Introduction
Osteosarcoma is one of the most common malignant
bone tumors and predominantly occurs in the long bones, including
the humerus, ulna, radius, femur, fibula and pelvis, of adolescents
and children. Although the mortality rate of osteosarcoma has
declined and the survival rate has increased dramatically over the
past decade with the improvement of chemotherapy and aggressive
surgical techniques (1), the
prognosis of patients with metastasis remains poor, with only a 20%
5-year survival rate (2). Thus, it
is important to further clarify the underlying molecular signaling
mechanisms that regulate osteosarcoma, and to develop effective
strategies to control osteosarcoma carcinogenesis and
metastasis.
The mechanisms of osteosarcoma metastasis are
complex, requiring multiple processes and various physiological
changes. Evidence has demonstrated that the degradation of the
extracellular matrix (ECM) is a pivotal process in the migration
and invasion of osteosarcoma cells. Numerous factors are associated
with the process of ECM degradation, such as matrix
metal-loproteinase-2 (MMP-2), which enables cancer cells to degrade
the ECM facilitating the migration and invasion of tumor cells
toward the bloodstream. MMP-2 production and activation is induced
by various cytokines, of which tumor necrosis factor-α (TNF-α) is
one of the most important mediators (3,4).
Ampelopsin (3,5,7,3′4′5′-hexahydroxyl 2,3 dihydrogen
flavonol), one of the most common flavonoids, is derived from the
tender stem and leaves of the plant species Ampelopsis
grossedentata, which has been widely used in traditional
Chinese medicine (5,6). Ampelopsin has been used in the
treatment of numerous diseases, including inflammation (5), oxidative stress (5), liver injury (7) and hyperlipidemia (8). Previous studies have investigated the
anticancer effect of ampelopsin in prostate (9), ovarian (10) and breast (11) cancer. However, to the best of our
knowledge there are no reports of the anticancer activity of
ampelopsin on osteosarcoma cells. The present study aimed to
investigate whether ampelopsin may exert an effect against
osteosarcoma cell migration and invasion.
Materials and methods
Reagents
Ampelopsin and recombinant human TNF-α were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse monoclonal
MMP-2 and GAPDH antibodies were purchased from Abcam (Cambridge,
UK; cat. nos. ab2462 and ab8245, respectively). Rabbit monoclonal
total-p38 mitogen activated protein kinase (MAPK) and
phospho-p38MAPK were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA; cat. nos. 8690 and 4511, respectively).
SB203580, a selective inhibitor of the p38MAPK signaling pathway,
was purchased from Sigma-Aldrich.
Cell culture
U2OS osteosarcoma cells were purchased from the
American Type Culture Collection (Manassas, VA, USA). U2OS cells
were seeded and maintained at 37°C in a humidified atmosphere of 5%
CO2 in Hyclone Dulbecco's modified Eagle's medium (DMEM;
GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
Hyclone fetal bovine serum (FBS; GE Healthcare Life Sciences), 100
U/ml penicillin and 100 μg/ml streptomycin
(Sigma-Aldrich).
Western blot analysis
To clarify the effect of TNF-α on the expression of
MMP-2, U2OS cells were cultured in 6-well plates, and were randomly
divided into 5 groups as follows: Control, physiological saline of
equal volume; 1, 10, 50 and 100 ng/ml TNF-α for 24 h. Furthermore,
U2OS cells were pretreated with ampelopsin (5, 10 and 50 μM)
for 2 h prior to TNF-α treatment (100 ng/ml) for 24 h, and the
relative expression of MMP-2 was determined. To ascertain the
effect of TNF-α on the p38MAPK signaling pathway, U2OS cells were
cultured and stimulated with TNF-α (100 ng/ml) for 5, 15, 30, 60
and 120 min. A different set of cells were treated with 50
μM ampelopsin for 2 h prior to TNF-α treatment (100 ng/ml)
for 5, 15, 30, 60 and 120 min, and the phosphorylation of p38MAPK
was detected using western blot analysis. In addition, SB203580 (10
mmol/l), an inhibitor of the p38 pathway, was used to pre-treat
another set of U2OS cells for 2 h prior to TNF-α (100 ng/ml)
treatment for 24 h.
For detection of the target proteins, ampelopsin,
SB203580 or TNF-α-treated cancer cells were collected and lysed for
30 min in a lysis buffer containing phenylmethylsulfonyl fluoride,
EDTA, pepstatin and leupeptin as protein inhibitors (Beyotime
Institute of Biotechnology, Haimen, China). Lysates were then
centrifuged at 21,000 × g at 4°C for 15 min to remove the insoluble
material. The concentrations of the extracted proteins were
measured with a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) following the manufacturer's
instructions. The protein lysates (10 μg) were subjected to
10% SDS-PAGE according to the concentrations at 90 V. Following
electrophoresis, the proteins were electrotransferred to
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA) at 200
mA for 2 h. In order to minimize the non-specific binding, the
blots were blocked in 5% non-fat milk for 2 h at room temperature.
The blots were then incubated with antibodies against MMP-2
(1:1,000), total-p38MAPK (1:1,500), phospho-p38MAPK (1:1,500) and
GAPDH (1:10,000) overnight at 4°C. The blots were washed and
exposed to horseradish peroxidase-conjugated goat anti-rabbit and
goat anti-mouse secondary antibodies (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.; OriGene Technologies, Inc.,
Rockville, MD, USA; cat. nos. ZDR-5306 and ZDR-5307, respectively)
for 2 h, and the levels of the target proteins were detected by the
FluorChem HD2 electrochemiluminescence detection system (Alpha
Innotech Corporation, Sa Jose, CA, USA) and an enhanced
chemiluminescence reagent (EMD Millipore). The quantification of
the scanned blots was performed using ImageJ software (imagej.nih.gov/ij/) and the results are expressed as
fold change relative to the control. Three independent experiments
were duplicated for each reaction.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
U2OS cells were cultured in 6-well plates, and were
randomly divided into 5 groups as follows: Control, physiological
saline of equal volume; 1, 10, 50 and 100 ng/ml TNF-α for 24 h.
