Introduction
Ischemia/reperfusion (I/R) injury, which is a common
pathophysiological condition in various tissues, occurs when blood
supply returns to the tissue following a period of ischemia. Renal
I/R damage is a major cause of acute kidney injury after partial
nephrectomy or renal transplantation (1,2),
which is associated with severe morbidity and mortality (3). Effective interventions or treatments
are required for the attenuation of renal I/R injury.
Renal I/R injury is a complex process that involves
oxidative stress (4), inflammation
(5), and apoptosis (6,7).
Increased levels of reactive oxygen species (ROS) and/or decreased
antioxidant enzyme activity are key events following renal I/R,
which may activate subsequent damage (8). Under renal I/R conditions, various
proinflammatory factors are elevated (9), and activation of the caspase pathway
and subsequent apoptotic cell death results in renal cytotoxicity
(10). These processes are
intertwined, leading to a vicious circle of damage (11). Antioxidant, anti-inflammatory, and
anti-apoptotic effects have been reported to ameliorate renal I/R
injury to a certain degree (9–13).
Oleanolic acid (OA) is a natural triterpenoid and an
aglycone of several saponins. OA is present in various food
products, including vegetable oils, and is a main constituent of
the leaves and roots of Olea europaea, Viscum album
L., Aralia chinensis L., and >120 other plant species
(14). In the past three decades,
OA has been used in Chinese medicine for the treatment of liver
disorders, including viral hepatitis. In vitro and in
vivo studies have demonstrated that OA possesses potent
antioxidant activities (15,16),
and anti-inflammatory effects (17), which contribute toward its
potential protective effects. However, whether OA exerts protective
effects against renal I/R injury, and the possible underlying
mechanism, remain unclear. The present study aimed to evaluate the
renal protective effects of OA against I/R injury, and to examine
the potential underlying molecular mechanism. The results indicated
that OA preconditioning effectively prevented renal I/R injury via
antioxidant, anti-inflammatory, and anti-apoptotic activities.
Activation of nuclear factor erythroid 2-related factor 2
(Nrf2)/γ-glutamylcysteine ligase (GCLc) signaling, and upregulation
of glutathione (GSH) may have an important role in the preventive
effects of OA against renal I/R injury.
Materials and methods
Animals and treatment
All animal experimental protocols were performed in
accordance with the standards established by the Animal Care and
Use Committee of the Medical College of Nanchang University
(Nanchang, China). The present study was approved by the ethics
committee of the Medical College of Nanjing University. Male adult
Wistar rats (weight, 180–200 g; age, 6–8 weeks) were obtained from
the Animal Center of the Medical College of Nanchang University and
housed in stainless steel cages with ad libitum access to
food and water. Two protocols were subsequently conducted, the OA
and buthionine sulphoximine (BSO) used were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Protocol 1: A total of 30 rats
were randomly divided into five groups: The sham group, which
received sham surgery; the I/R group; the 12.5 mg/kg OA + I/R
group, which received 12.5 mg/kg OA for 15 consecutive days prior
to the operation; the 25 mg/kg OA + I/R group, which received 25
mg/kg OA for 15 consecutive days prior to the operation; and the 50
mg/kg OA + I/R group, which received 50 mg/kg OA for 15 consecutive
days prior to the operation. Protocol 2: A total of 24 rats were
randomly divided into four groups: The sham group, which received
sham surgery; the I/R group; the 50 mg/kg OA + I/R group, which
received 50 mg/kg OA for 15 consecutive days prior to the
operation); and the 50 mg/kg OA + BSO + I/R group, which received
50 mg/kg OA and 10 mg/kg BSO for 15 consecutive days prior to the
operation. Rats were intraperitoneally injected with OA in sterile
saline containing 2% Tween 80.
Model of renal I/R injury
Rats were fasted overnight, and were then
anaesthetized with pentobarbital sodium (50 mg/kg; i.p.;
Sigma-Aldrich) prior to the operation. Renal IR injury was
established as previously described (11,18).
Briefly, a midline incision was made, and the kidneys and renal
pedicles containing the artery, vein and nerve of each kidney were
exposed. The bilateral renal pedicle was exposed and clamped with a
vascular clamp for 45 min, in order to induce ischemia. The clamps
were then removed to allow reperfusion for 6 h. Pale coloring was
considered to indicate ischemia of the kidneys, which returned to
red post-reperfusion. In the control rats, the same surgical
procedure was conducted without the clamping.
Following reperfusion, the rats were anaesthetized
with 50 mg/kg pentobarbital sodium and decapitated, and blood
samples were collected for serum separation, and the kidneys were
removed. Blood samples were stored overnight at 4°C and centrifuged
at 664 × g for 10 min to separate the serum. Serum and kidney
samples were stored at −70°C until further analysis.
Evaluation of renal function
The serum concentrations of blood urea nitrogen
(BUN) and creatinine (Cr) were detected to assess renal function
using a QuantiChromTM Urea assay kit and Creatinine Colorimetric
assay kit (Bioassay Systems LLC, Hayward, CA, USA), according to
the manufacturer's protocols. Kidney injury molecule-1 (KIM-1)
content was examined using a KIM-1 enzyme-linked immunosorbent
assay (ELISA) kit (USCN Life Science Inc., Wuhan, China), according
to the manufacturer's protocol. Serum lactate dehydrogenase (LDH)
activity was evaluated using a LDH Colorimetric assay kit
(BioVision Inc., Milpitas, CA, USA) according to the manufacturer's
protocols.
