Introduction
Diabetes is a common disease that poses a serious
threat to human health and has an increasing incidence (1). As a metabolic disorder, diabetes
results from an inadequate amount of functional β-cells (2). In type 1 diabetes (T1D), β-cells are
destroyed by the immune system (3), while type 2 diabetes (T2D) is
associated with insulin resistance and β-cell dysfunction (4). The reduction of the amount of β cells
is a common feature of T1D as well as T2D (5). According to Kim and Lee (6), apoptosis of islet β-cells has a key
role in the pathogenesis of diabetes. Glucose-stimulated insulin
secretion is one of the important physiological characteristics of
islet β-cells. Extracellular-regulated protein kinase 1/2 (ERK1/2)
is an important member of the mitogen-activated protein kinase
(MAPK) family. It is activated by multiple extracellular stimuli
and regulates cell growth, proliferation, differentiation and death
(7). A previous study showed that
glucose stimulation can activate the ERK1/2 signal transduction
pathway in islet β-cells, while the role of ERK1/2 activation in
insulin secretion has remained elusive (8). The present study aimed to investigate
the role of the ERK1/2 signal transduction pathway in
glucose-stimulated insulin secretion in β-TC6 mouse pancreatic
cells.
Subjects and methods
Cell culture
β-TC6 mouse pancreatic cells (Shanghai Cell Bank of
Chinese Academy of Sciences, Beijing, China) were cultured in
high-glucose Dulbecco's modified Eagle's medium (HyClone, Logan,
UT, USA) with fetal bovine serum, streptomycin and penicillin
(Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China)
at 37°C in a humidified atmosphere containing 5% CO2.
The medium was replaced once every three days. The cells were
passaged at a split ratio of 1:2 every 7–10 days. The survival rate
was >90% according to trypan blue staining.
Glucose-stimulated insulin secretion
β-TC6 cells were digested with 0.25% trypsin and
0.01% ethylenediaminetetraacetic acid (HyClone). The single-cell
suspension (4×104/ml) was seeded into 24-well plates.
After 48 h of growth, the cells were washed by phosphate-buffered
saline (PBS; Fuzhou Maixin Biotechnology Development Co., Ltd.) and
then cultured in serum- and sugar-free KRBH medium (4) (NaCl, 129 mM; KCl, 4.8 mM;
NaHCO3, 5 mM; MgSO4, 1.2 mM;
CaCl2, 2 M) at 37°C for 30 min. The cells were washed
with KRBH medium and further cultured at 37°C for 60 min in KRBH
medium containing glucose at a concentration of 0, 1.38, 5.5 or
11.1 mM, respectively (9).
Finally, the cell supernatant was collected and the insulin
concentration was measured using a radioimmunoassay.
Intervention with MAPK inhibitor
PD98059
β-TC6 cells were seeded into six-well plates and
incubated for 47.5 h. The MAPK inhibitor PD98059 (Cell Signaling
Technology, Inc., Danvers, MA, USA) was added to yield a final
concentration of 2, 10 or 50 µM was added, followed by
culture for 30 min. After washing with PBS, the cells were
incubated in KRBH medium containing 1.38 mM glucose for 60 min. The
cells were then lysed in lysis buffer (Fuzhou Maixin Biotechnology
Development Co., Ltd.) and the level of phosphorylated ERK1/2 was
measured by western blot analysis. Furthermore, insulin secretion
was measured in the supernatant of centrifuged lysate using a
radioimmunoassay.
Detection of insulin concentration in the
cell supernatant
The insulin concentration in culture supernatant was
detected using a radioimmunoassay employing a GC-1200
γ-radioimmunoassay instrument (USTC Chuangxin Co., Ltd., Hefei,
China) and an insulin radioimmunoassay kit (Science and Technology
Center, Beijing PLA General Hospital, Beijing, China) according to
the manufacturer's instructions. Each group comprised three tubes
(the smae supernatant aliquoted into three tubes) and the average
value was used as result.
Detection of ERK1/2 phosphorylation
levels
The level of ERK1/2 phosphorylation was detected by
western blot analysis. After culture with different concentrations
of glucose and optionally with PD98059, the β-TC6 cells were
collected and lysed with radioimmunoprecipitation assay buffer
mixed with protein phosphatase inhibitor (Fuzhou Maixin
Biotechnology Development Co., Ltd.), followed by centrifugation at
1,049 × g at 4°C for 5 min. The cell supernatant was collected and
the protein concentration was determined. Equal amounts of protein
were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis for 1.5 h to separate the protein, followed by
electrotransfer onto a nitrocellulose membrane (Sigma-Aldrich St.