Another set of cells were pretreated with SB203580 (10 mmol/l) or
ampelopsin (5, 10 and 50 μM) for 2 h prior to TNF-α
treatment for 24 h, and the expressions of MMP-2 at the mRNA level
were detected. Total RNA was extracted from the U2OS osteosarcoma
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Carlsbad, CA, USA), and measured using a SmartSpec 3000
UV/Vis spectrophotometer (Bio-Rad Laboratories, Inc., Beijing,
China). RT was performed using oligo (dT) primers (Boshang Biology
Technology Ltd., Shanghai, China; MMP-2 primers cat. no. H187580;
18s primers cat. no. H179060) and the RevertAid First Strand cDNA
Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) at 37°C for 1 h according to the
manufacturer's instructions. Briefly, reverse transcription was
performed at 37°C for 10 min using 1 μg RNA, 1 μl
oligo (dT) primers and 10 μl RNase-free water. Mixture was
incubated at 70°C for 10 min and 4 μl 5X reaction buffer, 1
μl ribonuclease inhibitor and 2 μl dNTP were added.
Following incubation at 37°C for 5 min, 2 μl reverse
transcriptase were added to the mixture, and the final 20 μl
reaction mixture was cultured at 37°C for 1 h. The enzymatic
activity was inactivated by heating at 65°C for 10 min at the end
of the incubation period.
RT-qPCR was subsequently performed with the SYBR
Green I kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's instructions and analyzed using a LightCycler (Roche
Diagnostics, Mannheim, Germany) with 18sRNA as a reference gene.
The thermal cycling conditions used for qPCR were as follows:
denaturing at 94° for 30 sec, annealing at 58°C for 30 sec and
extension at 72°C for 30 sec, a total of 37 cycles. The primer
sequences used for RT-qPCR were as follows: MMP-2, forward
5′-GGATGATGCCTTTGCTCG and reverse 5′-GGAGTCCGTCCTTACCGT (12); 18sRNA, forward
5′-CTTAGTTGGTGGAGCGATTTG-3′ and reverse 5′-GCTGAACGCCACTTGTCC-3′
(13). The melting curves were
assessed and the comparative 2−ΔΔCq method was used to
normalize the relative expression levels of the products generated
by each set of primers (14).
Scratch wound healing assay
A scratch wound healing assay was used to assess the
migratory ability of the U2OS osteosarcoma cells. U2OS cells were
cultured in 6-well plates (1×106 cells/well; Corning
Incorporated, Corning, NY, USA) with DMEM supplemented with 10%
FBS. A pipette tip was used to make straight scratches of the same
width in the monolayer of U2OS cells on the surface of each well.
U2OS cells were treated as follows: Control, physiological saline;
TNF-α, 100 ng/ml for 24 h; TNF-α + ampelopsin, 50 μM
ampelopsin for 2 h prior to TNF-α (100 ng/ml) incubation for 24 h;
ampelopsin, 50 μM treatment for 2 h prior to physiological
saline of equal volume for 24 h. Images were captured to measure
the wound healing under the Olympus IX71 microscope (Olympus
Corporation, Tokyo, Japan) (10).
Transwell assay
A Transwell assay was used to assess the effect of
ampelopsin on the invasive ability of U2OS osteosarcoma cells.
Briefly, U2OS cells (2×105 cells/well) were cultured in
modified Boyden chambers with 8-μm pore filter inserts
(Corning Incorporated). The upper chamber contained cells in DMEM
supplemented with 1% FBS, while the lower chamber contained DMEM
supplemented with 10% FBS. Following re-suspension in the upper
chamber, U2OS cells were treated as described above for the
control, TNF-α, TNF-α + ampelopsin and ampelopsin groups. The cells
remaining on the upper surface were gently wiped away with a cotton
swab and the cells on the lower surface were fixed with 95% ethanol
for 30 min and stained with 0.1% hexamethylpararosaniline following
methanol fixation (Sigma-Aldrich) at 37°C for 30 min (10). The number of cells on the lower
