Introduction
Diabetes mellitus is a chronic disease characterized
by the impairment of islet β-cell function, and dysfunctional
glucose and lipid metabolism. Approximately 10% of the adults in
China suffer from diabetes (1) and
the incidence rate is rapidly increasing. Patients with diabetes
mellitus suffer from macrovascular and microvascular complications
(2). The currently available
antidiabetic therapeutic agents exert significant hypoglycemic
effects, however, many of these therapeutic agents require further
development for an improved therapeutic outcome (3). The majority of hypoglycemic
therapeutic agents do not rehabilitate islet β-cell function
(4). For example, metformin (MF)
is one of the therapeutic agents prescribed first to improve
glucose metabolism; however, few studies have demonstrated
improvements in islet cell function (5). Dipeptidyl peptidase-4 inhibitors and
glucagon-like peptide-1 receptor agonists appear to have potential
effects on the restoration of islet cells, however, are
occasionally associated with severe side effects (6). Therapeutic agents that improve
extrapancreatic glucose metabolism and rehabilitate islet cell
function may provide a cure for type 1 or 2 diabetes.
Traditional Chinese medicines (TCMs) are a
significant alternative treatment modality, which may be
administered to prevent and treat diabetes (7). These medicinal plants have comparable
effects to common antidiabetic therapeutic agents and are consumed
at a lower cost. Astragalus membranaceus has been documented
in China as a tonic herb in TCM for thousands of years (8), and ~80% of the TCM formulas supplied
by the State Food and Drug Administration in China describe this
plant as an antidiabetic therapeutic agent (7). Fructus Crataegi Pinnatifidae
(hawthorn) is traditionally used to aid digestion and this plant is
prescribed in 50% of TCM formulas for the treatment of
hyperlipidemia (9). In addition,
hawthorn administration has antidiabetic effects (10). However, Astragalus or
hawthorn were frequently used, as crude or low-quality extracts, in
combination with other herbs to treat diabetes, which limited its
further application in patients with diabetes as a result of poor
quality controls and variable effects.
Combining two or more active components from TCMs,
rather than using crude or low-quality herb extracts, to create a
novel formula has become a popular method for developing
therapeutic agents to treat diseases (11). Astragalus polysaccharides
(APS) and Crataegus flavonoids (CF) are typical active
components of Astragalus membranaceus and Fructus
Crataegi Pinnatifidae, respectively. Furthermore,
polysaccharides and flavonoids are the two primary natural
components that exert potential antidiabetic effects. Combining the
different natural components may reduce the respective adverse
effects and enhance the common antidiabetic effects.
In China, Astragalus membranaceus and
Fructus Crataegi Pinnatifidae are the most frequently
administered herbs to treat diabetes and hyperlipidemia,
respectively; therefore the present study hypothesized that APS
combined with CF may have a synergic antidiabetic effect. The
current study demonstrated that APS + CF (AC), rather than the
combination of crude or low-quality extracts, yielded a
significant, synergic antidiabetic effect by restoring β-islet cell
function and enhancing the metabolism of liver tissues in
streptozotocin (STZ)-induced type 1 diabetic mice.
Materials and methods
Animals
Male NIH Swiss outbred mice (n=80, 18±2 g, 4 weeks
old) were purchased from the Guangdong Medical Laboratory Animal
Center (Foshan, China). The animals were kept in an environmentally
controlled breeding room (temperature, 20±2°C; humidity, 60±5%;
12-h light/dark cycle). The mice were fed with standard laboratory
chow and water ad libitum. The study was performed in strict
accordance with the recommendations of the Guide for the Care and
Use of Laboratory Animals by the Institutional Animal Care and Use
Committee of Tsinghua University (Beijing, China). The protocol was
approved by the Animal Welfare and Ethics Committee of Tsinghua
University. All animals were fasted from 09:00 to 15:00 prior to
starting the trials.
Therapeutic agents
The flavonoids (30%, g/g) from the CF fruits and
polysaccharides (50%, g/g) from the APS roots were purchased from
Nanjing Zelang Medical Technology Co., Ltd. (Nanjing, China). The
therapeutic agents were weighed for trials according to the net
weights of flavonoids and polysaccharides. APS and CF were combined
at the net weight ratio of 1 g polysaccharide to 1 g flavonoids,
forming a novel formula designated as AC: 1 g ÷ 30% = 3.33 g of CF
extracts for net CF flavonoids; 1 g ÷ 50% = 2 g of APS extracts for
net APS polysaccharides. In addition, MF was purchased from
Sigma-Aldrich (St. Louis, MO, USA). The therapeutic agents were
freshly suspended in distilled water prior to administration.
Experimental procedures
Male NIH Swiss outbred mice were fasted for 24 h
(weight, 18±2 g following fasting) and diabetes was induced by
intraperitoneal (i.p.) injection of 100 mg/kg STZ (Sigma-Aldrich).
STZ was freshly prepared in 0.1 M of ice-cold citric acid buffer
(pH 4.5; Tianjin Baishi Chemical Industry Co., Ltd., Tianjin,
China). One week after the STZ injection, the diabetic mice models
with fasting blood glucose >11.1 mmol/l were selected for
further trials. The selected mice were divided into six groups
(n=10) as follows: Normal and diabetic controls, and diabetic mice
treated with 200 mg/kg/day of APS, CF, AC and MF. Normal mice
received no treatment and were fed with the identical volume of
vehicles as diabetic controls. The therapeutic agents were freshly
prepared and orally administrated twice a day at 0.3 ml/mouse.
Normal and diabetic control mice were treated with the identical
volume of distilled water. Food and water intake were periodically
measured in metabolic cages within 24 h and blood glucose was
assayed once a week. Subsequent to 4 weeks of therapeutic agent
administration, mice were fasted for 6 h, anesthetized by i.p.
injection with 10% (g/ml) urethane (Sigma-Aldrich) in
phosphate-buffered saline (PBS) at a dosage of 0.1 ml/10 g body
weight and blood samples (~0.5 ml) were collected for further
biochemical assays. Subsequent to the mice sacrifice by cervical
dislocation, liver and pancreatic tissues were removed and stored
at −80°C, or soaked in 10% formalin solution (Tianjin Baishi
Chemical Industry Co., Ltd.) for further biochemical and
histopathological assays.