Evaluation of antioxidant defense
Renal tissues were homogenized for evaluation of
antioxidant defense. Methane dicarboxylic aldehyde (MDA) levels
were determined as described previously (19), using the colorimetric absorption of
the 2-thiobarbituric acid-MDA chromophore for detection of lipid
peroxidation. This was conducted using an MDA assay kit (Nanjing
Jiancheng Bioengineering Research Institute, Nanjing, China). Renal
superoxide dismutase (SOD) activity was assayed using a Total SOD
assay kit (Nanjing Jiancheng Bioengineering Research Institute)
based on reduction of nitro-blue tetrazolium (NBT) by
O•−2 produced by hydroxylamine ydrochloride
autoxidation, as described previously (20). One unit of SOD was defined as the
amount of protein that caused a 50% inhibition in the rate of NBT
reduction. A microplate reader was used to determine absorbance in
the MDA and SOD assays (Infinite 200; Tecan Group, Ltd., Männedorf,
Switzerland). Renal activity of catalase (CAT) was determined using
a CAT assay kit (Nanjing Jiancheng Bioengineering Research
Institute) according to the manufacturer's protocols. One unit of
CAT was defined as the amount required to decompose 1 μmol
H2O2 per second. Glutathione (GSH) and
glutathione peroxidase (GPx) activity was assayed using a GSH
Fluorometric assay kit and GPx assay kit (BioVision Inc.),
according to the manufacturer's protocols.
Determination of inflammation
Levels of interferon-γ (IFN-γ), interleukin (IL)-6
and IL-10 were detected in homogenized renal tissue using specific
CLIA kit for IL-6 and CLIA kit for IL-10 (USCN Life Science Inc.
and Eagle Biosciences, Inc., Nashua, NH, USA, respectively). Renal
myeloperoxidase (MPO) levels were also examined using a
Myeloperoxidase ELISA kit (ALPCO, Salem, NH, USA). These assays
were conducted according to the manufacturers' protocols.
Determination of apoptosis
Renal tissue was cut into 10-mm sections and fixed
in paraformaldehyde (Sigma-Aldrich) for use in determination of
apoptosis. A terminal deoxynucleotidyl transferase-mediated dUTP
nick-end labeling (TUNEL) assay was conducted to evaluate the rate
of apoptosis in renal sections using an In Situ Cell Death
Detection kit (Roche Diagnostics, Basel, Switzerland) according to
the manufacturer's protocols. The number of apoptotic cells was
counted in 10 random fields using a BX51TF microscope at
magnification ×200 (Olympus Corporation, Tokyo, Japan). Renal
caspase 3 content was detected in homogenized renal tissue using an
ELISA kit for Caspase 3 (USCN Life Science Inc.) according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was purified from renal tissue using
RNeasy Plus kit (Qiagen, Inc., Valencia, CA, USA), according to the
manufacturer's protocol. Total RNA (0.5 μg) was reverse
transcribed using the Fast SYBR® Green Master Mix
according to the manufacturer's instructions (Takara Bio, Inc.,