Louis, MO, USA). Following washing of the membrane with PBS (Fuzhou
Maixin Biotechnology Development Co., Ltd.), it was probed with the
primary antibody against phospho-p44/p42 MAPK antibody (1:1,000;
cat. no. sc9101S; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
or β-actin (l:1,000; cat. no. MX30002; Fuzhou Maixin Biotechnology
Development Co., Ltd., Fuzhou, China) at 4°C overnight. The
secondary antibody (horseradish peroxidase-linked anti-rabbit
immunoglobulin G; cat. no. MX3200-2; l:2,000 dilution; Fuzhou
Maixin Biotechnology Development Co., Ltd.) was added, followed by
incubation at room temperature for 2 h. After washing of the
membrane with PBS, it was incubated with NBT-BCIP solution (Fuzhou
Maixin Biotechnology Development Co., Ltd.) in the dark. Images
were captured and analyzed using the LAS3000 imaging system (Fuji
Film Co., Tokyo, Japan).
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS 11.5 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. One-way analysis of variance was
performed for comparison among multiple groups. The
independent-samples t-test was used for comparison between two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
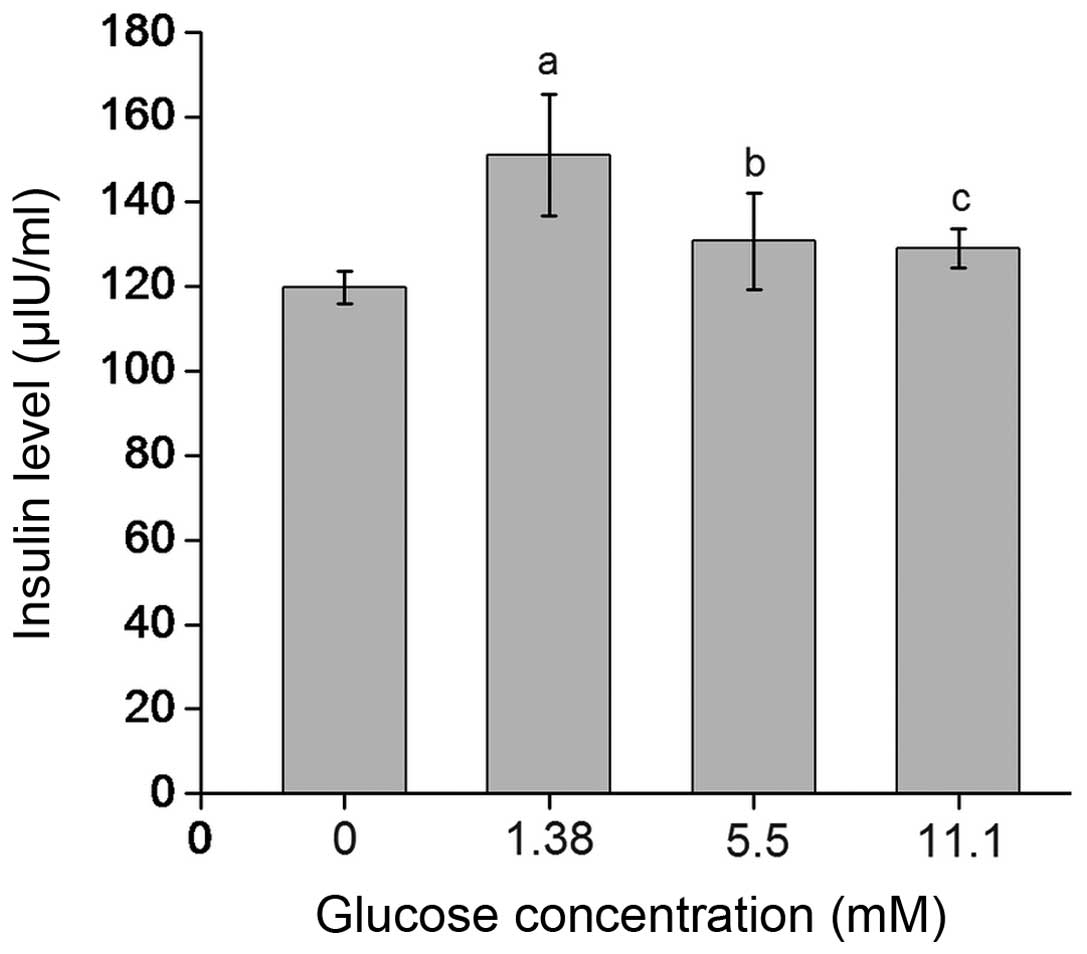
Glucose dose-dependently induces insulin
secretion by β-TC6 cells
The insulin levels in the supernatants of β-TC6
cells treated with 1.38, 5.5 and 11.1 mM glucose were 151.08±14.34,
130.67±11.35 and 129.05±4.71 µIU/ml, respectively, which
were significantly elevated compared with those in the in 0 mM
glucose group (119.77±3.89 µIU/ml; P<0.01 or P<0.05).
