Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-associated mortality and one of the most
lethal malignancies worldwide (1).
Early stage HCC with preserved liver function can be effectively
treated by resection, liver transplantation or percutaneously, and
with a 5 year survival rate (2).
Generally, HCC progression can be defined by a decrease in
differentiation, the loss of tissue-specific gene expression,
acceleration of cell proliferation and, ultimately, invasion
(3). Patients with HCC often
exhibit tumor cell invasion and metastasis prior to conventional
diagnosis (4). Therefore, it is
vital to study the molecular basis of HCC and explore novel
therapeutic agents.
Choline is an essential nutrient that is required to
make the major membrane phospholipid, phosphatidylcholine (PC)
(5). Choline has been suggested to
serve multiple roles in cancer development. Choline metabolites can
affect DNA methylation and lead to a disruption of DNA repair
(6). Choline can also modify cell
signaling that is mediated by intermediary phospholipid
metabolites, and can support the synthesis of cell membranes and,
therefore, cell proliferation (7).
In this sense, the identification and characterization of choline
transporters in cancer may offer a novel target for the design of
antitumor strategies. Therefore, it is important to identify
choline transporters in cancer cells.
The choline transport system has been categorized
into three transporter families: Polyspecific organic cation
transporters (OCTs/SLC22A1-2) with low affinity for choline,
high-affinity choline transporter 1 (CHT1/SLC5A7) and
intermediate-affinity choline transporter-like proteins
(CTLs/SLC44A1-5) (8). Previously,
the CTLs/SLC44A1-5 were shown to be present in various human
tissues (9). The presence of
SLC44A1 protein in the rat and human central nervous systems, where
it is found in neuronal, glial and endothelial cells, suggests that
malfunction of this transporter may have important implications in
nervous system development and repair following injury, and in
neurodegenerative diseases (10).
SLC44A2 is expressed as two isoforms, SLC44A2-P1 and SLC44A2-P2, in
the heart, colon, lung, kidney and liver, which suggests that
tissue-specific differences may influence its function in each
tissue (11). Moderate SLC44A3
expression is present in the kidney, ileum and colon, while a
notably strong SLC44A4 expression can be detected in the intestine,
stomach and kidney. Much fewer data are available regarding the
expression of SLC44A5, which was markedly low in the brain and
higher in the spinal cord, however, to a lesser extent than SLC44A1
(12). However, SLC44A3-5 remain
to be characterized functionally.
The gene expression suggests that SLC44A5 is
important in development and progression of HCC. The effect of
SLC44A5 knockdown on viability, cell cycle, apoptosis and invasion
of HCC cell lines were assessed and the possible mechanism was also
explored. The present study provided original documentation for the
upregulation of SLC44A5 in HCC and it may be an effective
therapeutic target for this disease.
Materials and methods
Clinical HCC samples
Specimens of HCC and paired non-cancerous tissues
were obtained from 35 patients with stage I-IV HCC, admitted to
Zhongnan Hospital of Wuhan University (Hebei, China), were enrolled
in the present study. Ethical approval for the present study was
provided by the independent Ethics Committee of Zhongnan Hospital
of Wuhan University (Hebei, China). Written informed consent was
obtained from all patients involved in the present study. All
research was performed in accordance with the Helsinki Declaration
of 1975 (13). No patient had
received radiotherapy or chemotherapy. The percentage of tumor
cellularity in the patients with HCC's tissue section was at least
70%, as determined by pathological examination of histology slides
in hospital patient's cohort. HCC and paired non-cancerous tissues
were immediately snap-frozen in liquid nitrogen and stored at −80°C
until the total RNA was extracted. Tumor samples were composed of
at least 80% of viable-appearing tumor cells on histological
assessment.
Cell cultures and transfection
The HCC cell lines, including SMMC-7721, BEL-7404,
MHCC-97H, MHCC-97L, HepG2 and HuH7, were obtained from the Cell
Bank of Academia Sinica (Shanghai, China) and grown in Dulbecco's
modified Eagle's medium (DMEM), supplemented with 10% fetal bovine
serum (FBS), 2 mM L-glutamine, 100 U/μl penicillin and 100
μg/μl streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a humidified incubator
containing 5% CO2 in air at 37°C. Short hairpin (sh)RNA
targeting position 1,946-1,968 (GUU GCA GUU ACA GAU GAA G) of human
SLC44A5 mRNA was cloned into a lentiviral vector (PLKO.1-EGFP). The
cells were transfected with shRNA (40 nM) using the Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according the
manufacturer's protocol. A non-specific scramble shRNA sequence was
used as negative control and the selective silencing of SLC44A5 was
confirmed by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). The cells were analyzed 48 h after
transfection.
RT-qPCR
The total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. cDNA was synthesized from RNA using a MMLV
RT reagent kit (Thermo Fisher Scientific, Inc.). The DyNAmo Flash
SYBR Green qPCR kit (Finnzymes Oy, Espoo, Finland) was used,
according to the manufacturer's instructions. qPCR was performed to
detect the mRNA expression levels of indicated genes. The primers
sequences are list in Table I.
Relative quantification of SLC44A5 expression levels was determined
using the 2−ΔΔCq method.
 | Table IPrimes sequences used in this
study. |
Table I
Primes sequences used in this
study.
| Primer | Sequence |
|---|
| SLC44A5 | |
| Forward |
5′-GACATCGGGATTCAGACTAAC-3′ |
| Reverse |
5′-ATAATGGCGACTCGGATTC-3′ |
| PCNA | |
| Forward |
5′-GCCTGACAAATGCTTGCTGAC-3′ |
| Reverse |
5′-TTGAGTGCCTCCAACACCTTC-3′ |
| CDK1 | |
| Forward |
5′-ACCATACCCATTGACTAAC-3′ |
| Reverse |
5′-ATAAGCACATCCTGAAGAC-3′ |
| Caspase-3 | |
| Forward |
5′-AACTGGACTGTGGCATTGAG-3′ |
| Reverse |
5′-AAACACATTAGGCACAATCC-3′ |
| Caspase-9 | |
| Forward |
5′-GGAAGAGGGACAGATGAATG-3′ |
| Reverse |
5′-TTGTTTGGCACCACTCAG-3′ |
| GAPDH | |
| Forward |
5′-CACCCACTCCTCCACCTTTG-3′ |
| Reverse |
5′-CCACCACCCTGTTGCTGTAG-3′ |
Western blot analysis
HCC tissues and cell lines transfected with SLC44A5
shRNA or negative controls vector were lysed using
radioimmunoprecipitation buffer, supplemented with protease
inhibitor (Beyotime Institute of Biotechnology, Inc., Shanghai,
China). The protein concentration was estimated using the
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.).
