Introduction
Lung cancer is a leading cause of cancer-associated
mortality worldwide, predominantly from cases of non-small cell
lung cancer (NSCLC) (1). The
prognosis for lung cancer is poor, particularly in the advanced
stages (2,3). To select the most effective
therapeutic strategy, prognostic assessment is required (4). Although there are diverse clinical
indicators for prognostic evaluation, patients with cancer who
exhibit similar clinical features nonetheless may experience
markedly different clinical outcomes. With the recent development
of gene profiling techniques, including microRNA (miRNA)
microarrays and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), novel molecular biomarkers may be used as
prognostic factors in addition to traditional clinical
characteristics (5–7).
miRNAs comprise a class of noncoding small RNAs that
participate in various biological processes. Usually, miRNAs
function at the post-transcriptional level and regulate hundreds of
target mRNAs, thus serving various roles in biological functions,
including tumorigenesis and cancer progression (8). miRNA expression profiles have been
identified in various tissues and diseases. Furthermore, miRNAs
have been identified as biomarkers for the diagnosis, treatment and
prognosis of various diseases (9–13).
A miRNA signature containing five miRNAs (miR-221,
let-7a, miR-137, miR-372 and miR-182) was previously reported to be
associated with overall survival (OS) and progression-free survival
(PFS) in patients with early stage NSCLC, following examination of
frozen specimens subsequent to surgical resection (14). Another miRNA signature comprising
four miRNAs (miR-486, miR-30d, miR-1 and miR-499) in serum has been
reported to be associated with outcome in early stage NSCLC
(15). However, whether the above
miRNAs can predict the clinical outcome of advanced stage NSCLC
remains unknown. In addition, the correlation between tissue and
serum miRNA expression remains to be elucidated.
To investigate these problems, the present study
detected the aforementioned miRNAs in serum, in order to analyze
their association with survival. Furthermore, the serum miRNAs
associated with PFS were detected in paired tissue samples, to
determine the expression correlation. Finally, the present study
attempted to verify the functions of relevant miRNAs in
vitro.
Materials and methods
Patient enrollment and sample
processing
The present study was approved by the Ethics
Committee of the Tongji Medical Department, Huazhong University of
Science and Technology (Wuhan, China). All participants provided
written informed consent to participate in the present study, and
the ethics committee approved this consent procedure. Serum miRNAs
were detected from 68 consecutive patients enrolled between
December 2012 and May 2013 at the Cancer Center, Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology. The final follow-up was in December 2013. The median
time of follow-up was 256 days. All of the patients were first
pathologically confirmed to suffer from locally advanced or
metastatic NSCLC, and developed progressive disease during
follow-up. Due to the high censor rate, OS statistics were not
calculated. Fresh tissue specimens were obtained from 24 patients
using computed tomography-guided percutaneous lung biopsy, and were
paired with the corresponding patient's serum, in order to analyze
the correlation between the two sample types. Following admission
into hospital, a 5 ml peripheral blood sample was collected into a
gold-top serum-separating tube. The lung cancer subtypes were
authenticated by the Pathology Department, Union Hospital, Tongji
Medical College, Huazhong University of Science and Technology,
according to the World Health Organization classification (16). The evaluation after treatment was
performed according to the Response Evaluation Criteria in Solid
Tumors Version 1.1 (17). The
objective tumor response was evaluated every 6–8 weeks. Clinical
data were retrieved from the electronic medical records database of
the Cancer Center, Union Hospital, Tongji Medical College, Huazhong
University of Science and Technology.
miRNA isolation and quantification
The serum specimens were incubated at room
temperature for 30 min-2 h and were centrifuged at 12,000 × g for
15 min at 4°C. Subsequently, the samples were dispensed into
Eppendorf tubes and were stored at −80°C until further use. Serum
total RNA was isolated using the mirVana™ PARIS™ kit
(Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Based
on reports that there are no favorable stably expressed genes in
serum, a synthetic Caenorhabditis elegans miRNA (cel-miR-39;
5′-UCACCGGGUGUAAAUCAGCUUG-3′; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) was selected as a control gene, of which 25 fmol
was added after the addition of denaturing solution to each sample
in the serum miRNA isolation procedure (18,19).
Fresh tissue specimens were immediately transferred
into RNAlater RNA Stabilization Reagent (Qiagen, Inc.,
Valencia, CA, USA) after being obtained and were stored at −80°C.
The tissue samples were homogenized prior to RNA extraction. The
E.Z.N.A®. Total RNA kit II (Omega Bio-tek, Norcross, GA,
USA) was used to extract total RNA from the tissue, and small
nuclear U6 RNA was used for normalization.
RT was performed on total RNA using a stem-loop RT
primer (Applied Biosystems; Thermo Fisher Scientific, Inc.;
Table I), and the TaqMan microRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The total reaction volume (15 µl) was
incubated in a TProfessional Basic Thermocycler (Biometra GmbH,
Göttingen, Germany). qPCR was performed using the SYBR®
Select Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and the Step One Plus system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). A volume of 2 µl diluted reverse
transcription product was mixed with 10 µl SYBR Select
Master Mix (2X; Applied Biosystems; Thermo Fisher Scientific,
Inc.), 0.8 µl forward and reverse primers (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and 6.4 µl
nuclease-free water to a final volume of 20 µl. All
reactions were performed in triplicate under the following thermal
cycling conditions: 50°C For 2 min and 95°C for 2 min, followed by
40 cycles at 95°C for 3 sec and 60°C for 30 sec.
 | Table IReverse transcription-quantitative
polymerase chain reaction primers. |
Table I
Reverse transcription-quantitative
polymerase chain reaction primers.
| Gene | Primer sequence
(5′–3′) |
|---|
| hsa-miR-1 | RT
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATAC
F GACATACATGGCAGGTGGAATGTAAAGAAGT
R CAGTGCAGGGTCCGAGGTAT |
| hsa-let-7a-5p | RT
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATAC
F GACAACTATGGTCGTGAGGTAGTAGGTTGTA
R CAGTGCAGGGTCCGAGGTAT |
| hsa-miR-30d-5p | RT
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATAC
F GACCTTCCACCTGTGTAAACATCCCCGAC
R CAGTGCAGGGTCCGAGGTAT |
| hsa-miR-221-3p | RT
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATAC
F GACGAAACCGGGAGCTACATTGTCTGCTGG
R CAGTGCAGGGTCCGAGGTAT |
|
hsa-miR-499a-5p | RT
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATAC
F GACAAACATCGGTGCTTAAGACTTGCAGTGA
R CAGTGCAGGGTCCGAGGTAT |
| hsa-miR-486-5p | RT
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATAC
F GACCTCGGGCGTCCTGTACTGAGCTGCC
R GTGCAGGGTCCGAGGT |
| cel-miR-39 | RT
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATAC
F GACCAAGCTGCGGTCACCGGGTGTAAATC
R GTGCAGGGTCCGAGGT |
| U6 | RT
CGAATTTGCGTGTCATCCT
F CTCGCTTCGGCAGCACATA
R CGAATTTGCGTGTCATCCT |
Cell culture and transfection
The H358 and PC9 human lung cancer cell lines were
obtained from the Laboratory of the Cancer Center, Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology. The cells were cultured in RPMI 1640 medium (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C in an atmosphere containing 5% CO2.