Otsu, Japan). The RT-qPCR assay was performed in triplicate in
reaction volumes of 20 μl containing 10 μl Fast SYBR
Green Master Mix (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 1 μl of sense and antisense primers (0.5 μM;
Augct DNA-Syn Biotechnology Co., Ltd., Beijing, China), 1 μl
cDNA (50 ng/20 μl) and 7 μl double distilled water.
The reactions were performed in a CFX96 Real-Time PCR system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The cycling
conditions used were: Initial activation for 5 min at 95°C; and 40
cycles of denaturation for 5 sec at 95°C and 10 sec of combined
annealing and extension at the individual primer temperature
between 55 and 62.5°C. The relative amount of each gene was
normalized to β-actin. The primer sequences used were as follows:
β-actin, sense 5′-AGA TCC TGA CCG AGC GTG GC-3′, antisense 5′-CCA
GGG AGG AAG AGG ATG CG-3′; Nrf2, sense 5′-TCA GCT ACT CCC AGG TTG
CCC A-3′, antisense 5′-GGC AAG CGA CTC ATG GTC ATC TAC-3′; and
GCLc, sense: 5′-AAC ACA GAC CCA ACC CAG AG-3′ and antisense 5′-CCG
CAT CTT CTG GAA ATGTT-3′. Gene expression levels were analyzed
using the 2−ΔΔCq method (21).
Statistical analysis
Statistical analysis of the data was performed using
GraphPad software, version 5.0 (GraphPad Software, Inc., La Jolla,
CA, USA). The results are presented as the mean ± standard error.
Statistical significance of the differences between the groups was
analyzed using one-way analysis of variance followed by
Newman-Keuls multiple comparison post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
OA preconditioning prevents renal I/R
injury
The present study consisted of two protocols. In
protocol 1, the dose-dependent effects of 12.5–50 mg/kg OA were
detected on the renal function of I/R rats. In protocol 2, BSO was
used to examine the role of GSH regulation in OA-induced prevention
of renal I/R injury. In order to evaluate renal function, several
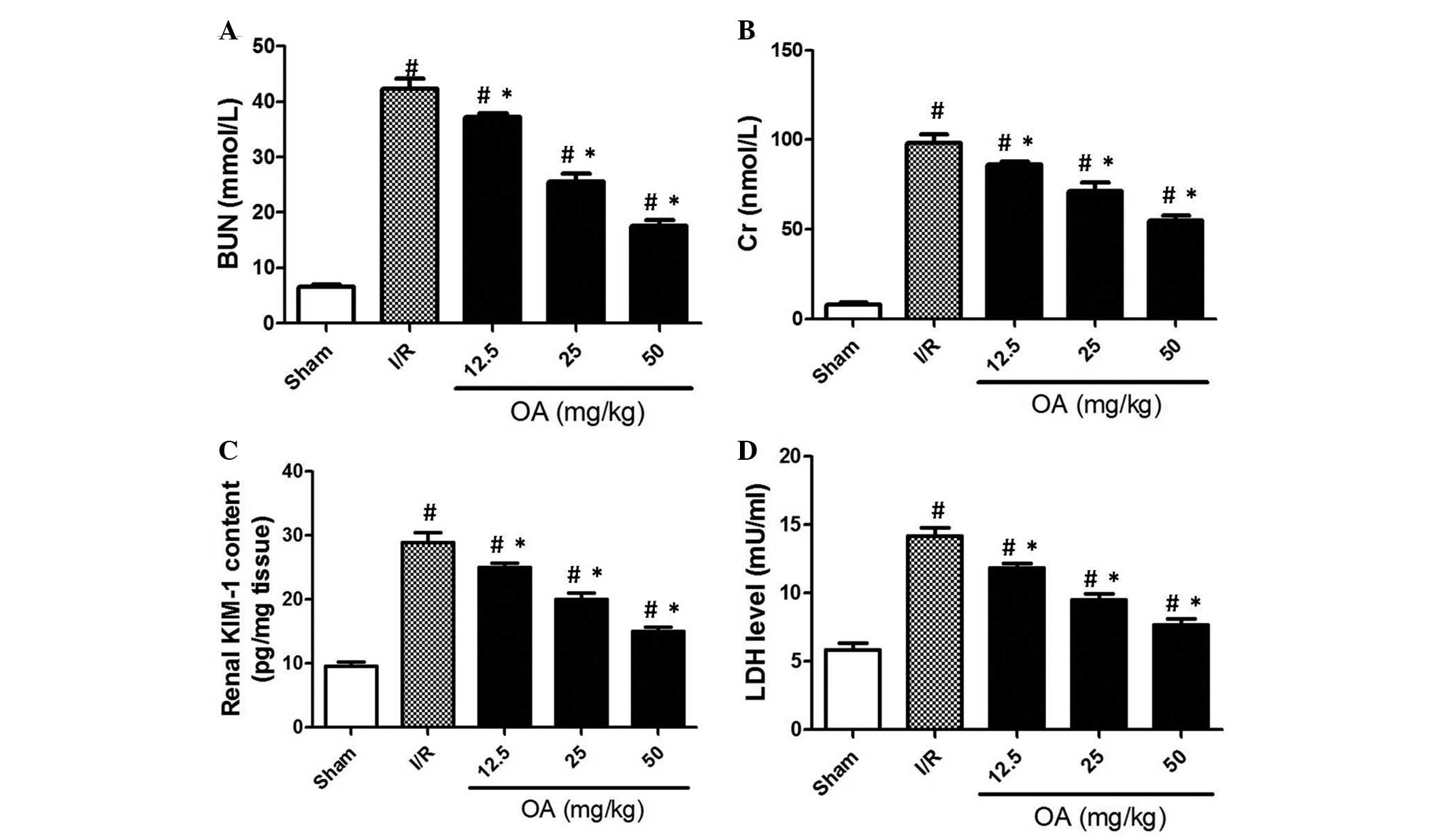
related parameters were determined. In the case of renal
insufficiency, BUN and Cr are retained in the blood, and their
excretion by the kidneys is impaired. As shown in Fig. 1A and B, BUN and Cr levels were
significantly increased ~5-fold in the I/R group compared with in
the sham control group (P=0.0008). Conversely, OA preconditioning
dose-dependently prevented the increased levels of BUN and Cr in
the I/R rats (Fig. 1A and B;
P=0.0001). KIM-1 is a novel biomarker for human renal proximal
tubule injury (22). The present
study demonstrated that in I/R rats, KIM-1 content was increased
~3-fold compared with in the sham control group (Fig. 1C; P=0.0004). OA preconditioning
significantly reduced KIM-1 content in I/R rats in a dose-dependent
manner (Fig. 1C). Following tissue
injury, LDH may be released into the bloodstream. In the