Oral glucose tolerance test (OGTT)
The OGTT was conducted as previously described with
slight modifications (12).
Following therapeutic agent administration for 4 weeks, the mice
(n=6) were fasted for 6 h. Blood samples (20–50 ul) were collected
from the tail veins to determine the blood glucose levels at 0,
0.5, 1 and 2 h following 2.5 g/kg glucose (Yongda Chemical Reagent
Development Center, Tianjin, China) administration. The blood
glucose concentration underwent temporal analysis, and the area
under the curve (AUC) of blood glucose and time was calculated as
follows: AUC0−2h = [(G0h +
G0.5h)×0.5+(G0.5h +
G1h)×0.5+(G1h + G2h)×1]/2, where G
is the blood glucose value.
Hematoxylin and eosin (H&E)
staining
Five mice were randomly selected from each group.
The pancreatic tissue mass (~2-mm diameter) of each mouse was fixed
with 10% formaldehyde (v/v; Tianjin Baishi Chemical Industry Co.,
Ltd.) at room temperature for 2 weeks, and processed for routine
paraffin-wax histology and staining with H&E by Dr Yunan Zhao
at the Nanjing University of Traditional Chinese Medicine (Nanjing,
China). Five continuous slices (10 μm thickness; distance
between each slice, 20 μm) were obtained to measure the
morphology of Langerhans islets in the pancreatic tissue of mice
using the ECLIPSE TE2000-E inverted microscope (Nikon Corporation,
Tokyo, Japan).
Western blotting
Pancreatic and liver tissues from five randomly
selected mice from each group were homogenized and lysed with a
mixture of 100 mM NaCl, 20 mM Tris-HCl, 0.5 mM EDTA (Sangon Biotech
Co., Ltd., Shanghai, China) and 0.5% (v/v) Nonidet P-40 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The lysates were centrifuged
at 10,000 × g at 4°C for 10 min. Supernatants were collected and
the protein concentration was determined using the bicinchoninic
acid assay kit (Nanjing Jiancheng Biotech Institute Co., Ltd.,
Nanjing, China). Western blot analysis was performed according to
the manufacturer's protocol. Antibodies against rabbit polyclonal
pancreatic and duodenal homeobox-1 (PDX-1; 1:500 to 1:1,000; Abcam,
Cambridge, UK; cat. no. ab47267), rabbit monoclonal adenosine
5′-monophosphate (AMP)-activated protein kinase (AMPK) and
phosphorylated AMPK (pAMPK; 1:500 to 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA; cat. nos. 5831 and 4188,
respectively), and mouse monoclonal β-actin (1:500 to 1:1,000;
Santa Cruz Biotechnology, Inc.; cat. no. sc-47778) were used for
overnight incubation at 4°C for protein targeting. Protein
expression was visualized with horseradish peroxidase-conjugated
goat anti-rabbit (1:2,000; Amersham Biosciences; GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA; cat. no. RPN4301) and goat
anti-mouse (1:5,000; Abcam; cat. no. ab97040) secondary antibodies,
and the enhanced LumiGLO chemiluminescent substrate kit (KPL, Inc.,
Gaithersburg, MD, USA). The protein expression of β-actin was used
as the reference expression for normalization and data were
analyzed using the ImageJ software, version 1.48 (National
Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Five mice were randomly selected from each group.
Total RNAs of pancreatic islet tissues (~50 mg) were extracted
using RNAiso Plus reagent (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's instructions. RT was
performed using the PrimeScript Reagent kit (Takara; cat. no.
DRR037A) according to the manufacturer's instructions. The RT
reaction solution (10 μl total) was consisted of 2 μl
5X PrimeScript Buffer, 0.5 μl PrimeScript RT Enzyme Mix I,
0.5 μl Oligo dT Primer (50 μM), 0.5 μl Random
6 mers (100 μM), 1 μg total RNA and RNase free water.
The procedure was performed with the Alpha Unit Block Assembly for
DNA Engine systems (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with a thermocycler program comprising steps at 42°C for 30 min,
85°C for 5 min, and a hold step at 4°C. The genes were selected for
RT-qPCR and the primers were synthesized by Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA; Table I). β-actin served as the internal
control for normalization. qPCR was conducted using the SYBR Green
I real-time PCR Master Mix according to the manufacturer's protocol
(Toyobo Co, Ltd., Osaka, Japan; cat. no. QPT-201) and using the
Applied Biosystems 7300 real-time PCR System (Thermo Fisher
Scientific, Inc.). The qPCR reaction solution (20 μl total)
was consisted of 6.4 μl distilled water, 10 μl SYBR
Green Realtime PCR Master Mix, 0.8 μl forward primer (10
μM), 0.8 μl reverse primer (10 μM) and 2
μl cDNA. The procedure involved pre-denaturation of cDNA
samples at 95°C for 60 sec (first stage) and the amplification of
the denatured cDNA samples with 40 cycles at 95°C for 15 sec, at
60°C for 15 sec and at 72°C for 45 sec (second stage). Data were
normalized using the β-actin gene and fold changes were calculated
using the 2−ΔΔCq normalization method (13).
 | Table IPrimer sequences for the analyzed
genes. |
Table I
Primer sequences for the analyzed
genes.
| Gene | NCBI accession
number | Primers
(5′→3′) | Size (bp) |
|---|
| Ins-1 | NM_008386 | F:
CACTTCCTACCCCTGCTGG
R: ACCACAAAGATGCTGTTTGACA | 81 |
| Ins-2 | NP_001172013.1 | F:
GCTTCTTCTACACACCCATGTC
R: AGCACTGATCTACAATGCCAC | 147 |
| Neurog3 | NM_009719.6 | F:
GAAGCAGAAGTGGGTGAC
R: TGGGGAGTAGATAGAGCC | 384 |
| Mafa | NM_194350.1 | F:
CAGCACCACCTGAACCCC
R: GGATGACCTCCTCCTTGC | 418 |
| IL-6 | NM_031168 | F:
CTGCAAGAGACTTCCATCCAG
R: AGTGGTATAGACAGGTCTGTTGG | 131 |
| TNF-α | NM_013693.2 | F:
GGGCTTCCAGAACTCCA
R: GCTACAGGCTTGTCACTCG | 213 |
| CCL2 | NM_011333 | F:
TTAAAAACCTGGATCGGAACCAA
R: GCATTAGCTTCAGATTTACGGGT | 121 |
| β-actin | NM_007393 | F:
GTGACGTTGACATCCGTAAAGA
R: GCCGGACTCATCGTACTCC | 245 |
Statistical analysis
Data are expressed as the mean ± standard deviation.