In addition, the insulin levels in the 1.38 mM glucose group were
significantly higher than those in the 11.1 mM glucose group
(P<0.05) (Fig. 1).
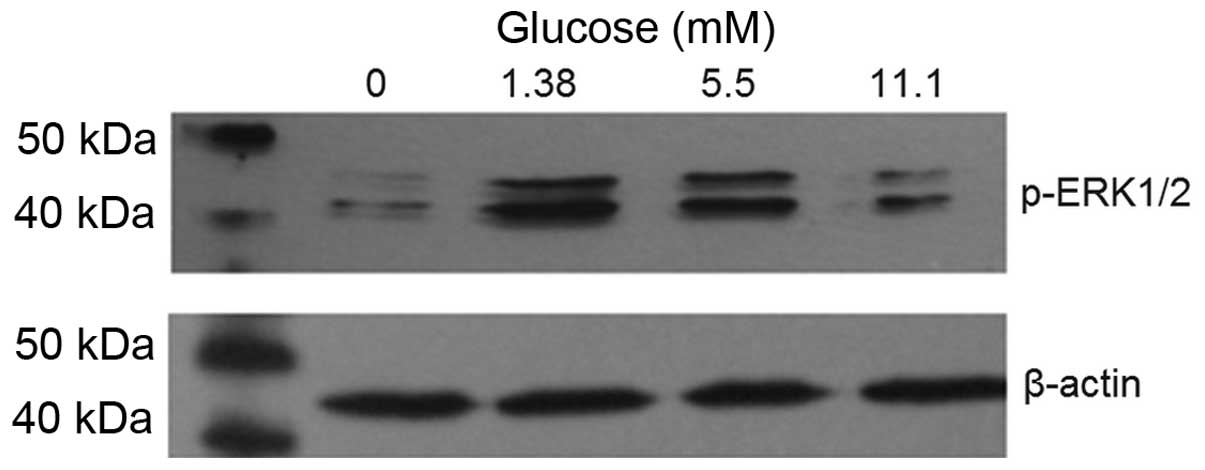
Glucose induces ERK1/2 phosphorylation in
β-TC6 cells
As shown in Fig. 2,
the level of ERK1/2 phosphorylation was increased in the 1.38, 5.5
and 11.1 mM glucose groups compared with that in the 0 mM glucose
group. The level of ERK1/2 phosphorylation was highest in the 1.38
mM glucose group. β-actin was used as the intrinsic parameter to
evaluate the amount of protein.
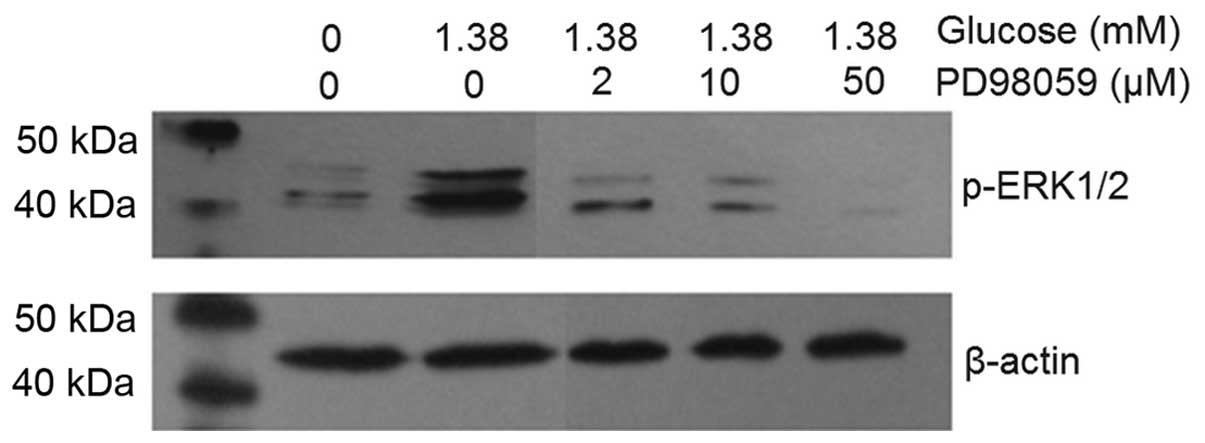
MAPK inhibitor PD98059 dose-dependently
reduces ERK1/2 phosphorylation induced by insulin
After intervention with 2, 10 and 50 µM
PD98059, the levels of ERK1/2 phosphorylation induced by 1.38 mM
glucose stimulation were decreased in a dose-dependent manner
(Fig. 3). Furthermore, in the 1.38
mM glucose + 50 µM PD98059 group, the phosphorylation of
ERK1/2 was almost completely inhibited and below the level in the
untreated control group. β-actin was used as the intrinsic
parameter to evaluate the amount of protein and to ensure equal
protein loading. Microscopic observation indicated that the growth,
viability and morphology of β-TC6 cells were not affected by
PD98059 (results not shown).
