Introduction
Lymph node metastasis is a hallmark of pancreatic
cancer (PC) progression and represents one of the most important
prognostic factors. The formation of new lymphatic vessels, known
as lymphangiogenesis, has an important role in this process
(1), although the molecular
mechanisms largely remain to be elucidated. Vascular endothelial
growth factor C (VEGF-C) is the central regulator of
lymphangiogenesis, and increased expression in various types of
primary tumor is correlated with the capacity of tumor cells to
disseminate to regional lymph nodes (2,3).
Implantation of cells overex-pressing VEGF-C has been shown to
induce tumor-associated lymphangiogenesis in several orthotopic
transgenic mouse models (4,5). In
addition, anti-VEGF-C treatment has been shown to inhibit
VEGF-C-induced lymphatic hyperplasia and tumor cell migration to
draining lymph nodes (6).
KAI1, also known as CD82, is a metastasis-suppressor
gene belonging to the tetraspanin family (7). The protein has four hydrophobic
transmembrane domains, two extracellular domains and three short
intracellular domains. Downregulation of KAl1 expression is often
observed in advanced stages of several types of human cancer. Loss
of KAI1 expression has been shown to be associated with increased
cell migration, reduced homotypic cell adhesion and altered ability
of tumor cells to bind specific extracellular proteins (8). These changes result in increased
invasiveness of tumor cells in vitro as well as a tendency
to metastasize in vivo; thus, the KAI1 gene is implicated in
the suppression of lymphatic metastasis.
In a previous study by our group, KAI1 was found to
be able to reduce lymphangiogenesis, as indicated by detection of
lymphatic vessel endothelial hyaluronan receptor-1 (9). Increased VEGF-C expression has also
been demonstrated to be closely associated with lymphangiogenesis
in PC invasion and lymphatic metastasis (10). Therefore, the present study
examined the association between KAI1 and VEGF-C expression and
investigated the signaling pathways involved in the KAI1-induced
decreases in VEGF-C expression and lymphatic metastasis the MIA
PaCa-2 and PANC1 PC cell lines.
Materials and methods
Tumor samples and
immunohistochemistry
Tumor samples were obtained from 28 patients with PC
who were treated at the General Hospital of Shenyang Military Area
(Shenyang, China) from September 2010 to September 2013. All of the
patients underwent local tumor resection and synchronal abdomen
dissection. The present study was approved by the ethics committee
of the General hospital of Shenyang Military Area (Shenyang,
China). Informed consent was obtained from all patients. Lymph node
(LN) metastases were found in 23 patients (82%), while seven
patients (25%) had metastases limited to node level (NL)1 (i.e.,
peripancreatic nodes). Metastases up to NL2 (nodes along main
arteries and the hepatic hilum) and NL3 (pre-aortic nodes) were
found in six (21%) and 10 (36%) patients, respectively, according
to the diagnostic criteria of the Japan Pancreas Society (11).
Immunohistochemistry
Paraffin-embedded tumor tissue sections (4
μm) were dewaxed and rehydrated. KAI1 and VEGF-C were
detected using specific primary antibodies [KAI1 (1:100 dilution;
BD Pharmingen, Heidelberg, Germany; VEGF-C (1:50 dilution; Zytomed,
Berlin, Germany]. The avidin-biotin technique was applied with
diaminobenzidine (BIOSS, Beijing, China) as the substrate for
visualization of KAI1 and VEGF-C immunoreactivity, which was graded
based on the intensity and the percentage of positive cells.
Hematoxylin (BIOSS) was used for counterstaining. The staining
intensity was scored from 0 to 3 (0 = no staining; 1 = weak, 2 =
moderate and 3 = strong staining intensity) using an inverted
fluorescent microscope (CKX41-A32PH, Olympus Corporation, Tokyo,
Japan). The evaluation and scoring of immunohistochemically stained
slides were carried out by 2 independent researchers
Cell culture and transfection
The MIA PaCa-2 and PANC1 human PC cell lines were
provided by the Shanghai Institute of Cell Biology, Chinese Academy
of Sciences (Shanghai, China). The cells were grown as
sub-confluent monolayers in Dulbecco's modified Eagle's medium
(DMEM; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal calf serum (FCS; GE Healthcare Life Sciences), 2
mmol/lL-glutamine, 100 IU/ml penicillin G, and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. KAI1 overexpression plasmid (pCMV-KAI1 DNA) was a
kind gift from Dr Jin-Tang Dong (Emory University School of
Medicine, Atlanta, Georgia, USA). Plasmid transfection was
performed according to a protocol of a previous study by our group
(9). The pCMV-KAI1-transfected
cells were renamed as MIA PaCa-2-K and PANC-1-K. The cells
transfected with empty pCMV vector (BIOSS) were renamed as MIA
PaCa-2-p and PANC-1-p.