Equal quantities of protein (30 μg) were subsequently
separated on 12% SDS-PAGE gels, and were subsequently transferred
onto nitrocellulose membranes (EMD Millipore, Billerica, MA, USA).
Following blocking, the membranes were immunoblotted overnight at
4°C with primary antibodies: polyclonal goat anti-SLC44A5 (1:1,000;
cat. no. sc-68054; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), monoclonal rabbit anti-proliferating cell nuclear antigen
(PCNA; 1:5,000; cat. no. ab92552; Abcam, Cambridge, MA, USA),
monoclonal rabbit anti-cyclin-dependent kinase (CDK)1 (1:1,000;
cat. no. ab32384; Abcam), monoclonal rabbit anti-caspase-3
(1:3,000; cat. no. ab32351; Abcam), polyclonal rabbit
anti-caspase-9 (1:1,000; cat. no. ab2014; Abcam) or monoclonal
rabbit anti-GAPDH (1:1,500; cat. no. 5174; Cell Signaling
Technology, Inc., Danvers, MA, USA). After washing, the membranes
were incubated with goat anti-rabbit (cat. no. A0208) or donkey
anti-goat (cat. no. A0181) horseradish peroxidase-conjugated
secondary antibodies (1:1,000; Beyotime Institute of Biotechnology,
Inc.) at 37°C for 1 h. The membranes were washed with Tris-buffered
saline containing 20% Tween 20 (Amresco, LLC, Solon, OH, USA)
Signals were detected using an enhanced chemiluminescence system
(Pierce, Rockford, IL, USA).
Cell viability assay
Cell viability was assessed using the Cell counting
kit (CCK)-8 (Dojindo, Kumamoto, Japan). Briefly, control, negative
control vector and SLC44A5 shRNA-treated cells were seeded into
96-well plates at an initial density of 3×103 cells/well
for 72 h. At specified time points, 100 μl CCK-8 solution
was added to each well of the plate and the plate was incubated for
1 h. Cell viability was determined by scanning with an iMark
microplate absorbance reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) at 450 nm. All samples were assessed in triplicate for
each group and the experiment was repeated at least twice.
Cell cycle analysis
A total of ~1×104 cells were removed at
specified time points, washed twice with phosphate-buffered saline
(PBS) and fixed in cold ethanol for 30 min. The cells were
subsequently incubated with propidium iodide (PI) and 0.5
μg/μl RNase A for 30 min. Thereafter, the cells were
analyzed on a BD Accuri C6 flow cytometer (BD Biosciences, San
Diego, CA, USA).
Apoptosis assays
Apoptosis was determined by flow cytometry using an
Annexin-V fluorescein isothiocyanate (FITC)/PI double-staining,
according to the manufacturer's protocol (BioVision, Mountain View,
CA, USA). Briefly, at 48 h after transfection, the cells were
collected and resuspended in 500 μl binding buffer,
containing 5 μl annexin-V/FITC and 5 μl PI, and
subsequently incubated for 5 min in the dark at room temperature.