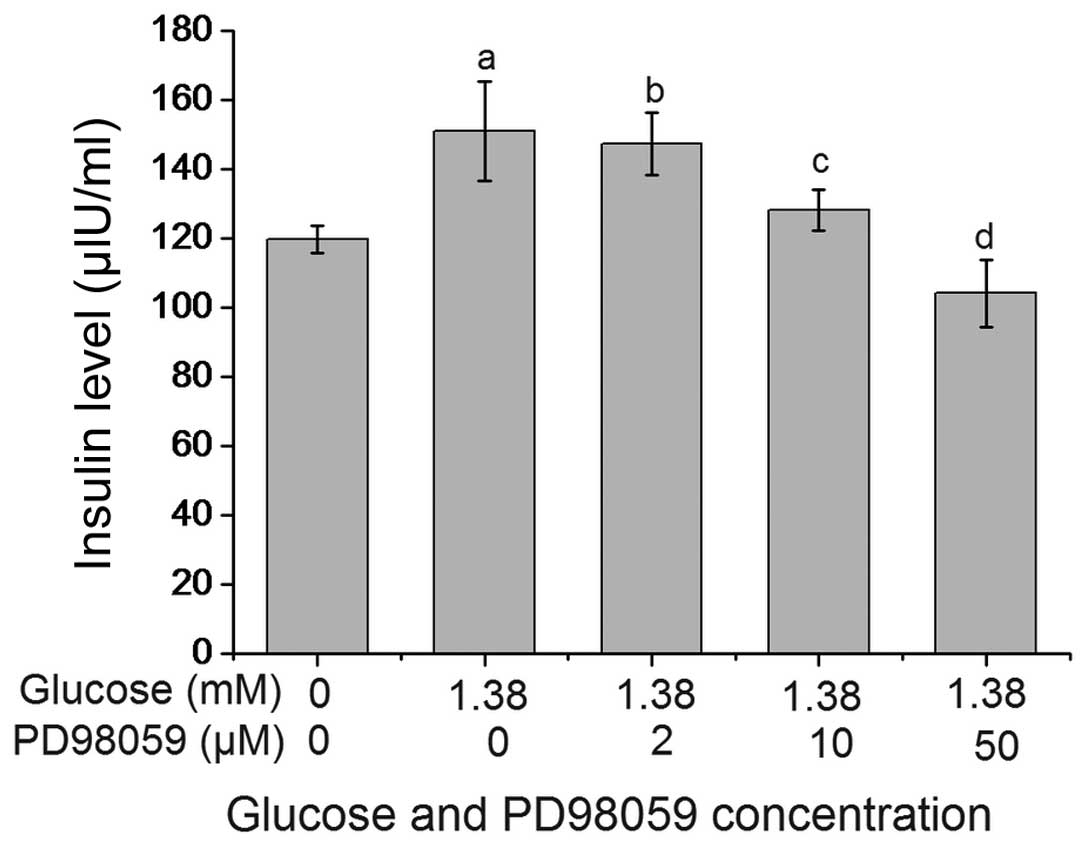
PD98059 inhibits glucose-induced insulin
secretion by β-TC6 cells
As shown in Fig. 4,
PD98059 suppressed glucose-stimulated insulin secretion by β-TC6
cells in a dose-dependent manner. The insulin levels in the 1.38 mM
glucose + 10 µM PD98059 group and the 1.38 mM glucose + 50
µM PD98059 group were 128.27±6.07 and 104.10±9.83
µIU/ml, respectively, which were significantly lower than
those in the 1.38 mM glucose + 0 µM PD98059 group
(151.08±14.34 U/ml; P<0.01). The insulin level in the 1.38
µM glucose + 50 µM PD98059 group was lower compared
with the untreated group, however, the difference was not
significant (P>0.05).
