Introduction
Chronic obstructive pulmonary disease (COPD) is
characterized by persistent and progressive airflow limitation,
which is associated with chronic airway inflammation and
parenchymal destruction (1). When
exposed to chronic irritants such as cigarette smoke (a common risk
factor of COPD) repeated injury and repair, and chronic
inflammatory response of the airway wall result in structural
changes termed airway remodeling (2,3).
Airway smooth muscle (ASM) is one of the important structures of
airway wall, and contributes to the chronic inflammatory response
and airway remodeling in COPD due to its proliferation and
synthetic capabilities (4).
Many cytokines, chemokines and growth factors
promote ASM cell (ASMC) proliferation (5) and exposure to cigarette smoke extract
(CSE) induces a proliferative phenotype of cultured ASMCs (6). In addition, ASMCs provide cytokines
in the airway by releasing eotaxin, chemokine (C-C motif) ligand 5,
monocyte chemoattractant proteins, interleukin-6 (IL-6) and IL-8
(7). Pro-inflammatory cytokines,
such as tumor necrosis factor-α (TNF-α), and CSE lead to increased
expression of various cytokines from ASM (8,9). The
release of IL-8, an important neutrophil chemoattractant, from
ASMCs and inflammatory cells is important in the pathogenesis of
COPD (10). However, the
underlying mechanisms of ASM proliferation and synthetic function
induced by cigarette smoke exposure have not been elucidated.
Although a wide variety of ion channels are
expressed in ASMCs to regulate its biological activities, the
precise function of the majority of channels requires
investigation. Transient receptor potential cation channel
subfamily M member 7 (TRPM7) is a non-selective ion channel
permeable to various divalent cations (such as Mg2+ and
Ca2+) with a distinctive serine/threonine protein kinase
domain in its C-terminal (11–13).
This channel is highly expressed in the brain, heart, liver, kidney
and lung of mammals (14,15) and it has been determined that TRPM7
is important for cellular survival and proliferation of numerous
cell types (16). Additionally, in
our previous study, it was identified that inhibition of TRPM7
reduced the release of cytokines in rat bone marrow-derived mast
cells (17). However, to the best
of our knowledge, the association between TRPM7 and ASM with
cigarette smoke exposure has not previously been investigated. The
aim of the present study was to detect the expression of TRPM7 in
ASMCs from rats exposed to cigarette smoke. Furthermore, the role
of TRPM7 in cell proliferation and IL-8 release was investigated in
the ASMCs.
Materials and methods
Animals
A total of 24 five- to six-week-old male
Sprague-Dawley (SD) rats (weight, 150–180 g) were obtained from the
Laboratory Animal Center of Sun Yat-sen University (Guangdong
Experimental Animal Testing, Guangzhou, China; certificate no.
0101668). The rats were housed in a temperature-controlled room
(26±1°C) and maintained on a 12-h light/dark cycle with free access
to water and food. All the experiments described were performed in
accordance with the regulations of the Centre of Animal Experiments
of Sun Yat-sen University. Ethical approval for this investigation
was obtained from the Research Ethics Committee, Sun Yat-sen
University (Guangzhou, China).
Cigarette smoke exposure
The rats were randomly divided into the
smoke-exposed group and control groups (n=12). The rats of the
smoke-exposed group were placed in a plastic chamber and exposed to
3R4F research cigarette smoke (University of Kentucky, Lexington,
KY, USA), one cigarette twice a day, with one day off every week,
for 90 days. The control rats were placed in another plastic
chamber and exposed to fresh air over the same time period. All
rats were sacrificed by cervical dislocation following
CO2 narcosis after the 90-day period for CSE (or fresh
air for the control group) was completed. CSE was prepared by
burning two 3R4F research cigarettes without a filter and passing
the smoke to a 50 ml conical tube containing 20 ml culture medium
(Guangzhou Whiga Technology Co., Ltd., Guangzhou, China) at a rate
of 5 min/cigarette. The obtained solution was filtered through a
0.22-μm filter and was referred to as 100% strength CSE.
Lung histology and immunohistochemical
staining
Lung tissue samples (5 μm) of right lobe was
removed from the rats. The tissue samples were fixed with 10%
neutral buffered formalin (Guangzhou Whiga Technology Co., Ltd.)
and the specimens were dehydrated (by incubating sequentially for 2
h in 75%, 85%, 95% and 100% alcohol, in turn) and embedded in
paraffin (Guangzhou Whiga Technology Co., Ltd.). Fixed embedded
tissue sections (size, 5 μm) were sliced, placed on glass
slides and deparaffinized. Subsequently, α-smooth muscle actin
immunohistochemical staining with monoclonal mouse anti-α-smooth
muscle actin antibody (1:100; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to determine smooth muscle cells in the
lung. The ASM areas were stained brown as a result of the α-smooth
muscle actin immunohistochemical staining. The ASM areas
(μm2) of eight independent bronchioles were
measured using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). The results are expressed
as a proportion of the total airway area [i.e. the lumen plus the
airway wall for the external area at the adventitial border
(μm2)].
Isolation and culture of ASMCs
ASMCs were obtained from the trachea and bronchi of
smoke-exposed and control SD rats as previously described (18,19).
Briefly, the trachea and main bronchi were removed and separated
from the lung, bronchial vessels and connective tissue, then
dissected into cubes (dimension, 1×1×1 mm). All segments were
cultured with Gibco Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc.) supplemented with 20% fetal calf
serum (FCS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin (Guangzhou Whiga Technology Co., Ltd., Guangzhou, China)
and 100 mg/ml streptomycin (Guangzhou Whiga Technology Co., Ltd.)
in conditions of 37°C and 5% CO2 for 5 days. Cells were
grown to 80% confluence (observed under an inverted phase contrast
microscope; Leica Microsystems Wetzlar GmbH, Wetzlar, Germany), the
medium was removed, the explants were washed twice with Gibco
Hank's Balanced Salt Solution (Thermo Fisher Scientific, Inc.) and
trypsinized (in 1 ml of a 0.25% trypsin solution; Guangzhou Whiga
Technology Co., Ltd.) for 1 min. Next, ASMCs were cultured in DMEM
medium supplemented with penicillin and streptomycin at the
above-mentioned concentrations and 10% FCS. The medium was replaced
every 2 days. ASMCs were identified by the characteristic
hill-and-valley appearance and α-smooth muscle actin
immunocytochemical staining. Experiments were performed on freshly
isolated ASMCs.
Immunocytochemistry
ASMCs were identified by α-smooth muscle actin
immunocytochemical staining, as previously described (18,19).
Briefly, ASMCs were seeded onto sterile glass coverslips and grown
to 70% confluence. Cells were fixed in 4% paraformaldehyde for 20
min, and the sections were reacted with 3%
H2O2 (Guangzhou Whiga Technology Co., Ltd.)
After a rinse in phosphate-buffered saline (PBS), the sections were
blocked with 2% bovine serum albumin (BSA; Guangzhou Whiga
Technology Co., Ltd.) and incubated with monoclonal mouse
anti-α-smooth muscle actin antibody (1:300; Thermo Fisher
Scientific, Inc.) at 4°C overnight for immunolabeling.
Subsequently, the sections were incubated with a secondary
horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG
(1:300; Cell Signaling Technology, Inc., Danvers, MA, USA) for 30
min at 37°C and then washed three times with PBS. The peroxidase
activity was visualized by a color reaction using
3,3′-diaminobenzidine (1 ml; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) as the substrate. The slides were then
counterstained with hematoxylin (Wuhan Boster Biological
Technology, Ltd.), mounted and examined under a microscope (Olympus
Corp., Tokyo, Japan) using AxioVision 4.1 software (Carl Zeiss
Microscopy, LLC, Thornwood, NY, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from ASMCs according to the
instructions of the TRIzol Reagent kit (Invitrogen; Thermo Fisher
Scientific, Inc.). RT-PCR was performed using the SuperScript
One-step RT-PCR system (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. There were 35 cycles of
90°C for 35 sec, 56°C for 30 sec and 72°C for 30 sec. β-actin
served as an internal control. The primers were as follows:
Forward, 5′-CTGAAGAGGAATGACTACAC-3′ and reverse,
5′-ACAGGAAAAAGAGAGGGAG-3′ for TRPM7 [(GenBank, AC000071)
Invitrogen; Thermo Fisher Scientific, Inc.]; forward,
5′-TGAGCTGCGTGTGGCCCCTGAG-3′ and reverse,
5′-GGGGCATCGGAACCGCTCATTG-3′ for β-actin [(GenBank, AC000080)
Applied Biosystems; Thermo Fisher Scientific, Inc.].