surface of the membranes was quantified using the Olympus IX71
microscope.
Statistical analysis
All data in the present study were evaluated with
SPSS predictive analytics software, version 18.0 (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance was used to analyze
the normally distributed data, while Mann-Whitney U test was used
to analyze the non-parametric variables with post-hoc test used for
multiple comparisons between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
TNF-α upregulates MMP-2 expression at the
protein and mRNA levels
In a previous study, MMP-2 was correlated with
aggressive tumor progression and low survival rates (15). Thus, the present study investigated
the effect of TNF-α on the expression levels of MMP-2 in
osteosarcoma cells. U2OS cells were cultured in 6-well plates and
randomly divided into 5 groups, which were treated with 1, 10, 50
or 100 ng/ml TNF-α for 24 h, while treatment with physiological
saline of equal volume was used as the control group. As
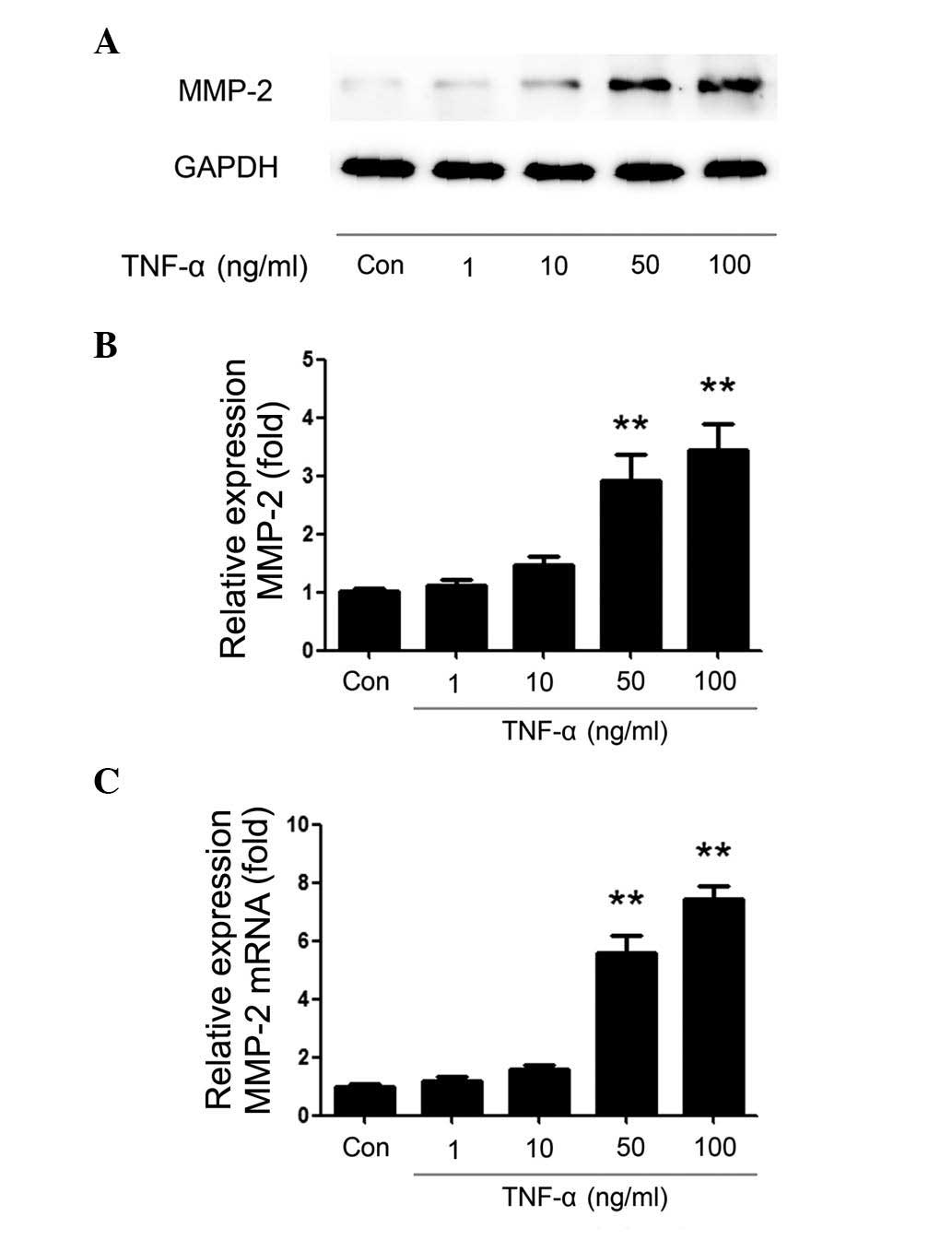
demonstrated in Fig. 1A, TNF-α
increased MMP-2 protein expression levels in a
concentration-dependent manner. A significant effect was observed
at a concentration of 50 ng/ml compared with the control group
(P=0.0021) and peak MMP-2 expression was reached at 100 ng/ml TNF-α
treatment (Fig. 1B; P=0.0017). The
results indicated that TNF-α may induce the migration of U2OS cells
via the increased expression levels of MMP-2.
Additionally, the same TNF-α stimulations were
performed and RT-qPCR was used to examine the mRNA levels of MMP-2.
As demonstrated in Fig. 1C,
following TNF-α treatment, the relative expression of MMP-2 mRNA
was increased in a concentration-dependent manner. The MMP-2 mRNA
expression level was significantly increased compared with the
control group following stimulation with 50 and 100 ng/ml TNF-α
(P<0.00001).
Similar changes to MMP-2 protein and mRNA levels
were observed, and thus, the data suggested that TNF-α induces
MMP-2 production in a concentration-dependent manner at the protein
and mRNA levels.
TNF-α increases the phosphorylation of
p38MAPK, with the effect abolished by ampelopsin
The current study aimed to elucidate the mechanism
by which TNF-α induced the expression of MMP-2. In previous
studies, the p38MAPK pathway was demonstrated to be an important
regulator of MMP-2 following stimulation with a variety of
cytokines in multiple cell types (16–19).
TNF-α was previously reported regulate a number of biological
functions via the activation of the p38MAPK pathway (20–22).
Therefore, it was speculated that TNF-α may stimulate the
expression of MMP-2 via the phosphorylation of p38MAPK. Following
stimulation with TNF-α (100 ng/ml) for 5, 15, 30, 60 and 120 min,
the proteins were extracted from U2OS cells. As demonstrated in
Fig. 2A, the relative expression
of phospho-p38 was significantly upregulated, compared with the
control group, after 15 min stimulation (P=0.0014). Maximal
activity was observed at 30 min of stimulation (P=0.0011), which
gradually decreased after 60 and 120 min of stimulation (P=0.0018
and P=0.0237, respectively). Additionally, 50 μM ampelopsin
was cultured with U2OS cells for 2 h prior to TNF-α stimulation
(100 ng/ml) for 5, 15, 30, 60 and 120 min. As demonstrated in
Fig. 2B, ampelopsin markedly
inhibited the enhancement of p38 phosphorylation that was induced
by TNF-α stimulation, and no significant differences in the levels
of phospho-p38 were observed compared with the control group
(P>0.05). These results demonstrated that TNF-α increased
phosphorylation of p38MAPK, and that ampelopsin abolished the
effect.