For the inhibition experiments, 1.0×106
cells were plated in 10-cm dishes and maintained in DMEM with 10%
FCS. When the cells reached 70–80% confluence, the cell culture
medium was replaced with serum-free minimum essential medium (5 ml
MEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing the 100 μM Src inhibitor (incubated for 48 h;
PP2; EMD Millipore, San Diego, CA, USA) and 100 μM STAT3
inhibitor (incubated for 16 h; AG490; EMD Millipore). Cell lysates
were harvested after 24 h for western blot analysis.
Western blot analysis
Cells were harvested in a lysis buffer obtained from
Biogoodland (Wuxi, China) (10 mmol/l Tris/HCl, 5 mmol/l
ethylenediaminetetraacetic acid, 50 mmol/l NaCl, 30 mmol/l
Na3PO4, 50 mmol/l NaF, 0.1 mmol/l
Na3VO4, 1 mmol/l phenylmethylsulfonyl
fluoride, 5 mg/ml aprotinin and 0.1% Triton X-100; pH 7.6) and
total protein was quantified using the bicinchoninic acid method
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). Equal amounts of protein (30 μg) were subjected to
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(Beijing Solarbio Science & Technology Co., Ltd.) under
reducing conditions. The separated proteins were transferred onto
polyvinylidene difluoride membranes (EMD Millipore), which were
subsequently incubated in blocking buffer [Tris-buffered saline and
0.1% Tween 20 (TBST) containing 5% non-fat dry milk) for 2 h at
room temperature. Subsequently, the membranes were incubated at 4°C
overnight with the following primary antibodies: Polyclonal rabbit
anti-KAI1 (1:200 dilution; cat. no. sc-1087; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), polyclonal rabbit
anti-VEGF-C (1:500 dilution; cat. no. sc-25783; Santa Cruz
Biotechnology, Inc.), rabbit polyclonal anti-Src (1:50 dilution;
cat. no. bs-10604R-AP; BIOSS), rabbit polyclonal anti-phospho
(p)-Src (1:50 dilution; cat. no. bs-1730R-Bio; BIOSS), rabbit
polyclonal anti-STAT3 (1:200 dilution; cat no. sc-7179; Santa Cruz
Biotechnology, Inc.), rabbit polyclonal anti-p-STAT3 (1:200
dilution; cat no. sc-71792; Santa Cruz Biotechnology, Inc.) or
rabbit polyclonal anti-β-actin (1:100 dilution; Abcam, Cambridge,
UK). After three washes in TBST, the membranes were then incubated
with horseradish peroxidase-conjugated secondary antibody rabbit
immunoglobulin G (1:2,000 dilution; cat. no. sc-2749; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Immunoreactive
bands were visualized using an Western Lightning Chemiluminescence
Reagent Plus kit (Perkin-Elmer Life Science, Boston, MA, USA). The
signals were detected using a Las-4000 mini CCD camera (GE
Healthcare).
Statistical analysis
Values are expressed as the mean ± standard
deviation from at least three independent experiments. Student's
paired t-test was used to analyze statistical significance.
