + Z.
seed oil-treated. Quantitative analysis of (B) p-c-fos, (C)
p-c-JUN, (D) p-NF-κB p65 and (E) p-ATF-2 expression levels. Protein
expression levels were normalized to β-actin. Data are expressed as
the mean ± standard deviation (n=3). *P<0.05 vs. the
OVA-treated asthmatic mice. OVA, ovalbumin; Z. seed oil,
Zanthoxylum bungeanum seed oil; d, days; p-NF-κB, nuclear
factor-κB; ATF, activating transcription factor. |
Discussion
Asthma is a chronic disease of the lungs
characterized by bronchial inflammation and AHR. Lung biopsies from
asthmatic patients demonstrate significant infiltration of
eosinophils, lymphocytes, macrophages and mast cells into the
bronchial airway. This inflammatory response is accompanied by
structural changes, including thickening of the epithelial lining
of the airway, sub-epithelial fibrosis, and hyperplasia of goblet
cells and airway smooth muscle cells (airway remodeling).
Considering the prominent inflammatory response in asthma, the
majority of asthma therapies employ anti-inflammatory agents
(30–32).
In the current study, the therapeutic potential and
anti-inflammatory effect of Z. seed oil was examined in mice with
asthma induced by sustained OVA challenge. Intraperitoneal
injection of OVA followed by sustained challenge with intranasal
administration of OVA is known to induce the expansion of the Th2
lymphocyte population and production of Th2 cytokines, leading to
AHR and inflammation. This inflammation is typically characterized
by eosinophil infiltration, production of OVA-specific antibodies
(IgE) (33), and production of
IL-4, IL-5, IL-9 and IL-13 (34).
The present study identified that sustained OVA exposure induces a
massive influx of lymphocytes, eosinophils, neutrophils and
mononuclear cells in the lung tissue of mice. Furthermore, OVA
exposure led to the induction of the pro-inflammatory cytokines
TNF-α, IL-4 and IL-5, and reduced levels of IFNγ in the BALF of
asthmatic mice. These cytokines serve an important role in the
initiation and pathophysiology of asthma, including airway
remodeling, IgE production and eosinophil function (35). Administration of Z. seed oil to the
asthmatic mice effectively inhibited the influx of inflammatory
cells into the lungs, as the total leucocyte and eosinophil
populations in the lung tissue were significantly reduced. The
reduction in infiltration and damage was accompanied by a decrease
in IL-4 and IL-5 levels, and an increase in the IFNγ levels in the
BALF of Z. seed-treated mice. These results suggest that Z. seed
oil suppresses the Th2 cell population in asthmatic lung tissue,
while the elevated IFNγ levels are consistent with a reduction in
the eosinophil population. The Z. seed oil-induced reduction of
T-helper lymphocytes and increase of IFNγ production may reflect an
imbalance in the Th1 and Th2 cytokine profile of the lungs. Thus,
Z. seed oil may reduce atopic inflammation in asthma by inhibiting
Th2 cell activity, resulting in decreased IL-4 and IL-5
production.
Chemotaxis is an important step in the migration of
inflammatory cells to sites of inflammation. Although the precise
role of IL-8 in lung disease is unclear, this cytokine serves a
pivotal role in innate immunity by recruiting immune cells, such as
neutrophils and monocytes, to the sites of inflammation (36). Furthermore, IL-8 is associated with
neutrophil inflammation in the respiratory tract, particularly in
cases of severe, persistent asthma (37). Eosinophils are attracted to the
site of inflammation in the bronchial airway by chemokines, such as
eotaxin and chemokine (C-C motif) ligand 5 [also known as regulated
on activation, normal T cell expressed and secreted], which bind to
the chemokine receptor CCR-3 (38). These chemokines and their receptors
may serve a significant role in the pathogenesis of asthma.
The current study demonstrated that OVA-induced
asthma promotes the expression of IL-8, MCP-1, CCR-3 and LTC4S,
suggesting that these genes are involved in the development of
asthma following OVA exposure. These results were consistent with
the increased inflammatory response observed in the mouse model.