The hsa-mir-221-3p mimic, hsa-mir-221-3p inhibitor,
hsa-mir-486-5p mimic and hsa-mir-486-5p inhibitor were purchased
from Guangzhou RiboBio Co., Ltd.. Cells were plated to 50%
confluence in each well, and were transfected with 50 nM mimic or
mimic negative control (mimic NC), or with 100 nM inhibitor or
inhibitor NC using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in Opti-MEM (Gibco; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol.
MTT assay
The viable cell numbers were measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich, St. Louis, MO, USA). The H358 and PC9 cells
were seeded at a density of 5×103 cells/well in 96-well
plates, and were transfected with miR-221 mimic, miR-221 inhibitor,
miR-486 mimic, miR-486 inhibitor, mimic NC or inhibitor NC. The
cells were incubated for 24, 48 or 72 h post-transfection. The MTT
assay was conducted according to the manufacturer's protocol. A
total of 20 µl MTT (5 mg/ml) was added to each well, and the
plates were incubated for 4 h at 37°C in an atmosphere containing
5% CO2. Subsequently, the supernatant was discarded, and
200 µl dimethyl sulfoxide was added to each well, in order
to dissolve the formazan. The optical density was evaluated by
measuring the absorbance of each well at 570 nm using a
spectrophotometer (M450; Bio-Rad Laboratories, Inc., Hercules, CA,
USA). All experiments were performed in triplicate.
Wound healing assay
The H358 and PC9 cells (~5×105) suspended
in 2 ml complete medium (Hyclone; GE Healthcare Life Sciences) were
seeded into 6-well plates, and cell confluence reached ~80% 24 h
post-transfection. A scratch was created by drawing a straight line
in each well using a 200-µl pipette tip. Any debris was
washed off using phosphate-buffered saline. The 6-well plates were
then incubated in serum-free medium for 24 h, and subsequently
three fields were randomly picked from each scratch wound and
analyzed at 200× magnification (CKX41; Olympus Corporation). The
experiments were performed in triplicate.
Transwell migration and invasion
assay
The H358 and PC9 cells were resuspended in RPMI 1640
medium 24 h post-transfection. For the transwell migration assay,
4×104 seeded H358 cells (200 µl) or
2×104 seeded PC9 cells (200 µl) were plated in
the top chamber with the non-coated membrane (24-well insert; pore
size, 8 µm; BD Biosciences, Franklin Lakes, NJ, USA). For
the invasion assay, cells were plated in the top chamber with
extracellular matrix gel (Sigma-Aldrich)-coated membranes (24-well
insert; pore size, 8 µm; BD Biosciences). The lower regions
were filled with 800 µl RPMI 1640 containing 20% FBS as a
chemoattractant. Following a 24 h incubation, the cells on the
upper surface of the membrane were removed with a cotton swab. The
cells on the lower surface of the membrane were fixed with methyl
alcohol for 20 min, and were stained with 0.1% crystal violet for
15 min. The number of invading cells was counted in three randomly
selected visual fields using a microscope (CKX41; Olympus
Corporation, Tokyo, Japan) at 200× magnification. The experiments
were performed in triplicate.
Statistical analysis
The RT-qPCR data were analyzed using the comparative
quantification cycle (Cq) method. Standard curves were created
prior to analysis of the specimens. The correlation coefficients
were >0.995, and the amplification efficiencies were in the
range of 1±20%. The expression level of each miRNA was classified
according to the median relative quantification measured by
2−ΔΔCq (ΔCq=CqmiRNA expression −
Cqcontrol expression) (20). During the pre-experiment, three
(miR-137, miR-182, and miR-372) of the nine miRNAs exhibited
unstable or undetermined expression in serum; therefore, these
three miRNAs were not analyzed.
A cross-validation method was used to investigate
the value of the miRNAs for predicting PFS. The 68 serum specimens
were randomly assigned to a training set (n=34; used to establish a
model) or a testing set (n=34; used to verify the model). In the
training set, a risk score formula was established based on a
linear combination of the miRNA expression level weighted by the
regression coefficient derived from a univariate Cox regression
analysis. Student's t-test and Chi-squared test were used to
evaluate the differences in clinical characteristics. The risk
score was calculated as follows: Risk score = (−0.918 × miR-1
expression level) + (1.336 × miR-30d expression level) + (0.864 ×
miR-221 expression level) + (−0.712 × miR-486 expression level).
Patients with higher risk scores were expected to have shorter
PFS.