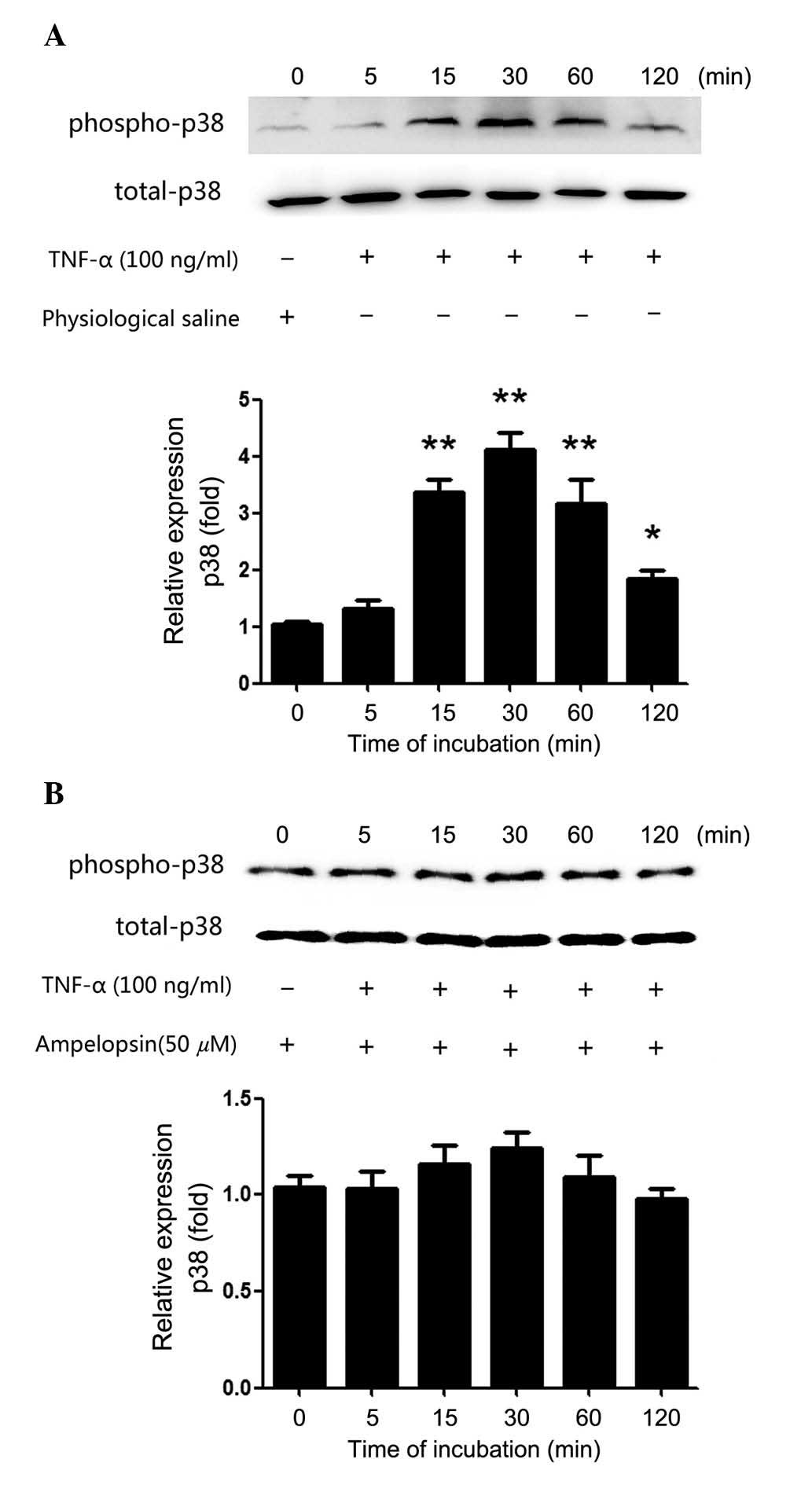 | Figure 2Phosphorylation of p38MAPK increased
following TNF-α treatment, while ampelopsin abolished the effect.
(A) U2OS osteosarcoma cells were treated with TNF-α (100 ng/ml) for
5, 15, 30, 60 and 120 min, with the same volume of physiological
saline used as the control group. Western blot assay was performed
to observe the change of phospho-p38MAPK protein expression. (B)
Ampelopsin (50 μM) was cultured with U2OS cells for 2 h
prior to TNF-α (100 ng/ml) addition to the supernatant for 5, 15,
30, 60 or 120 min, with the same volume of physiological saline
used as the control group. Western blot was performed to measure
the change to phospho-p38MAPK protein expression.
*P<0.05 and **P<0.01 vs. the control
group. Data are presented as the mean ± the standard error from
three independent experiments in duplicate. Phospho,
phosphorylated; p38MAPK, p38 mitogen-activated protein kinase;
TNF-α, tumor necrosis factor-α. |
TNF-α-induced expression of MMP-2 is
mediated by the p38MAPK pathway and suppressed by ampelopsin
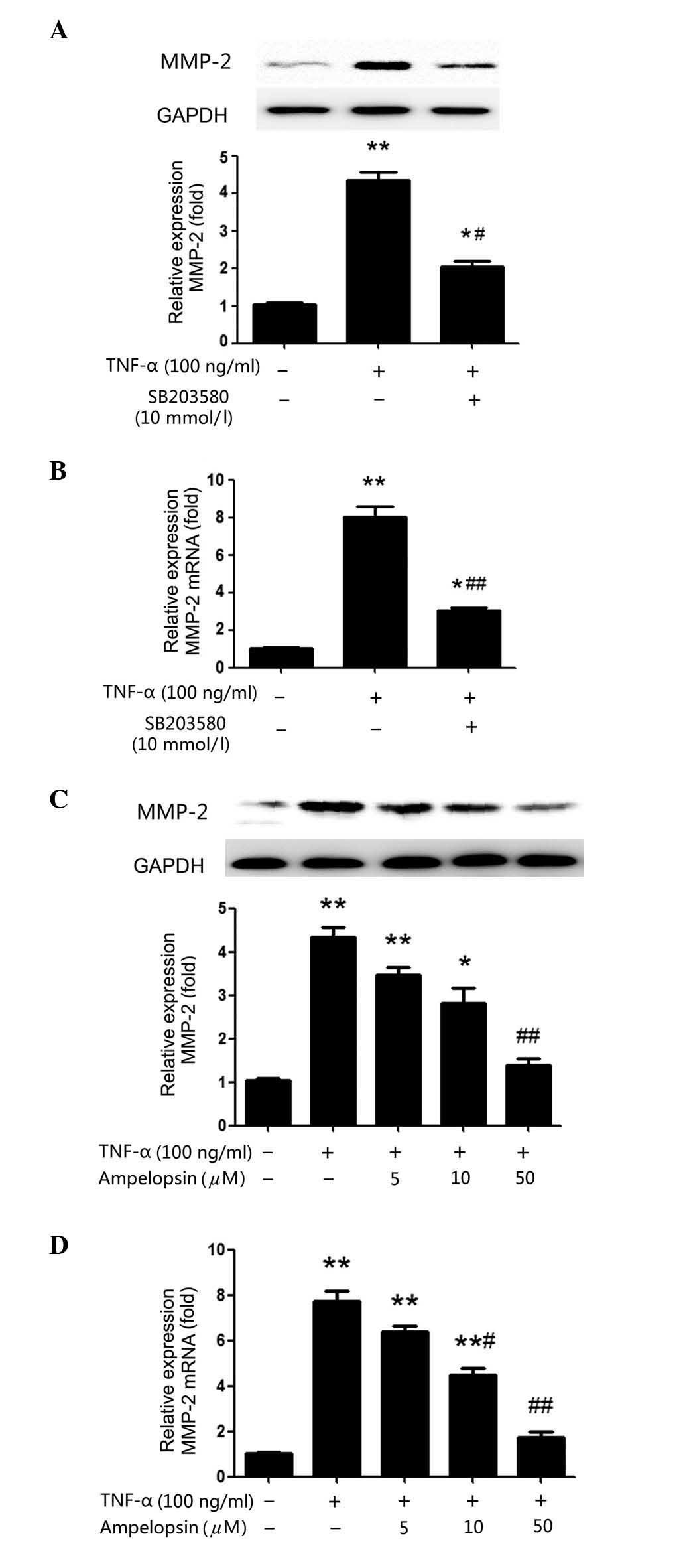
In order to further understand the importance of the
p38MAPK pathway the upregulation of MMP-2 expression levels by
TNF-α, SB203580 (10 mmol/l), a specific inhibitor of p38, was used
to pre-treat U2OS cells for 2 h prior to TNF-α treatment for a
further 24 h. As presented in Fig. 3A
and B, pre-treatment with SB203580 inhibited the TNF-α-induced
production of MMP-2, compared with the TNF-α only treatment group,
at the protein (P=0.0191) and mRNA (P<0.00001) level, which
strongly suggested that TNF-α increases MMP-2 production via the
p38MAPK signaling pathway.