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used for all
statistical analyses. All experiments were repeated three times.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
KAI1 expression is inversely associated
with VEGF-C expression in pancreatic tumor samples
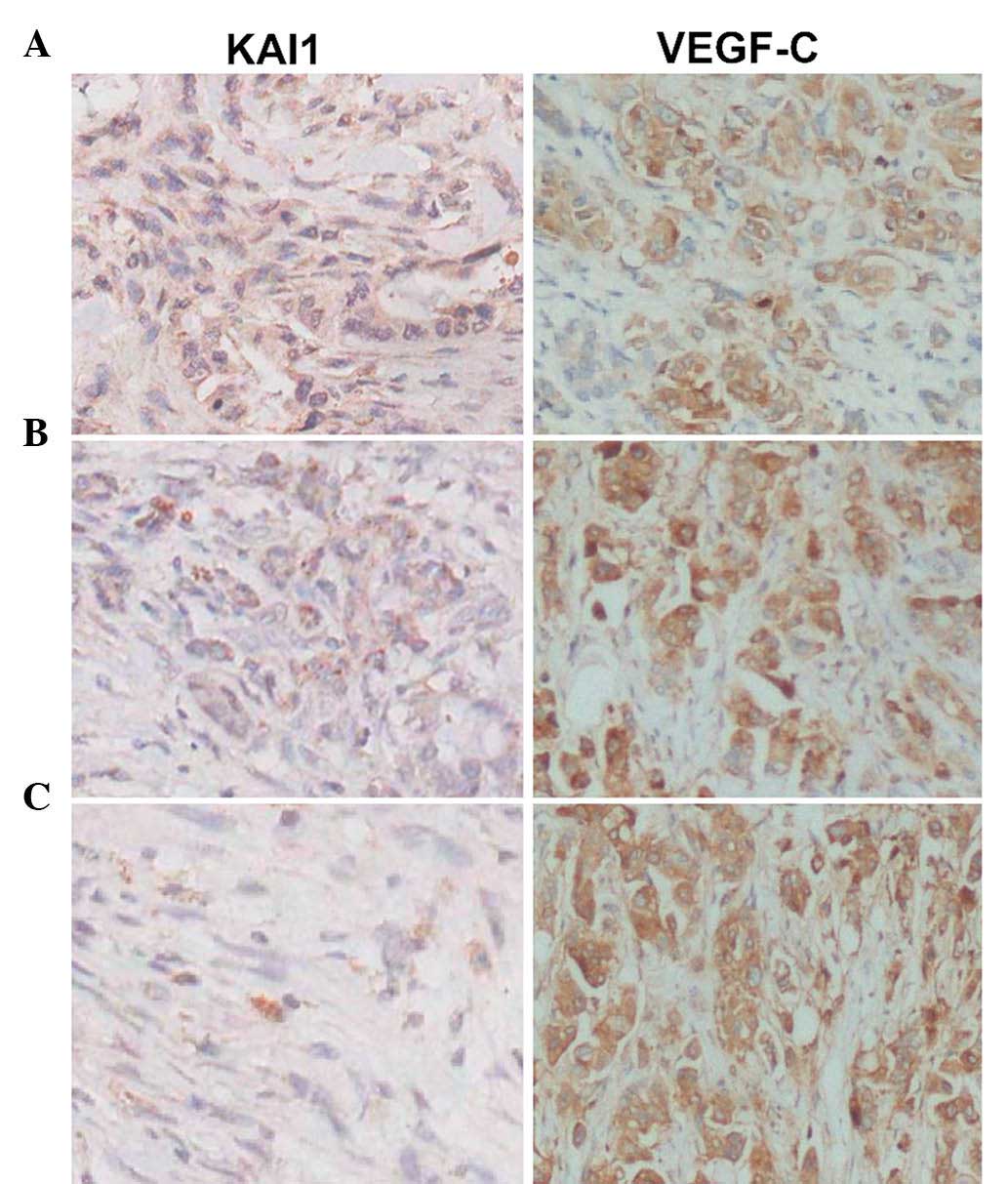
The present study evaluated 28 PC tumor samples by
immunohistochemistry (Fig. 1A–C).
VEGF-C expression was detected in most tumors with LN metastasis
and a negative association was identified between the expression of
KAI1 and VEGF-C in tumors with or without node metastasis
(P<0.05) (Fig. 1A–C). Tumors
from patients with metastases at NL1 to NL3 and with low KAI1
expression showed high VEGF-C expression (P<0.05).