The induction of IL-8 MCP-1, CCR-3, LTC4S and TNF-α were
effectively inhibited by the Z. seed oil treatment. TNF-α promotes
inflammation, leukocyte infiltration and stimulates cytokine
production (39,40). Thus, Z. seed oil may prevent the
induction of genes regulating the cytokine response, which likely
contributes to its anti-inflammatory effect.
The current study indicated that ICAM-1 expression
was upregulated in the lung tissue of asthmatic mice, and may
contribute to the inflammatory response by promoting adhesion of
inflammatory cells. ICAM-1 is an adhesion protein that is expressed
in various types of cells, including endothelial and epithelial
cells, leukocytes, and fibroblasts. In particular, inflammatory
cytokines can promote ICAM-1 expression, thus augmenting the immune
response and leukocyte accumulation (41,42).
Administration of Z. seed oil attenuated the induction of TNF-α and
ICAM-1 following OVA-induced asthma. These results suggest that Z.
seed oil may suppress the induction of adhesion molecules that
contribute to eosinophil and neutrophil infiltration into the
bronchial airway pathway, consequently inhibiting the development
of asthma.
TLRs are an evolutionarily conserved family of cell
surface molecules that participate in the innate immune recognition
of pathogens (43,44). There are two major pathways
involved in TLR signal transduction. The MyD88-dependent pathway
recruits IL-1 receptor-associated kinase (IRAK) 4, IRAK1 and
TNF-R-associated factor 6 to activate transcription factors, and
trigger the release of pro- and anti-inflammatory cytokines
(1). The present study
demonstrated that TLR2 and TNF-R1 expression levels were
significantly increased in the lung tissue of asthmatic mice,
compared with the TLR4 and CD14 expression levels. The results
indicate that TLR2 and TNF-R1 were selectively activated in
response to the sustained OVA challenge, implicating these pathways
in the pro-inflammatory signaling of asthma.
The MAPK signaling pathway serves a role in the
immune response by regulating gene expression, as well as cell
proliferation, survival, death and mobility (45). The phosphorylation of numerous
components of the MAPK system (ERK, p38 MAPK and JNK) are
upregulated in animal models of asthma. In turn, MAPK enhances
transcriptional activation protein-1, ATF-2 and c-JUN, leading to
cytokine production, and differentiation and proliferation of
inflammatory cells (12). The
ERK1/2 selective inhibitor U0126 significantly blocks
antigen-induced airway inflammatory cell infiltration, and the
production of IL-4, IL-5, eotaxin, serum antigen-specific IgE and
mucus in BALF, while decreasing VCAM-1 expression and AHR in a
dose-dependent manner (28).
In the current study, p-ERK1/2 and p-JNK levels were
significantly increased in the lung tissue of OVA-induced asthmatic
mice. Furthermore, nuclear ERK1/2 phosphorylation was demonstrated
to be induced and p38 MAPK was downregulated by OVA treatment.
These results suggest that the transcriptional activity of ERK and
JNK, but not p38 MAPK, are induced in OVA-stimulated asthmatic lung
tissue. Downstream of ERK1/2 and JNK, c-fos and c-JUN levels were
increased, while downstream of p38 MAPK, p-ATF-2 levels were
decreased. However, no significant change in NF-κB expression was
observed in the asthmatic lung tissue. The upregulation of the
ERK1/2 and JNK expression levels possibly occurred at the
transcriptional level, as an increase in the mRNA expression levels
was detected for the genes of interest in the asthmatic lung
tissue. These data suggest that the ERK1/2 and JNK signaling
cascades, but not p38 MAPK, may provide a therapeutic target for
the treatment of asthma. Additionally, administration of Z. seed
oil significantly reduced ERK1/2 and JNK phosphorylation following
OVA exposure. Consistent with the reduced phosphorylation, the
nuclear levels of c-fos and c-JUN were downregulated in the lung
tissue of Z. seed oil-treated mice. The inhibition of ERK and JNK
signaling may be responsible for the reduction of inflammatory
cytokine production (IL-8, MCP-1 and TNF-α) and ICAM-1 expression
in the lung tissue following Z. seed oil treatment. The present
study demonstrated that Z. seed oil elevated p-p38 MAPK and p-ATF-2
levels, suggesting that there is a selective contribution of each
of the MAPKs in the inflammatory response of OVA-induced
asthma.