Additionally, ampelopsin was used to investigate
whether it inhibits the effects of TNF-α. U2OS cells were
pretreated with various concentrations of ampelopsin (5, 10 and 50
μM) for 2 h before TNF-α (100 ng/ml) was added to the cells
for a further 24 h. As demonstrated in Fig. 3C and D, the enhanced MMP-2
expression level induced by TNF-α was gradually reduced with
increasing ampelopsin concentrations. At 50 μM ampelopsin,
the expression of MMP-2 mRNA and protein was significantly reduced
compared with the TNF-α group (P<0.00001 and P=0.0072,
respectively). The results confirmed the importance of the p38MAPK
pathway and the inhibitory effect of ampelopsin on TNF-α-stimulated
MMP-2 production.
TNF-α induces the migration and invasion
of osteosarcoma cells
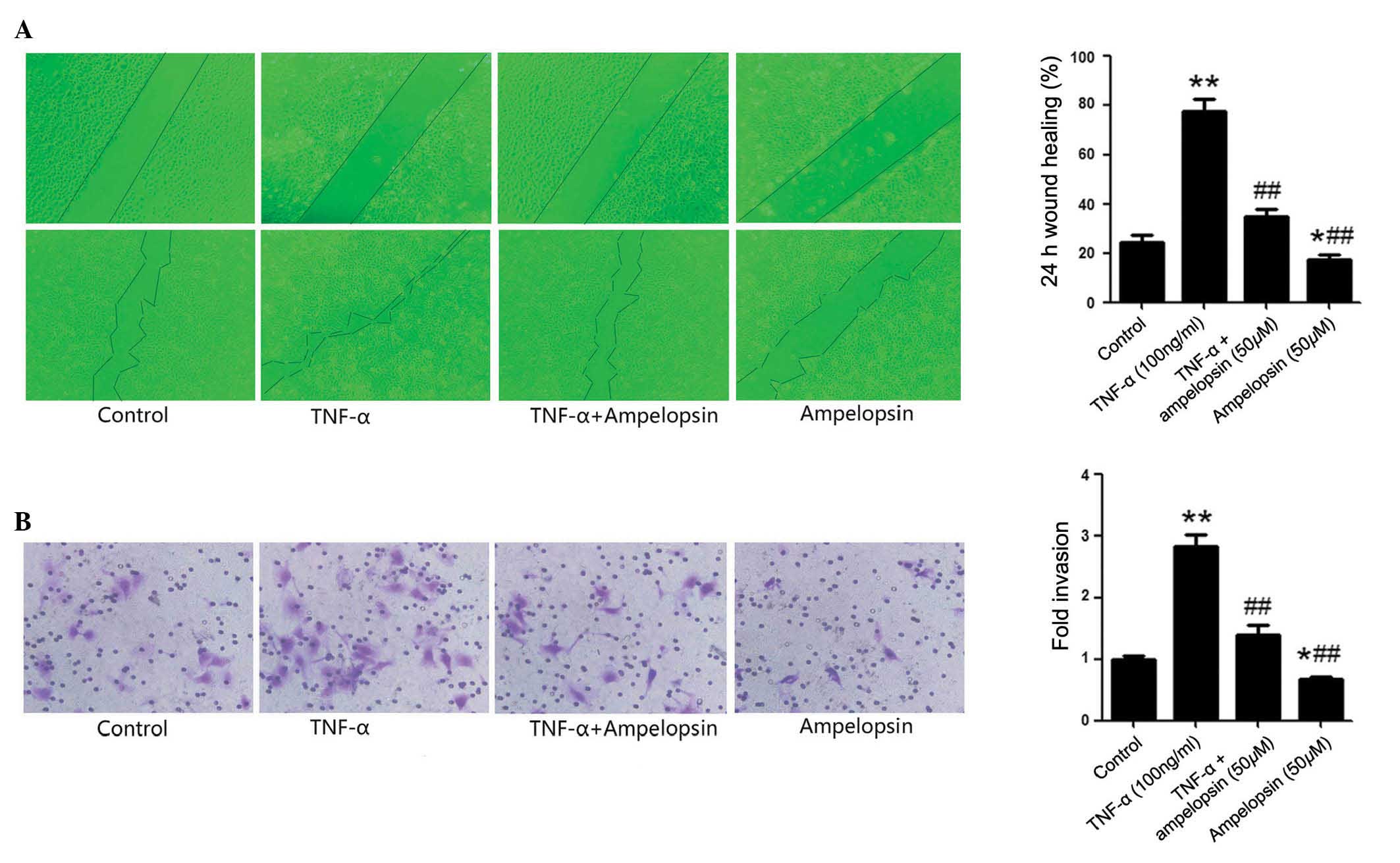
Wound healing and Transwell assays were used to
assess the effects of TNF-α on the migration and invasion of U2OS
osteosarcoma cells, respectively. U2OS cells were cultured with 100
ng/ml TNF-α for 24 h in the TNF-α group, or physiological saline of
equal volume was used in the control group. As presented in
Fig. 4A, the wound healing assay
demonstrated that TNF-α significantly enhanced the healing of the
scratch wound compared with the control group (P<0.00001). The
results indicated that TNF-α promotes the migration of U2OS cells.