KAI1 inhibits VEGF-C expression in PC
cell lines
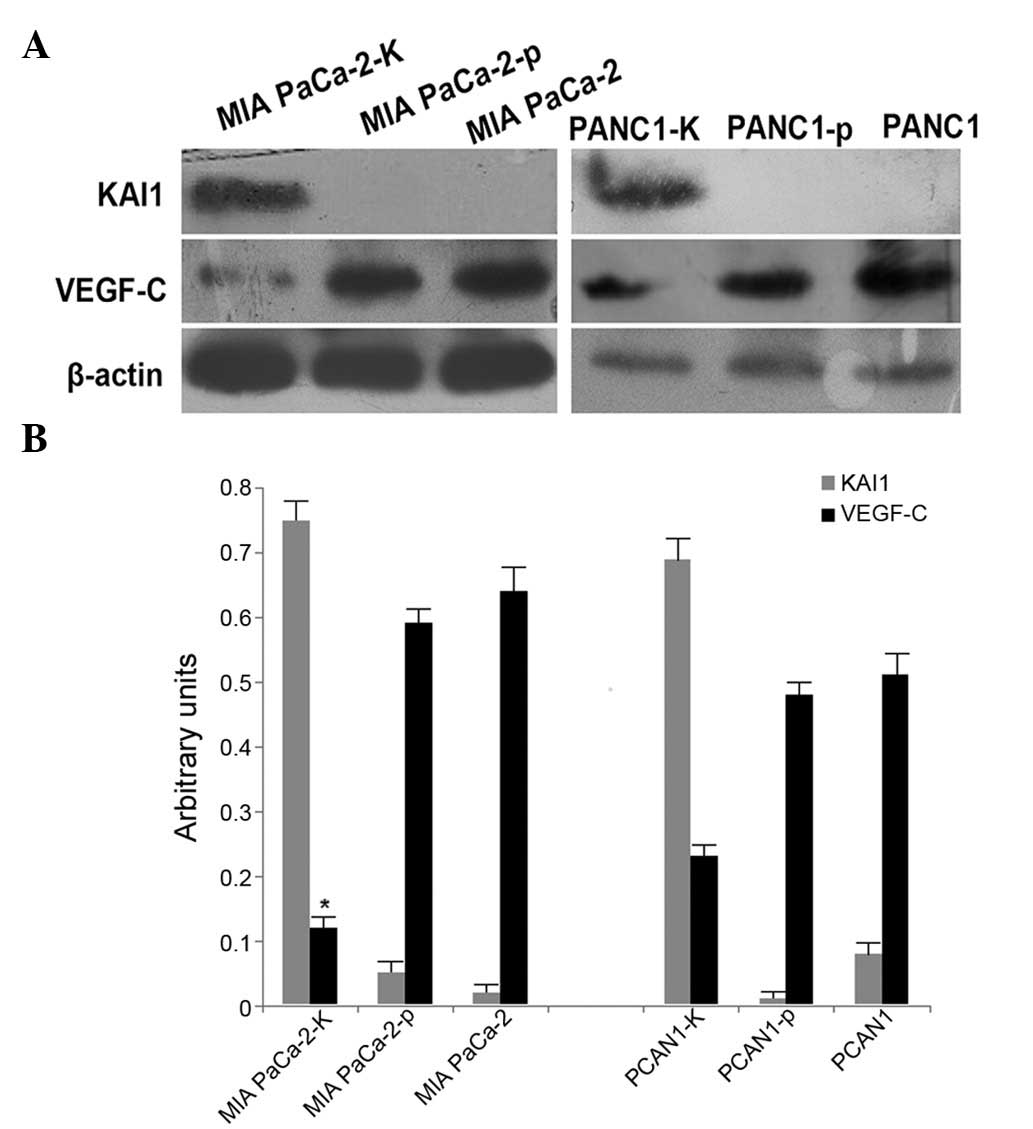
Western blot analysis was used to examine the
expression of KAI1 in MIA PaCa-2 and PANC-1 cells. While KAI1
protein was highly expressed in MIA PaCa-2-K and PANC-1-K cells, it
was almost undetectable in MIA PaCa-2, MIA PaCa-2-p, PANC-1 and
PANC-1-p cells (Fig. 2A).
Furthermore, reduced VEGF-C expression was observed in MIA PaCa-2-K
and PANC1-K cells compared with that in MIA PaCa-2, MIA PaCa-2-p,
PANC1 and PANC1-p cells (Fig. 2A).
The reduction of VEGF-C expression was more significant in MIA
PaCa-2 cells than that in PANC1 cells (Fig. 2B).