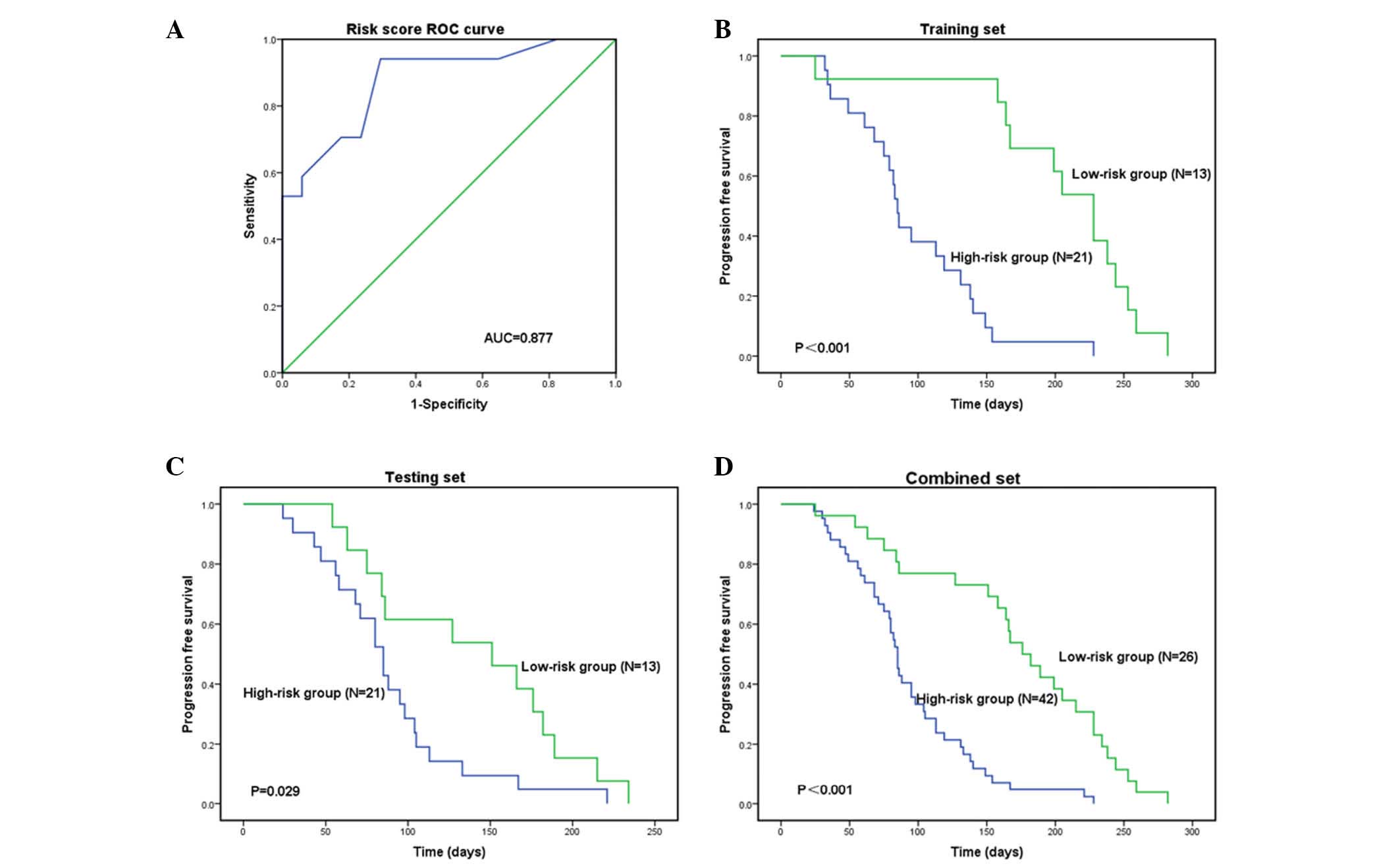
According to the risk score, a receiver operating
characteristics analysis was used to determine a cutoff point
(0.518) with an area under the curve of 0.877 (the sensitivity was
0.941, and the specificity was 0.706). Hence, the patients in the
training set were divided into a high-risk group and a low-risk
group. The same statistical models were applied to the testing set
and the combined set. Survival differences between the high-risk
and low-risk groups were estimated using a Kaplan-Meier analysis
and 2-sided log-rank tests. A Cox proportional hazards regression
analysis was performed to assess the independent contributions of
the risk score and clinicopathological characteristics to survival
prediction.
To analyze the correlation between serum and tissue
miRNAs, the four survival-associated miRNAs were detected in 24
fresh tissue samples paired with 24 serum samples. In the
pre-experiment, the expression of miR-1 was undetermined;
therefore, three miRNAs were ultimately tested. A Pearson analysis
was used to test the correlation.
All statistical analyses were performed using SPSS
version 21.0 (IBM SPSS, Armonk, NY, USA). All experiments were
performed in triplicate. All tests were two-tailed, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Detection of serum miRNAs in the training
set
Six miRNAs were detected in the training set serum
specimens (n=34), and a Cox proportional hazards regression was
applied to each of the six miRNAs to determine the PFS-associated
miRNA signature. Four of the miRNAs were identified to be
associated with PFS. Subsequently, a signature was constructed
using the risk score method. As shown in Table II, two miRNAs (miR-1 and miR-486)
were revealed to be protective, whereas the other two (miR-30d and
miR-221) were identified as risk factors (Table II).
 | Table IIUnivariate Cox regression analysis of
the training set. |
Table II
Univariate Cox regression analysis of
the training set.
| miRNA | Univariate Cox
regression analysis
|
|---|
| β | HR | 95% CI | P |
|---|
| hsa-miR-1 | −0.918 | 0.4 | 0.197–0.809 | 0.011 |
| hsa-let-7a-5p | 0.345 | 1.412 | 0.698–2.860 | 0.337 |
| hsa-miR-30d-5p | 1.338 | 3.812 | 1.655–8.781 | 0.002 |
| hsa-miR-221-3p | 0.846 | 2.331 | 1.122–4.846 | 0.023 |
|
hsa-miR-499a-5p | 0.242 | 1.274 | 0.633–2.565 | 0.498 |
| hsa-miR-486-5p | −0.712 | 0.491 | 0.242–0.997 | 0.049 |
Four-miRNA signature and patient survival
in the training set
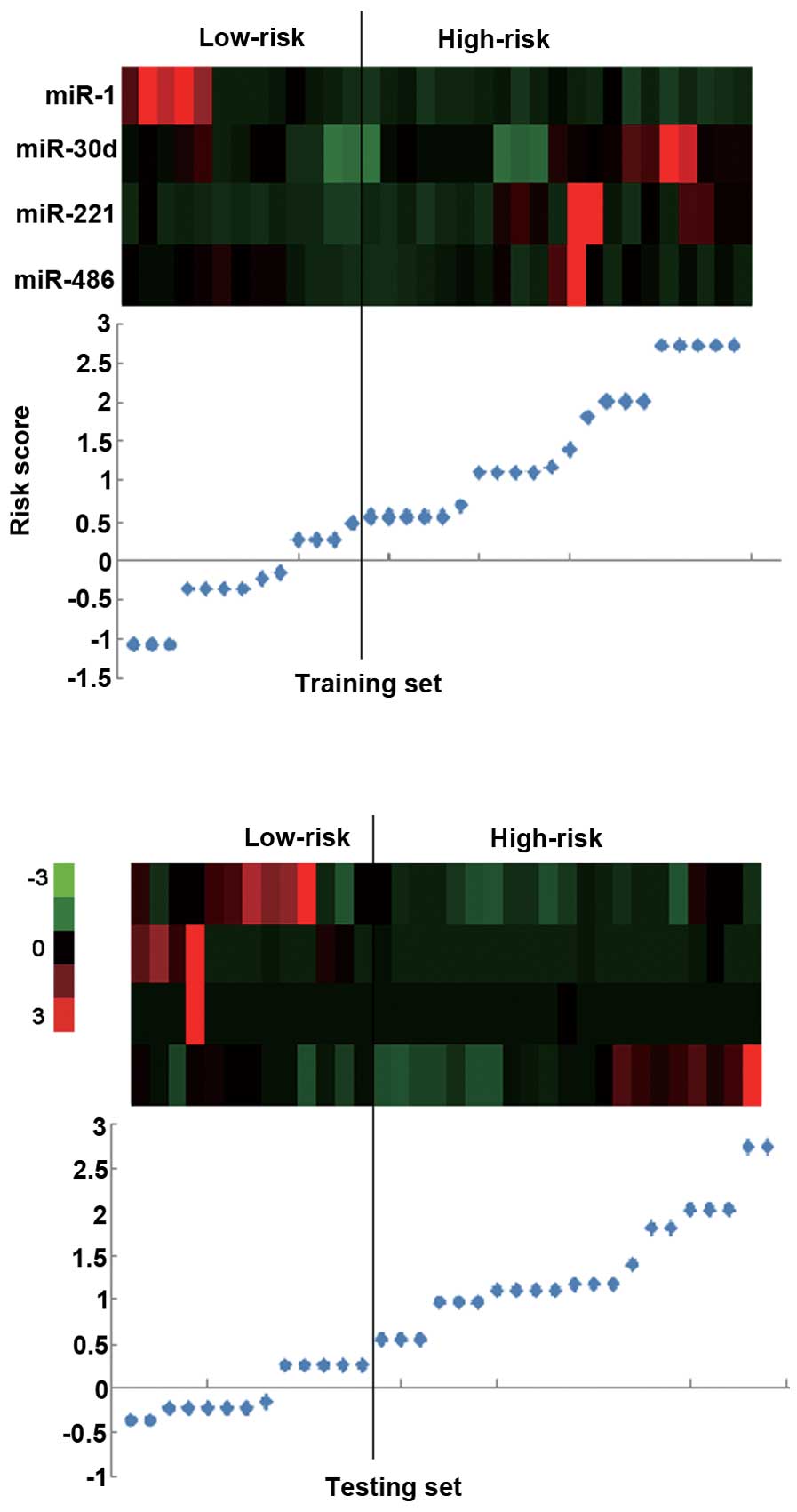
The risk score formula was used to calculate the
risk score in the training set, and to divide the set into a
high-risk group and a low-risk group according to a cutoff value.