Additionally, as presented in Fig.
4B, the Transwell assay also demonstrated that treatment with
TNF-α significantly increased the invasion cells compared with the
control group (P=0.0087).
Ampelopsin suppresses TNF-α-induced
increases to osteosarcoma cell migration and invasion
Subsequently, to further clarify the effect of
ampelopsin on the migration and invasion of osteosarcoma cells,
U2OS cells were pretreated with 50 μM ampelopsin prior to
TNF-α stimulation. As demonstrated in Fig. 4A, the wound healing assay
demonstrated that ampelopsin significantly inhibited the effect of
TNF-α on wound healing areas compared with the TNF-α group
(P=0.0032). As presented in Fig.
4B, the Transwell assay also demonstrated that pretreatment
with ampelopsin significantly decreased the invasion of U2OS cells
compared with the TNF-α group (P=0.0069). These results indicated
that ampelopsin suppresses the migration and invasion of
osteosarcoma cells that is promoted by TNF-α. The current study
also investigated the effects of ampelopsin on the osteosarcoma
cell migration and invasion when used alone, without TNF-α
stimulation. The results presented in Fig. 4 demonstrated that ampelopsin
treatment alone also had the ability to suppress U2OS cells
migration compared with the control group (P=0.0251) or the TNF-α
group (P<0.0000), and invasion compared with the control group
(P=0.0376) or the TNF-α group (P<0.0000).
Discussion
Cancer metastasis is a complex biological process
requiring multiple steps, including degradation of the ECM,
activation of cell adhesion, migration and angiogenesis. The
interactions between cells, cytokines, proteinases and the ECM that
result in cell migration and tumor invasion are not fully
clarified, however, TNF-α has been demonstrated to be important in
inflammatory reactions, angiogenesis, cell proliferation and
apoptosis via the regulation of various signaling pathways,
including the phosphorylation of MAPKs (23,24).
TNF-α has been demonstrated to promote tumor angiogenesis, invasion
and metastasis via upregulating the expression of angiogenic
factors (25) and stimulating
multiple cytokines and chemokines that accelerate the mobilization
of tumor cells and their colonization to other tissues (26). Previous clinical reports have
observed that the TNF-α concentration in serum was increased in
patients with various types of cancer, including chronic
lymphocytic leukemia (27),
ovarian (28) and prostate
(29) cancer. Simultaneously,
chemotherapy significantly decreased the serum TNF-α concentration
(28,29). The ability of TNF-α to promote
tumor progression has also been confirmed in animal models.
Additional pathways associated with TNF-α upregulation of tumor
growth include the vascular endothelial growth factor (VEGF)
(25), hepatocyte growth factor
(30), epithelial-mesenchymal
transition (31), macrophage
migration-inhibitory factor, chemoattractant protein-1 (24) and MMP (32) pathways. However, TNF-α was also
reported to promote cancer cell death and exhibit an anti-oncogenic
effect on certain tumors (33).
Thus, the present study aimed to clarify the specific effects of
TNF-α on the metastasis of osteosarcoma.
The human osteosarcoma U2OS cell line, which was
initially derived from a moderately differentiated sarcoma of a
15-year-old female in 1964, has been widely used in biomedical
research. The U2OS cell line has the lowest level of chromosomal
numerical variations compared with other osteosarcoma cell lines.
In addition, according to previous reports, only 2% of U2OS cells
have multipolar mitoses, which is similar to normal control
fibroblasts (34). Thus, in the
present study, the U2OS cell line was selected to represent
osteosarcoma in vitro. The results confirmed that TNF-α
significantly induced the migration and invasion of U2OS
osteosarcoma cells. This result was in accordance with an
investigation by Mori et al (35), which also demonstrated that TNF-α
was required for the tumorigenesis of AX osteosarcoma cells. It is
suggested that although TNF-α exerts a cytotoxic effect on
osteosarcoma, osteosarcoma cells may be resistant to the
cytotoxicity.
The mechanisms of osteosarcoma metastasis are
complex, involving multiple signaling pathways and various
physiological changes. MMP-2 is an important member of the MMP
super-family, the members of which act by digesting ECM molecules
and promoting the invasion and metastasis of various types of
cancer. The present study addressed one aspect of the multiple
interactions of TNF-α during tumorigenesis by determining the
effects of TNF-α on an ECM remod-eling enzyme, MMP-2. Previous
reports have demonstrated that TNF-α activates the expression of
MMP-2 in multiple cell types, including synovial fibroblasts,
endothelial cells, dermal fibroblasts and corneal epithelial cells
(36–38). The data of the present study
confirmed the importance of TNF-α on the promotion of MMP-2
expression in U2OS osteosarcoma cells and provided further support
for the theory that TNF-α increases MMP-2 activation via the
phosphorylation of p38MAPK. In previous studies, the p38MAPK
pathway was demonstrated to be an important regulator of MMP-2
following stimulation of several cell types with a variety of
cytokines (16–19). The present study confirmed that p38
and MMP-2 proteins were highly expressed in U2OS osteosarcoma
cells. Simultaneously, the results demonstrated that stimulation
with TNF-α (100 ng/ml) at different time points significantly
upregulated the phosphorylation of p38MAPK and exhibited an
abnormal time-dependent increase, with the peak activity observed
at 30 min. The application of SB203580 (10 mmol/l), a specific
inhibitor of the p38 pathway, reduced the protein expression level
of MMP-2 that was increased by TNF-α, further confirming the theory
that TNF-α promotes the expression of MMP-2 via the p38MAPK
signaling pathway. Overall, these results suggested that TNF-α
activates p38MAPK and increases MMP-2 expression through this
pathway.