KAI1-induced decreases in VEGF-C
expression are mediated via Src/STAT3 in MIA PaCa-2 cells
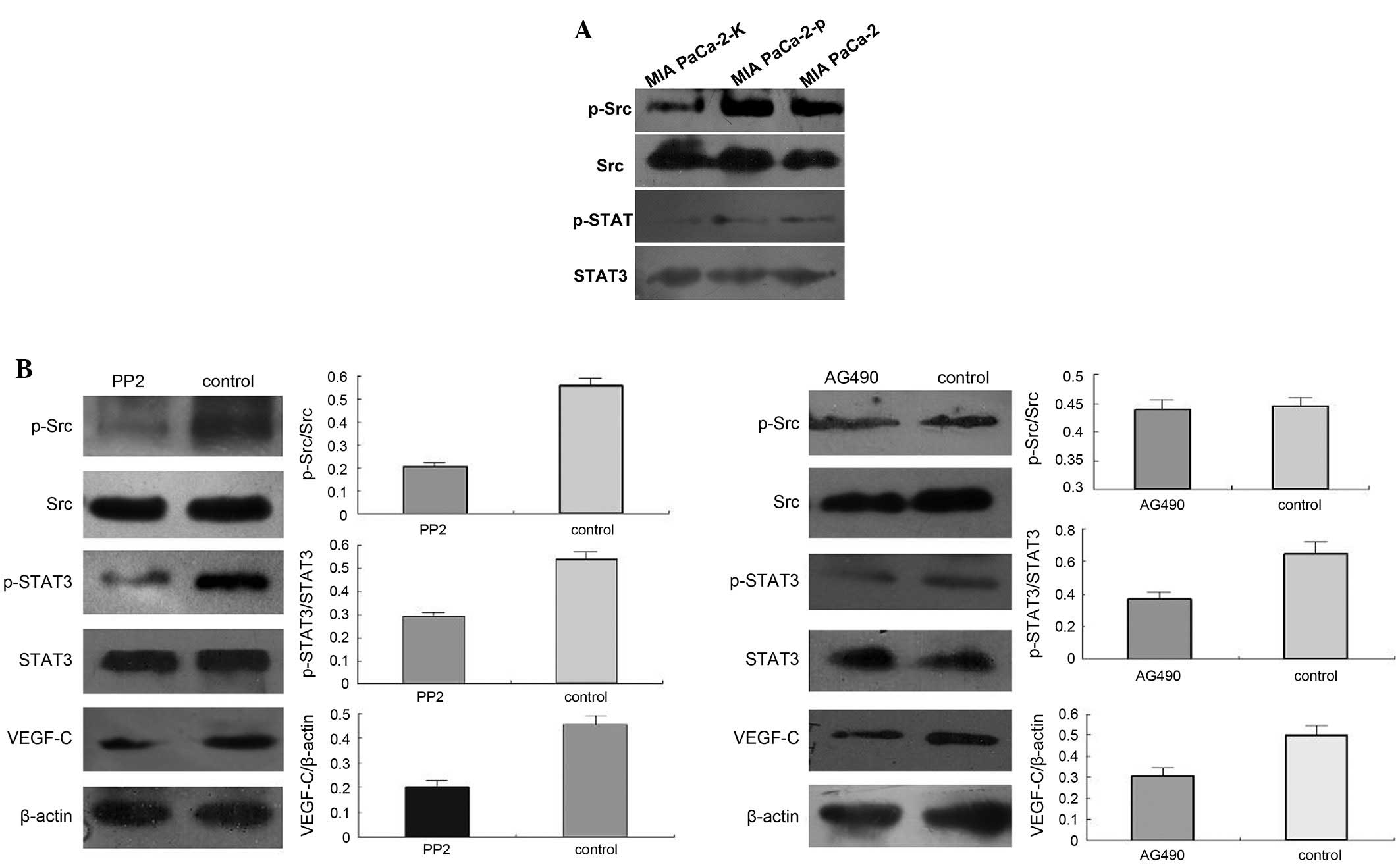
Western blot analysis indicated that Src and STAT3
phosphorylation was markedly decreased in MIA PaCa-2-K cells
compared with that in MIA PaCa-2 and MIA PaCa-2-p cells (Fig. 3A). Specific inhibitors of Src (PP2)
and STAT3 (AG490) activity were used to determine whether Src and
STAT3 activation is required for the KAl1-induced downregulation of
VEGF-C expression. Pre-treatment with PP2 efficiently reversed the
enhancement of Src and STAT3 phosphorylation and VEGF-C expression
(Fig. 3A). Furthermore,
pre-treatment with AG490 efficiently reversed the KAI1-induced
enhancement of STAT3 phosphorylation and VEGF-C expression, but had
no effect on Src phosphorylation (Fig.