The comparison of the mean values for the groups was performed
using one-way analysis of variance. Newman-Keuls comparisons were
used to determine the source of significant differences and Pearson
correlation analysis was conducted using SPSS software (version
12.0; SPSS, Inc., Chicago, IL, USA) when appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight, and food and water
intake
The diabetic syndromes in the STZ-induced mice were
investigated. The body weights of diabetic mice were significantly
reduced at 0 (P<0.01), 17 and 24 days (P<0.05) compared with
the normal control (Fig. 1A).
AC-treated mice exhibited significantly increased body weights at
10 days, and APS- and CF-treated mice demonstrated significantly
reduced body weights at 21 and 24 days, and 24 and 28 days,
respectively, compared with the diabetic controls (Fig. 1A; P<0.05). AC and CF treatment
was not able to effectively reverse the decrease of body weights in
this diabetic model, as AC and CF likely exert a similar
body-lowering effect as MF treatment.
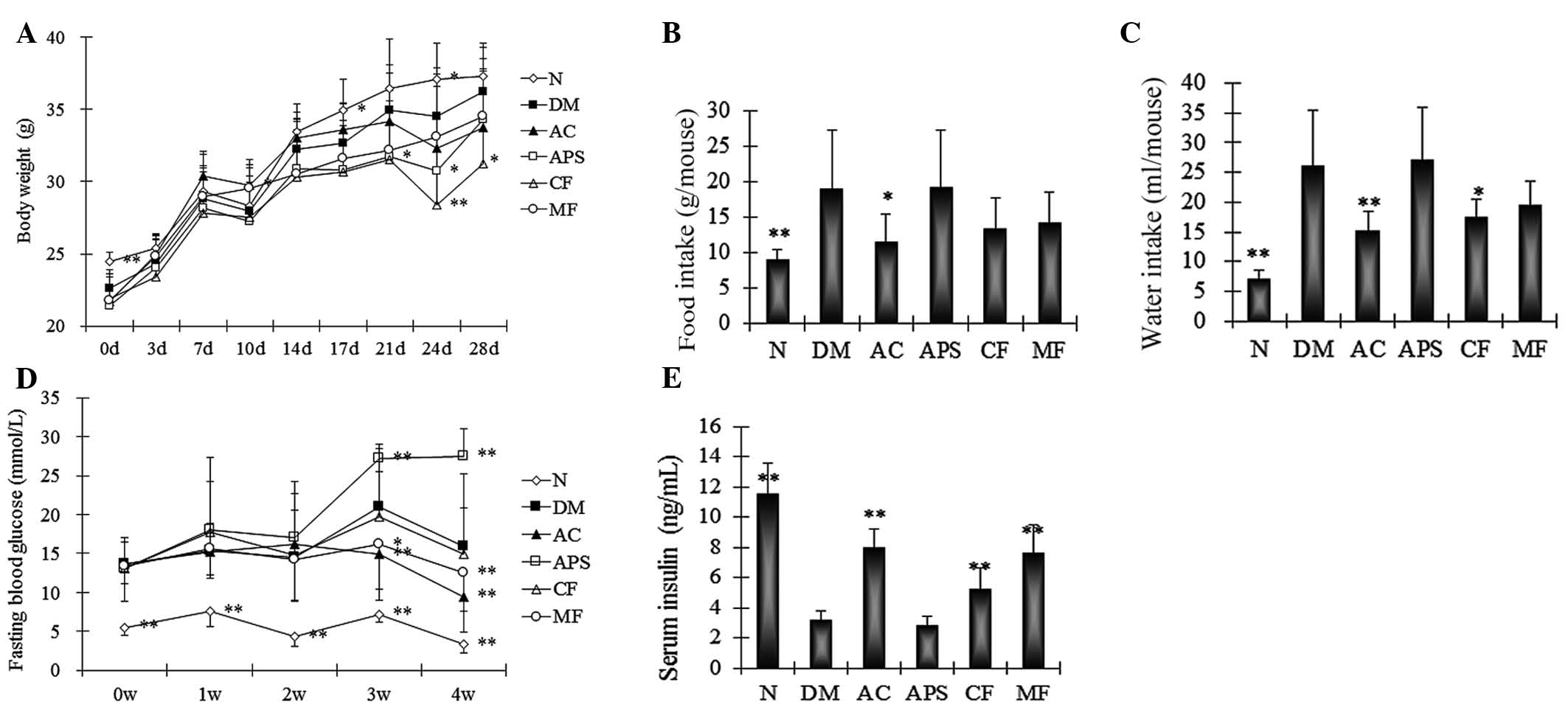 | Figure 1Changes in the (A) body weight, (B)
diet, (C) water intake, (D) fasting blood glucose and (E) serum
insulin levels of the mice following 4 weeks of treatment. Data are
expressed as the mean ± standard deviation (n=10).
*P<0.05, **P<0.01 vs. the DM group. N,
normal control; DM, diabetic control; AC, APS + CF-treated; APS,
Astragalus polysaccharides; CF, Crataegus flavonoids;
MF, metformin. |
Furthermore, the diabetic mice demonstrated an
increase in food intake after 7 days when compared with the normal
controls (Fig. 1B). In addition,
the AC-, CF- and MF-treated mice attenuated the increase in food
intake following 7 days of treatment (Fig. 1B). The APS-treated mice did not
demonstrate a significant change in food intake compared with the
diabetic control (Fig. 1B;
P>0.05). When comparing the normal control and diabetic mice a
significant increase in water intake was demonstrated during the
period 4 weeks of observation (Fig.
1C). The AC- and CF-treated mice significantly attenuated the
increase in the water intake of diabetic mice following 10 days of
treatment compared with the diabetic controls (Fig. 1C; P<0.01 and P<0.05,
respectively). The MF- and APS-treated mice did not demonstrate
significant changes in the water intake.