Patients in the high-risk group had a shorter PFS compared with
those in the low-risk group: 85 days vs. 228 days (P<0.001;
Fig. 1A and B). The patients in
the high-risk and low-risk groups exhibited similar clinical
characteristics (Table III).
 | Table IIIClinical characteristics in the
training set, testing set and combined set. |
Table III
Clinical characteristics in the
training set, testing set and combined set.
| Characteristic | High risk | Low risk | P |
|---|
| Training set | 21 | 13 | |
| Age | 57.81±7.369 | 54.69±9.187 | 0.283a |
| Gender | | | |
| Male | 17 | 8 | |
| Female | 4 | 5 | 0.254b |
| Smoking
status | | | |
| No | 11 | 8 | |
| Yes | 10 | 5 | 0.728b |
| Pathology | | | |
|
Adenocarcinoma | 18 | 10 | |
| Squamous
carcinoma | 3 | 3 | 0.653b |
| Clinical
stage | | | |
| IIIb | 2 | 2 | |
| IV | 19 | 11 | 0.627b |
| Testing set | 21 | 13 | |
| Age | 60.19±10.186 | 63.23±8.584 | 0.377a |
| Gender | | | |
| Male | 14 | 8 | |
| Female | 7 | 5 | 1.000b |
| Smoking
status | | | |
| No | 10 | 5 | |
| Yes | 11 | 8 | 0.728b |
| Pathology | | | |
|
Adenocarcinoma | 19 | 11 | |
| Squamous
carcinoma | 2 | 2 | 0.627b |
| Clinical
stage | | | |
| IIIb | 3 | 2 | |
| IV | 18 | 11 | 1.000b |
| Combined | 42 | 26 | |
| Age | 59.00±8.859 | 58.96±9.739 | 0.987a |
| Gender | | | |
| Male | 31 | 16 | |
| Female | 11 | 10 | 0.418b |
| Smoking
status | | | |
| No | 21 | 13 | |
| Yes | 21 | 13 | 1.000b |
| Pathology | | | |
|
Adenocarcinoma | 37 | 21 | |
| Squamous
carcinoma | 5 | 5 | 0.489b |
| Clinical
stage | | | |
| IIIb | 5 | 4 | |
| IV | 37 | 22 | 0.723b |
Validation of the four-miRNA signature
for survival prediction in the testing and combined set
The same risk-score formula and cutoff value
obtained from the training set were used to analyze the testing and
combined sets. Following calculation of the risk score, the
patients were classified into a high-risk group and a low-risk
group. Similar findings were revealed in this analysis; the
high-risk group had a shorter PFS compared with that of the
low-risk group: 85 Days vs. 151 days in the testing set (P=0.029)
and 85 days vs. 176 days (P<0.001) in the combined set (Figs. 1C and D, and 2). The low-risk group exhibited high
expression levels of protective miRs and low expression of
risk-related miRs, and the high-risk group exhibited low expression
levels of protective miRs and high expression of risk-related miRs
(Figs. 1C and D, and 2).
To investigate whether this serum four-miRNA
signature is an independent predictor of PFS, a multivariate Cox
regression analysis was performed. The results indicate that the
miRNA signature (P<0.001) and smoking status (P=0.037) were
significantly associated with PFS (Table IV).
 | Table IVCox regression analyses of the
combined set. |
Table IV
Cox regression analyses of the
combined set.