In conclusion, Z. seed oil has a profound
therapeutic effect on airway inflammation and AHR in the murine
model of asthma used in the current study. Z. seed oil
significantly attenuates the inflammatory response to sustained OVA
exposure, including inflammatory cell infiltration into the
bronchial airway, and induction of inflammatory cytokines and
adhesion molecules. Z. seed oil appears to exert its
anti-inflammatory effect partially through the suppression of ERK
and JNK signaling. Thus, the therapeutic activity of Oriental
medicines that employ Z. seed oil may be partially due to: i)
Immuno-modulatory agents contained in Z. seed oil that reduce Th2
cell immune responses; or ii) reduced production of
pro-inflammatory cytokines, chemokines and adhesion molecules that
attenuate the recruitment of inflammatory cells to OVA exposed lung
tissue. Thus, the present study demonstrated that Z. seed oil may
serve as a therapeutic agent for the prevention or treatment of
allergen-induced asthma disease and that future studies may provide
novel therapeutic strategies for asthma.
Abbreviations:
|
Z. seed oil
|
Zanthoxylum bungeanum seed oil
|
|
OVA
|
ovalbumin
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
IL
|
interleukin
|
|
IFNγ
|
interferon γ
|
|
LTC4S
|
leukotriene C4 synthase
|
|
CCR-3
|
chemokine (C-C motif) receptor-3
|
|
VCAM-1
|
vascular cell adhesion molecule-1
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
TNF-α/R
|
tumor necrosis factor-α/receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-jun N-terminal kinase
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
TLR
|
toll-like receptor
|
|
ATF-2
|
activating transcription factor-2
|
|
IRAK
|
interleukin-1 receptor-associated
kinase
|
References
|
1
|
Takeda M, Ito W, Tanabe M, Ueki S, Kato H,
Kihara J, Tanigai T, Chiba T, Yamaguchi K, Kayaba H, et al:
Allergic airway hyper-responsiveness, inflammation, and remodeling
do not develop in phosphoinositide 3-kinase γ-deficient mice. J
Allergy Clin Immunol. 123:805–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akbari O, Faul JL, Hoyte EG, Berry GJ,
Wahlström J, Kronenberg M, DeKruyff RH and Umetsu DT:
CD4+ invariant T-cell-receptor+ natural
killer T cells in bronchial asthma. N Engl J Med. 354:1117–1129.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elias JA, Lee CG, Zheng T, Ma B, Homer RJ
and Zhu Z: New insights into the pathogenesis of asthma. J Clin
Invest. 111:291–297. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Expert Panel Report 3 (EPR-3): Guidelines
for the diagnosis and management of asthma-summary report 2007. J
Allergy Clin Immunol. 120:S94–S138. 2007. View Article : Google Scholar
|
|
5
|
Torén K and Hermansson BA: Incidence rate
of adult-onset asthma in relation to age, sex, atopy and smoking: A
Swedish population-based study of 15813 adults. Int J Tuberc Lung
Dis. 3:192–197. 1999.PubMed/NCBI
|
|
6
|
de Marco R, Locatelli F, Cazzoletti F,
Bugianio M, Carosso A and Marinoni A: Incidence of asthma and
mortality in a cohort of young adults: A 7-year prospective study.
Respir Res. 6:952005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karjalainen A, Kurppa K, Martikainen R,
Klaukka T and Karjalainen J: Work is related to a substantial
portion of adult-onset asthma incidence in the Finnish population.
Am J Respir Crit Care Med. 164:565–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Heart Blood and Lung Institute:
Guidelines for the diagnosis and management of asthma. National
Institutes of Health; Bethesda, USA: 2002
|
|
9
|
Duan W, Chan JH, Wong CH, Leung BP and
Wong WS: Anti-inflammatory effects of mitogen-activated protein
kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol.