Western blot analysis
ASMCs were lysed with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). The extracts were collected and protein concentrations were
measured using a bicinchoninic acid protein assay kit (Wuhan Boster
Biological Technology, Ltd.). Equal quantities (40 μg) of
protein were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (Wuhan Boster
Biological Technology, Ltd.) and blotted onto polyvinylidene
fluoride (PVDF) membrane (Merck Millipore, Bedford, MA, USA). The
PVDF membrane was blocked for nonspecific protein binding with
Tris-buffered saline/Tween-20 (TBST; Guangzhou Whiga Technology
Co., Ltd.) and 5% non-fat milk (Guangzhou Whiga Technology Co.,
Ltd.) at room temperature for 2 h. Then, the membrane was incubated
overnight at 4°C with primary goat anti-TRPM7 (1:500; Abcam,
Cambridge, UK) and primary mouse anti-β-actin (1:1,000;
Sigma-Aldrich, St. Louis, MO, USA) monoclonal antibodies. Next, the
membranes were washed with TBST, incubated with HRP-conjugated
rabbit anti-goat IgG (1:500) and rabbit anti-mouse IgG (1:1,000)
secondary antibodies (Cell Signaling Technology, Inc.) for 1 h, and
then washed three times with TBST. The proteins were detected using
an enhanced chemiluminescence system (a SignalBoost™ Immunoreaction
Enhancer kit; Merck Millipore).
Detection of TRPM7 protein levels in
ASMCs using immunofluorescence
ASMCs were fixed in cold acetone (4°C) for 5 min and
washed with 0.01 mol/l PBS (Guangzhou Whiga Technology Co., Ltd.)
and immersed in 0.3% Triton X-100 (Guangzhou Whiga Technology Co.,
Ltd.) in PBS. Subsequently, ASMCs were blocked with 1% BSA in PBS
for 1 h at room temperature and incubated with primary monoclonal
goat anti-TRPM7 antibody (1:100; Abcam) in PBS containing 3% BSA
for 24 h (4°C). Following a wash with PBS at 4°C, ASMCs were
labeled with the fluorescein isothiocyanate-conjugated secondary
monoclonal donkey anti-goat IgG antibody (1:100; Jackson
ImmunoResearch Laboratories, Baltimore, MA, USA) 1 h at room
temperature. The nuclei of ASMCs were stained with Hoechst stain
(Beyotime Institute of Biotechnology, Shanghai, China).
Design of shRNA against rat TRPM7
Short hairpin RNA (shRNA) targeting the rat
TRPM7 gene (TRPM7-shRNA) was designed and synthesized by
Guangzhou Forevergen Co., Ltd. (Guangzhou, China) and the sequences
were as follows: Forward,
5′-GATCCCCGTCGTTTCTTCCAGAGGTGTTCAAGAGACACCTCTGGAAGAAACGACTTTTTA-3′
and reverse,
5′-AGCTTAAAAAGTCGTTTCTTCCAGAGGTGTCTCTTGAACACCTCTGGAAGAAACGACGGG-3′.
The control shRNA contained scrambled sequences (scramble-shRNA) as
follows: Forward,
5′-GATCCCCGCCAGCTTAGCACTGACTCTTCAAGAGAGAGTCAGTGCTAAGCTGGCTTTTTA-3′
and reverse,
5′-AGCTTAAAAAGCCAGCTTAGCACTGACTCTCTCTTGAAGAGTCAGTGCTAAGCTGGCGGG-3′.
Generation of lentivirus vectors and
transduction of ASMCs
The lentivirus vectors were constructed as
previously described (17).
Briefly, 293T human kidney cells (Invitrogen; Thermo Fisher
Scientific) in 10-cm culture dishes were cotransfected with 10
μg pLV vector, 4.8 μg pGag-Pol, 1.8 μg pRev
and 2.7 μg pMDG (Guangzhou Forevergen Co., Ltd.) using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The supernatants were collected 48 and 72 h after
transfection, filtered through a 0.4-μm membrane and the
viruses were concentrated using an Amicon Ultra-15 100 kDa filter
(Merck Millipore). Transduction of ASMCs with lentivirus vectors
was performed in the presence of 10 μg/ml polybrene
(Sigma-Aldrich). Using pLV vectors with puromycin marker (a coding
sequence of the lentivirus vector, obtained from Guangzhou
Forevergen Co., Ltd.), cells were selected with 2 μg/ml
puromycin (Merck Millipore) following transduction for 24 h. The
floating cells were removed, and the remaining attached cells were
analyzed and collected for further experiments.
[3H]-thymidine incorporation
assay
ASMCs were plated in 24-well cluster plates at a
density of 1×105 cells/well in a medium containing 10%
FCS and grown to 95% confluence. ASMCs were stimulated with CSE (5
or 15%) or TNF-α (1 ng/ml; Sigma-Aldrich) for 24 h. Then the cells
were cultured with [3H]-thymidine (0.25 μCi/ml;
GE Healthcare Life Sciences, Chalfont, UK) for 24 h, harvested onto
glass-fiber filters (Merck Millipore), and washed twice with PBS
and twice with ice-cold 5% trichloroacetic acid (Guangzhou Whiga
Technology Co., Ltd.). Subsequently, the cells were dissolved in
0.5 ml NaOH (1 M). Incorporated [3H]-thymidine was
quantified by liquid-scintillation counting (Beckman Coulter Inc.,
Brea, CA, USA).
Cell number determination
ASMCs were plated in 24-well cluster plates at a
density of 1×105 cells/well in medium containing 10% FCS
and grown to 95% confluence. Then the cells were stimulated with
CSE (5 or 15%) or TNF-α (1 ng/ml) for 24 h. ASMCs were harvested
and viable cells were counted in duplicate, using a hemocytometer
(Guduo Biotechnology Co., Ltd., Shanghai, China). Trypan blue
exclusion assay was performed by mixing 80 μl cell
suspension and 20 μl 0.4% Trypan Blue Solution
(Sigma-Aldrich) for 2 min. Blue cells were counted as dead cells
and the cells that did not absorb the dye were counted as living
cells.
Measurement of IL-8 in ASMCs
ASMCs were plated in 24-well cluster plates, washed
with PBS and stimulated with CSE (5 or 15%) for 24 h or TNF-α (1
ng/ml) for 1 h. Release of IL-8 in the ASMCs culture supernatants
was measured using a specific IL-8 enzyme-linked immunosorbent
assay (ELISA) kit (Sanquin, Amsterdam, Netherlands) according to
the manufacturers' protocol.
Statistical analysis
Data are presented as mean ± standard error of the
mean. Statistical significance was determined by Student's
t-test for unpaired observations or one-way analysis of
variance followed by Bonferroni's post-hoc test for independent
observations. P<0.05 was considered to indicate a statistically
significant difference.