| Characteristic | Univariate Cox
regression analysis
| Multivariate Cox
regression analysis
|
|---|
| HR | 95% CI | P | HR | 95% CI | P |
|---|
| Age | 1.012 | 0.986–1.038 | 0.378 | | | 0.913 |
| Gender | 0.584 | 0.337–1.014 | 0.054 | | | 0.264 |
| Smoking status | 1.443 | 0.860–2.420 | 0.165 | 1.760 | 1.033–2.996 | 0.037 |
| Pathology | 0.772 | 0.392–1.521 | 0.454 | | | 0.321 |
| Clinical stage | 1.061 | 0.519–2.172 | 0.870 | | | 0.141 |
| Risk score | 2.326 | 1.759–3.172 | <0.001 | 2.545 | 1.859–3.484 | <0.001 |
Correlation between serum and tissue
miRNAs
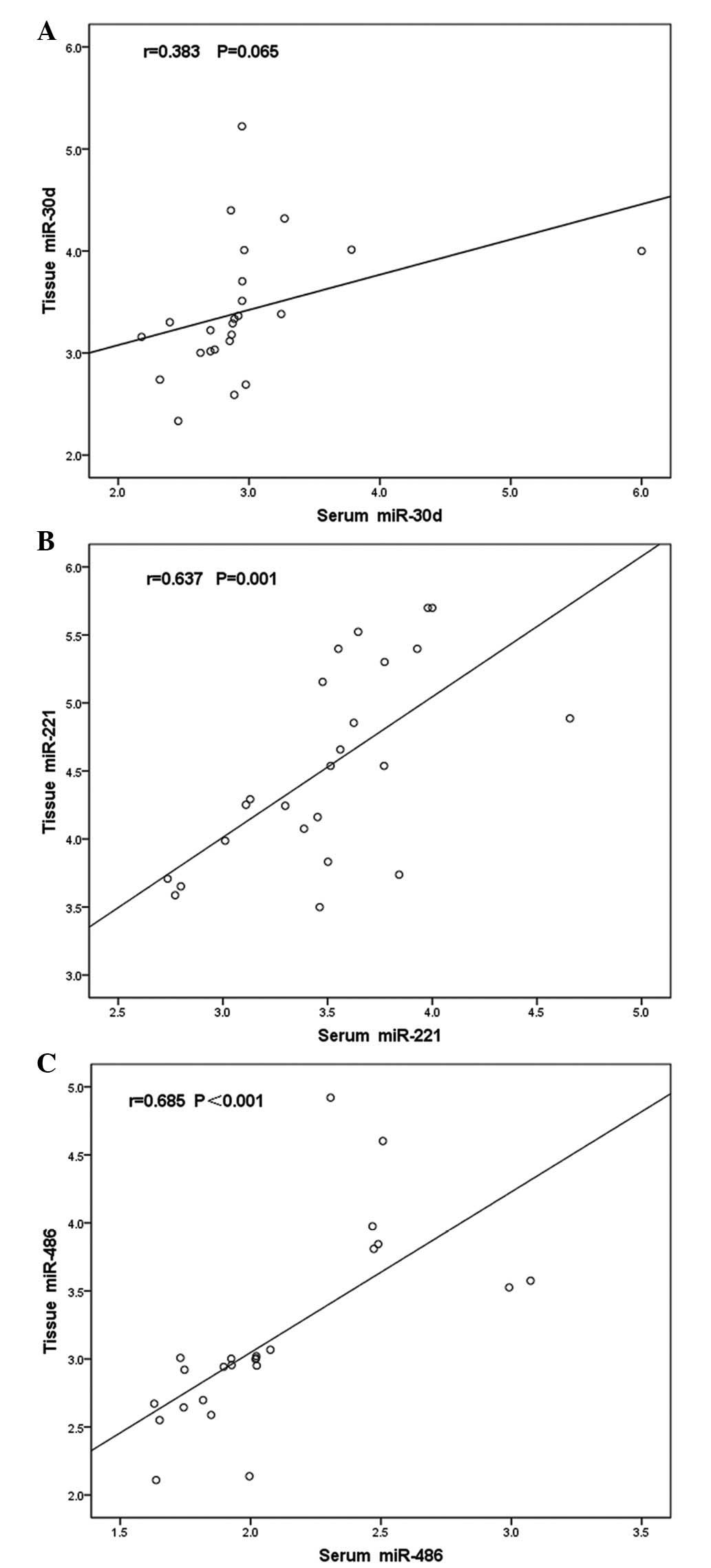
The four miRNAs were subsequently detected in the
corresponding fresh tissue specimens; however, miR-1 was expression
was undetermined. A Pearson correlation analysis was performed to
analyze the relative quantification of the serum and tissue miRNAs.
Two of the three miRNAs exhibited correlations between the serum
and tissue levels. The correlation coefficients for miR-30d,
miR-221 and miR-486 were 0.383 (P=0.065), 0.673 (P=0.001) and 0.685
(P<0.001), respectively (Fig.
3A–C).
Upregulation of miR-221 or downregulation
of miR-486 promotes cell proliferation, migration and invasion in
lung cancer cells
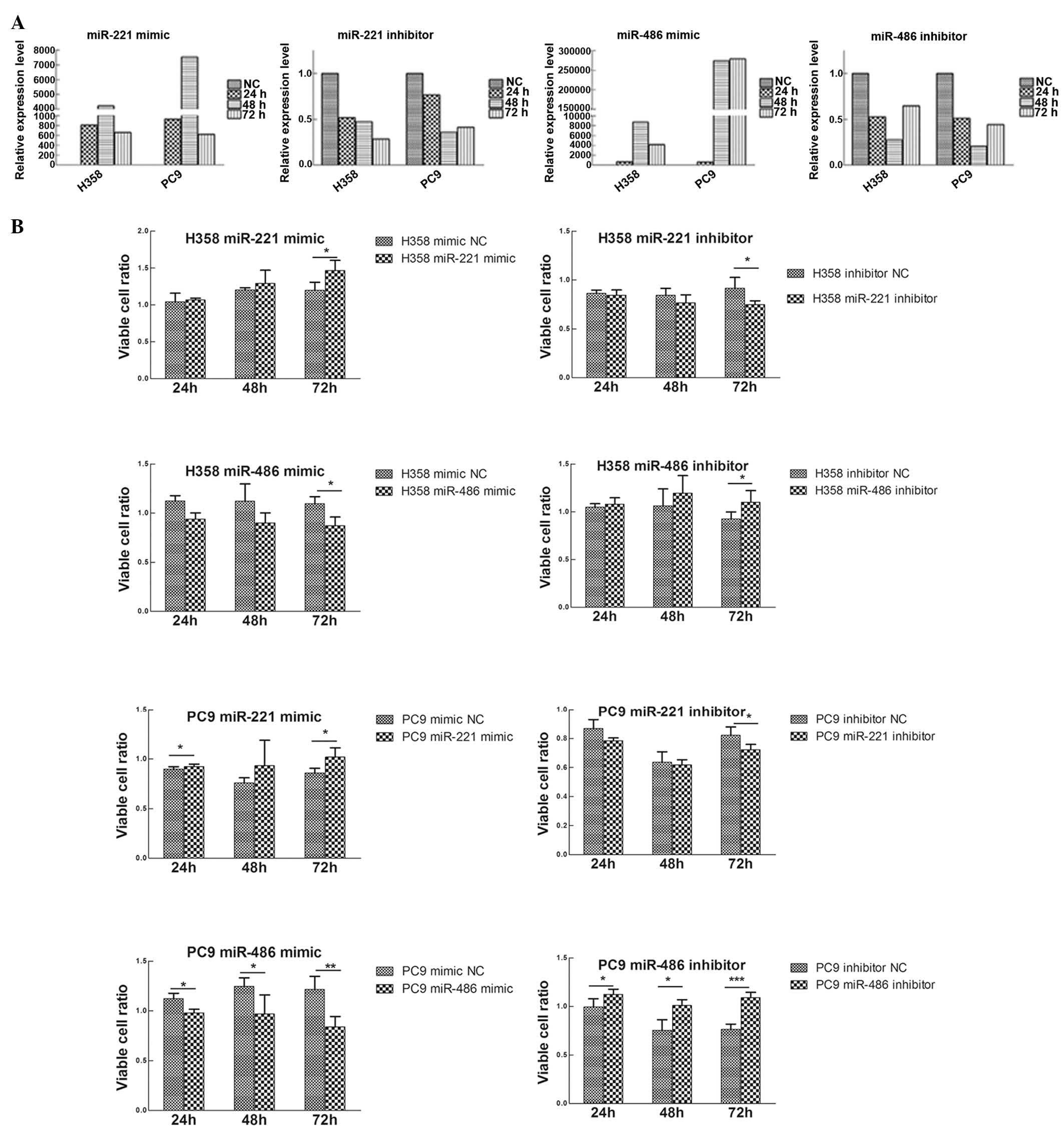
To study the biological roles of miR-221 and
miR-486, transiently transfected H358 and PC9 cell lines were
constructed. miR-221 mimic and inhibitor, miR-486 mimic and
inhibitor, and mimic NC and inhibitor NC were transfected into the
H358 and PC9 cell lines (Fig.