172:7053–7059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pelaia G, Cuda G, Vatrella A, Gallelli L,
Caraglia M, Marra M, Abbruzzese A, Caputi M, Maselli R, Costanzo
FS, et al: Mitogen-activated protein kinases and asthma. J Cell
Physiol. 202:642–653. 2005. View Article : Google Scholar
|
|
11
|
Kumar A, Lnu S, Malya R, Barron D, Moore
J, Corry DB and Boriek AM: Mechanical stretch activates nuclear
factor-kappaB, activator protein-1, and mitogen-activated protein
kinases in lung parenchyma: Implications in asthma. FASEB J.
17:1800–1811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong C, Davis RJ and Flavell RA: MAP
kinases in the immune response. Annu Rev Immunol. 20:55–72. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atherton HC, Jones G and Danahay H:
IL-13-induced changes in the goblet cell density of human bronchial
epithelial cell cultures: MAP kinase and phosphatidylinositol
3-kinase regulation. Am J Physiol Lung Cell Mol Physiol.
285:L730–L739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamashita M, Kimura M, Kubo M, Shimizu C,
Tada T, Perlmutter RM and Nakayama T: T cell antigen
receptor-mediated activation of the Ras/mitogen-activated protein
kinase pathway controls interleukin 4 receptor function and type-2
helper T cell differentiation. Proc Natl Acad Sci USA.
96:1024–1029. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rahman MT and Alimuzzaman M:
Antinociceptive and antidiarrhoeal activity of Zanthoxylum rhetsa.
Fitoterapia. 73:340–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang CT, Doong SL, Tsai IL and Chen IS:
Coumarins and anti-HBV constituents from Zanthoxylum schionifolium.
Phytochemistry. 45:1419–1422. 1997. View Article : Google Scholar
|
|
17
|
Bracke ME, Depypere HT, Boterberg T, Van
Marck VL, Veenekens KM, Vanleuchene E, Nuytinck M, Serreyn R and
Mareel MM: Influence of tangeretin on tamoxifen's therapeutic
benefit in mammary cancer. J Nat Cancer Inst. 91:354–359. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka S, Sato T, Akimoto N, Yano M and
Ito A: Prevention of UVB-induced photoinflammation and photoaging
by a polymethoxy flavonoid, nobiletin, in human keratinocytes in
vivo and in vitro. Biochem Pharmacol. 68:433–439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan GT, Pezzuto JM, Kinghorn AD and Hughes
SH: Evaluation of natural products as inhibitors of human
immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J Nat
Prod. 54:143–154. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia L, You J, Li G, Sun Z and Suo Y:
Compositional and antioxidant activity analysis of Zanthoxylum
bungeanum seed oil obtained by supercritical CO2 fluid extraction.
J Am Oil Chem Soc. 88:23–32. 2011. View Article : Google Scholar
|
|
21
|
Myou S, Leff AR, Myo S, et al: Blockade of
inflammation and airway hyperresponsiveness in immune-sensitized
mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med
17. 198(10): 1573–82. 2003. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
the relative gene expression data using real-time quantitative PCR
and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Murphy PM: Chemokine receptors: Structure,
function and role in microbial pathogenesis. Cytokine Growth Factor
Rev. 7:47–64. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Foessmann UUM, Loetscher P, Dahinden CA,
Langen H, Thelen M and Baggiolini M: Eotaxin-2s like eotaxin on
human eosinophil and receptor. J Exp Med. 183:2349–2354. 1997.
|
|
25
|
Alam R, York J, Boyars M, Stafford S,
Grant JA, Lee J, Forsythe P, Sim T and Ida N: Increased MCP-1,
RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic
asthmatic patients. Am J Respir Crit Care Med. 153:13981404.