Discussion
β-TC6 cells are derived from the insulinoma cells of
a transgenic mouse and express SV40 t-antigen under the control of
the insulin promoter. β-TC6 cells produce pro-insulin I and II, and
effectively secret insulin and a small amount of glucagon (10). The threshold of β cells refers to
the amount of glucose required for stimulation of insulin
production. The maximum threshold of β-TC6 cells regarding glucose
stimulation is lower than that of normal β-cells, with 1.3–3.0 mM
glucose stimulating insulin secretion at peak levels, which are 1.6
times those of sugar-free insulin levels (stimulation index, 1.6)
(9). In the present study,
stimulation with 1.38 mM glucose led to insulin secretion at peak
levels, which were 1.26 times those of sugar-free insulin levels
(stimulation index, 1.26). Insulin secretion was stimulated to a
lesser extent by higher concentrations of glucose (5.5 and 11.1
mM), indicating that 1.38 mM was the most suitable glucose
concentration to stimulate insulin secretion.
MAPKs are a class of serine/threonine protein
kinases which exist in most cells (11). ERK1/2 are important signaling
proteins of the MAPK family that can be activated by extracellular
stimuli such as ultraviolet irradiation, high osmotic pressure,
heat shock and cytokines (12). A
previous study reported that glucose stimulation can activate the
ERK1/2 signal transduction pathway (13). Longuet et al (14) found that glucose stimulation can
activate the ERK1/2 signal transduction pathway in rats, with the
degree of activation regulating the concentration of glucose. In
addition, glucose stimulation of the INS-1 rat pancreatic β-cell
line and the MIN6 mouse pancreatic β-cell line, has been shown to
activate the ERK1/2 signal transduction pathway (8). In line with these results, the
present study reported that glucose stimulation can activate ERK1/2
signal transduction pathway in β-TC6 mouse pancreatic cells.
The association between the activation of the ERK1/2
signal transduction pathway and insulin secretion in β-TC6 cells
has not been previously reported, to the best of our knowledge. A
study from 1997 indicated that the ERK1/2 signal transduction
pathway is not required for glucose-stimulated insulin secretion
(15). However, it has been
demonstrated that is found that in MIN6 mouse pancreatic β-cells
and primary rat islet β cells, blocking of the ERK1/2 signaling
pathway reduced glucose-stimulated insulin secretion (14,16).
Furthermore, Vlacich et al (17) reported that the protein kinase Pim3
can inhibit the activation of the ERK1/2 signal pathway through
suppressor of cytokine-induced signaling 6 and regulates
glucose-stimulated insulin secretion. The present study showed that
MAPK inhibitor PD98059 dose-dependently inhibited the activation of
the ERK1/2 signaling pathway and decreased insulin secretion
stimulated by glucose. Thus, it is concluded that the activation of
the ERK1/2 signal transduction pathway is may be associated with
insulin secretion in β-TC6 cells. In addition, a previous study
(18) indicates that inhibition of
the ERK1/2 pathway is associated with the apoptosis of β cells. The
apoptosis induced by glucose via this pathway may be an underlying
mechanism of diabetes development.
Defects of pancreatic β-cell function and reduced
insulin sensitivity are important pathophysiological features
during the onset of diabetes (19). When β-cell defects appear, the
pancreatic island mass and/or volume cannot steadily maintain the
glucose metabolism, resulting in high blood glucose levels
(20). The present study indicated
the role of the ERK1/2 signal transduction pathway in
glucose-stimulated insulin secretion, which may represent an
important underlying mechanism of the development of diabetes.
However, this mechanism remains to be further elucidated and
confirmed by future studies.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81560135).
References
|
1
|
Xu Y, Wang L, He J, et al: Prevalence and
control of diabetes in Chinese adults. JAMA. 310:948–959. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shaikh SR, Haas KM, Beck MA and Teague H:
The effects of diet-induced obesity on B cell function. Clin Exp
Immunol. 179:90–99. 2015. View Article : Google Scholar
|
|
3
|
Szablewski L: Role of immune system in
type 1 diabetes mellitus pathogenesis. Int Immunopharmacol.