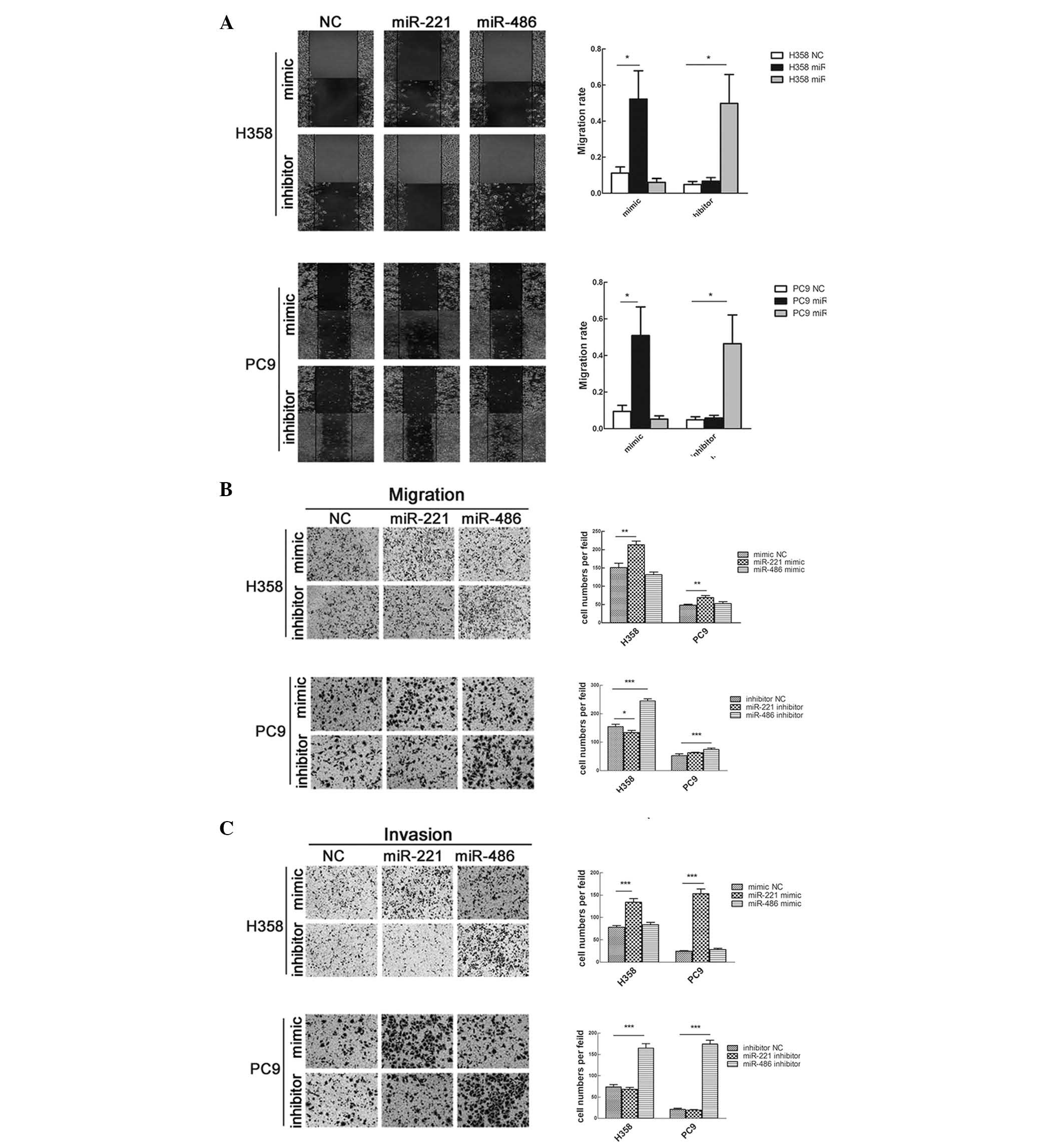
4A).
Using the MTT assay, the effects of miR-221 and
miR-486 on the lung cancer cell lines were evaluated. Upregulation
of miR-221 or downregulation of miR-486 promoted cell
proliferation, whereas the reverse processes inhibited cell
proliferation (Fig. 4B).
The effects of miR-221 and miR-486 on cell migration
and invasion were also studied. Transfection with miR-221 mimic or
miR-486 inhibitor significantly enhanced cell migration; however,
transfection with miR-221 inhibitor or miR-486 mimic did not affect
cell migration (Fig. 5A and B).
Consistent with these findings, the results of a Matrigel invasion
assay demonstrated that overexpression of miR-221 or knockdown of
miR-486 strengthened the invasive capacity of the cells (Fig. 5C).
Discussion
Due to the lack of reliable biomarkers for the early
detection and prognosis of malignancy, the identification of
biomarkers is of great importance. The serum expression of miRNAs
was initially described by Chim et al (21); subsequently, miRNAs have been
demonstrated to tolerate degradation, freezing, thawing and extreme
pH conditions (22,23). In 2008, it was reported that miRNAs
may be considered a novel class of cancer biomarkers (22,24).
At present, the use of miRNAs has been widely reported in cancer
diagnosis, clinical characteristics, individualized treatment and
prognosis (8,9,25,26).
NSCLC is a heterogeneous disease. The current
standard of treatment for patients with advanced or stage IV NSCLC
is 4–6 cycles of chemotherapy, which is followed by maintenance
therapy in a subgroup of patients without progression. Analysis of
the clinical characteristics of patients with lung cancer,
including patient age, and the number, size and location of
metastatic sites, may have reached the limit of its usefulness for
predicting outcomes; therefore, molecular biomarkers may add value
to this analysis. The ability to more accurately identify subgroups
of patients may refine prognostic models and lead to more
personalized lung cancer treatments. This advancement may help
determine which groups of patients require more aggressive therapy,
such as 6 cycles of chemotherapy plus maintenance therapy.