Ampelopsin is a type of flavonoid extracted from
Ampelopsis grossedentata, a plant predominantly distributed
in South China. The flavonoid is widely used to treat cold and
tinea corporis, and has exhibited multiple biological functions in
the processes of inflammation, oxidation, liver injury and
hyperlipidemia. In previous studies, ampelopsin was demonstrated to
possess anticancer properties in several types of malignant tumors.
Ampelopsin has been reported to inhibit the proliferation of
prostate cancer cells via the downregulation of B-cell lymphoma 2
expression, which is associated with cell apoptosis, and suppress
migration and invasion via the downregulation of chemokine (C-X-C
motif) receptor 4 expression (9).
Ampelopsin was also observed to reduce breast carcinogenesis by
inhibiting the mechanistic target of rapamycin signaling pathway
(39). Ampelopsin also exhibits
the ability to inhibit the secretion of VEGF and basic fibroblast
growth factor, thus, inhibiting the development of hepatocellular
carcinoma (40). In a previous
study, it was confirmed that ampelopsin reduces the migration and
invasion of ovarian cancer cells via inhibiting the
epithelial-to-mesenchymal transition (10). Ampelopsin has demonstrated
anti-carcinogenesis characteristics, however, direct evidence of
ampelopsin involvement in osteosarcoma and the detailed molecular
mechanisms have not been reported. The current study provided
insight into the mechanisms by which ampelopsin regulates
osteosarcoma cell migration and invasion.
In the present study, pretreatment with ampelopsin
for 2 h significantly inhibited the increases to MMP-2 expression
stimulated by TNF-α in a concentration-dependent manner.
Additionally, following pre-treatment with ampelopsin, the changes
to the phosphorylation of p38 were no longer clear. As presented in
Fig. 3A, it was demonstrated that
ampelopsin significantly reduced the effect of TNF-α on the
phosphorylation of p38. Therefore, in the current study, ampelopsin
was demonstrated to be an inhibitor of the p38 signaling pathway,
which mediated the expression of MMP-2. The results suggested that
ampelopsin exerts an inhibitory effect on TNF-α-induced MMP-2
production. The present study also investigated the effect of
ampelopsin on TNF-α-induced osteosarcoma cell migration and
invasion; the results demonstrated that ampelopsin significantly
reduced the effect of TNF-α on migration and invasion.
In summary, the present study demonstrated that
ampelopsin inhibits TNF-α-induced osteosarcoma cell migration and
invasion, and the effect of ampelopsin was mediated by
p38MAPK/MMP-2 signaling. According to the results of the present
study, the invasive properties of osteosarcoma cells may be reduced
by ampelopsin via the inhibition of TNF-α/p38MAPK/MMP-2 signaling,
and further in vivo studies should be performed in the
future to confirm these findings.
Acknowledgments
The present study was supported by the Science
Foundation of Shandong Province (no. ZR2014HP005).
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis:
Clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han YP, Tuan TL, Wu H, Hughes M and Garner
WL: TNF-alpha stimulates activation of pro-MMP2 in human skin
through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci.
114:131–139. 2001.
|
|
4
|
Séguin CA, Pilliar RM, Madri JA and Kandel
RA: TNF-alpha induces MMP2 gelatinase activity and MT1-MMP
expression in an in vitro model of nucleus pulposus tissue
degeneration. Spine. 33:356–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L
and Yin Z: Ampelopsin reduces endotoxic inflammation via repressing
ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int
Immunopharmacol. 12:278–287. 2012. View Article : Google Scholar
|
|
6
|
Zhou WM, He RR, Ye JT, Zhang N and Liu DY:
Synthesis and biological evaluation of new
5-fluorouracil-substituted ampelopsin derivatives. Molecules.
15:2114–2123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murakami T, Miyakoshi M, Araho D, Mizutani
K, Kambara T, Ikeda T, Chou WH, Inukai M, Takenaka A and Igarashi
K: Hepatoprotective activity of tocha, the stems and leaves of
Ampelopsis grossedentata, and ampelopsin. Biofactors. 21:175–178.
2004. View Article : Google Scholar
|
|
8
|
Sayama K, Lin S, Zheng G and Oguni I:
Effects of green tea on growth, food utilization and lipid
metabolism in mice. In Vivo. 14:481–484. 2000.PubMed/NCBI
|
|
9
|
Ni F, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Flavonoid ampelopsin inhibits the growth and metastasis of
prostate cancer in vitro and in mice. PLoS One. 7:388022012.
View Article : Google Scholar
|
|
10
|
Liu T, Liu P, Ding F, Yu N, Li S, Wang S,
Zhang X, Sun X, Chen Y, Wang F, et al: Ampelopsin reduces the
migration and invasion of ovarian cancer cells via inhibition of
epithelial-to-mesenchymal transition. Oncol Rep. 33:861–867.
2015.
|
|
11
|
Zhou Y, Liang X, Chang H, Shu F, Wu Y,
Zhang T, Fu Y, Zhang Q, Zhu JD and Mi M: Ampelopsin-induced
autophagy protects breast cancer cells from apoptosis through
Akt-mTOR pathway via endoplasmic reticulum stress. Cancer Sci.
105:1279–1287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsai TT, Ho NY, Lin YT, Lai PL, Fu TS, Niu
CC, Chen LH, Chen WJ and Pang JH: Advanced glycation end products
in degenerative nucleus pulposus with diabetes. J Orthop Res.
32:238–244. 2014. View Article : Google Scholar
|
|
13
|
Ren P, Sun D, Xin D, Ma W, Chen P, Gao H,
Zhang S and Gong M: Serum amyloid A promotes osteosarcoma invasion
via upregulating αvβ3 integrin. Mol Med Rep. 10:3106–3112.