Results
ASMC proliferation in rats exposed to
cigarette smoke
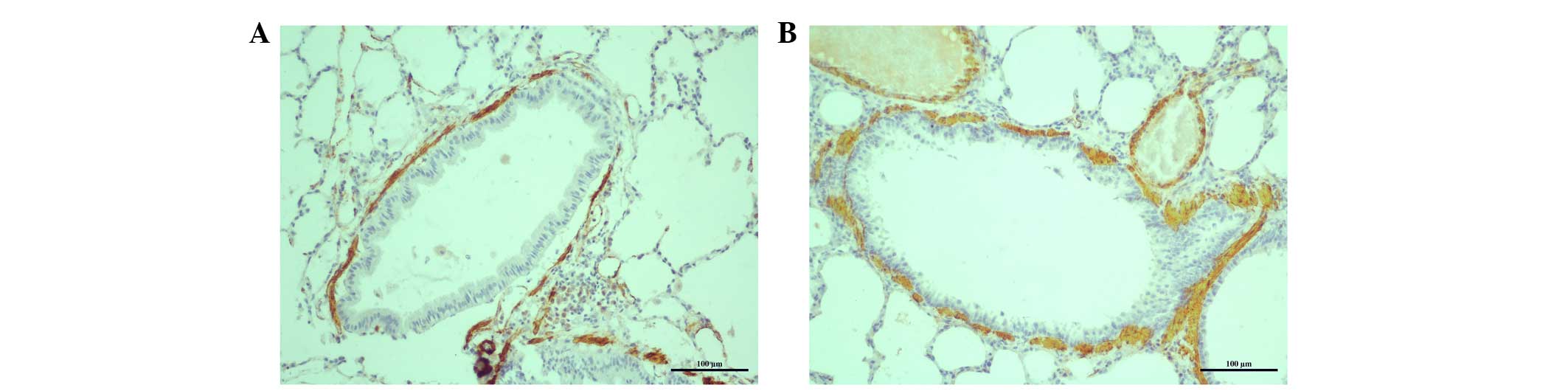
The lung tissue of rats was collected for
pathological analysis and α-smooth muscle actin immunohistochemical
staining. Morphological observations were performed under a light
microscope (Fig. 1). The structure
of the alveoli was mostly undamaged, the bronchioles were clear
(i.e. there was no infiltration of inflammatory cells in
bronchioles of control rats without CSE), and the bronchioles of
normal control rats exhibited eumorphism in terms of their cell
structure and histology. By contrast, pulmonary emphysema
manifested as alveolar wall thinning, alveolar structure
disruption, and alveolar space enlargement and fusion were observed
in rats that had been exposed to cigarette smoke. Additionally,
bronchial epithelial hyperplasia, submucosal inflammatory cell
infiltration, and airway wall and ASM thickening were observed in
rats exposed to cigarette smoke.
An increase in ASM mass was observed in rats exposed
to cigarette smoke when compared with normal rats [the ratio of the
ASM area to the total airway area was 0.1645±0.01016 vs.
0.0479±0.00889 (n=8) for CSE rats compared with normal rats;
respectively; t=70.139; P<0.001].
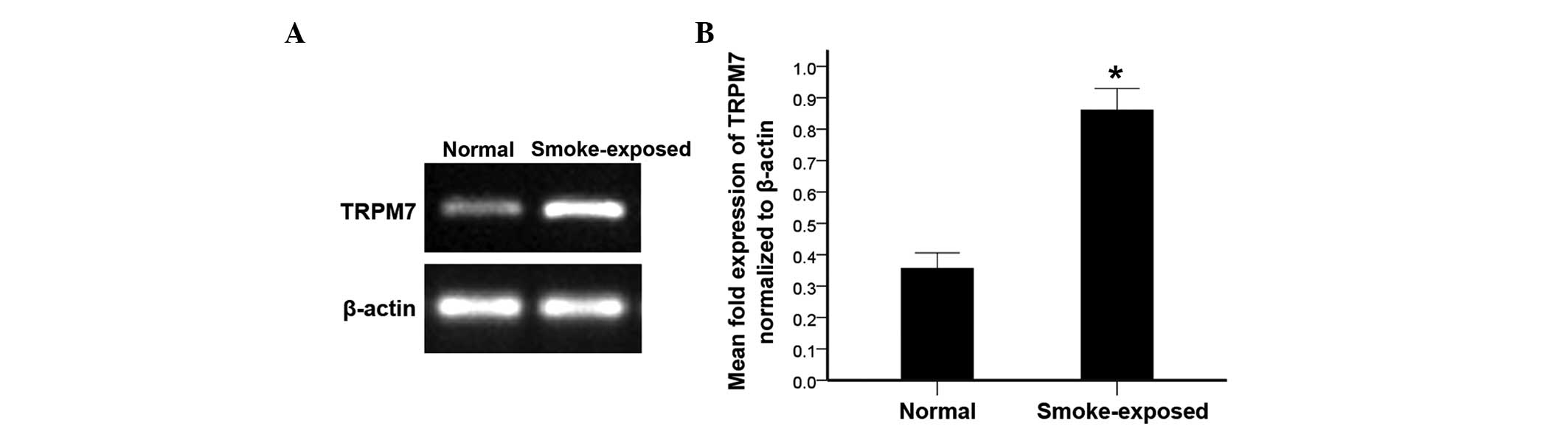
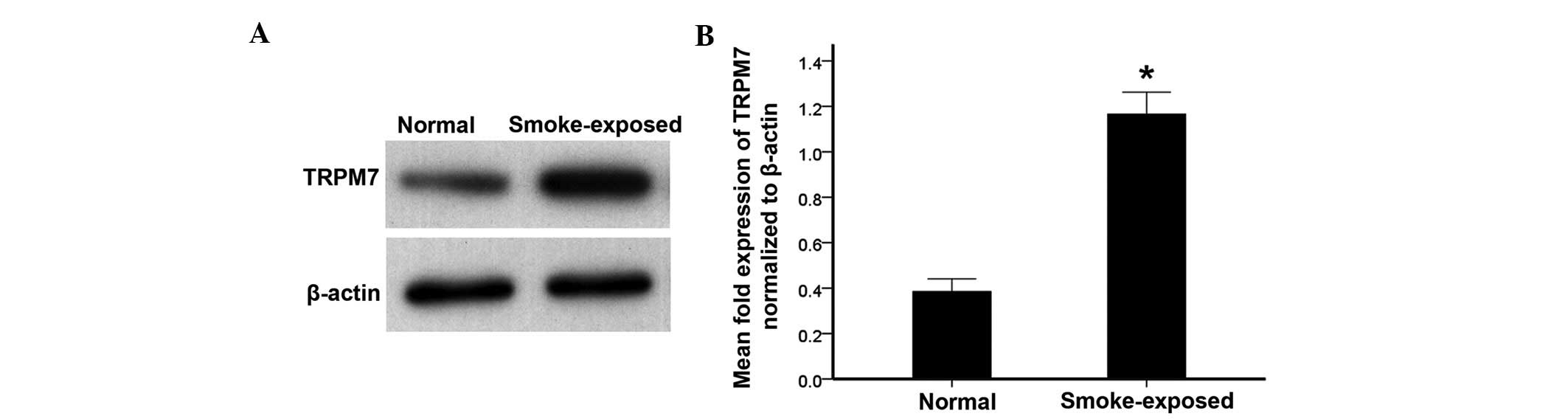
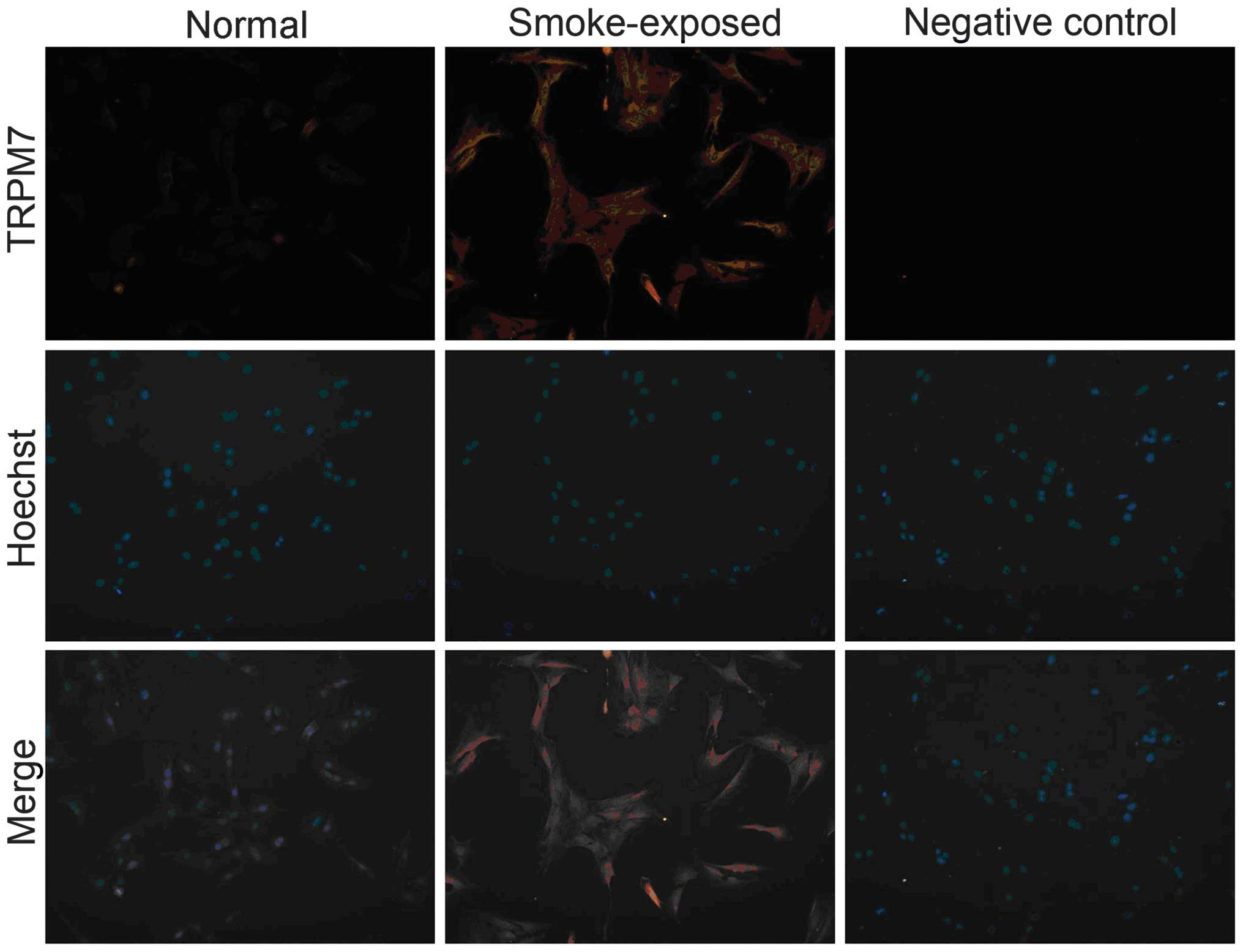
TRPM7 is upregulated in ASMCs from
smoke-exposed rats
The expression of TRPM7 in primary ASMCs was
detected by RT-PCR, western blot analysis and immunofluorescence.