circulation of I/R rats, LDH release was markedly increased.
Conversely, OA preconditioning markedly prevented this increase in
LDH release (Fig. 1D; P=0.002).
These results indicate that OA preconditioning may effectively
prevent I/R-induced renal injury in rats. Since 50 mg/kg exhibited
the most significant protective effect on these indices, this dose
was used in protocol 2.
Antioxidative effects of OA
preconditioning are associated with the prevention of renal I/R
injury
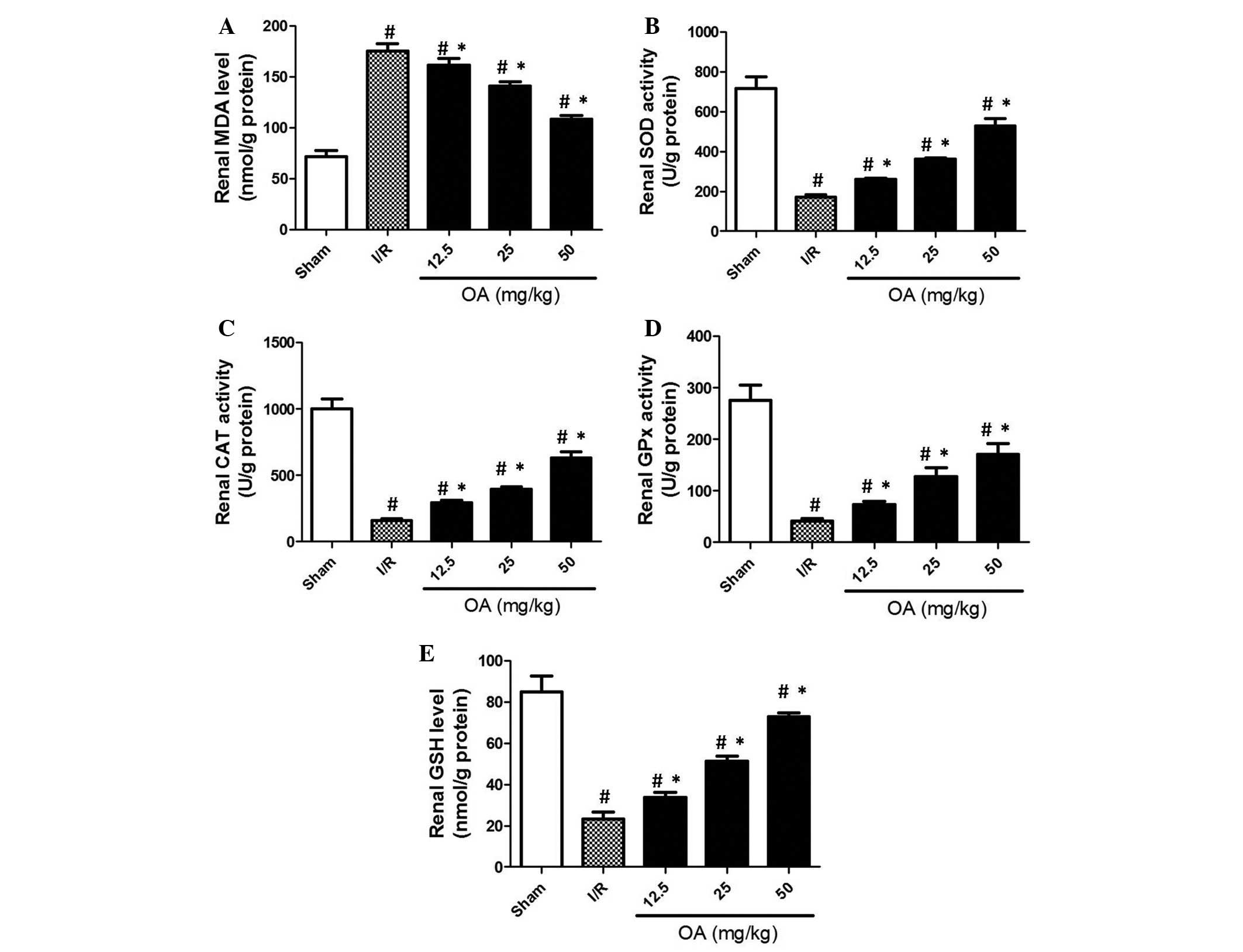
To evaluate antioxidant injury in I/R rats, several
key markers of antioxidant activity were detected. MDA is an
important end-point marker of lipid peroxidation. As shown in
Fig. 2A, MDA levels were increased
>2-fold in the kidneys of I/R rats compared with in the sham
control group. OA preconditioning markedly prevented the increased
levels of MDA in a dose-dependent manner (Fig. 2A; P=0.0009). SOD is an important
antioxidant enzyme, which catalyzes the dismutation of superoxide
to oxygen and hydrogen peroxide. In turn, hydrogen peroxide may be
broken down by CAT. The results of the present study demonstrated
that in I/R rats, the serum activities of SOD and CAT were markedly
decreased (Fig. 2B and C). As
expected, OA preconditioning prevented I/R-induced decreased renal
activity of SOD and CAT (Fig. 2B and
C; P=0.0003 and P=0.001, respectively). Furthermore, GPx is a
key antioxidant enzyme that eliminates organic peroxide using GSH
as a reductive equivalent. As shown in Fig. 2D, I/R induced a significant
decrease in GPx activity, which was prevented by OA preconditioning
(P=0.003). Furthermore, GSH is a major cellular antioxidant in
cells, which is able to detoxify various oxidative products
directly, or under the catalyzation of GPx or glutathione
S-transferase. The results demonstrated that I/R resulted in a
marked reduction in GSH renal content (Fig. 2E; P=0.0045). OA preconditioning
markedly prevented the I/R-induced decreased renal content of GSH.
These results suggest that I/R induced oxidative stress in the rat
kidneys, and the preventive effects of OA may be due to its
antioxidant activity.
Anti-inflammatory effects of OA
preconditioning are associated with the prevention of renal I/R
injury
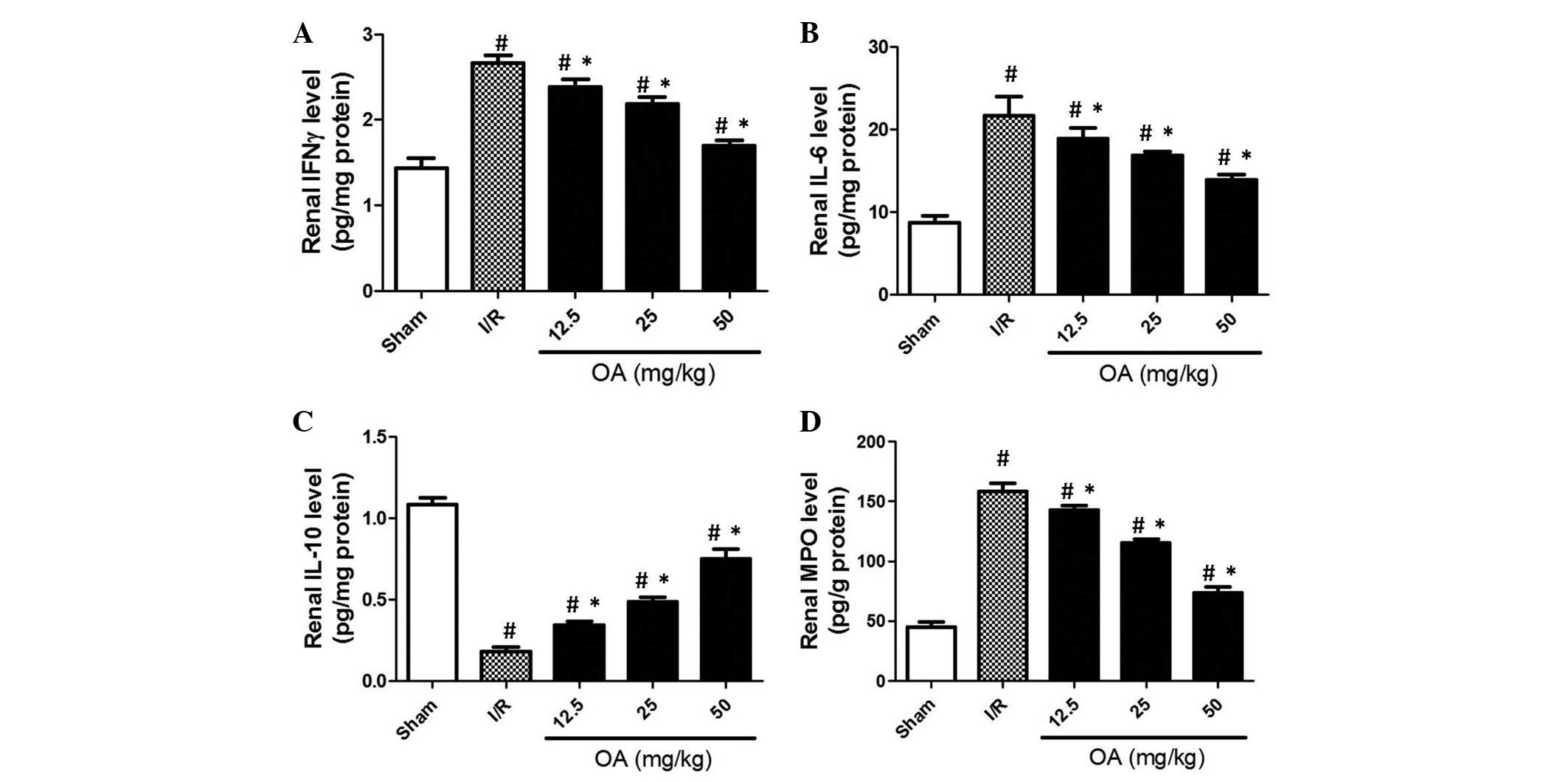
The process of inflammation is associated with
I/R-induced injury in various tissues. The present study examined
inflammation via the detection of several cytokines and an
important enzyme. As shown in Fig. 3A
and B, renal levels of IFN-γ and IL-6 were elevated in the I/R
rat group. OA preconditioning markedly prevented the increased
levels of IFN-γ and IL-6 in I/R rats (Fig. 3A and B; P=0.0045 and P=0.0013,
respectively). Furthermore, IL-10, a cytokine that exerts
protective effects against inflammatory injury (23), was decreased following I/R
operation (Fig. 3C; P=0.0062).