The present study reproducibly validated previously
identified early stage NSCLC prognosis-associated miRNAs using an
RT-qPCR analysis. These miRNAs were previously revealed to be
associated with the PFS and OS of patients with early stage NSCLC.
miR-137, miR-372, miR-182, miR-221 and let-7a were tested in
quick-frozen tissue samples from surgery (14), and miR-486, miR-30d, miR-1 and
miR-499 were tested in serum (15). All of the specimens were obtained
from patients with stage I–III NSCLC. In the present study, these
miRNAs were detected in serum obtained from patients with advanced
stage NSCLC. Subsequently, the PFS-associated serum miRNAs were
detected in fresh tissue samples, in order to analyze the
correlation between the expression of these miRNAs in serum and
tissue.
It has been definitively demonstrated that the
functions of genes are not isolated. Function-related genes may
have similar expression profiles, and biological functions result
from cooperation between genes. In addition, gene expression levels
exhibit space-time specificity; therefore, a gene expression
signature appears to be more suitable as a prognostic factor than a
single biomarker.
In the serum miRNA analysis, a risk score formula
was constructed using a Cox regression analysis and its predictive
function was validated using cross-validation methods. Therefore,
the PFS-associated miRNA expression level was transformed into a
calculable risk score, which may have clinical application value.
Repeated validation and distensible specimen detection are the key
steps during biomarker identification. In the present analysis, an
elementary validation was performed to establish a miRNA signature
as a prognostic factor.
From the risk score formula, it was revealed that
miR-1 and miR-486 exerted protective effects, whereas miR-30d and
miR-221 were risk factors. miR-1 has previously been reported to
act as a tumor suppressor by reducing migration and invasion, thus
inhibiting growth in NSCLC (27)
and head and neck squamous cell carcinoma (28). In addition, miR-486 is
downregulated in the plasma and tissues of patients with NSCLC
(29). A similar function for
miR-486 has been reported in gastric carcinoma (30). The expression levels of miR-30d
have been reported to be dysregulated in chronic lymphocytic
leukemia (31) and hepatocellular
carcinoma (HCC). According to a study by Yao et al (32), the increased expression levels of
miR-30d enhanced cell migration and invasion in HCC. Furthermore,
Li et al (33) reported
that miR-30d overexpression significantly increased cell growth,
thus acting as an oncomir. Regarded as a novel family of oncogenes,
the overexpression of miR-221 may contribute to the oncogenesis and
progression of prostate carcinoma (34,35),
and its overexpression is associated with tumor multifocality and
reduced time to recurrence after surgery in HCC (36).
In the aforementioned studies regarding early stage
NSCLC specimens, miR-137, miR-182 and miR-372 tissue levels, but
not serum levels, were reported to be associated with prognosis;
and the same phenomenon was observed during serum detection in the
present study. Therefore, it may be hypothesized that there are
some differences in miRNA secretion. Since the majority of
circulating miRNAs are released by cells, the authors of the
present study are convinced that there is a connection between
tissue expression and circulating miRNAs. There are three
hypotheses regarding the release of miRNAs into the circulation:
They may be released directly, packed into microparticles, or
packed into exosomes (37).
Indeed, the majority of previous studies have demonstrated the same
trend of differences between circulating and tissue miRNAs.
However, a different trend has also been observed. Wulfken et
al (38) reported that only 36
of 109 high-level serum miRNAs were upregulated in tissues.
Furthermore, Pigati et al (39) demonstrated that only 66% of
extracellular miRNAs closely reflected cellular miRNAs, according
to a miRNA microarray analysis. miR-544 expression was
downregulated in serum but showed only a weak positive correlation
with tissue expression (40). In
the present study, miR-221 and miR-486 exhibited a significant
correlation between serum and tissue levels. Conversely, miR-1 was
detected as a risk factor in serum, but not in tissues. The
correlation between serum and tissue miRNAs supports the hypothesis
that circulating miRNAs can serve as a monitor for malignant
lesions. Notably, because of the simplicity and reproducibility of
obtaining blood samples, noninvasive, easily testable serum miRNAs
may serve as a more promising biomarker for lung cancer
prognosis.
Through the correlation analysis, the present study
demonstrated that two miRNAs were relevant in both the cell
environment and the non-cell environment. This finding provided the
theoretical foundation for an in vitro study. In relation to
PFS, which represents short-term survival, the proliferative,
migratory and invasive capacities were studied in cell lines. It
was observed that miR-221 was identified as an oncogenic risk
factor, whereas miR-486 exerted protective effects against cancer
cell proliferation, migration and invasion.
In conclusion, the present study identified a
four-miRNA signature that independently contributed to PFS in
patients with advanced NSCLC. The serum expression levels of
miR-221 and miR-486 were positively correlated with those in
tissue. Furthermore, the roles of miR-221 and miR-486 were verified
in vitro.
Acknowledgments
The present study was funded by the National Natural
Science Foundation of China (grant no. 81101690) and the major
program of Hubei Provincial Health Department (grant no.
JX6A02).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R,
Grannis FW Jr, et al: National comprehensive cancer network:
Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw.
11:645–653. 2013.
|
|
3
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim ES, Herbst RS, Wistuba II, Lee JJ,
Blumenschein GR Jr, Tsao A, Stewart DJ, Hicks ME, Erasmus J Jr,
Gupta S, et al: The BATTLE trial: Personalizing therapy for lung
cancer. Cancer Discov. 1:44–53. 2011. View Article : Google Scholar
|
|
5
|
Endoh H, Tomida S, Yatabe Y, Konishi H,
Osada H, Tajima K, Kuwano H, Takahashi T and Mitsudomi T:
Prognostic model of pulmonary adenocarcinoma by expression
profiling of eight genes as determined by quantitative real-time
reverse transcriptase polymerase chain reaction. J Clin Oncol.
22:811–819. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Director's Challenge Consortium for the
Molecular Classification of Lung Adenocarcinoma; Shedden K, Taylor
JM, Enkemann SA, Tsao MS, Yeatman TJ, Gerald WL, Eschrich S,
Jurisica I, Giordano TJ, et al: Gene expression-based survival
prediction in lung adenocarcinoma: A multi-site, blinded validation
study. Nat Med. 14:822–827. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morin P Jr: MiRNAs in cancer: Non-coding
RNAs as appealing biomarkers for malignancy. Cancer Biomark.
11:227–228. 2012.PubMed/NCBI
|
|
9
|
Steer CJ and Subramanian S: Circulating
microRNAs as biomarkers: A new frontier in diagnostics. Liver
Transpl. 18:265–269. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kleivi Sahlberg K, Bottai G, Naume B,
Burwinkel B, Calin GA, Børresen-Dale AL and Santarpia L: A serum
microRNA signature predicts tumor relapse and survival in
triple-negative breast cancer patients. Clin Cancer Res.