Fasting blood glucose and serum
insulin
The diabetic mice demonstrated a significant
increase of fasting blood glucose compared with the normal control
(Fig. 1D; P<0.05). AC- and
MF-treated mice demonstrated a significant decrease in fasting
blood glucose compared with the diabetic controls at 3 and 4 weeks
of treatment (Fig. 1D; P<0.05
and P<0.01, respectively). The APS-treated mice demonstrated a
significant increase compared with the diabetic controls at 3 and 4
weeks of treatment (Fig. 1D;
P<0.01). No significant changes were observed in the CF-treated
mice following 2 weeks of treatment (Fig. 1D; P>0.05).
In addition, the serum insulin levels were
determined. The diabetic mice demonstrated a significant decrease
in the serum insulin levels compared with the normal controls
(Fig. 1E; P<0.01). Compared
with the diabetic control, the AC-, CF- and MF-treated mice
demonstrated significant increases in serum insulin levels
(Fig. 1E; P<0.01). No
significant difference was observed between the insulin levels of
APS-treated mice those of the diabetic control mice (Fig. 1E; P>0.05).
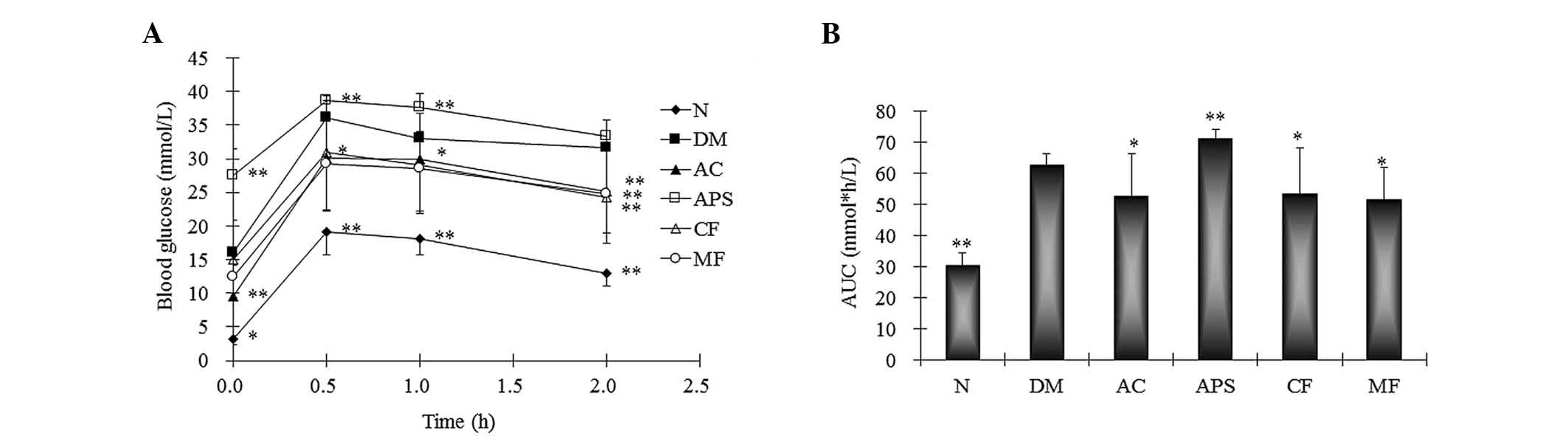
OGTT
The OGTT effectively mimics the islet function and
glucose metabolism. Following the oral administration of glucose,
the diabetic mice demonstrated a significant increase in the AUC
for blood glucose when compared with the normal controls (Fig. 2; P<0.01). However, this increase
was attenuated by AC, CF and MF treatment (Fig. 2; P<0.05 vs. the diabetic
control). The APS-treated group demonstrated a significant increase
in the AUC compared with the diabetic control following the oral
administration of glucose in the diabetic mice (Fig. 2; P<0.01). Taken together with
the above results, the diabetic mice demonstrated the specific
syndromes of type 1 diabetes, however AC treatment attenuated these
syndromes and exerted synergic antidiabetic effects.
H&E staining
The primary function of the islet β-cells is to
store and release insulin, to regulate glucose metabolism.
Therefore, following treatment, the pathological changes of islet
β-cells were assessed using H&E staining. In the current study,
STZ induced islet β-cells toxicity in the diabetic mice
contributing to islet cell DNA damage compared with the normal
control mice (Fig. 3A). Diabetic
mice demonstrated a significant decrease in the size of islets of
Langerhans. AC-, CF- and MF-treated mice demonstrated protective
effects on the islet β-cells when compared with the diabetic
control (Fig. 3A). However, no
marked effect was demonstrated on the islet cells in APS-treated
mice. Although Hawthorn demonstrated glucose-lowering effects in
high-fat, diabetic mice in a previous study (10), no effects of restoring islet
function were recorded. Therefore, the present results are the
first, to the best of our knowledge, to demonstrate that CF induced
the protective effects on islet cells.