3B).
Discussion
Metastasis is a hallmark of cancer and contributes
to~90% of cancer-associated mortalities (12). As metastastization of cancers
largely occurs via the lymphatic system, an understanding of the
underlying molecular mechanisms of lymphangiogenesis is of high
importance for the development of novel treatment strategies. The
present study reported on the specific roles of KAI1 in regulating
Src/STAT3/VEGF-C signaling in lymphangiogenesis in PC.
Accumulating evidence has demonstrated that KAI1 is
a metastasis-suppressor gene that functions without affecting
primary tumorigenicity (8,13). KAI1 signaling pathways and
functions are mediated through the activities of a complex
molecular network, the expression and activities of which are
tightly controlled under physiological conditions. Changes in this
delicate balance may trigger a cascade of molecular events that
ultimately lead to malignancy. A previous study by our group
examined the role of KAI1 in lymphangiogenesis in mice and
indicated a negative association with the expression of VEGF-C
(9).
VEGF-C secretion by tumor cells can induce the
activation of VEGF receptor-3 in the vascular endothelium, thereby
inducing the formation of new lymphatic vessels. VEGF-C ha an
important stimulatory function in lymphangiogenesis (14), with high VEGF-C expression being
significantly associated with LN metastasis, high
tumor-nodes-metastasis stage and poor outcome in patients with PC
(15). The present study
identified a significant negative association between KAI1 and
VEGF-C protein expression in pancreatic tumor samples and PC cells.
These results confirmed the hypothesis that KAI1 has the potential
to suppress the expression of VEGF-C and is its upstream regulator,
as posed by a previous study by our group (9). Furthermore, the effect of KAI1 on
VEGF-C was more obvious in the highly metastatic MIA PaCa-2 cell
line as compared to that in the PANC1 cell line. The result that
KAI1 has a more significant inhibitory effect on VEGF-C expression
in highly metastatic cells represents a novel characteristic of
KAI1. The present study therefore investigated the underlying
molecular mechanisms of the effects of KAI1 on VEGF-C to control
lymphangiogenesis in the human PC cell lines MIA PaCa-2 and PANC1.
Src signaling is an important component of VEGF-C-induced
lymphangiogenesis (16). In
addition, Src has been indicated to regulate VEGF via STAT3 in
human breast cancer as well as in head and neck carcinoma (17). The present study showed that KAI1
inhibits Src and STAT3 phosphorylation as well as VEGF-C
expression. However, pre-treatment with the Src inhibitor PP2
efficiently reversed KAI1-induced enhancement of Src and STAT3
phosphorylation and VEGF-C expression. In addition, pre-treatment
with the STAT3 inhibitor AG490 efficiently reversed KAI1-induced
enhancement of STAT3 phosphorylation and VEGF-C expression, but had
no effect on the enhancement of Src phosphorylation in MIA PaCa-2
cells. These results indicated that KAI1 inhibits VEGF-C via the
Src/STAT3 signaling pathway. However, density comparison on
low-quality blots is not conclusive.
In conclusion, the present study provided the first
pre-clinical evidence that KAI1 has the potential to suppress
VEGF-C expression in pancreatic tumor samples and the human MIA
PaCa-2 and PANC1 PC cell lines. Furthermore, it was demonstrated
that KAI1 downregulates VEGF-C expression via the Src/STAT3
signaling pathways in MIA PaCa-2 PC cells. An understanding of the
signal transduction mechanisms involved in the
KAI1/Src/STAT3/VEGF-C axis may provide a novel approach for the
treatment of lymphatic metastasis in PC.