Conversely, OA preconditioning markedly prevented I/R-induced
reductions in IL-10 levels (Fig.
3C). MPO is an enzyme that is stored in azurophilic granules of
polymorphonuclear neutrophils and macrophages, and is released into
the extracellular fluid in response to I/R-induced inflammation
(24). The present study
demonstrated that MPO levels were increased ~3-fold in the I/R
group compared with in the sham control group (Fig. 3D; P=0.0012). Conversely, MPO levels
were inhibited by OA preconditioning in a dose-dependent manner
(Fig. 3D). These results indicate
that I/R induced inflammatory injury in the rat kidneys, and the
preventive effects of OA may be caused by its anti-inflammatory
effects.
Anti-apoptotic effects of OA
preconditioning are associated with the prevention of renal I/R
injury
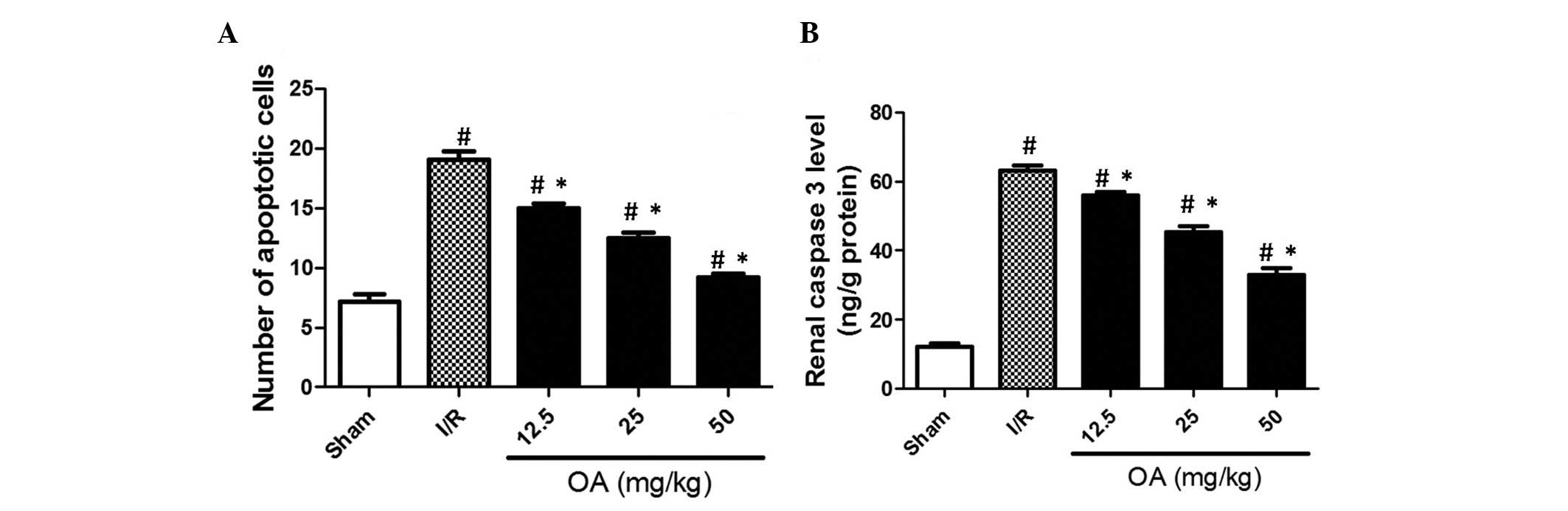
The present study also examined the effects of OA
preconditioning and I/R on apoptotic cell death in the rat kidneys.
As shown in Fig. 4A, apoptotic
cells were detected by TUNEL staining and presented as the number
of apoptotic cells. The results demonstrated that I/R significantly
increased the number of apoptotic cells in the kidneys (Fig. 4A; P=0.0006). OA preconditioning
notably and dose-dependently reduced the number of apoptotic cells
(Fig. 4A). In addition, caspase 3,
a pivotal member of the apoptotic pathway, was increased following
I/R injury (Fig. 4B; P=0.0009).
Conversely, OA preconditioning decreased caspase 3 content
(Fig. 4B). These results indicate
that the anti-apoptotic effects of OA may be associated with the
prevention of I/R-induced renal injury.