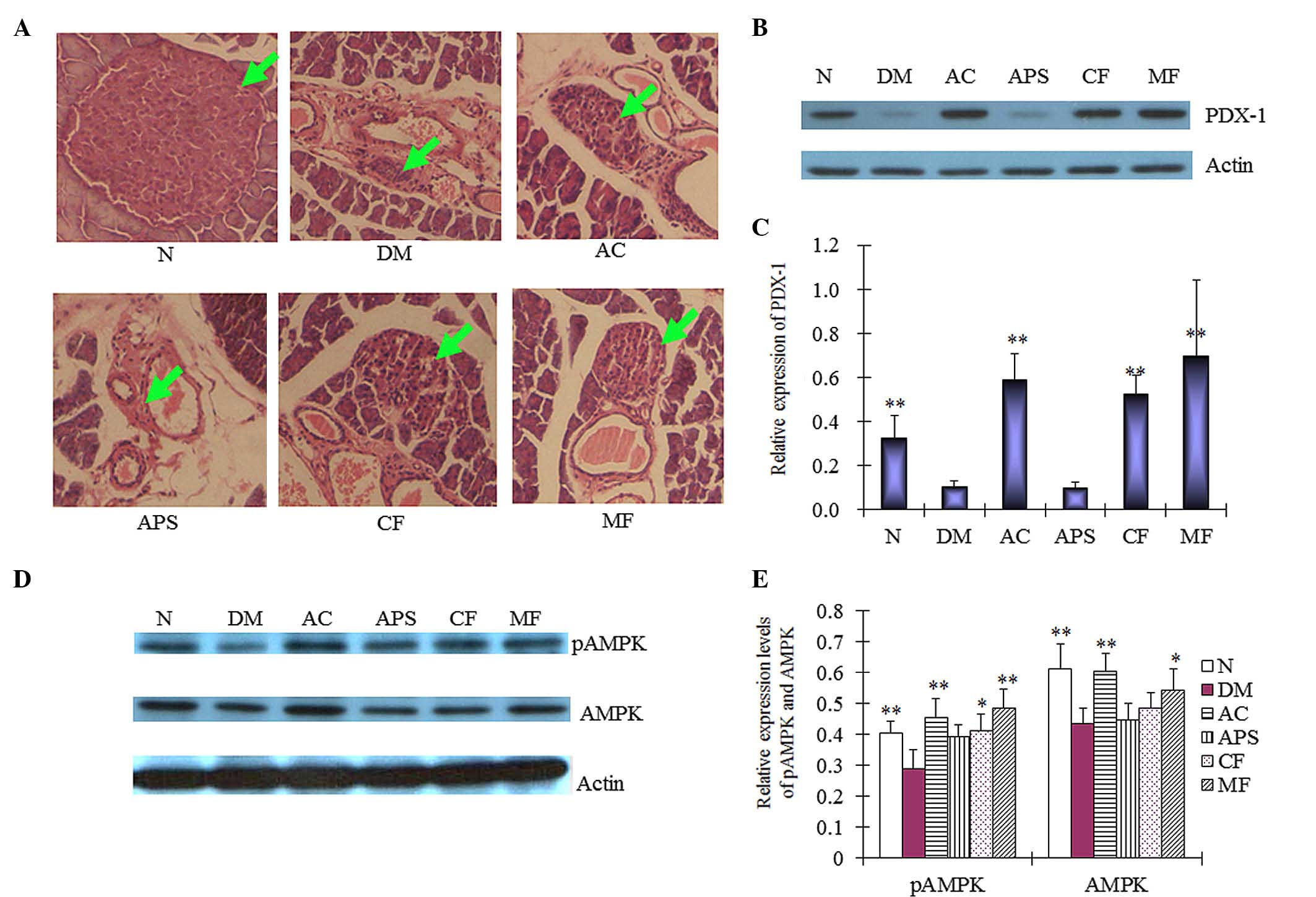 | Figure 3(A) Pathological changes (green arrows
represent the islets of Langerhans) and (B and C) expression levels
of PDX-1 in the islet cells of pancreatic tissue samples of mice
following 4 weeks of treatment. (D and E) Expression levels of AMPK
and pAMPK in mice liver tissue as determined by western blotting
following 4 weeks of treatment. Data are expressed as the mean ±
standard deviation (n=6). *P<0.05,
**P<0.01 vs. the DM group. N, normal control; DM,
diabetic control; AC, APS + CF-treated; APS, Astragalus
polysaccharides; CF, Crataegus flavonoids; MF, metformin;
PDX-1, pancreatic and duodenal homeobox-1; AMPK, adenosine
5′-monophosphate-activated protein kinase; p, phosphorylated. |
Protein expression
In addition, the molecular mechanisms of the
protective effects of AC on islet cells were determined at the
protein level. PDX-1 in involved in pancreatic development, and
β-cell function and survival (14). In the present study, the diabetic
mice demonstrated significantly decreased PDX-1 protein expression
levels in the pancreatic tissue when compared with the normal
control (Fig. 3B and C;
P<0.01). The AC-, CF- and MF-treated mice demonstrated
significant increases in the PDX-1 protein expression levels
compared with the diabetic control mice (Fig. 3B and C; P<0.01). However, no
significant difference was observed between the protein expression
levels of PDX-1 in the APS-treated mice and the diabetic control
mice (Fig. 3B and C; P>0.05).
These results indicated that the islet-restoring effect of AC may
be mediated by upregulating PDX-1 expression.
In addition to the pancreatic islet cells, liver
tissue cells are important in the regulation of glucose metabolism.
pAMPK is an active form of AMPK that is responsible for enhancing
glucose metabolism (15). In the
present study, the expression level of pAMPK was significantly
increased in the liver tissue of the AC-treated mice compared with
the diabetic controls (Fig. 3D and
E; P<0.01), a comparable increase was observed in the
MF-treated mice (P<0.01). Furthermore, administration of CF
demonstrated an increase in the pAMPK protein expression levels in
the liver tissue of the diabetic mice (P<0.05). APS treatment,
however, did not demonstrate a significant effect when compared
with the diabetic control (Fig. 3D and
E). The results indicate that the antidiabetic effects of AC
may be mediated by the expression of pAMPK.
mRNA expression levels
The gene expression of islet function- and
inflammation-associated mRNAs in the pancreatic tissue of the mice
was assessed using RT-qPCR. AC- and CF-treated mice demonstrated a
significant increase in the mRNA expression levels of Neurog3,
Mafa, Ins-1 and Ins-2 in the pancreatic tissue compared with the
diabetic control mice (Fig. 4A;
P<0.01). APS treatment demonstrated an increase in the mRNA
expression levels of Neurog3 and Mafa when compared with the
diabetic control, however had less of an effect when compared with
the AC- and CF-treated mice (Fig.
4A). Furthermore, administration of MF demonstrated an increase
in the Mafa, Ins-1 and Ins-2 mRNA expression levels compared with
the diabetic control (Fig. 4A).
Neurog3 and Mafa are responsible for islet development (16), and Ins-1 and Ins-2 promote the
synthesis of proinsulin. These results indicated that the
restorative effects of AC on islet cells are mediated by
upregulating the expression levels of islet function-associated
genes.