Analysis was immediately performed using a flow cytometer.
In vitro invasion assay
The upper well of the Transwell (Corning, Corning,
NY, USA) was coated with Matrigel (BD Biosciences) at 37°C in a 5%
CO2 incubator for 1 h. The indicated cells were serum
starved for 24 h. Subsequently, 5×104 cells in 500
μl serum-free DMEM were seeded into the upper well of the
Transwell chamber. Culture medium, supplemented with 10% FBS (750
μl) was added into the lower well of the chamber. After 48 h
incubation, the cells in the upper well were removed with a cotton
swab and the cells that migrated into the lower well were washed
with PBS, fixed in 3.7% paraformaldehyde and stained with 0.2%
crystal violet. Images of the cells were captured and cell number
was counted using an Olympus CX41RF microscope (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using the
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). The comparison of different groups was analyzed using
two-tailed Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
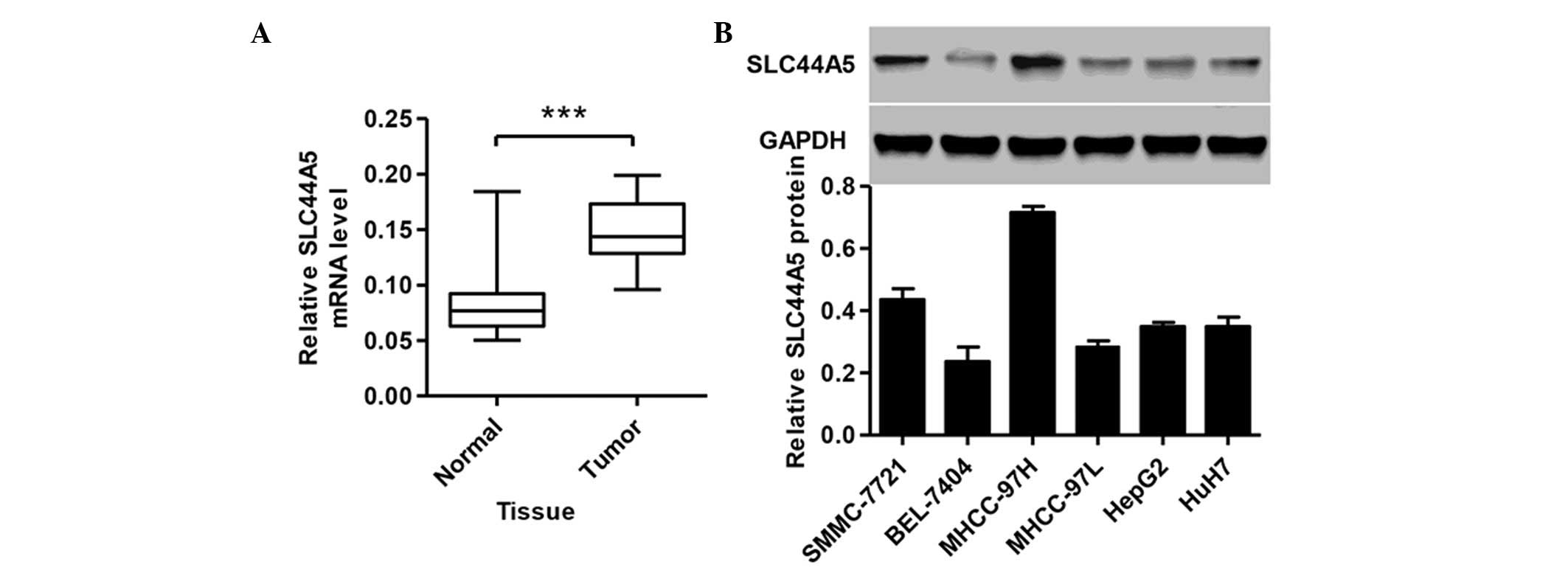
SLC44A5 is upregulated in HCC tissues and
cell lines
To study the biological role of SLC44A5 in HCC,
RT-qPCR was performed to detect the expression levels of SLC44A5 in
tissues from patients with HCC. A total of 35 HCC tissue samples
and 35 normal tissue samples were collected from Zhongnan Hospital
of Wuhan University. As shown in Fig.
1A, the expression of SLC44A5 was higher in the HCC tissues
compared with the normal control tissue (P<0.001). The protein
expression levels of SLC44A5 were next determined in the HCC cell
lines, SMMC-7721, BEL-7404, MHCC-97H, MHCC-97L, HepG2 and HuH7, by
western blotting. SLC44A5 was expressed at a higher level in
MHCC-97H and SMMC-7721 cell lines compared with other four cell
lines (Fig. 1B). Therefore,
MHCC-97H and SMMC-7721 cell lines were selected for further
investigation.
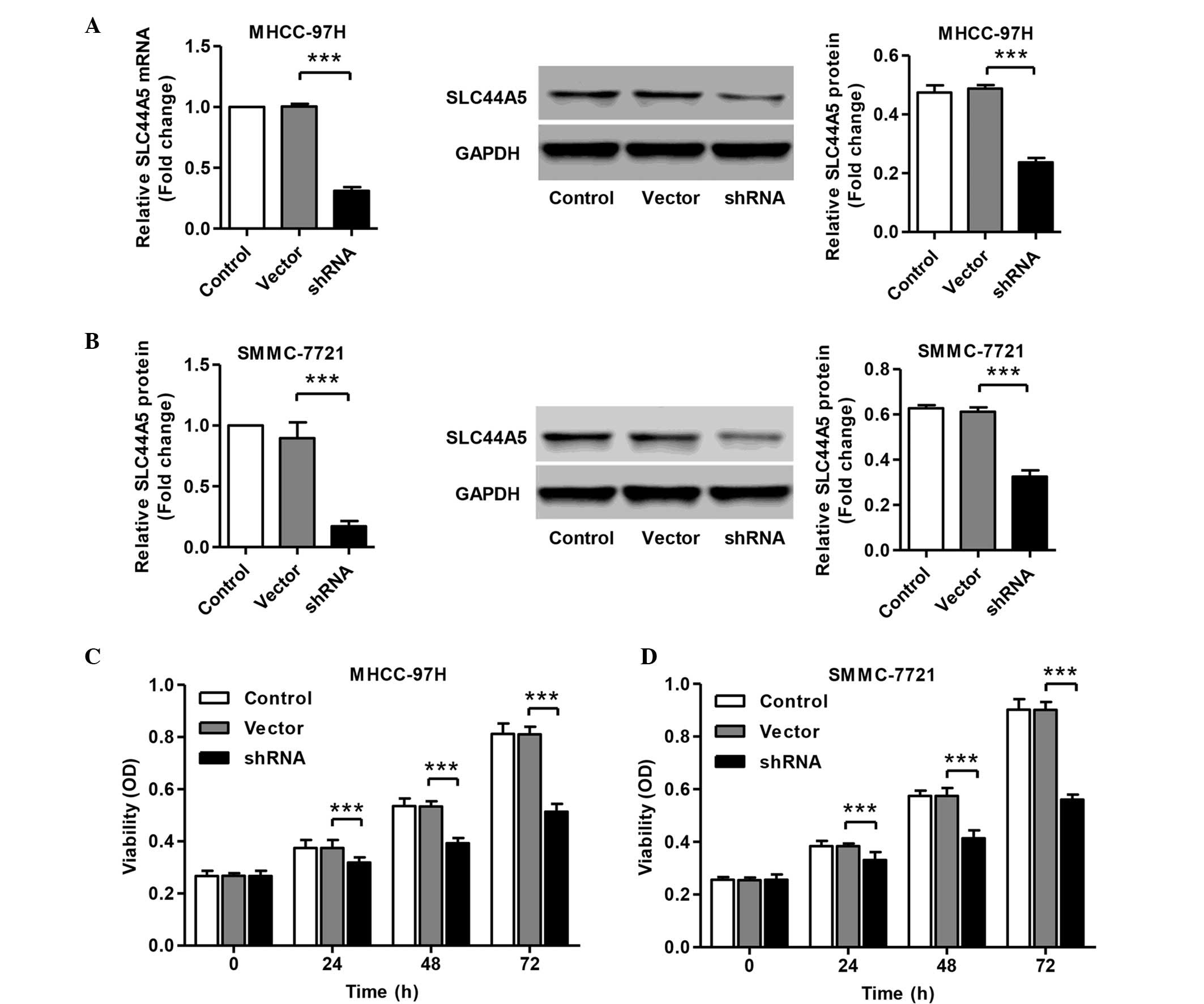
Knockdown of SLC44A5 represses HCC cell
viability
To investigate the functions of SLC44A5 on HCC,
shRNA was designed and transfected into MHCC-97H and SMMC-7721
cells. The mRNA and protein expression levels of SLC44A5 in
response to specific shRNA were assessed. The mRNA and protein
expression levels of SLC44A5 were remarkably reduced in MHCC-97H
and SMMC-7721 cells transfected with shRNA (Fig. 2A–D). No apparent change was
observed in the cells with the negative control vector. To
determine the role of SLC44A5 on the viability of HCC cell lines,
the viability of MHCC-97H and SMMC-7721 cells transfected SLC44A5
shRNA was assessed by CCK-8 assay. As shown in Fig. 2E and F, 37±0.6 and 38±0.1%
inhibition of cell viability was observed 72 h after shRNA
transfection in MHCC-97H and SMMC-7721 cells, respectively.