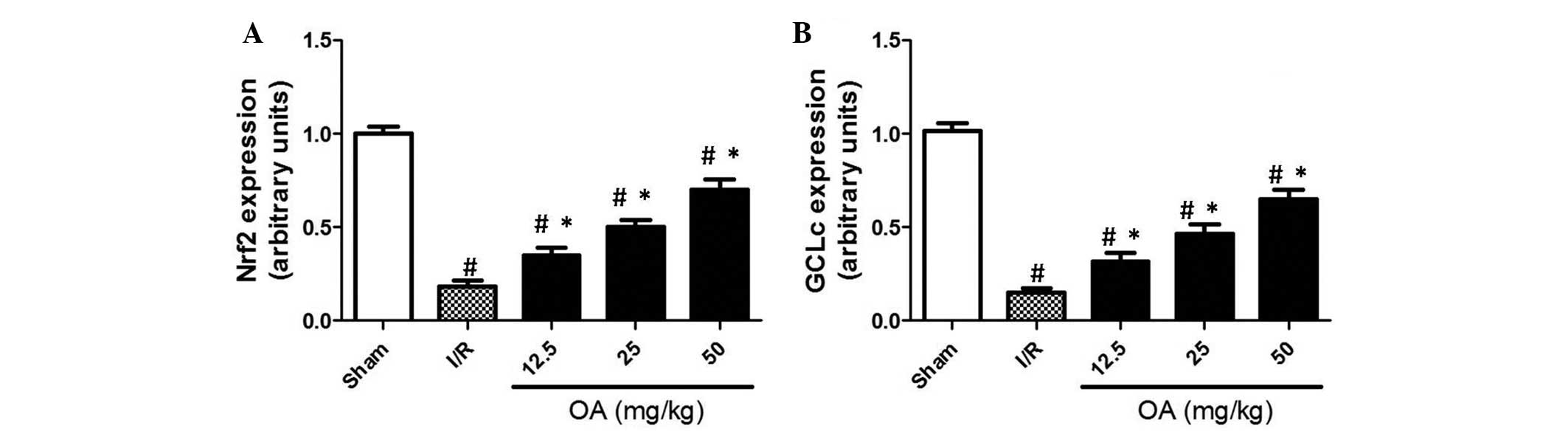
Effects of OA preconditioning on Nrf2 and
GCLc expression
In order to examine the mechanism underlying the
protective effects of OA preconditioning against renal I/R injury
in rats, the mRNA expression levels of Nrf2 and the catalytic
subunit of GCLc were determined by RT-qPCR. Nrf2 is a pivotal
transcription factor, which controls redox balance by regulating
various antioxidant enzymes, including GCLc. GCLc is rate-limiting
subunit of enzymes responsible for GSH synthesis. The present study
demonstrated that I/R resulted in a significant decrease in the
expression levels of Nrf2 and GCLc (Fig. 5; P=0.0015). Conversely, OA
preconditioning exerted a notable inhibition on I/R-induced
reductions in Nrf2 and GCLc expression (Fig. 5; P=0.0021). These results indicate
that stabilization of Nrf2/GCLc signaling, and subsequent
maintenance of the GSH pool, may be associated with the preventive
effects of OA preconditioning against renal I/R injury.
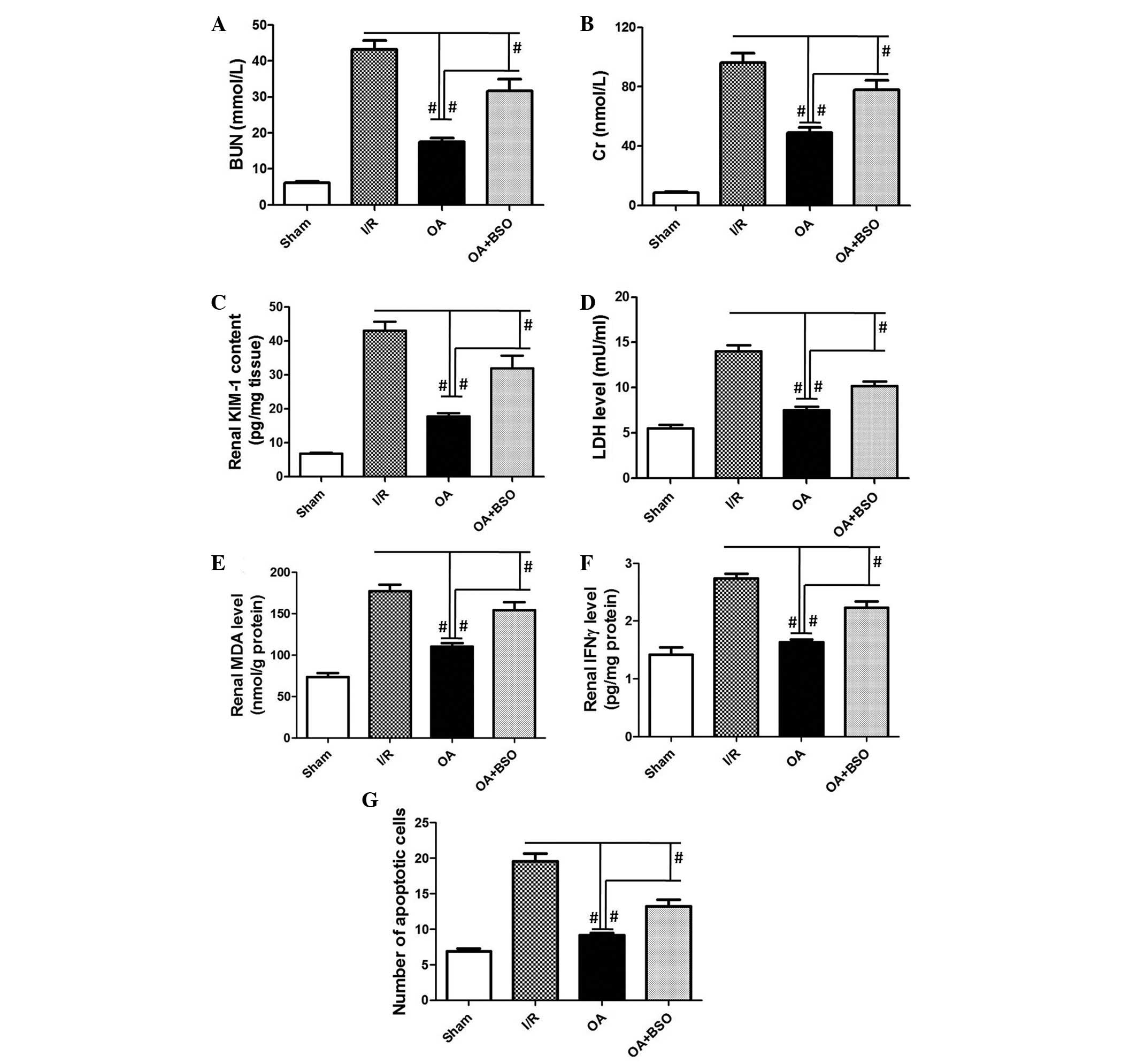
Maintenance of the GSH pool is associated
with the preventive effects of OA preconditioning against renal I/R
injury
In order to determine the role of the GSH pool in
OA-induced prevention of renal I/R injury, I/R rats were
pre-treated with BSO, an inhibitor of GSH synthesis. As shown in
Fig. 6, OA-induced inhibition of
BUN, Cr, KIM-1, LDH, MDA, IFN-γ, and apoptosis, which were
increased by I/R, was significantly suppressed by BSO (P=0.0002,
P=0.0003, P=0.0001, P=0.0002, P=0.0001, P=0.006, P=0.001,
respectively). These results indicate that maintenance of the GSH
pool is critical for OA preconditioning-induced prevention of renal
I/R injury. The antioxidant activity of OA may be considered the
primary capability, which contributes to the anti-inflammatory and
anti-apoptotic effects of OA preconditioning (Fig. 7).