As indicated by Fig. 2, the TRPM7
mRNA level was elevated in ASMCs of smoke-exposed rats when
compared with normal rats. Furthermore, the western blot analysis
indicated increased TRPM7 protein levels in ASMCs in smoke-exposed
rats compared with normal rats (Fig.
3). Additionally, immunofluorescence demonstrated increased
expression levels of TRPM7 proteins in ASMCs from smoke-exposed
rats when compared with normal rats (Fig. 4).
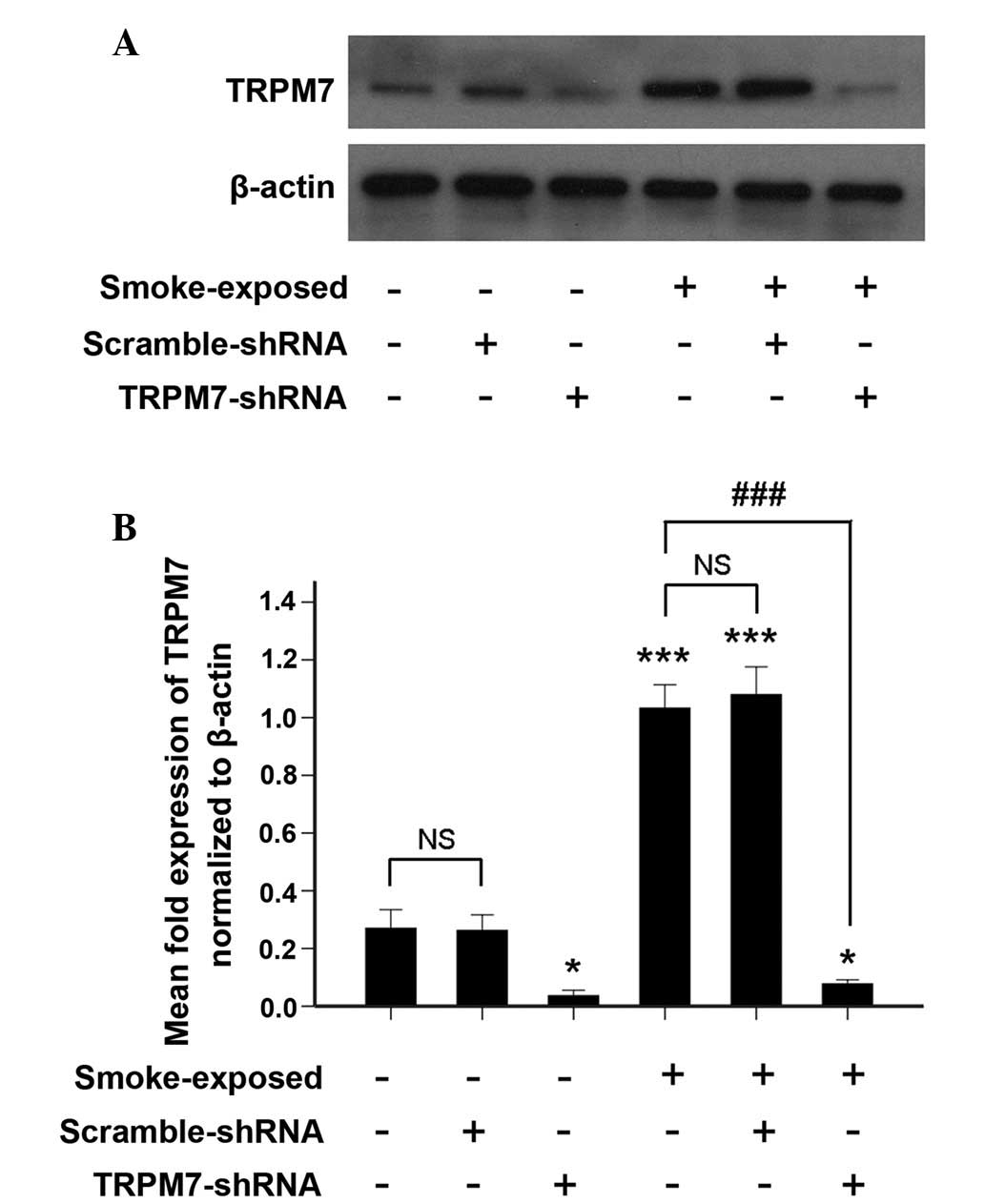
Silencing of TRPM7 in ASMCs by
TRPM7-shRNA lentivirus vector
TRPM7 protein expression was detected by western
blotting in ASMCs infected with the TRPM7-shRNA lentivirus vector.
As indicated by Fig. 5, treatment
with TRPM7-shRNA lentivirus vector resulted in a significant
decrease in TRPM7 protein expression.