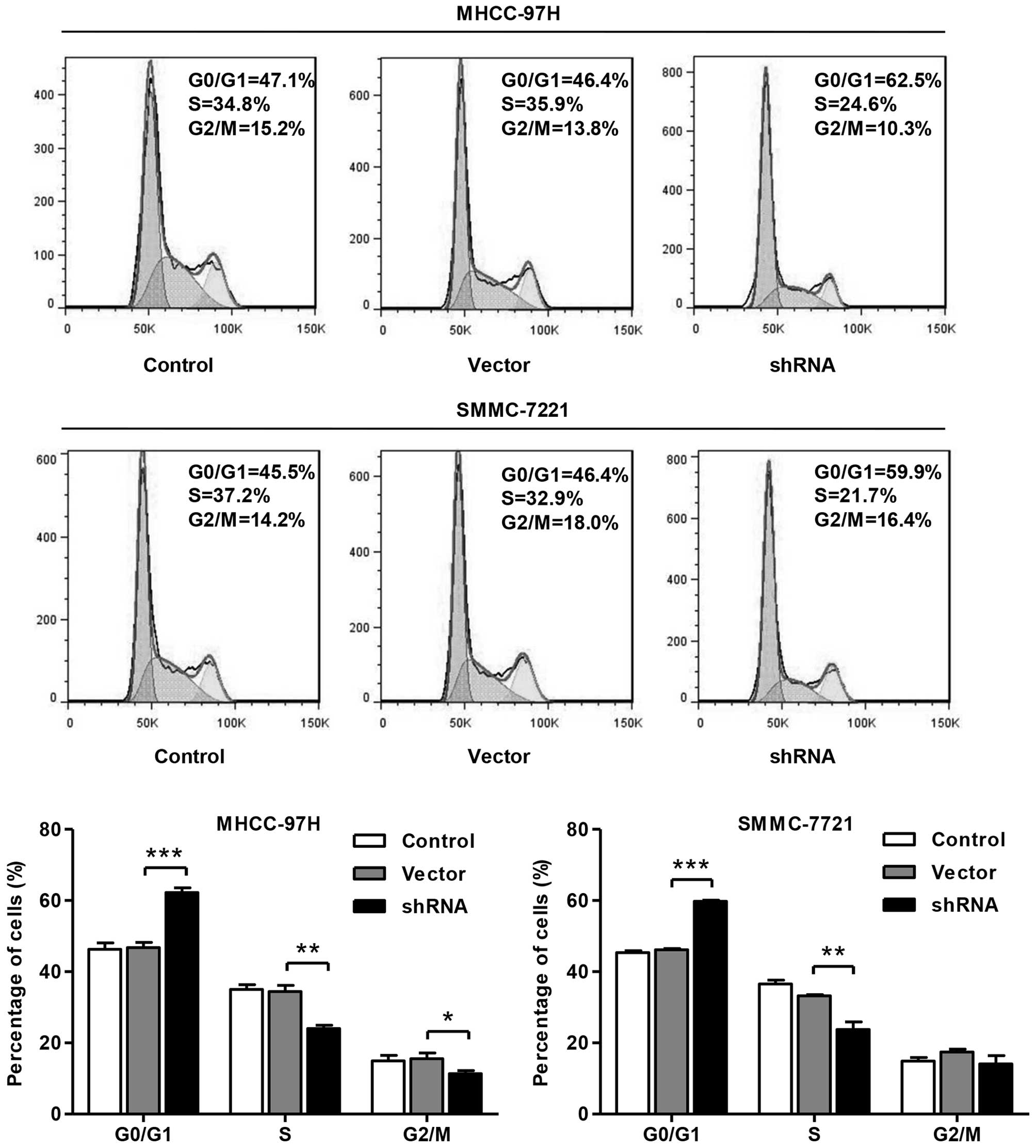
Knockdown of SLC44A5 induces HCC cell
cycle arrest at G0/G1 phase
To further validate the inhibition of cell viability
by SLC44A5 shRNA, the cell cycle was analyzed in MHCC-97H and
SMMC-7721 cells (Fig. 3). Cell
cycle analysis revealed that knockdown of SLC44A5 with shRNA
notably increased the rate of G0/G1 phase cells and reduced the
S-phase cell population in both cell lines. These results indicated
that knockdown of SLC44A5 in HCC cells may inhibit cell viability
by arresting cell cycle progression at G0/G1 phase.