Discussion
The present study examined the effects of OA
preconditioning on I/R-induced renal injury. In protocol 1, the
rats were intraperitoneally injected with 12.5–50 mg/kg OA for 15
consecutive days prior to the induction of renal I/R. In protocol
2, the rats were intraperitoneally injected with 50 mg/kg OA, with
or without 10 mg/kg BSO, for 15 consecutive days prior to the
induction of renal I/R.
The results demonstrated that OA preconditioning was
able to prevent I/R-induced renal injury, as evidenced by decreased
serum levels of BUN, Cr and LDH, and renal levels of KIM-1,
compared with the I/R group. Furthermore, OA preconditioning
exhibited antioxidant effects in I/R-operated rats, as reflected by
decreased MDA levels, increased SOD, CAT and GPx activities, and
increased GSH content compared with the I/R rats. OA
preconditioning also exerted anti-inflammatory effects in
I/R-operated rats, as shown by decreased IFN-γ, IL-6 and MPO
levels, and increased IL-10 levels compared with the I/R rats. In
addition, OA preconditioning exhibited anti-apoptotic effects
against renal I/R injury, as reflected by decreased apoptosis and
caspase 3 content. However, proapoptotic effects of OA have also
been reported. For example, Wang et al (25) demonstrated that OA inhibited
hepatocellular carcinoma via mitochondrial-dependent apoptosis. The
discrepancy of the apoptotic effects of OA may be attributed to
tissue-specific effects, or may be due to different pathological
and experimental conditions. The results of the present study
indicated that OA preconditioning prevented I/R-induced renal
injury in rats, and the antioxidant, anti-inflammatory, and
anti-apoptotic effects of OA may be associated with the prevention
of renal I/R injury.
The present study also evaluated the molecular
mechanism underlying the antioxidant, anti-inflammatory, and
anti-apoptotic activities of OA. OA administration was able to
inhibit I/R-induced decreases in Nrf2 and GCLc expression. Nrf2 is
a well-known crucial regulator of cellular redox homeostasis, which
has important roles in the protection against ROS damage via its
ability to induce the expression of various antioxidant enzymes
that detoxify ROS, and other antioxidant proteins (26). GCLc is a main target of Nrf2
transcriptional regulation (27).
The regulation of GCLc by Nrf2 is associated with modulation of the
GSH pool. GCLc is a subunit of the rate-limiting enzyme for de
novo synthesis of GSH. GSH, which is a thiol tripeptide, is
important in numerous cellular functions, including detoxifying
electrophiles, scavenging ROS, maintaining the essential thiol
status of proteins, and providing a reservoir for cysteine
(28).
The present study examined the role of the GSH pool
in the protective effects of OA preconditioning against renal I/R
injury. In the presence of BSO, the protective effects of OA
preconditioning on renal function, and the antioxidant,
anti-inflammatory, and anti-apoptotic effects of OA preconditioning
were significantly suppressed. The present study hypothesized that
OA preconditioning stabilized Nrf2/GCLc signaling and resulted in
maintenance of the GSH pool, thus defending tissues against
I/R-induced renal injury. The antioxidant activity of OA may
therefore be considered the major mechanism underlying the
preventive effects of OA preconditioning against renal I/R injury,
and may contribute to the resultant anti-inflammatory and
anti-apoptotic effects.
In conclusion, the results of the present study
demonstrated that OA preconditioning effectively prevented
I/R-induced renal injury in rats, and this protection may be due to
the antioxidant, anti-inflammatory, and anti-apoptotic effects of
OA. OA-induced stabilization of Nrf2/GCLc signaling and maintenance
of the GSH pool was shown to have a major role in the preventive
effects of OA preconditioning against renal I/R injury. The present
study provided initial evidence regarding the protective effects of
OA preconditioning against renal I/R injury.
References
|
1
|
Gueler F, Shushakova N, Mengel M, Hueper
K, Chen R, Liu X, Park JK, Haller H, Wensvoort G and Rong S: A
novel therapy to attenuate acute kidney injury and ischemic
allograft damage after allogenic kidney transplantation in mice.
PLoS One. 10:e01157092015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agrawal M and Swartz R: Acute renal
failure. Am Fam Physician. 61:2077–2088. 2000.PubMed/NCBI
|
|
3
|
Tuttle KR: Ischemic nephropathy. Curr Opin
Nephrol Hypertens. 10:167–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simone S, Rascio F, Castellano G, Divella
C, Chieti A, Ditonno P, Battaglia M, Crovace A, Staffieri F,
Oortwijn B, et al: Complement-dependent NADPH oxidase enzyme
activation in renal ischemia/reperfusion injury. Free Radic Biol
Med. 74:263–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niu J, Wu J, Li X and Zhang F: Association
between endothelin-1/endothelin receptor A and inflammation in
mouse kidneys following acute ischemia/reperfusion. Mol Med Rep.