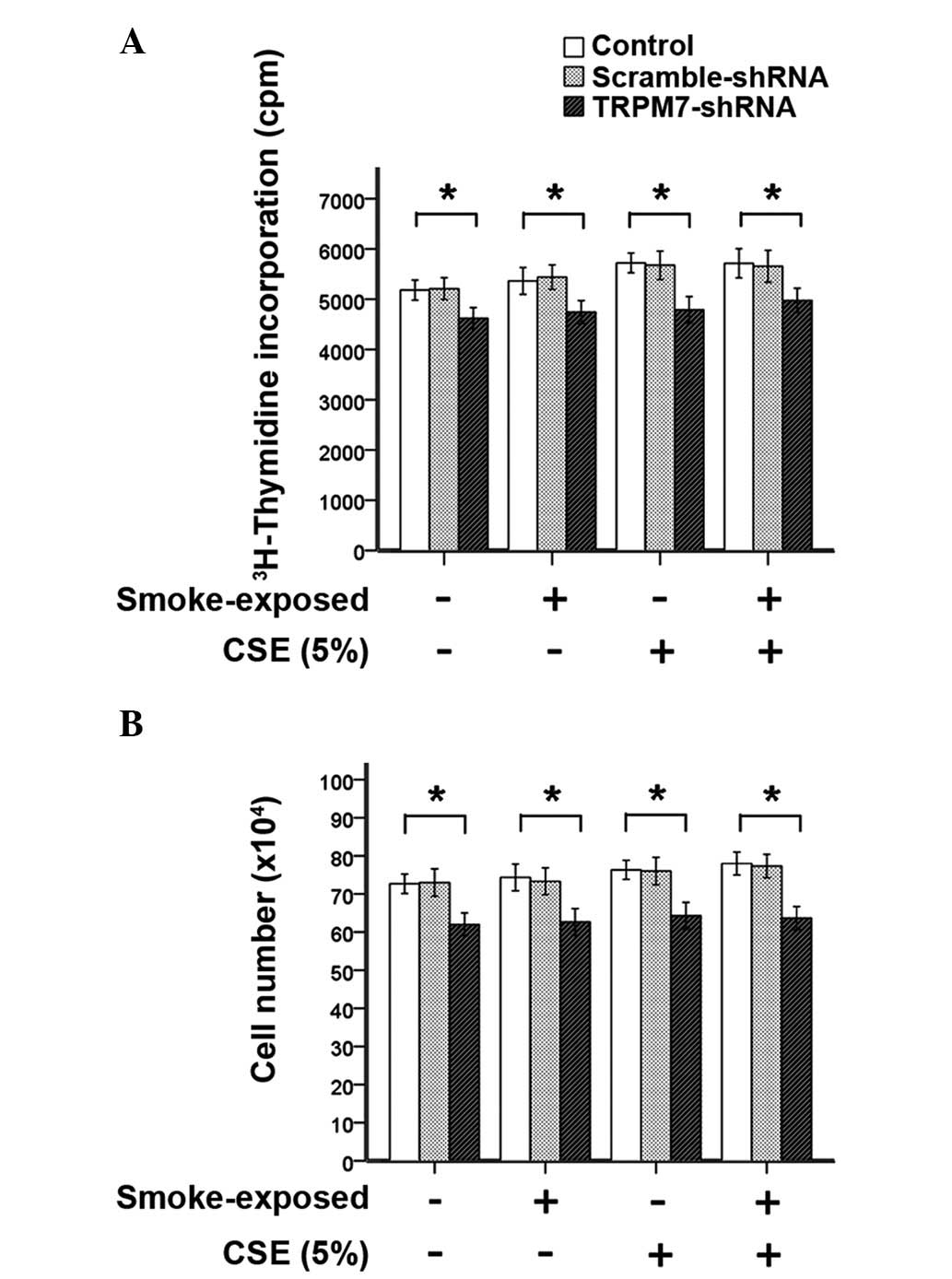
Upregulation of TRPM7 augments
proliferation of ASMCs from smoke-exposed rats
ASMCs were stimulated with CSE (5% or 15%) or TNF-α
(1 ng/ml) for 24 h. To assess the proliferation of ASMCs, DNA
synthesis and cell number of ASMCs were determined by
[3H]-thymidine incorporation assay and with a
hemocytometer, respectively. As indicated by Fig. 6, stimulation with CSE (5%) did not
induce increased DNA synthesis or ASMC number. However, treatment
with TRPM7-shRNA lentivirus vector significantly reduced
methyl-[3H]-thymidine incorporation and the cell number
of ASMCs compared with ASMCs that were not treated with the
TRPM7-shRNA lentivirus vector. These observations indicated that
TRPM7 is required for ASMC proliferation.
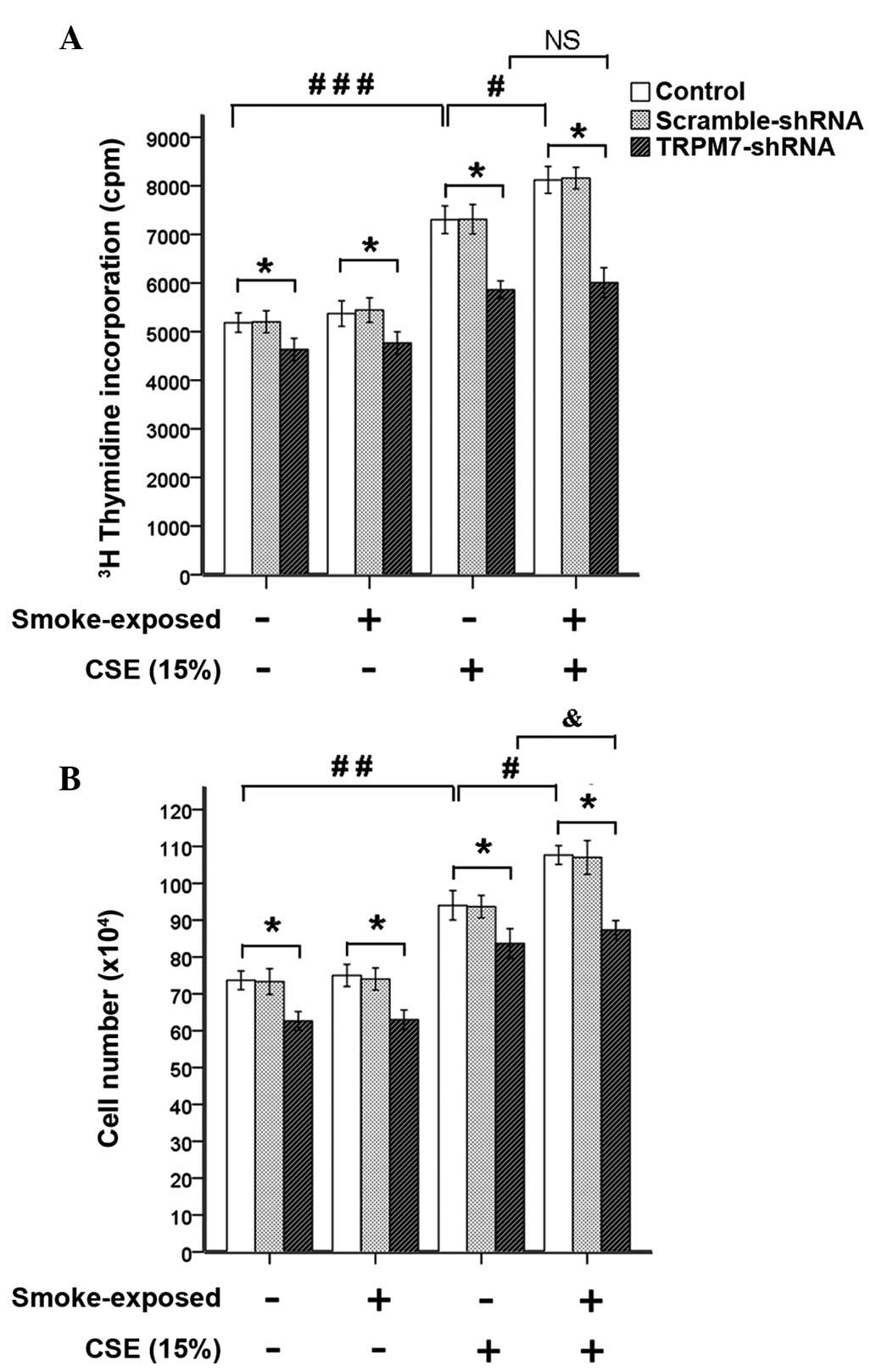
Stimulation with CSE (15%) induced significantly
increased DNA synthesis and ASMC number, which was not alleviated
by treatment with TRPM7-shRNA lentivirus vector (Fig. 7). When compared with ASMCs from
normal rats, the CSE (15%)-induced increases of
methyl-[3H]-thymidine incorporation and cell number were
significantly enhanced in ASMCs from smoke-exposed rats, as
demonstrated by greater expression levels of TRPM7. Treatment with
TRPM7-shRNA lentivirus vector reduced
methyl-[3H]-thymidine incorporation and cell number of
ASMCs. These results indicated that upregulation of TRPM7 augmented
proliferation of ASMCs induced by CSE (15%) in smoke-exposed rats.
However, in ASMCs treated with TRPM7-shRNA lentivirus vector,
stimulation with CSE (15%) induced significantly increased cell
number of ASMCs from smoke-exposed rats when compared with normal
rats.