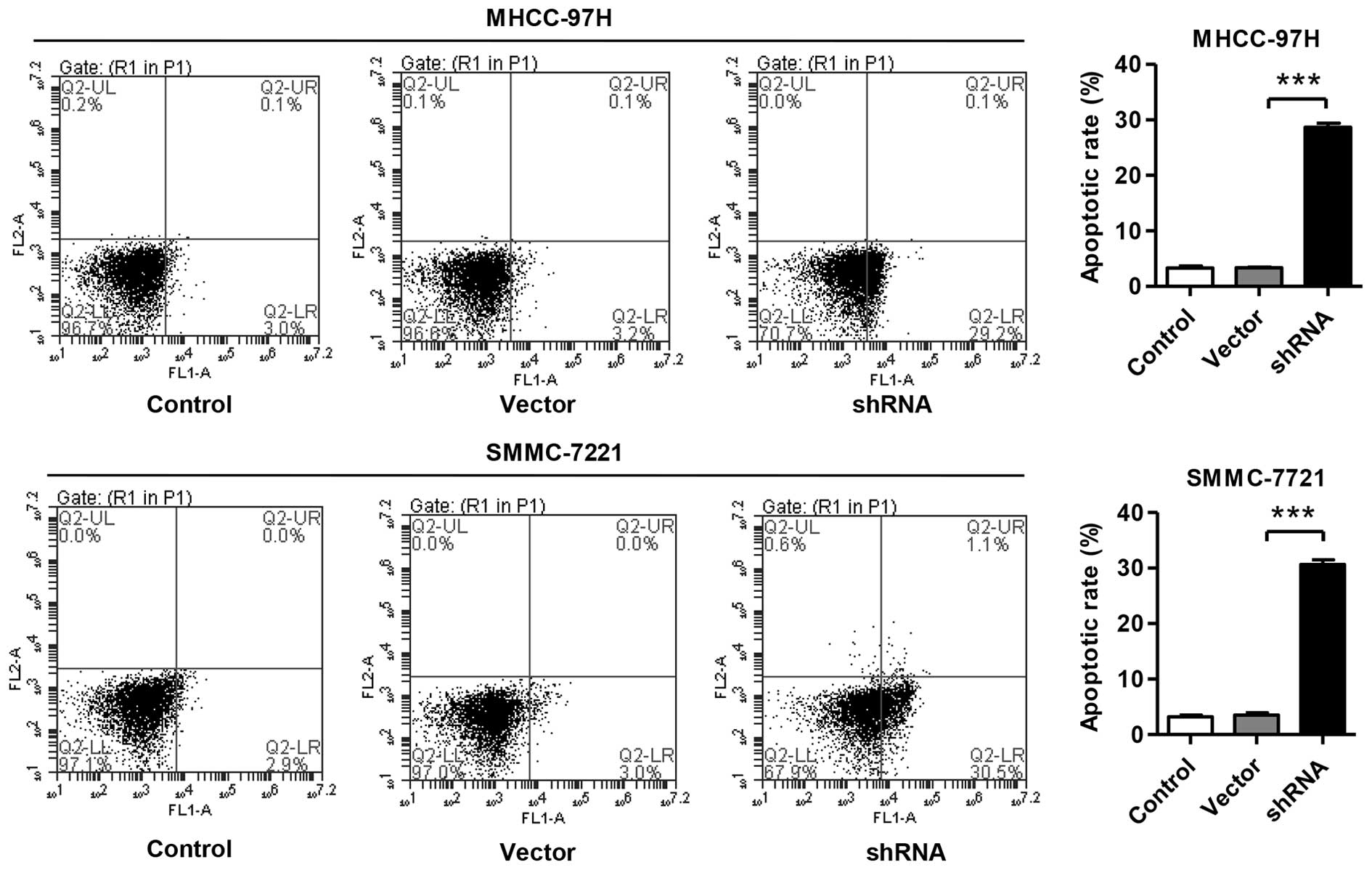
Knockdown of SLC44A5 induces HCC cell
apoptosis
The apoptotic function of SLC44A5 was then assessed
in MHCC-97H and SMMC-7721 cells using an annexin V-FITC/PI staining
assay. As shown in Fig. 4, flow
cytometry analysis revealed that inhibition of SLC44A5 in MHCC-97H
cells significantly induced cell apoptosis by 29% compared with the
corresponding cells transfected with negative control vector.
Increasing cell apoptosis was also observed in SMMC-7721 cells
transfected with SLC44A5 shRNA.
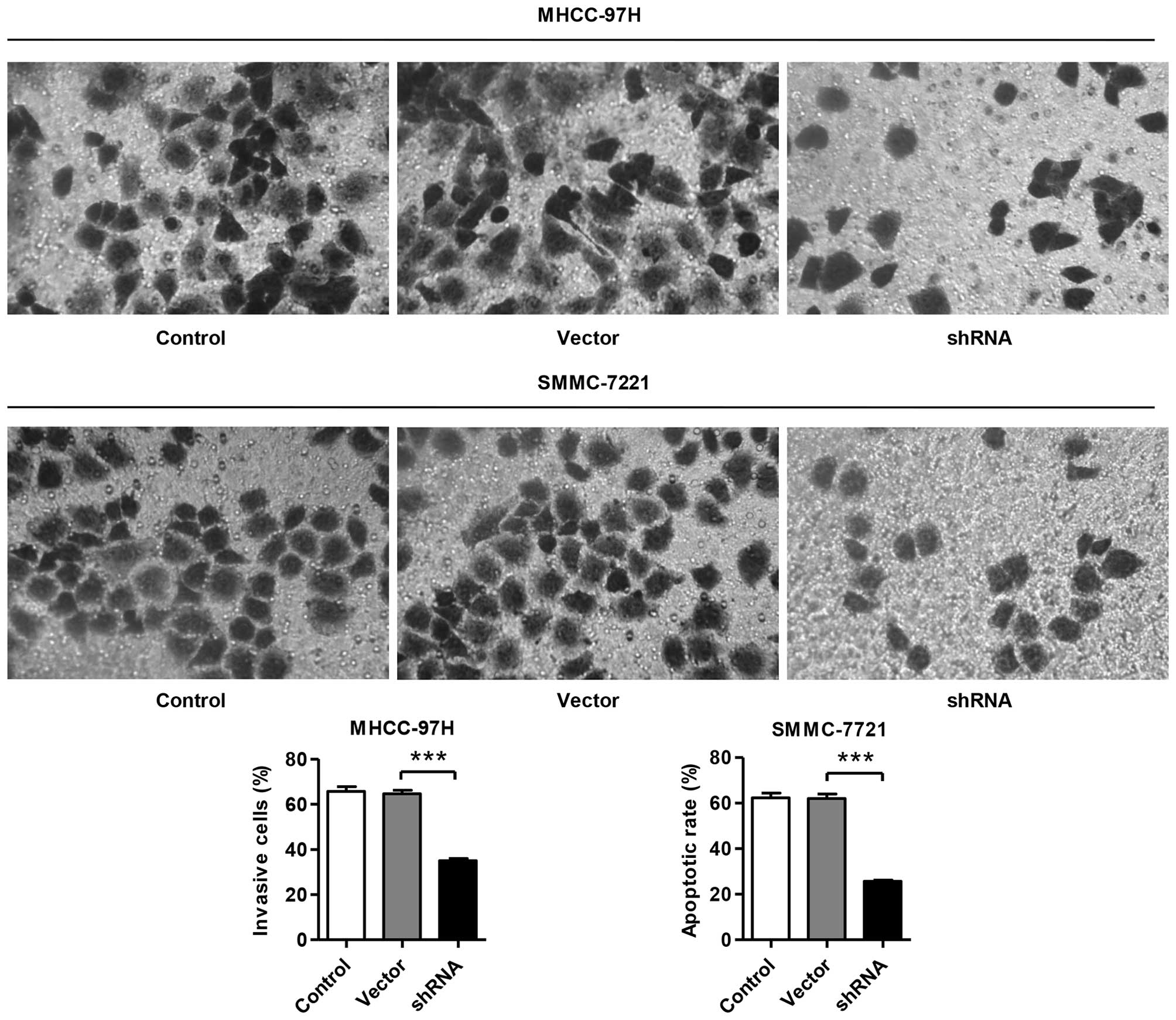
Knockdown of SLC44A5 induces HCC cell
invasion
To assess whether SLC44A5 affected the invasive
ability of HCC cells, Matrigel-coated membrane chamber invasion
assays were performed. As shown in Fig. 5, a marked reduction in the invasive
ability was observed in the SLC44A5 knockdown MHCC-97H and
SMMC-7721 cells compared with the negative control vector group.