11:3981–3987. 2015.PubMed/NCBI
|
|
6
|
Wang L, Liu X, Chen H, Chen Z, Weng X, Qiu
T and Liu L: Effect of picroside II on apoptosis induced by renal
ischemia/reperfusion injury in rats. Exp Ther Med. 9:817–822.
2015.PubMed/NCBI
|
|
7
|
Dong B, Zhou H, Han C, Yao J, Xu L, Zhang
M, Fu Y and Xia Q: Ischemia/reperfusion-induced CHOP expression
promotes apoptosis and impairs renal function recovery: The role of
acidosis and GPR4. PLoS One. 9:e1109442014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cruthirds DL, Novak L, Akhi KM, Sanders
PW, Thompson JA and MacMillan-Crow LA: Mitochondrial targets of
oxidative stress during renal ischemia/reperfusion. Arch Biochem
Biophys. 412:27–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang B, Jain S, Pawluczyk IZ, Imtiaz S,
Bowley L, Ashra SY and Nicholson ML: Inflammation and caspase
activation in long-term renal ischemia/reperfusion injury and
immunosuppression in rats. Kidney Int. 68:2050–2067. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu HH, Hsiao TY, Chien CT and Lai MK:
Ischemic conditioning by short periods of reperfusion attenuates
renal ischemia/reperfusion induced apoptosis and autophagy in the
rat. J Biomed Sci. 16:192009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El Morsy EM, Ahmed MA and Ahmed AA:
Attenuation of renal ischemia/reperfusion injury by açaí extract
preconditioning in a rat model. Life Sci. 123:35–42. 2015.
View Article : Google Scholar
|
|
12
|
Ilhan H, Eroglu M, Inal V, Eyi YE, Arziman
I, Yildirim AO, Tansel A, Uzun G and Yamanel L: Hyperbaric oxygen
therapy alleviates oxidative stress and tissue injury in renal
ischemia/reperfusion injury in rats. Ren Fail. 34:1305–1308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu
H and Chen Z: Ozone oxidative preconditioning inhibits inflammation
and apoptosis in a rat model of renal ischemia/reperfusion injury.
Eur J Pharmacol. 581:306–314. 2008. View Article : Google Scholar
|
|
14
|
Pérez-Camino MC and Cert A: Quantitative
determination of hydroxy pentacyclic triterpene acids in vegetable
oils. J Agric Food Chem. 47:1558–1562. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Ye XL, Liu R, Chen HL, Bai H,
Liang X, Zhang XD, Wang Z, Li WL and Hai CX: Antioxidant activities
of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases.
Chem Biol Interact. 184:328–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H
and Hai C: Oleanolic acid co-administration alleviates
ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing
modulating in rats. Chem Biol Interact. 221:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nkeh-Chungag BN, Oyedeji OO, Oyedeji AO
and Ndebia EJ: Anti-inflammatory and membrane-stabilizing
properties of two semisynthetic derivatives of oleanolic acid.
Inflammation. 38:61–69. 2015. View Article : Google Scholar
|
|
18
|
Bischoff A, Bucher M, Gekle M and Sauvant
C: Differential effect of COX1 and COX2 inhibitors on renal
outcomes following ischemic acute kidney injury. Am J Nephrol.
40:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wills ED: Mechanisms of lipid peroxide
formation in animal tissues. Biochem J. 99:667–676. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Hai CX, Liang X, Yu SX, Zhang W
and Li YL: The protective effects of Acanthopanax senticosus Harms
aqueous extracts against oxidative stress: Role of Nrf2 and
antioxidant enzymes. J Ethnopharmacol. 127:424–432. 2010.
View Article : Google Scholar
|
|
21
|
Grimholt RM, Urdal P, Klingenberg O and
Piehler AP: Rapid and reliable detection of α-globin copy number
variations by quantitative real-time PCR. BMC Hematol. 14:42014.
View Article : Google Scholar
|
|
22
|
Han WK, Bailly V, Abichandani R, Thadhani
R and Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel
biomarker for human renal proximal tubule injury. Kidney Int.
62:237–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vázquez-Frias R, Gutiérrez-Reyes G,
Urbán-Reyes M, Velázquez-Guadarrama N, Fortoul-van der Goes TI,
Reyes-López A and Consuelo-Sánchez A: Proinflammatory and
anti-inflammatory cytokine profile in pediatric patients with
irritable bowel syndrome. Rev Gastroenterol Mex. 80:6–12.
2015.PubMed/NCBI
|
|
24
|
Loria V, Dato I, Graziani F and Biasucci
LM: Myeloperoxidase: A new biomarker of inflammation in ischemic
heart disease and acute coronary syndromes. Mediators Inflamm.
2008:1356252008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Bai H, Zhang X, Liu J, Cao P, Liao
N, Zhang W, Wang Z and Hai C: Inhibitory effect of oleanolic acid
on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest
and mitochondrial-dependent apoptosis. Carcinogenesis.
34:1323–1330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwak MK, Wakabayashi N, Itoh K, Motohashi
H, Yamamoto M and Kensler TW: Modulation of gene expression by
cancer chemopreventive dithiolethiones through the Keap1-Nrf2
pathway. Identification of novel gene clusters for cell survival. J
Biol Chem. 278:8135–8145. 2003. View Article : Google Scholar
|
|
27
|
Lu YF, Liu J, Wu KC, Qu Q, Fan F and
Klaassen CD: Overexpression of Nrf2 protects against
microcystin-induced hepatotoxicity in mice. PLoS One. 9:e930132014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arrick BA and Nathan CF: Glutathione
metabolism as a determinant of therapeutic efficacy: A review.
Cancer Res. 44:4224–4232. 1984.PubMed/NCBI
|