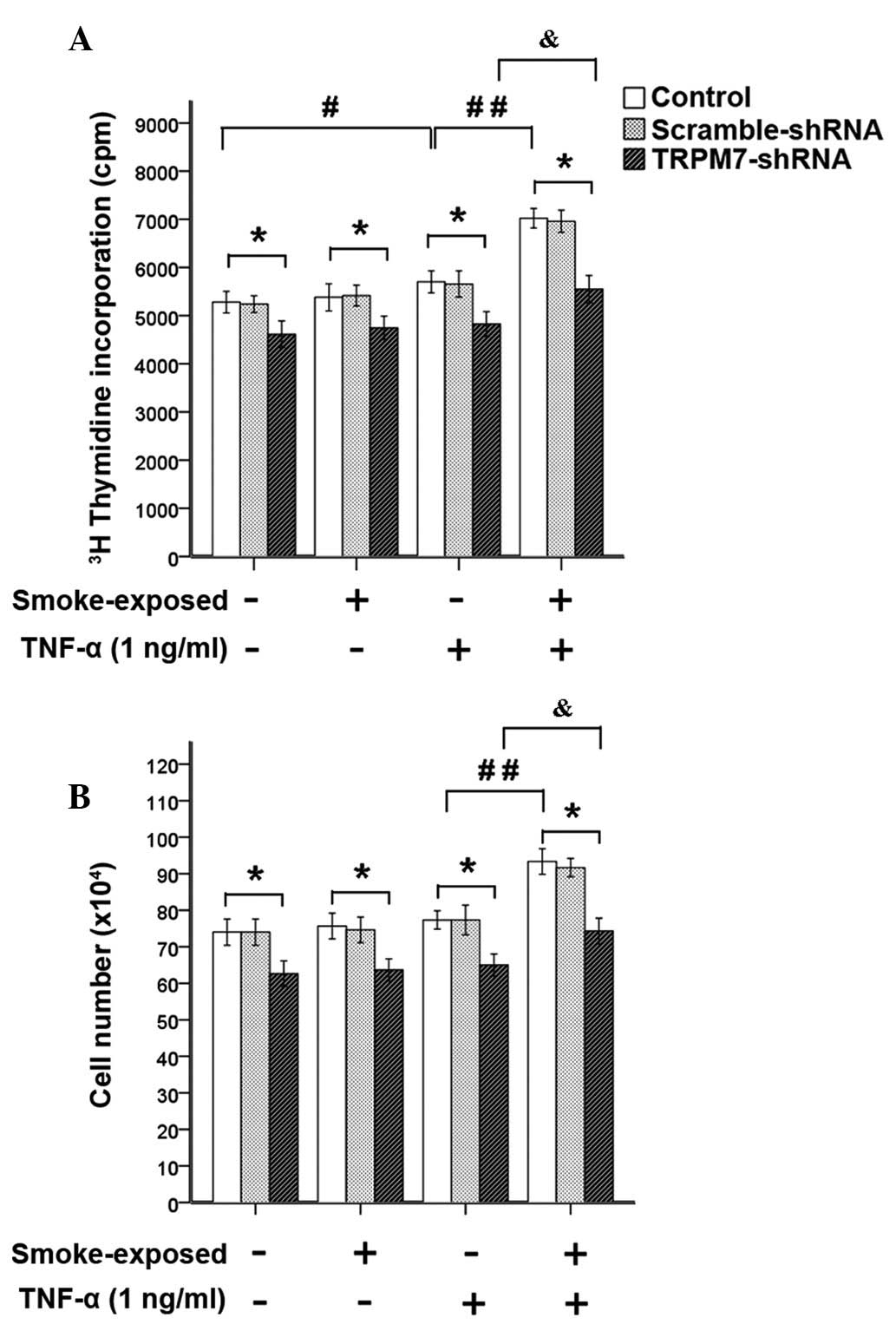
As indicated by Fig.
8, stimulation with TNF-α (1 ng/ml) led to increased DNA
synthesis in ASMCs, this increase was enhanced significantly in
ASMCs from smoke-exposed rats with higher expression levels of
TRPM7. However, stimulation with TNF-α (1 ng/ml) induced a
significant increase in ASMC number in smoke-exposed rats, but not
in ASMC number in normal rats. Treatment with TRPM7-shRNA
lentivirus vector reduced methyl-[3H]-thymidine
incorporation and cell number of ASMCs. This indicated that
upregulation of TRPM7 augmented proliferation of ASMCs induced by
TNF-α (1 ng/ml) in smoke-exposed rats. However, increased
methyl-[3H]-thymidine incorporation and cell number of
ASMCs from smoke-exposed rats induced by TNF-α was not completely
inhibited by treatment with TRPM7-shRNA lentivirus vector.
Upregulation of TRPM7 augments IL-8
secretion in ASMCs from smoke-exposed rats
ASMCs were stimulated with CSE (5% or 15%) for 24 h
or TNF-α (1 ng/ml) for 1 h and the IL-8 release was determined
using an ELISA. Stimulation with CSE (5%) did not increase IL-8
release significantly and treatment with TRPM7-shRNA lentivirus
vector did not affect IL-8 release in the ASMCs.
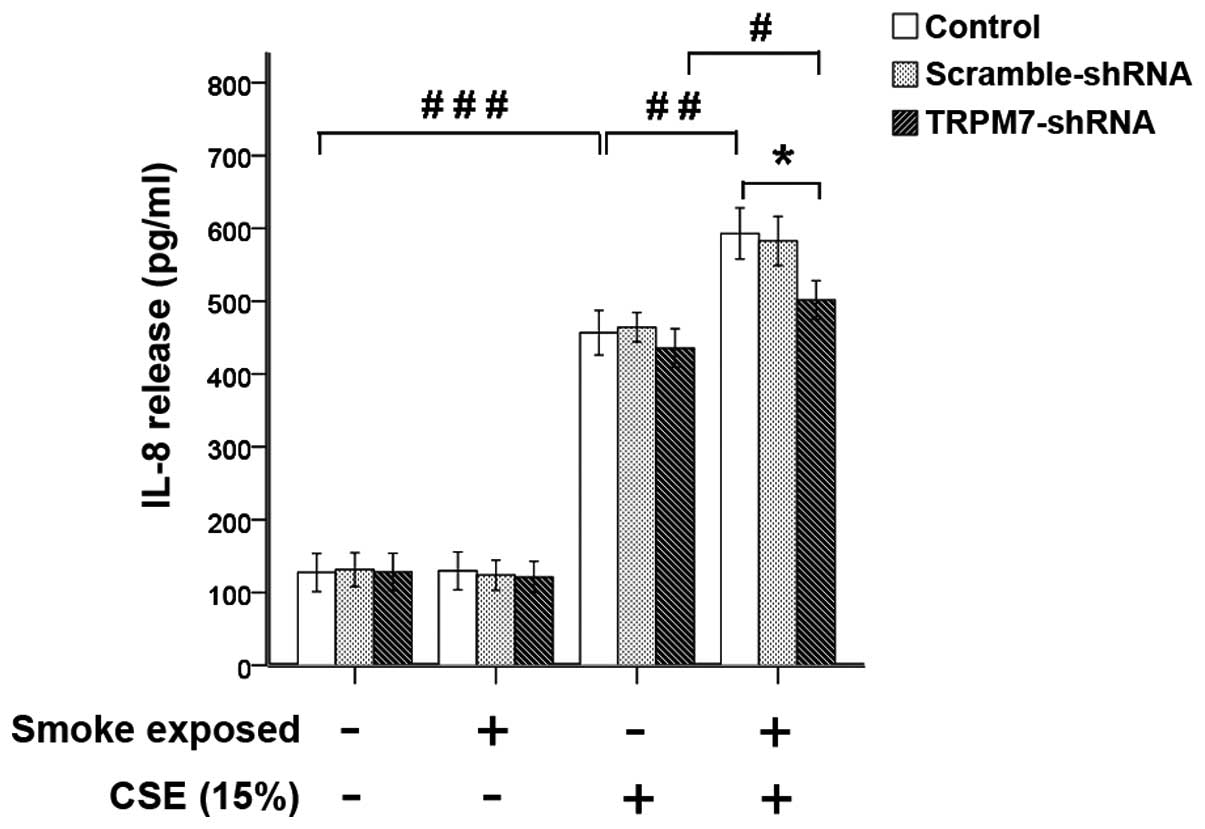
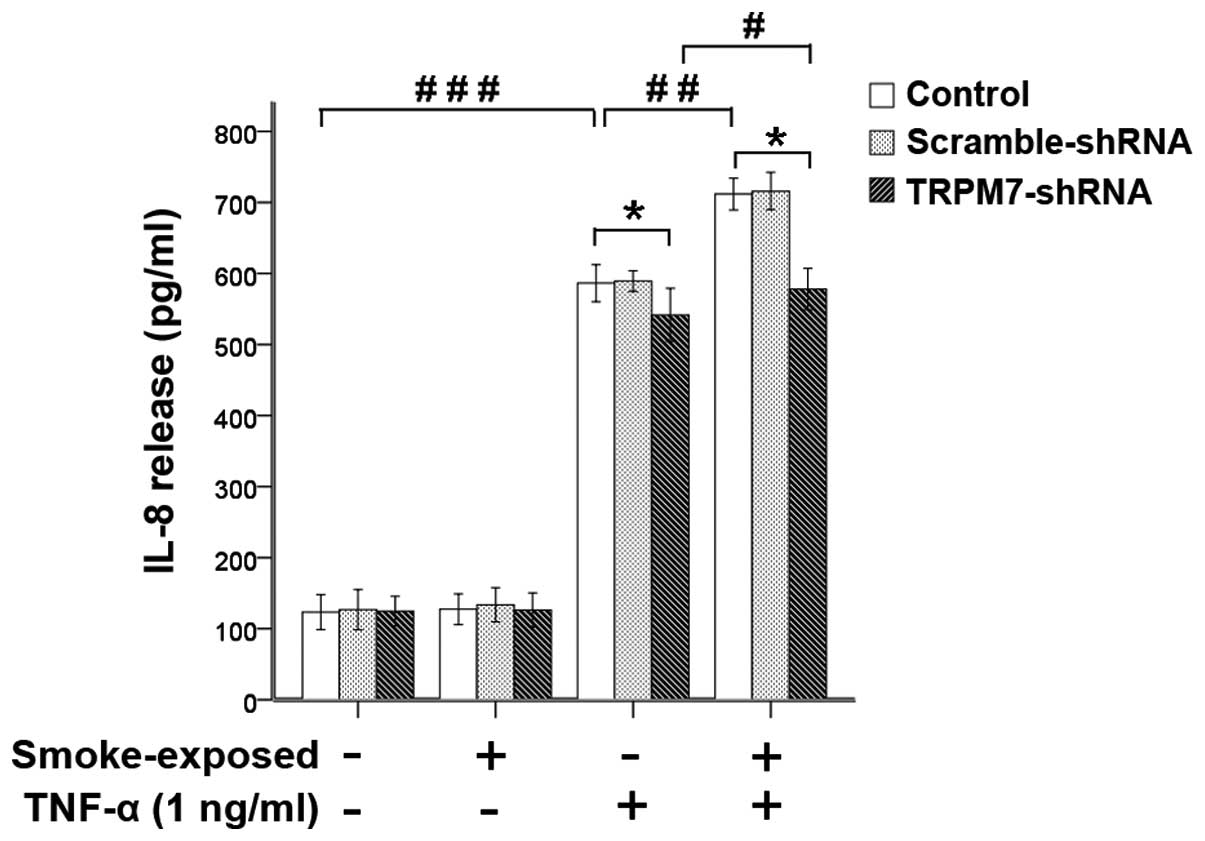
Stimulation with CSE (15%) or TNF-α (1 ng/ml)
significantly increased the secretion of IL-8 in ASMCs was not
inhibited by treatment with TRPM7-shRNA lentivirus vector (Figs. 9 and 10). Compared with ASMCs from normal
rats, the increase of IL-8 secretion induced by CSE (15%) or TNF-α
was greater in ASMCs from smoke-exposed rats with higher levels of
TRPM7 expression. Treatment with TRPM7-shRNA lentivirus vector
reduced IL-8 release, which was induced by CSE (15%) and TNF-α in
ASMCs. These results indicated that upregulation of TRPM7 augmented
CSE (15%)- or TNF-α-induced IL-8 secretion in ASMCs from
smoke-exposed rats. However, in ASMCs treated with the TRPM7-shRNA
lentivirus vector, stimulation with CSE (15%) or TNF-α also induced
a significant increase in IL-8 secretion in ASMCs from
smoke-exposed rats when compared with normal rats.
Discussion
The present study demonstrated that TRPM7 was
upregulated in ASMCs from rats exposed to cigarette smoke. The TRP
channel is a superfamily of ion channels that control the passage
of various ions across membranes (11). These channels are homo- or
heterotetramers formed by proteins, which contain six putative
transmembrane domains and a pore-forming loop between the fifth and
sixth segments. TRPM7 is a widely expressed member of the TRP
superfamily. Previous studies have indicated that TRPM7 is
expressed in various cell types, including lymphocytes (20), neurons (21), vascular smooth muscle cells
(22), vascular endothelia
(23), mast cells (24), fibroblasts (25), kidney cells (26) and adipocytes (27). In the present study, RT-PCR,
western blot assays and immunofluorescence demonstrated that TRPM7
was expressed in ASMCs from normal rats and the expression levels
of TRPM7 were markedly higher in ASMCs from smoke-exposed rats.
The results of the current study identified that
upregulation of TRPM7 augmented proliferation in ASMCs from
smoke-exposed rats. The function of TRPM7 in cellular proliferation
varies between different cell types. In certain types of cells,
TRPM7 exhibits a cell death function; for example, TRPM7 leads to
cell death in anoxic neurons (21). However, TRPM7 is also required for