The number of invasive SLC44A5 shRNA MHCC-97H and SMMC-7721 cells
was 47±0.5 and 59±1.9% of that of the negative control vector
group, respectively.
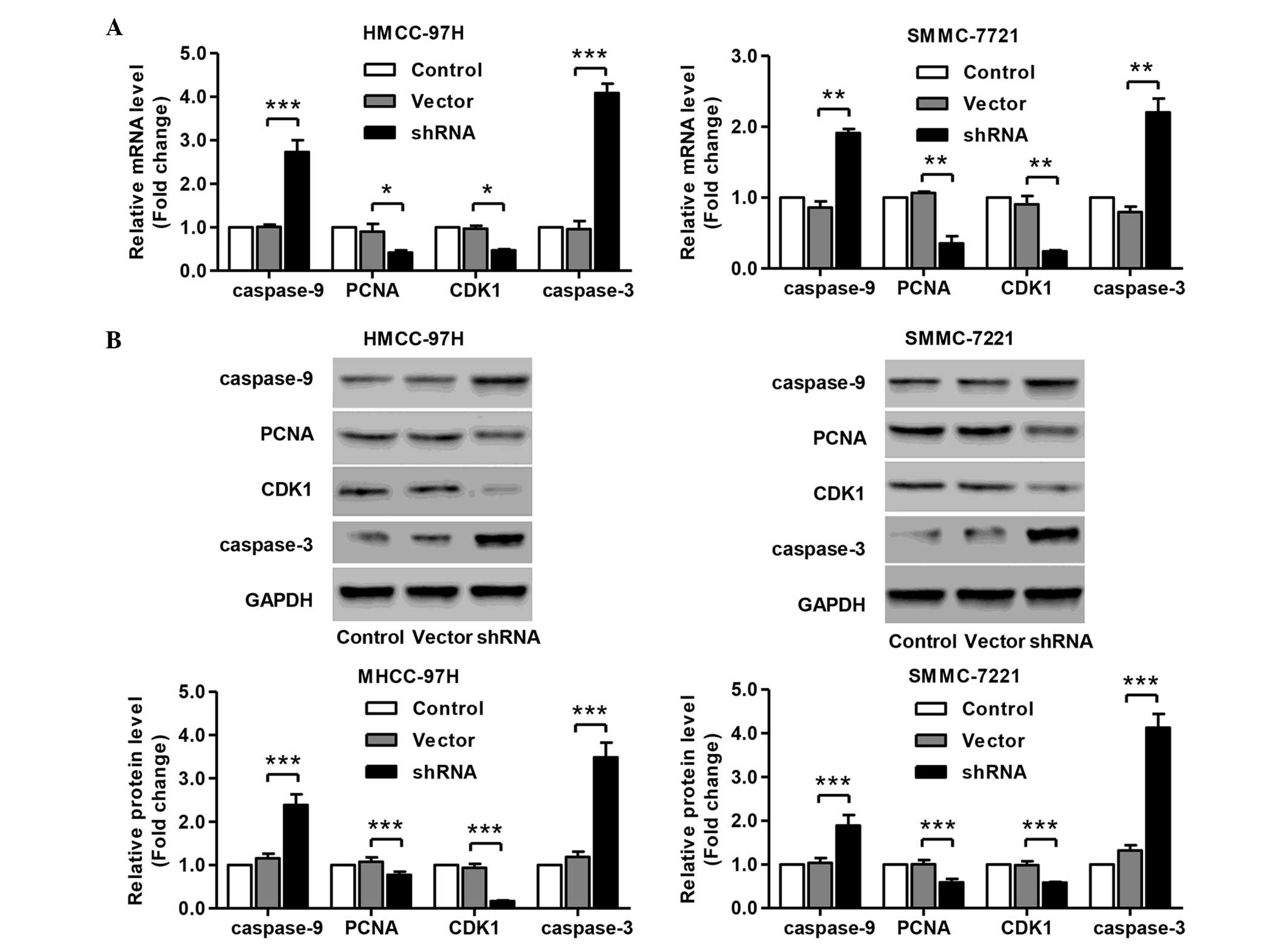
Knockdown of SLC44A5 represses the
expression of cell cycle and apoptosis markers
Having documented significantly induced cell cycle
arrest and apoptosis of SLC44A5 knockdown in HCC cell lines, the
present study wondered how SLC44A5 affects HCC cell cycle and
apoptosis. To investigate this, the expression of cell cycle and
apoptosis markers in MHCC-97H and SMMC-7721 cells were determined
by RT-qPCR and western blotting. The results revealed that SLC44A5
knockdown resulted in a significant reduction in the mRNA and
protein expression levels of PCNA, CDK1, caspase-3 and caspase-9,
compared with the negative control vector group in MHCC-97H and
SMMC-7721 cells (Fig. 6A and B).