View Article : Google Scholar
|
|
26
|
Parent C and Eichacker PQ: Neutrophil and
endothelial cell interactions in sepsis. The role of adhesion
molecules. Infect Dis Clin North Am. 13:427–447. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shanley TP, Warner RL and Ward PA: The
role of cytokines and adhesion molecules in the development of
inflammatory injury. Mol Med Today. 1:40–45. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asea A, Kraeft SK, Kurt-Jones EA,
Stevenson MA, Chen LB, Finberg RW, Koo GC and Calderwood SK: HSP70
stimulates cytokine production through a CD14-dependant pathway,
demonstrating its dual role as a chaperone and cytokine. Nat Med.
6:435–442. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duan W and Wong WS: Targeting
mitogen-activated protein kinases for asthma. Curr Drug Targets.
7:691–698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Talati M, Meyrick B, Peebles RS Jr, Davies
SS, Dworski R, Mernaugh R, Mitchell D, Boothby M, Roberts LJ II and
Sheller JR: Oxidant stress modulates murine allergic airway
responses. Free Radic Biol Med. 40:1210–1219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohn L, Elias JA and Chupp GL: Asthma:
Mechanisms of disease persistence and progression. Annu Rev
Immunol. 22:789–815. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cameron EJ, McSharry C, Chaudhuri R,
Farrow S and Thomson NC: Long-term macrolide treatment of chronic
inflammatory airway diseases: Risks, benefits and future
developments. Clin Exp Allergy. 42:1302–1312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Lamm WJ, Albert RK, Chi EY,
Henderson WR Jr and Lewis DB: Influence of the route of allergen
administration and genetic background on the murine allergic
pulmonary response. Am J Respir Crit Care Med. 155:661–669. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wills-Karp M: Interleukin-13 in asthma
pathogenesis. Immunol Rev. 202:175–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho JY, Miller M, Baek KJ, Han JW, Nayar
J, Lee SY, McElwain K, McElwain S, Friedman S and Broide DH:
Inhibition of airway remodeling in IL-5-deficient mice. J Clin
Invest. 113:551–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huber AR, Kunkel SL, Todd RF III and Weiss
SJ: Regulation of transendothelial neutrophil migration by
endogenous interleukin-8. Science. 254:99–102. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jatakanon A, Uasuf C, Maziak W, Lim S,
Chung KF and Barnes PJ: Neutrophilic inflammation in severe
persistent asthma. Am J Respir Crit Care Med. 160:1532–1539. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lukacs NW, Miller AL and Hogaboam CM:
Chemokine receptors in asthma: Searching for the correct immune
targets. J Immunol. 171:11–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
ten Hove W, Houben LA, Raaijmakers JAM,
Bracke M and Koenderman L: Differential regulation of TNF and
GM-CSF induced activation of P38 MAPK in neutrophils and
eosinophils. Molecular Immunology. 44:2492–2496. 2007. View Article : Google Scholar
|
|
40
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee T and Sousa A: Immunoinflammatory role
of the airway epithelial cell in asthma. Eur Respir Rev. 4:368–370.
1994.
|
|
42
|
Kampen GT, Stafford S, Adachi T, Jinquan
T, Quan S, Grant JA, Skov PS, Poulsen LK and Alam R: Eotaxin
induces degranulation and chemotaxis of eosinophils through the
activation of ERK2 and p38 mitogen-activated protein kinases.
Blood. 95:1911–1917. 2000.PubMed/NCBI
|
|
43
|
Carl VS, Brown-Steinke K, Nicklin MJH and
Smith MFS Jr: Toll-like receptor 2 and 4 (TLR2 and TLR4) agonists
differentially regulate secretory interleukin-1 receptor antagonist
gene expression in macrophages. J Biol Chem. 277:17448–17456. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moon EY, Kang JS, Han SH, Yang KH, Pyo S,
Lee MY, Lee HK and Yu DY: Differential role of peroxiredoxin II
(PrxII) on the expression of toll-like receptor 4 (TLR4) and B-cell
activating factor (BAFF) in ovalbumin (OVA)-induced mouse asthma.
Int Immunopharmacol. 8:935–944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arbabi S and Maier RV: Mitogen-activated
protein kinases. Crit Care Med. 30(Suppl): S74–S79. 2002.
View Article : Google Scholar : PubMed/NCBI
|