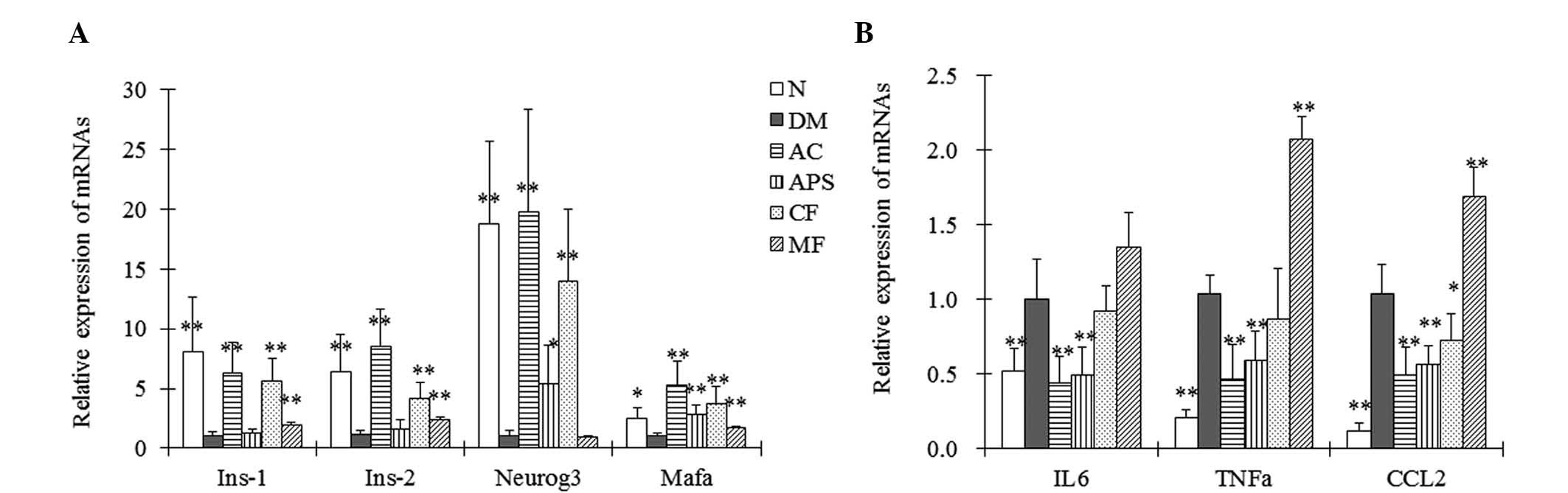 | Figure 4Relative mRNA expression levels of (A)
Ins-1, Ins-2, Neurog3, Mafa and (B) IL-6, TNF-α and CCL2 in the
pancreatic tissues of the mice assayed via reverse
transcription-quantitative polymerase chain reaction following oral
administration of the therapeutic agents for 4 weeks. Data are
expressed as the mean ± standard deviation (n=5).
*P<0.05, **P<0.01 vs. the DM group. N,
normal control; DM, diabetic control; AC, APS + CF-treated; APS,
Astragalus polysaccharides; CF, Crataegus flavonoids;
MF, metformin; Ins-1, insulin 1; neurog3, neurogenin 3; mafa, v-maf
musculoaponeurotic fibrosarcoma oncogene family, protein A; IL-6,
interleukin 6; TNF-α; tumor necrosis factor-α; CCL2, chemokine (C-C
motif) ligand 2. |
Furthermore, the diabetic mice demonstrated
significant increases in interleukin-6 (IL-6), tumor necrosis
factor-α (TNF-α) and chemokine (C-C motif) ligand 2 (CCL2) mRNA
expression levels in the pancreatic tissues when compared with the
normal control (Fig. 4B;
P<0.01). However, administration of AC and APS significantly
reduced the expression levels of IL-6, TNF-α and CCL2 in the
pancreatic tissues of the diabetic mice compared with those of the
diabetic control mice (Fig. 4B;
P<0.01). Furthermore, CF treatment significantly reduced the
mRNA expression levels of CCL2 compared with the diabetic control
(Fig. 4B; P<0.01). However, MF
treatment significantly increased the mRNA expression levels of
TNF-α and CCL2 compared with the diabetic control. These results
indicated that the restorative effects of AC on islet cells involve
the attenuation of inflammatory stress.
Discussion
Astragalus and Hawthorn extracts in Chinese
formulas are considered to have poor quality controls and variable
clinical therapeutic effects. The present study selected refined
active components to form a stable formula to guarantee a good
quality control and stable therapeutic effects. In the current
study, the combination treatment (AC) comprising APS and CF
demonstrated synergic antidiabetic effects. Astragalus
membranaceus and Hawthorn are the most frequently used
traditional Chinese formulas to treat diabetes and hyperlipidemia,
respectively (7,9). However, in the current study
Astragalus membranaceus or Hawthorn alone (using the
typically prescribed active components) did not demonstrate the
required antidiabetic effect. This indicates that active components
from a single herb or other natural product may not be effective
treatments; however, combining active components from various herbs
or other natural sources may be a more promising strategy. Diabetes
is a disease with complicated dysfunctions and requires combined
therapeutic strategies. Therefore, it is hypothesized that an
appropriate combination of different active components from natural
products may aid in the prevention and treatment of diabetes.
In the current study, the antidiabetic effects and
mechanisms of action of the combination therapy, APS + CF were
investigated. The results demonstrated that AC had significant
antidiabetic effects and it is proposed that the antidiabetic
mechanisms of AC involve the restoration of pancreatic islet
function and the improvement of glucose metabolism in the liver.
PDX-1 serves an important role in pancreatic β-cell survival. The
restoration effects of AC on β-islet cells may be mediated by the
regulated expression of PDX-1. In addition, previous studies
demonstrated that Hawthorn extracts and APS significantly increased
AMPK phosphorylation (10,17). However, in the present study, APS
did not demonstrate a significant effect, which may have been due
to variances in the different methods of extraction or dosages
administered. In addition, AC significantly increased the
expression level of AMPK in the samples of liver tissue from
diabetic mice compared with the diabetic control mice. Thus, the AC
combination treatment appeared to increase AMPK expression and
promote phosphorylation of AMPK. Overall, this combination yielded
synergic antidiabetic effects, however, the potential molecular
mechanisms require further investigation.
Diabetes is an inflammatory disease (18). In the current study, AC and APS
significantly decreased the mRNA expression levels of inflammatory
factors, including IL-6, TNF-α and CCL2 in the pancreatic tissues.