2014.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Bjørnland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad O and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng YC, Kuo WW, Wu HC, Lai TY, Wu CH,
Hwang JM, Wang WH, Tsai FJ, Yang JJ, Huang CY and Chu CH: ZAK
induces MMP-2 activity via JNK/p38 signals and reduces MMP-9
activity by increasing TIMP-1/2 expression in H9c2 cardiomyoblast
cells. Mol Cell Biochem. 325:69–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong J, Gencay MM, Bubendorf L, Burgess
JK, Parson H, Robinson BW, Tamm M, Black JL and Roth M: ERK1/2 and
p38 MAP kinase control MMP-2, MT1-MMP, and TIMP action and affect
cell migration: A comparison between mesothelioma and mesothelial
cells. J Cell Physiol. 207:540–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schram K, De Girolamo S, Madani S, Munoz
D, Thong F and Sweeney G: Leptin regulates MMP-2, TIMP-1 and
collagen synthesis via p38 MAPK in HL-1 murine cardiomyocytes. Cell
Mol Biol Lett. 15:551–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan F, Ma S, Cao W, Liu H, Chen F, Chen X
and Shi R: SDF-1α upregulation of MMP-2 is mediated by p38 MAPK
signaling in pancreatic cancer cell lines. Mol Biol Rep.
40:4139–4146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YP, Chen Y, John J, Moylan J, Jin B,
Mann DL and Reid MB: TNF-alpha acts via p38 MAPK to stimulate
expression of the ubiquitin ligase atrogin1/MAFbx in skeletal
muscle. FASEB J. 19:362–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stjernberg-Salmela S, Ranki A, Karenko L,
Siitonen S, Mustonen H, Puolakkainen P, Sarna S, Pettersson T and
Repo H: Low TNF-induced NF-kappaB and p38 phosphorylation levels in
leucocytes in tumour necrosis factor receptor-associated periodic
syndrome. Rheumatology (Oxford). 49:382–390. 2010. View Article : Google Scholar
|
|
22
|
Bibikova E, Youn MY, Danilova N, Ono-Uruga
Y, Konto-Ghiorghi Y, Ochoa R, Narla A, Glader B, Lin S and Sakamoto
KM: TNF-mediated inflammation represses GATA1 and activates p38 MAP
kinase in RPS19-deficient hematopoietic progenitors. Blood.
124:3791–3798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wajant H: The role of TNF in cancer.
Results Probl Cell Differ. 49:1–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X and Lin Y: Tumor necrosis factor
and cancer, buddies or foes? Acta Pharmacol Sin. 29:1275–1288.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nabors LB, Suswam E, Huang Y, Yang X,
Johnson MJ and King PH: Tumor necrosis factor alpha induces
angiogenic factor up-regulation in malignant glioma cells: A role
for RNA stabilization and HuR. Cancer Res. 63:4181–4187.
2003.PubMed/NCBI
|
|
26
|
Kulbe H, Thompson R, Wilson JL, Robinson
S, Hagemann T, Fatah R, Gould D, Ayhan A and Balkwill F: The
inflammatory cytokine tumor necrosis factor-alpha generates an
autocrine tumor-promoting network in epithelial ovarian cancer
cells. Cancer Res. 67:585–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferrajoli A, Keating MJ, Manshouri T,
Giles FJ, Dey A, Estrov Z, Koller CA, Kurzrock R, Thomas DA, Faderl
S, et al: The clinical significance of tumor necrosis factor-alpha
plasma level in patients having chronic lymphocytic leukemia.
Blood. 100:1215–1219. 2002.PubMed/NCBI
|
|
28
|
Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson
JL, Wilbanks GD, Burke F and Balkwill FR: Expression and regulation
of tumor necrosis factor alpha in normal and malignant ovarian
epithelium. Mol Cancer Ther. 5:382–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Michalaki V, Syrigos K, Charles P and
Waxman J: Serum levels of IL-6 and TNF-alpha correlate with
clinicopathological features and patient survival in patients with
prostate cancer. Br J Cancer. 90:2312–2316. 2004.PubMed/NCBI
|
|
30
|
Tomita Y, Yang X, Ishida Y, Nemoto-Sasaki
Y, Kondo T, Oda M, Watanabe G, Chaldakov GN, Fujii C and Mukaida N:
Spontaneous regression of lung metastasis in the absence of tumor
necrosis factor receptor p55. Int J Cancer. 112:927–933. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bates RC and Mercurio AM: Tumor necrosis
factor-alpha stimulates the epithelial-to-mesenchymal transition of
human colonic organoids. Mol Biol Cell. 14:1790–1800. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hagemann T, Robinson SC, Schulz M, Trumper
L, Balkwill FR and Binder C: Enhanced invasiveness of breast cancer
cell lines upon co-cultivation with macrophages is due to TNF-alpha
dependent up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villeneuve J, Tremblay P and Vallières L:
Tumor necrosis factor reduces brain tumor growth by enhancing
macrophage recruitment and microcyst formation. Cancer Res.
65:3928–3936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niforou KM, Anagnostopoulos AK, Vougas K,
Kittas C, Gorgoulis VG and Tsangaris GT: The proteome profile of
the human osteosarcoma U2OS cell line. Cancer Genomics Proteomics.
5:63–78. 2008.PubMed/NCBI
|
|
35
|
Mori T, Sato Y, Miyamoto K, Kobayashi T,
Shimizu T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Tando T, et al:
TNFα promotes osteosarcoma progression by maintaining tumor cells
in an undifferentiated state. Oncogene. 33:4236–4341. 2014.
View Article : Google Scholar
|
|
36
|
Cooney R, Iocono J, Maish G, Smith JS and
Ehrlich P: Tumor necrosis factor mediates impaired wound healing in
chronic abdominal sepsis. J Trauma. 42:415–420. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kitzis V, Engrav LH and Quinn LS:
Transient exposure to tumor necrosis factor-alpha inhibits collagen
accumulation by cultured hypertrophic scar fibroblasts. J Surg Res.
87:134–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang YN, Wang F, Zhou W, Wu ZQ and Xing
YQ: TNF-α stimulates MMP-2 and MMP-9 activities in human corneal
epithelial cells via the activation of FAK/ERK signaling.
Ophthalmic Res. 48:165–170. 2012. View Article : Google Scholar
|
|
39
|
Chang H, Peng X, Bai Q, Zhou Y, Yu X,
Zhang Q, Zhu J and Mi M: Ampelopsin suppresses breast
carcinogenesis by inhibiting the mTOR signalling pathway.
Carcinogenesis. 35:1847–1854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo GQ, Zeng S and Liu DY: Inhibitory
effects of ampelopsin on angiogenesis. Zhong Yao Cai. 29:146–150.
2006.In Chinese. PubMed/NCBI
|