cell survival and proliferation in various cell types. Previously,
it was reported that TRPM7 suppression by retrovirus-mediated siRNA
targeting the TRPM7 gene decreased the survival rate of
RBL-2H3 cells (28). Additionally,
TRPM7 is required for the proliferation of various types of normal
and cancer cell, including human mast (24), B lymphocyte (20), human head and neck carcinoma
(29), breast cancer (30), hepatocellular carcinoma (31), gastric cancer (32) and prostate cancer (33) cells. Consistent with the majority
of results regarding the underlying mechanisms of cellular
proliferation, in the current study, silencing of TRPM7 reduced DNA
synthesis and cell number of ASMCs, and upregulation of TRPM7
augmented cell proliferation in ASMCs from rats exposed to
cigarette smoke.
To the best of our knowledge, this is the first
study regarding the importance of TRPM7 in cytokine secretion by
ASMCs. In our previous study, it was determined that knockdown of
TRPM7 reduced the release of cytokines in rat bone marrow-derived
mast cells (17). Therefore, the
present study determined that silencing of TRPM7 with TRPM7-shRNA
lentivirus vector reduced IL-8 release in ASMCs induced by CSE
(15%) and TNF-α in ASMCs from rats exposed to cigarette smoke.
Furthermore, the increase of IL-8 secretion induced by CSE and
TNF-α was enhanced in ASMCs from rats exposed to cigarette smoke,
as demonstrated by higher expression levels of TRPM7. This suggests
that upregulation of TRPM7 augmented the release of IL-8 in ASMCs
from rats exposed to cigarette smoke. As IL-8 is important in
neutrophil recruitment, the upregulation of TRPM7 in ASMCs from
rats exposed to cigarette smoke may contribute to the inflammatory
response of the airway.
The present study investigated the proliferation of
ASMCs and IL-8 release induced by TNF-α due to the association
between cigarette smoke exposure and TNF-α. The level of TNF-α is
often associated with smoking status, systemic inflammation and
airflow limitation in patients with COPD (34,35).
In animal models, mice with knocked-out TNF-α receptors did not
develop an inflammatory response following acute cigarette smoke
exposure (36). Furthermore, it
was previously demonstrated that TNF-α accounts for the majority of
inflammatory cell infiltration in a mouse model with 6-month smoke
exposure (37). However, the
mitogenic effect of TNF-α on ASMCs is controversial. A previous
study reported that TNF-α promotes ASMC proliferation, which was
mediated via the phosphatidylinositol 3-kinase signaling pathway,
and the p38 and extracellular signal-regulated kinase 1/2
mitogen-activated protein kinase (MAPK) signaling pathway (5). Furthermore, it was previously
proposed that TNF-α did not induce the proliferation of ASMCs
(38) and may inhibit
proliferation induced by other growth factors (39). This may be due to differences in
species used for the experiments, the concentration of TNF-α,
exposure duration and culture medium. In the present study, a
positive mitogenic effect of TNF-α on ASMCs was observed and the
upregulation of TRPM7 led to a proliferative effect of TNF-α. The
underlying mechanism of TRPM7 regulating the proliferation of ASMCs
may contribute to a potential interaction between its distinctive
serine/threonine protein kinase domain, and the PI3K and MAPK
signaling pathway, which consists of a cascade reaction of
serine/threonine kinases (40);
however, further research is required to elucidate this
further.