CF treatment markedly decreased the expression level of CCL2,
however, MF treatment increased the expression levels of IL-6,
TNF-α and CCL2. A previous study demonstrated the direct inhibition
of APS on the lipopolysaccharide-induced inflammatory responses in
monocytes or macrophages (19). In
the present study, however, APS did not protect the β-cells as
previously reported (20). This
result may be due to the fact that a lower dose of APS could not
control the fasting blood glucose, thus, the islet β-cells were
subjected to long-term, severe glucotoxicity. However, the present
study proposes that the antidiabetic effects of AC are mediated by
its anti-inflammatory effects.
The administration of CF demonstrated no significant
effects on the inhibition of the expression of inflammatory factors
IL-6 and TNF-α in the present study, although the flavonoids from
Hawthorn leaves exerted anti-inflammatory effects in a previous
study (21). Flavonoids extracted
from different sources or analyzed at different dosages may have
variable components and effects. In the current study, CF appeared
to upregulate the expression levels of PDX-1, Mafa, Neurog3, Ins-1
and Ins-2. A previous study demonstrated that the combination of
PDX-1, Mafa and Neurog3 markedly induced insulin biosynthesis in
various non-β-cells (22). The
upregulation of Ins-1 or Ins-2 may be triggered by the increased
expression levels of Mafa, PDX-1 and Neurog3. Therefore, CF may
restore the islet cell function by regulating the expression of the
above-mentioned factors. Thus, the anti-inflammatory activities of
APS combined with the islet-restoring activities of CF contributed
to improved antidiabetic effects of AC treatment, when compared
with APS or CF treatment alone.
In conclusion, to the best of our knowledge, this is
the first study to suggest that combining APS and CF may be a
promising treatment for diabetes, as demonstrated by the synergic
antidiabetic effects. This combination may facilitate the
rehabilitation of islet cell function by upregulating PDX-1
expression and promoting the metabolism of liver glucose via the
promotion of AMPK phosphorylation (Fig. 5). These effects of AC may be useful
for the treatment of type 1 or 2 diabetes, as the patients are
frequently subjected to impaired islet cell function and disordered
extrapancreatic metabolism. In addition, the anti-inflammatory
activity of APS and the islet-restoring effects of CF contributed
to the synergic effects of AC on protecting islet function. Further
investigations are required to establish the potential molecular
mechanisms or compounds that contribute to this effect.
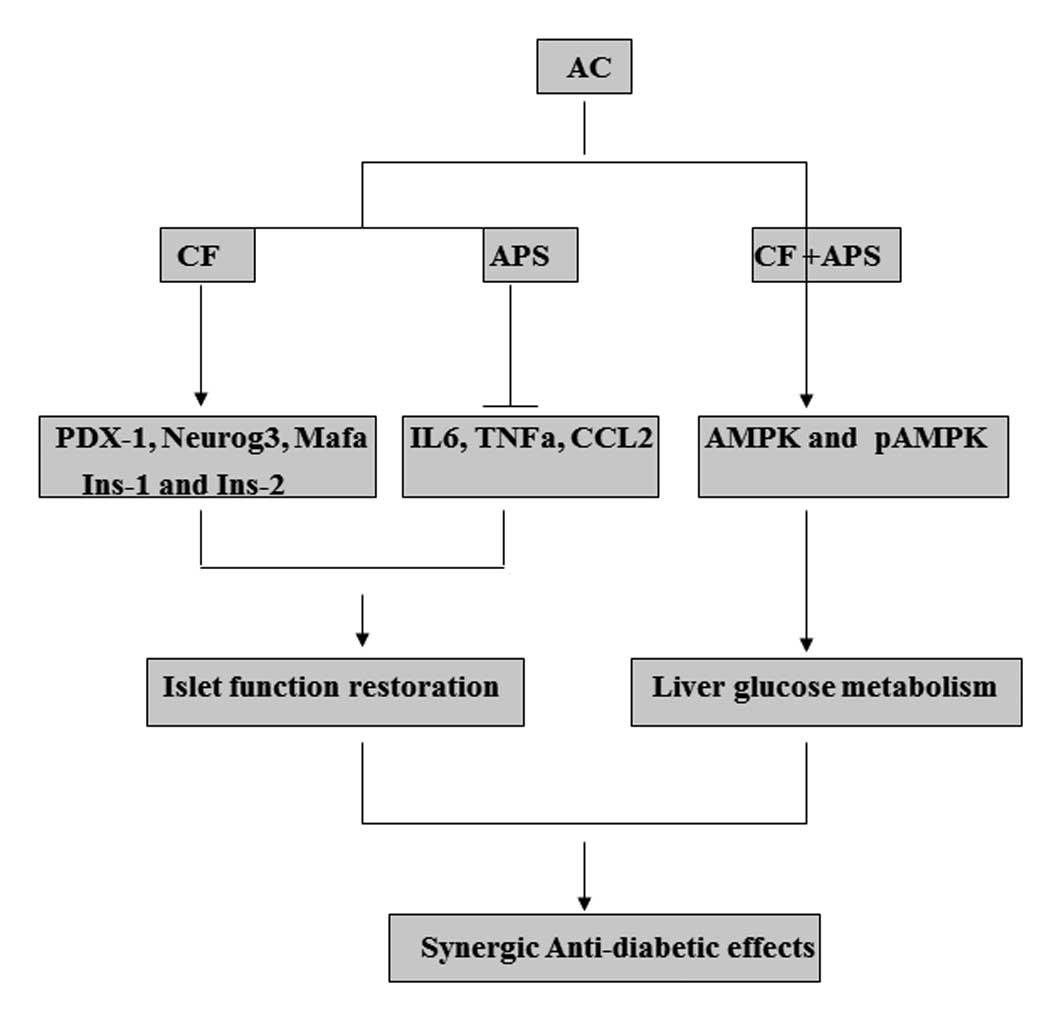 | Figure 5Synergic antidiabetic effects and
potential mechanisms of action of APS and CF in the treatment of
diabetes. AC, APS + CF-treated; APS, Astragalus
polysaccharides; CF, Crataegus flavonoids; PDX-1, pancreatic
and duodenal homeobox-1; neurog3, neurogenin 3; mafa, v-maf
musculoaponeurotic fibrosarcoma oncogene family, protein A;
Ins-1/2, insulin 1/2; IL-6, interleukin 6; TNF-α; tumor necrosis
factor-α; CCL2, chemokine (C-C motif) ligand 2; AMPK, adenosine
5′-monophosphate-activated protein kinase; p, phosphorylated. |
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81373460), the
Natural Science Foundation of Guangdong Province (grant no.