The present data suggested that knockdown of SLC44A5 inhibits the
expression of cell cycle and apoptosis-associated markers, which
may contribute to the induction of G1 cell cycle arrest and
apoptosis.
Discussion
HCC is one of the most highly malignant and lethal
cancer types. The development and progression of HCC is a
complicated process that involves the deregulation of multiple
genes that are essential for cell biological processes (14,15).
Previously, a distinct choline transporter called the SLC44A1-5
family was shown to be present in various human cancer cells
(16). Using RT-qPCR, the mRNA
expression profiles of SLC44A5 were measured in various cancer cell
lines, including NCI-H69 (small cell lung carcinoma), HT-29 (colon
adenocarcinoma), Jurkat (acute T-cell leukemia), SH-SY5Y
(dopaminergic, cholinergic, glutamatergic and adenosinergic
neuroblastoma) and LA-N-2 (cholinergic neuroblastoma). Among these
cell lines, SLC44A5 RNA was marginally expressed in HT-29 and
Jurkat cells, however, it was notably expressed in NCI-H69, SH-SY5Y
and LA-N-2 cells. Therefore, the expression of pattern of choline
transporters differed according to the cancer cell type.
Additionally, in the non-tumorigenic human mammary epithelial cell
line, MCF-10A, the expression levels of SLC44A5 were very low
compared with those in other cancer cells. The present study found
that SLC44A5 mRNA levels were consistently upregulated in HCC
clinical tissues compared with normal adjacent tissues; however,
SLC44A5 protein levels varied in the six HCC cell lines, including
SMMC-7721, BEL-7404, MHCC-97H, MHCC-97L, HepG2 and HuH7, suggesting
that SLC44A5 expression differed according to the HCC cell type.
Furthermore, it was shown that knockdown of SLC44A5 expression
inhibited cell viability and invasion and promoted apoptosis in
SMMC-7721 and MHCC-97H cells, indicating its role as an essential
oncogene during HCC tumorigenesis.
Notably, the SLC44A1-5 family has been shown to be
functionally important in the development of human lung, prostate
and colon carcinoma (17–20), however, the mechanisms underlying
the effects of the SLC44A1-5 family remain to be elucidated. One of
these proteins, SLC44A1, has previously been found to stimulate
NCI-H69 cell growth and choline uptake, suggesting that SLC44A1 may
function as a lung carcinogenic gene (17). The possible correlation between
choline uptake and viability was also assessed (the effect of
choline transporter inhibitors on the survival of various cancer
cells). It has been reported that quinine, quinidine and
desipramine inhibit choline uptake in various cell lines (20–22).
These drugs can inhibit cell viability in various cancer cell
lines, suggesting that cell viability may require an increased
supply choline and induce cell death by obstructing the function of
choline transporters. Such a potential oncogenic function of
endogenous SLC44A5 in HCC had not previously shown in vitro,
and the molecular mechanisms were also unknown. The present results
revealed that knockdown of SLC44A5 effectively decreased the
viability of SMMC-7721 and MHCC-97H cells, thus providing novel
insights into the role of SLC44A5 in HCC development and
progression.
Cell cycle regulation is frequently abnormal in most
common malignancies, resulting in aberrant cell viability (23,24).
Knockdown of SLC44A5 by shRNA significantly induced G0/G1 cell
cycle arrest in SMMC-7721 and MHCC-97H cells, which indicated that
the inhibition of cell viability in HCC cells is due to the arrest
of cell cycle progression. Cell cycle is mediated, directly or
indirectly, by misregulation of cyclin-dependent kinases (CDKs)
(25). In the present study, mRNA
and protein expression levels of the cell cycle markers, CDK1 and
PCNA, were notably reduced in SMMC-7721 and MHCC-97H cells treated
with SLC44A5 shRNA, which was consistent with the results of
induction of arrest G0/G1 cell cycle in SMMC-7721 and MHCC-97H
cells treated with SLC44A5 shRNA, indicating an association between
SLC44A5 function and the regulation of DNA replication and cell
cycle progression in HCC cells.
G1-phase arrest of cell cycle progression provides
an opportunity for cells to either undergo repairing or follow the
apoptosis process. The effects of SLC44A5 knockdown on the
induction of apoptosis were subsequently determined in SMMC-7721
and MHCC-97H cells. The flow cytometry data indicated that
knockdown of SLC44A5 resulted in significant induction of apoptosis
via increasing the mRNA and protein expression levels of the cell
apoptosis markers, caspase-3 and caspase-9, which was consistent
with previous studies showing that a gradual reduction in choline
supplementation initially causes apoptosis in rat hepatocytes
(8). In addition, knockdown of
SLC44A5 also inhibited the cell invasion of SMMC-7721 and MHCC-97H
cells. Due to its antiapoptosis and anti-invasion functions in HCC,
SLC44A5 may be a potential therapeutic target worth further
investigation.