21:1207–1214. 2015. View Article : Google Scholar
|
|
12
|
Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei
RR, Zhang MY, Sun Y, Huang BJ, Chen M, et al: Prognostic value of a
microRNA signature in nasopharyngeal carcinoma: A microRNA
expression analysis. Lancet Oncol. 13:633–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hur K, Toiyama Y, Schetter AJ, Okugawa Y,
Harris CC, Boland CR and Goel A: Identification of a
metastasis-specific MicroRNA signature in human colorectal cancer.
J Natl Cancer Inst. 107:dju4922015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu SL, Chen HY, Chang GC, Chen CY, Chen
HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS, et al: MicroRNA
signature predicts survival and relapse in lung cancer. Cancer
Cell. 13:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu
Y, Chen Y, Xu L, Zen K, Zhang C and Shen H: Serum microRNA
signatures identified in a genome-wide serum microRNA expression
profiling predict survival of non-small-cell lung cancer. J Clin
Oncol. 28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Travis WD: Pathology of lung cancer. Clin
Chest Med. 23:65–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
18
|
Kroh EM, Parkin RK, Mitchell PS and Tewari
M: Analysis of circulating microRNA biomarkers in plasma and serum
using quantitative reverse transcription-PCR (qRT-PCR). Methods.
50:298–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahn R, Heukamp LC, Rogenhofer S, von
Ruecker A, Müller SC and Ellinger J: Circulating microRNAs (miRNA)
in serum of patients with prostate cancer. Urology. 77:1265.e9–e16.
2011. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Chim SS, Shing TK, Hung EC, Leung TY, Lau
TK, Chiu RW and Lo YM: Detection and characterization of placental
microRNAs in maternal plasma. Clin Chem. 54:482–490. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gilad S, Meiri E, Yogev Y, Benjamin S,
Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed
N, et al: Serum microRNAs are promising novel biomarkers. PloS One.
3:e31482008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu Y, Govindan R, Wang L, Liu PY, Goodgame
B, Wen W, Sezhiyan A, Pfeifer J, Li YF, Hua X, et al: MicroRNA
profiling and prediction of recurrence/relapse-free survival in
stage I lung cancer. Carcinogenesis. 33:1046–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu R, Chen X, Du Y, Yao W, Shen L, Wang
C, Hu Z, Zhuang R, Ning G, Zhang C, et al: Serum microRNA
expression profile as a biomarker in the diagnosis and prognosis of
pancreatic cancer. Clin Chem. 58:610–618. 2012. View Article : Google Scholar
|
|
27
|
Nasser MW, Datta J, Nuovo G, Kutay H,
Motiwala T, Majumder S, Wang B, Suster S, Jacob ST and Ghoshal K:
Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression
of tumorigenic property of lung cancer cells and their
sensitization to doxorubicin-induced apoptosis by miR-1. J Biol
Chem. 283:33394–33405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nohata N, Sone Y, Hanazawa T, Fuse M,
Kikkawa N, Yoshino H, Chiyomaru T, Kawakami K, Enokida H, Nakagawa
M, et al: MiR-1 as a tumor suppressive microRNA targeting TAGLN2 in
head and neck squamous cell carcinoma. Oncotarget. 2:29–42.
2011.PubMed/NCBI
|
|
29
|
Boeri M, Verri C, Conte D, Roz L, Modena
P, Facchinetti F, Calabrò E, Croce CM, Pastorino U and Sozzi G:
MicroRNA signatures in tissues and plasma predict development and
prognosis of computed tomography detected lung cancer. Proc Natl
Acad Sci USA. 108:3713–3718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh HK, Tan AL, Das K, Ooi CH, Deng NT, Tan
IB, Beillard E, Lee J, Ramnarayanan K, Rha SY, et al: Genomic loss
of miR-486 regulates tumor progression and the OLFM4 antiapoptotic
factor in gastric cancer. Clin Cancer Res. 17:2657–2667. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marton S, Garcia MR, Robello C, Persson H,
Trajtenberg F, Pritsch O, Rovira C, Naya H, Dighiero G and Cayota
A: Small RNAs analysis in CLL reveals a deregulation of miRNA
expression and novel miRNA candidates of putative relevance in CLL
pathogenesis. Leukemia. 22:330–338. 2008. View Article : Google Scholar
|
|
32
|
Yao J, Liang L, Huang S, Ding J, Tan N,
Zhao Y, Yan M, Ge C, Zhang Z, Chen T, et al: MicroRNA-30d promotes
tumor invasion and metastasis by targeting Galphai2 in
hepatocellular carcinoma. Hepatology. 51:846–856. 2010.PubMed/NCBI
|
|
33
|
Li N, Kaur S, Greshock J, Lassus H, Zhong
X, Wang Y, Leminen A, Shao Z, Hu X, Liang S, et al: A combined
array-based comparative genomic hybridization and functional
library screening approach identifies mir-30d as an oncomir in
cancer. Cancer Res. 72:154–164. 2012. View Article : Google Scholar
|
|
34
|
Zheng C, Yinghao S and Li J: MiR-221
expression affects invasion potential of human prostate carcinoma
cell lines by targeting DVL2. Med Oncol. 29:815–822. 2012.
View Article : Google Scholar
|
|
35
|
Galardi S, Mercatelli N, Giorda E,
Massalini S, Frajese GV, Ciafrè SA and Farace MG: MiR-221 and
miR-222 expression affects the proliferation potential of human
prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem.
282:23716–23724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gramantieri L, Fornari F, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L and
Negrini M: MicroRNA-221 targets Bmf in hepatocellular carcinoma and
correlates with tumor multifocality. Clin Cancer Res. 15:5073–5081.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zen K and Zhang CY: Circulating microRNAs:
A novel class of biomarkers to diagnose and monitor human cancers.
Med Res Rev. 32:326–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wulfken LM, Moritz R, Ohlmann C,
Holdenrieder S, Jung V, Becker F, Herrmann E, Walgenbach-Brünagel
G, von Ruecker A, Müller SC and Ellinger J: MicroRNAs in renal cell
carcinoma: Diagnostic implications of serum miR-1233 levels. PLoS
One. 6:e257872011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pigati L, Yaddanapudi SC, Iyengar R, Kim
DJ, Hearn SA, Danforth D, Hastings ML and Duelli DM: Selective
release of microRNA species from normal and malignant mammary
epithelial cells. PloS One. 5:e135152010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma R, Zhang G, Wang H, Lv H, Fang F and
Kang X: Downregulation of miR-544 in tissue, but not in serum, is a
novel biomarker of malignant transformation in glioma. Oncol Lett.
4:1321–1324. 2012.PubMed/NCBI
|