22:182–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mukai E, Toyoda K, Kimura H, et al: GLP-1
receptor antagonist as a potential probe for pancreatic beta-cell
imaging. Biochem Biophys Res Commun. 389:523–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathis D, Vence L and Benoist C: Beta-cell
death during progression to diabetes. Nature. 414:792–798. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KA and Lee MS: Recent progress in
research on beta-cell apoptosis by cytokines. Front Biosci
(Landmark ed). 14:657–664. 2009. View
Article : Google Scholar
|
|
7
|
Kumar P, Rao GN, Pal BB and Pal A:
Hyperglycemia-induced oxidative stress induces apoptosis by
inhibiting PI3-kinase/Akt and ERK1/2 MAPK mediated signaling
pathway causing downregulation of 8-oxoG-DNA glycosylase levels in
glial cells. Int J Biochem Cell Biol. 53:302–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lawrence M, Shao C, Duan L, McGlynn K and
Cobb MH: The protein kinases ERK1/2 and their roles in pancreatic
beta cells. Acta Physiol (Oxf). 192:11–17. 2008. View Article : Google Scholar
|
|
9
|
Ohtani M, Oka T, Badyuk M, Xiao Y, Kellar
KJ and Daly JW: Mouse beta-TC-6 insulinoma cells: High expression
of functional alpha3beta4 nicotinic receptors mediating membrane
potential, intracellular calcium and insulin release. Mol
Pharmacol. 69:899–907. 2006.
|
|
10
|
Mokhtari D, Al-Amin A, Turpaev K, Li T,
Idevall-Hagren O, Li J, Wuttke A, Fred RG, Ravassard P, Scharfmann
R, et al: Imatinib mesilate-induced phosphatidylinositol 3-kinase
signalling and improved survival in insulin-producing cells: Role
of Src homology 2-containing inositol 5′-phosphatase interaction
with c-Abl. Diabetologia. 56:1327–1338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anbazhagan K, Rabbind Singh A, Isabelle P,
Stella I, Céline AD, Bissac E, Bertrand B, Rémy N, Naomi T, Vincent
F, et al: Human pre-B cell receptor signal transduction: Evidence
for distinct roles of PI3kinase and MAP-kinase signalling pathways.
Immun Inflamm Dis. 1:26–36. 2013. View
Article : Google Scholar
|
|
12
|
Xu S and Kang UG: Cocaine induces
ubiquitination of Egr-1 in the rat dorsal striatum. Neuroreport.
25:1362–1367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Zhang J, Liu X, Liu S and Tian J:
Tribbles 3 regulates the fibrosis cytokine TGF-β 1 through
ERK1/2-MAPK signaling pathway in diabetic nephropathy. J Immunol
Res. 2014:2403962014. View Article : Google Scholar
|
|
14
|
Longuet C, Broca C, Costes S, Hani EH,
Bataille D and Dalle S: Extracellularly regulated kinases 1/2
(p44/42 mitogen-activated protein kinases) phosphorylate synapsin I
and regulate insulin secretion in the MIN6 beta-cell line and
islets of Langerhans. Endocrinology. 146:643–654. 2005. View Article : Google Scholar
|
|
15
|
Khoo S and Cobb MH: Activation of
mitogen-activating protein kinase by glucose is not required for
insulin secretion. Proc Natl Acad Sci USA. 94:5599–5604. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guerra ML, Wauson EM, McGlynn K and Cobb
MH: Muscarinic control of MIN6 pancreatic β cells is enhanced by
impaired amino acid signaling. J Biol Chem. 289:14370–14379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vlacich G, Nawijn MC, Webb GC and Steiner
DF: Pim3 negatively regulates glucose-stimulated insulin secretion.
Islets. 2:308–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Z and Xu S: ERK1/2 MAP kinases in cell
survival and apoptosis. IUBMB Life. 58:621–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiochi H, Ohkura T, Fujioka Y, Sumi K,
Yamamoto N, Nakanishi R, Matsuzawa K, Izawa S, Ohkura H, Inoue K,
et al: Bezafibrate improves insulin resistance evaluated using the
glucose clamp technique in patients with type 2 diabetes mellitus:
A small-scale clinical study. Diabetol Metab Syndr. 6:1132014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Donath MY, Størling J, Maedler K and
Mandrup-Poulsen T: Inflammatory mediators and islet beta-cell
failure: A link between Type 1 and Type 2 diabetes. J Mol Med
(Berl). 81:455–470. 2003. View Article : Google Scholar
|