Acknowledgments
Partial results of the present study have been
published in the conference of Gastro 2013 APDW/WCOG in the form of
a 'Meeting Abstract'. The authors were Xiao Zhong Guo, Xu Liu,
HongYu Li, XiaoDong Shao, ZhongMin Cui, under the title is
'Src/STAT3 signaling pathways are involved in KAI1-reduced VEGF-C
down-regulation in PC' pg 881, abstract no. 160. The study was
supported by the China Postdoctoral Science Foundation (grant no.
2014M552691).
References
|
1
|
Qian CN, Berghuis B, Tsarfaty G, Bruch M,
Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, et
al: Preparing the ῾soil᾽: The primary tumor induces vasculature
reorganization in the sentinel lymph node before the arrival of
metastatic cancer cells. Cancer Res. 66:10365–10376. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stacker SA, Achen MG, Jussila L, Baldwin
ME and Alitalo K: Lymphangiogenesis and cancer metastasis. Nat Rev
Cancer. 2:573–583. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stacker SA, Farnsworth RH, Karnezis T,
Shayan R, Smith DP, Paavonen K, Davydova N, Caesar C, Inder R,
Baldwin ME, et al: Molecular pathways for lymphangiogenesis and
their role in human disease. Novartis Found Symp. 281:38–43. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karpanen T, Wirzenius M, Mäkinen T,
Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B,
Ylä-Herttuala S and Alitalo K: Lymphangiogenic growth factor
responsiveness is modulated by postnatal lymphatic vessel
maturation. Am J Pathol. 169:708–718. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skobe M, Hawighorst T, Jackson DG, Prevo
R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K and Detmar
M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoshida T, Isaka N, Hagendoorn J, di
Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP and Jain RK:
Imaging steps of lymphatic metastasis reveals that vascular
endothelial growth factor-C increases metastasis by increasing
delivery of cancer cells to lymph nodes: Therapeutic implications.
Cancer Res. 66:8065–8075. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu WM and Zhang XA: KAI1/CD82, a tumor
metastasis suppressor. Cancer Lett. 240:183–194. 2006. View Article : Google Scholar
|
|
8
|
Miranti CK: Controlling cell surface
dynamics and signaling: How CD82/KAI1 suppresses metastasis. Cell
Signal. 21:196–211. 2009. View Article : Google Scholar
|
|
9
|
Liu X, Guo XZ, Li HY, Chen J, Ren LN and
Wu CY: KAI1 inhibits lymphangiogenesis and lymphatic metastasis of
pancreatic cancer in vivo. Hepatobiliary Pancreat Dis Int.
13:87–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng P, Jin G, Hu X, Shi M, Zhang Y, Liu
R, Zhou Y, Shao C, Zheng J and Zhu M: Analysis of tumor-induced
lymphangiogenesis and lymphatic vessel invasion of pancreatic
carcinoma in the peripheral nerve plexus. Cancer Sci.
103:1756–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Japan Pancreas Society: Classification of
pancreatic carcinoma. 3rd English edn. Kanehara, Tokyo: 2003
|
|
12
|
Yan J, Yang Q and Huang Q: Metastasis
suppressor genes. Histol Histopathol. 28:285–292. 2013.PubMed/NCBI
|
|
13
|
Liu X, Guo XZ, Zhang WW, Lu ZZ, Zhang QW,
Duan HF and Wang LS: KAI1 inhibits HGF-induced invasion of
pancreatic cancer by sphingosine kinase activity. Hepatobiliary
Pancreat Dis Int. 10:201–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsumoto M, Roufail S, Inder R, Caesar C,
Karnezis T, Shayan R, Farnsworth RH, Sato T, Achen MG, Mann GB and
Stacker SA: Signaling for lymphangiogenesis via VEGFR-3 is required
for the early events of metastasis. Clin Exp Metastasis.
30:819–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurahara H, Takao S, Maemura K, Shinchi H,
Natsugoe S and Aikou T: Impact of vascular endothelial growth
factor-C and -D expression in human pancreatic cancer: Its
relationship to lymph node metastasis. Clin Cancer Res.
10:8413–8420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ischenko I, Seeliger H, Camaj P, Kleespies
A, Guba M, Eichhorn ME, Jauch KW and Bruns CJ: Src tyrosine kinase
inhibition suppresses lymphangiogenesis in vitro and in vivo. Curr
Cancer Drug Targets. 10:546–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|