2014A030313744), the Shenzhen Science and Technology R&D
Foundation (grant no. SGLH20121008144756945) and the China
Scholarship Council (grant no. 201308440130).
Abbreviations:
|
AC
|
Astragalus polysaccharides
combined with Crataegus flavonoids
|
|
AMPK
|
adenosine 5′-monophosphate-activated
protein kinase
|
|
APS
|
Astragalus polysaccharides
|
|
AUC
|
area under the curve
|
|
CCL2
|
chemokine (C-C motif) ligand 2
|
|
CF
|
Crataegus flavonoids
|
|
IL-6
|
interleukin 6
|
|
PDX-1
|
pancreatic and duodenal homeobox-1
|
|
pAMPK
|
phosphorylated AMPK
|
|
OGTT
|
oral glucose tolerance test
|
|
STZ
|
streptozotocin
|
|
TCM
|
traditional Chinese medicine
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: China National Diabetes and
Metabolic Disorders Study Group: Prevalence of diabetes among men
and women in China. N Engl J Med. 362:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konig M, Lamos EM, Stein SA and Davis SN:
An insight into the recent diabetes trials: What is the best
approach to prevent macrovascular and microvascular complications?
Curr Diabetes Rev. 9:371–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gajos G, Piłaciński S and
Zozulińska-Ziółkiewicz D: Controversies in diabetes in 2013 - a
brief update. Adv Clin Exp Med. 22:777–784. 2013.
|
|
4
|
Vaz JA and Patnaik A: Diabetes mellitus:
Exploring the challenges in the drug development process. Perspect
Clin Res. 3:109–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Diabetes Association: Approaches
to glycemic treatment. Diabetes Care. 38(Suppl 1): S41–48. 2015.
View Article : Google Scholar
|
|
6
|
Sun Y, Fan L, Meng J, Zhang F, Zhang D and
Mei Q: Should GLP-1 receptor agonists be used with caution in high
risk population for colorectal cancer? Med Hypotheses. 82:255–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie W, Zhao Y and Zhang Y: Traditional
Chinese medicines in treatment of patients with type 2 diabetes
mellitus. Evid Based Complement Alternat Med. 2011:7267232011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agyemang K, Han L, Liu E, Zhang Y, Wang T
and Gao X: Recent advances in Astragalus membranaceus antidiabetic
research: Pharmacological effects of its phytochemical
constituents. Evid Based Complement Alternat Med. 2013:6546432013.
View Article : Google Scholar
|
|
9
|
Xie W, Zhao Y and Du L: Emerging
approaches of traditional Chinese medicine formulas for the
treatment of hyperlipidemia. J Ethnopharmacol. 140:345–367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shih CC, Lin CH, Lin YJ and Wu JB:
Validation of the antidiabetic and hypolipidemic effects of
Hawthorn by assessment of gluconeogenesis and lipogenesis related
genes and AMP-activated protein kinase phosphorylation. Evid Based
Complement Alternat Med. 2013:5970672013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Wang YY and Yang G: Methods and
modes about the theory of traditional Chinese prescription
composition. Zhongguo Zhong Yao Za Zhi. 30:6–8. 112005.In
Chinese.
|
|
12
|
Xie W, Zhao Y, Gu D, Du L, Cai G and Zhang
Y: Scorpion in Combination with Gypsum: Novel Antidiabetic
Activities in Streptozotocin-Induced Diabetic Mice by Up-Regulating
Pancreatic PPARγ and PDX-1 Expressions. Evid Based Complement
Alternat Med. 2011:6835612011. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Hayes HL, Moss LG, Schisler JC, Haldeman
JM, Zhang Z, Rosenberg PB, Newgard CB and Hohmeier HE: Pdx-1
activates islet α- and β-cell proliferation via a mechanism
regulated by transient receptor potential cation channels 3 and 6
and extracellular signal-regulated kinases 1 and 2. Mol Cell Biol.
33:4017–4029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Winder WW: AMP-activated protein kinase:
Possible target for treatment of type 2 diabetes. Diabetes Technol
Ther. 2:441–448. 2000. View Article : Google Scholar
|
|
16
|
Zaldumbide A, Carlotti F, Gonçalves MA,
Knaän-Shanzer S, Cramer SJ, Roep BO, Wiertz EJ and Hoeben RC:
Adenoviral vectors stimulate glucagon transcription in human
mesenchymal stem cells expressing pancreatic transcription factors.
PLoS One. 7:e480932012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou F, Mao XQ, Wang N, Liu J and Ou-Yang
JP: Astragalus polysaccharides alleviates glucose toxicity and
restores glucose homeostasis in diabetic states via activation of
AMPK. Acta Pharmacol Sin. 30:1607–1615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie W and Du L: Diabetes is an
inflammatory disease: Evidence from traditional Chinese medicines.
Diabetes Obes Metab. 13:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Chen X, Zhang Y, Xu J, Zhang L, Li
Z, Liu W, Ouyang J, Han S and He X: Astragalus polysaccharide
induces anti-inflammatory effects dependent on AMPK activity in
palmitate-treated RAW264.7 cells. Int J Mol Med. 31:1463–1470.
2013.PubMed/NCBI
|
|
20
|
Li RJ, Qiu SD, Chen HX, Tian H and Liu GQ:
Effect of Astragalus polysaccharide on pancreatic cell mass in type
1 diabetic mice. Zhongguo Zhong Yao Za Zhi. 32:2169–2173. 2007.In
Chinese.
|
|
21
|
Fu JH, Zheng YQ, Li P, Li XZ, Shang XH and
Liu JX: Hawthorn leaves flavonoids decreases inflammation related
to acute myocardial ischemia/reperfusion in anesthetized dogs. Chin
J Integr Med. 19:582–588. 2013. View Article : Google Scholar
|
|
22
|
Kaneto H, Matsuoka TA, Katakami N and
Matsuhisa M: Combination of MafA, PDX-1 and NeuroD is a useful tool
to efficiently induce insulin-producing surrogate beta-cells. Curr
Med Chem. 16:3144–3151. 2009. View Article : Google Scholar : PubMed/NCBI
|