Although choline has already been reported to be
associated with HCC carcinogenesis, the present study revealed a
critical role for choline transporter, SLC44A5, as a promoter of
cell viability and invasion, and an inhibitor of apoptosis in HCC
cells. Notbaly, the present study indicated the important role of
SLC44A5 as a tumor promoter in HCC through the inhibition of
choline uptake, suggesting that SLC44A5 may be a potential target
for HCC therapy.
Acknowledgments
The present study was supported by The Fundamental
Research Funds for the Central Universities.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin ZZ, Shau WY, Hsu C, Shao YY, Yeh YC,
Kuo RN, Hsu CH, Yang JC, Cheng AL and Lai MS: Radiofrequency
ablation is superior to ethanol injection in early-stage
hepatocellular carcinoma irrespective of tumor size. PloS One.
8:e802762013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lazarevich NL, Cheremnova OA, Varga EV,
Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI,
Engelhardt NV and Duncan S: Progression of HCC in mice is
associated with a downregulation in the expression of hepatocyte
nuclear factors. Hepatology. 39:1038–1047. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashiguchi M, Ueno S, Sakoda M, Iino S,
Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et
al: Clinical implication of ZEB-1 and E-cadherin expression in
hepatocellular carcinoma (HCC). BMC Cancer. 13:5722013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisel SH: Choline deficiency. J Nutr
Biochem. 1:332–349. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson OS, Sant KE and Dolinoy DC:
Nutrition and epigenetics: An interplay of dietary methyl donors,
one-carbon metabolism and DNA methylation. J Nutr Biochem.
23:853–859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio E, Mezzanzanica D, Alberti P,
Spadaro F, Ramoni C, D'Ascenzo S, Millimaggi D, Pavan A, Dolo V,
Canevari S and Podo F: Alterations of choline phospholipid
metabolism in ovarian tumor progression. Cancer Res. 65:9369–9376.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michel V, Yuan Z, Ramsubir S and Bakovic
M: Choline transport for phospholipid synthesis. Exp Biol Med
(Maywood). 231:490–504. 2006.
|
|
9
|
O'Regan S, Traiffort E, Ruat M, Cha N,
Compaore D and Meunier FM: An electric lobe suppressor for a yeast
choline transport mutation belongs to a new family of
transporter-like proteins. Proc Natl Acad Sci USA. 97:1835–1840.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Machová E, O'Regan S, Newcombe J, Meunier
FM, Prentice J, Dove R, Lisá V and Dolezal V: Detection of choline
transporter-like 1 protein CTL1 in neuroblastoma × glioma cells and
in the CNS, and its role in choline uptake. J Neurochem.
110:1297–1309. 2009. View Article : Google Scholar
|
|
11
|
Kommareddi PK, Nair TS, Thang LV, Galano
MM, Babu E, Ganapathy V, Kanazawa T, McHugh JB and Carey TE:
Isoforms, expression, glycosylation and tissue distribution of
CTL2/SLC44A2. Protein J. 29:417–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Traiffort E, Ruat M, O'Regan S and Meunier
FM: Molecular characterization of the family of choline
transporter-like proteins and their splice variants. J Neurochem.
92:1116–1125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shephard DA: The 1975 Declaration of
Helsinki and consent. Can Med Assoc J. 115:1191–1192.
1976.PubMed/NCBI
|
|
14
|
Kunter I, Erdal E, Nart D, Yilmaz F,
Karademir S, Sagol O and Atabey N: Active form of AKT controls cell
proliferation and response to apoptosis in hepatocellular
carcinoma. Oncol Rep. 31:573–580. 2014.
|
|
15
|
Hong X, Song R, Song H, Zheng T, Wang J,
Liang Y, Qi S, Lu Z, Song X, Jiang H, et al: PTEN antagonises
Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to
hepatocarcinogenesis. Gut. 63:1635–1647. 2014. View Article : Google Scholar
|
|
16
|
Inazu M: Choline transporter-like proteins
CTLs/SLC44 family as a novel molecular target for cancer therapy.
Biopharm Drug Dispos. 35:431–449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inazu M, Yamada T, Kubota N and Yamanaka
T: Functional expression of choline transporter-like protein 1
(CTL1) in small cell lung carcinoma cells: A target molecule for
lung cancer therapy. Pharmacol Res. 76:119–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang T, Li J, Chen F, Zhao Y, He X, Wan D
and Gu J: Choline transporters in human lung adenocarcinoma:
Expression and functional implications. Acta Bioch Bioph Sin
(Shanghai). 39:668–674. 2007. View Article : Google Scholar
|
|
19
|
Awwad HM, Geisel J and Obeid R: The role
of choline in prostate cancer. Clin Biochem. 45:1548–1553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kouji H, Inazu M, Yamada T, Tajima H, Aoki
T and Matsumiya T: Molecular and functional characterization of
choline transporter in human colon carcinoma HT-29 cells. Arch
Biochem Biophys. 483:90–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inazu M, Takeda H and Matsumiya T:
Molecular and functional characterization of an Na+-independent
choline transporter in rat astrocytes. J Neurochem. 94:1427–1437.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yabuki M, Inazu M, Yamada T, Tajima H and
Matsumiya T: Molecular and functional characterization of choline
transporter in rat renal tubule epithelial NRK-52E cells. Arch
Biochem Biophys. 485:88–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng
L, Zhou H and Zhao RC: miR-145 inhibits breast cancer cell growth
through RTKN. Int J Oncol. 34:1461–1466. 2009.PubMed/NCBI
|
|
24
|
Sevli S, Uzumcu A, Solak M, Ittmann M and
Ozen M: The function of microRNAs, small but potent molecules, in
human prostate cancer. Prostate Cancer Prostatic Dis. 13:208–217.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malumbres M and Barbacid M: Mammalian
cyclin-dependent kinases. Trends Biochem Sci. 30:630–641. 2005.
View Article : Google Scholar : PubMed/NCBI
|