In the current study; however, treatment with
TRPM7-shRNA lentivirus vector did not completely inhibit the effect
of cell proliferation and IL-8 secretion that was induced by CSE
(15%) or TNF-α. Stimulation with CSE (15%) or TNF-α increased cell
proliferation and IL-8 secretion in ASMCs from smoke-exposed rats
when compared with normal rats in the presence of TRPM7-shRNA
lentivirus vector treatment. This indicated that there is an
alternative mechanism inducing the response to cigarette smoke or
TNF-α in ASMCs.
In conclusion, the present study revealed that TRPM7
expression levels were elevated in ASMCs from rats exposed to
cigarette smoke, and that upregulation of TRPM7 augmented cell
proliferation and IL-8 secretion in ASMCs from smoke-exposed rats.
ASMC proliferation and IL-8 secretion are considered to be
important in the development of COPD; therefore, the current study
indicates that TRPM7 may be a potential target for the treatment of
COPD.
Acknowledgments
The present study was supported by the National
Science Foundation of China (grant nos. 81000015 and 81370120).
References
|
1
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management
and prevention of chronic obstructive pulmonary disease: gold
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar
|
|
2
|
Saetta M, Di Stefano A, Turato G, Facchini
FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A and Fabbri LM: CD8+
T-lymphocytes in peripheral airways of smokers with chronic
obstructive pulmonary disease. Am J Respir Crit Care Med.
157:822–826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hogg JC, Chu F, Utokaparch S, Woods R,
Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson
HO and Paré PD: The nature of small-airway obstruction in chronic
obstructive pulmonary disease. N Engl J Med. 350:2645–2653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung KF: The role of airway smooth muscle
in the pathogenesis of airway wall remodeling in chronic
obstructive pulmonary disease. Proc Am Thorac Soc. 2:347–354;
discussion 371–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stamatiou R, Paraskeva E, Gourgoulianis K,
Molyvdas PA and Hatziefthimiou A: Cytokines and growth factors
promote airway smooth muscle cell proliferation. ISRN Inflamm.
2012:7314722012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pera T, Gosens R, Lesterhuis AH, Sami R,
van der Toorn M, Zaagsma J and Meurs H: Cigarette smoke and
lipopolysaccharide induce a proliferative airway smooth muscle
phenotype. Respir Res. 11:482010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Howarth PH, Knox AJ, Amrani Y, Tliba O,
Panettieri RA Jr and Johnson M: Synthetic responses in airway
smooth muscle. J Allergy Clin Immunol. 114(Suppl 2): S32–S50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manetsch M, Seidel P, Heintz U, Che W,
Hughes JM, Ge Q, Sukkar MB and Ammit AJ: TLR2 ligand engagement
upregulates airway smooth muscle TNFα-induced cytokine production.
Am J Physiol Lung Cell Mol Physiol. 302:L838–L845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baarsma HA, Meurs H, Halayko AJ, Menzen
MH, Schmidt M, Kerstjens HA and Gosens R: Glycogen synthase
kinase-3 regulates cigarette smoke extract- and IL-1β-induced
cytokine secretion by airway smooth muscle. Am J Physiol Lung Cell
Mol Physiol. 300:L910–L919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gosens R, Rieks D, Meurs H, Ninaber DK,
Rabe KF, Nanninga J, Kolahian S, Halayko AJ, Hiemstra PS and
Zuyderduyn S: Muscarinic M3 receptor stimulation increases
cigarette smoke-induced IL-8 secretion by human airway smooth
muscle cells. Eur Respir J. 34:1436–1443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clapham DE: TRP channels as cellular
sensors. Nature. 426:517–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitz C, Perraud AL, Johnson CO, Inabe
K, Smith MK, Penner R, Kurosaki T, Fleig A and Scharenberg AM:
Regulation of vertebrate cellular Mg2+ homeostasis by
TRPM7. Cell. 114:191–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Runnels LW, Yue L and Clapham DE:
TRP-PLIK, a bifunctional protein with kinase and ion channel
activities. Science. 291:1043–1047. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fonfria E, Murdock PR, Cusdin FS, Benham
CD, Kelsell RE and McNulty S: Tissue distribution profiles of the
human TRPM cation channel family. J Recept Signal Transduct Res.
26:159–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunert-Keil C, Bisping F, Krüger J and
Brinkmeier H: Tissue-specific expression of TRP channel genes in
the mouse and its variation in three different mouse strains. BMC
Genomics. 7:1592006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Visser D, Middelbeek J, van Leeuwen FN and
Jalink K: Function and regulation of the channel-kinase TRPM7 in
health and disease. Eur J Cell Biol. 93:455–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang L, Ng NM, Chen M, Lin X, Tang T,
Cheng H, Yang C and Jiang S: Inhibition of TRPM7 channels reduces
degranulation and release of cytokines in rat bone marrow-derived
mast cells. Int J Mol Sci. 15:11817–11831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M, Shi JT, Lv ZQ, Huang LJ, Lin XL,
Zhang W, Liang RY, Li YQ and Jiang SP: Triptolide inhibits TGF-β1
induced proliferation and migration of rat airway smooth muscle
cells by suppressing NF-κB but not ERK1/2. Immunology. Sep
29–2014.Epub ahead of print. View Article : Google Scholar
|
|
19
|
Chen M, Lv Z, Huang L, Zhang W, Lin X, Shi
J, Zhang W, Liang R and Jiang S: Triptolide inhibits TGF-β1-induced
cell proliferation in rat airway smooth muscle cells by suppressing
Smad signaling. Exp Cell Res. 331:362–368. 2015. View Article : Google Scholar
|
|
20
|
Sahni J, Tamura R, Sweet IR and
Scharenberg AM: TRPM7 regulates quiescent/proliferative metabolic
transitions in lymphocytes. Cell Cycle. 9:3565–3574. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aarts M, Iihara K, Wei WL, Xiong ZG,
Arundine M, Cerwinski W, MacDonald JF and Tymianski M: A key role
for TRPM7 channels in anoxic neuronal death. Cell. 115:863–877.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Touyz RM: Transient receptor potential
melastatin 6 and 7 channels, magnesium transport and vascular
biology: Implications in hypertension. Am J Physiol Heart Circ
Physiol. 294:H1103–H1118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng Z, Inoue K, Sun H, Leng T, Feng X,
Zhu L and Xiong ZG: TRPM7 regulates vascular endothelial cell
adhesion and tube formation. Am J Physiol Cell Physiol.
308:C308–C318. 2015. View Article : Google Scholar :
|
|
24
|
Wykes RC, Lee M, Duffy SM, Yang W, Seward
EP and Bradding P: Functional transient receptor potential
melastatin 7 channels are critical for human mast cell survival. J
Immunol. 179:4045–4052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu M, Huang C, Huang Y, Wu X, Li X and Li
J: Inhibition of TRPM7 channels prevents proliferation and
differentiation of human lung fibroblasts. Inflamm Res. 62:961–970.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng Z, Cao R, Wang Y, Cao H, Liu T, Yang
Z and Wang X: Suppression of renal TRPM7 may alleviate kidney
injury in the renal transplantation. World J Urol. 32:1303–1311.
2014. View Article : Google Scholar
|
|
27
|
Che H, Yue J, Tse HF and Li GR: Functional
TRPV and TRPM channels in human preadipocytes. Pflugers Arch.
466:947–959. 2014. View Article : Google Scholar
|
|
28
|
Ng NM, Jiang SP and Lv ZQ:
Retrovirus-mediated siRNA targeting TRPM7 gene induces apoptosis in
RBL-2H3 cells. Eur Rev Med Pharmacol Sci. 16:1172–1178.
2012.PubMed/NCBI
|
|
29
|
Jiang J, Li MH, Inoue K, Chu XP, Seeds J
and Xiong ZG: Transient receptor potential melastatin 7-like
current in human head and neck carcinoma cells: Role in cell
proliferation. Cancer Res. 67:10929–10938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guilbert A, Gautier M, Dhennin-Duthille I,
Haren N, Sevestre H and Ouadid-Ahidouch H: Evidence that TRPM7 is
required for breast cancer cell proliferation. Am J Physiol Cell
Physiol. 297:C493–C502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mishra R, Rao V, Ta R, Shobeiri N and Hill
CE: Mg2+- and MgATP-inhibited and Ca2+/calmodulin-sensitive
TRPM7-like current in hepatoma and hepatocytes. Am J Physiol
Gastrointest Liver Physiol. 297:G687–G694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim BJ, Kim SY, Lee S, Jeon JH, Matsui H,
Kwon YK, Kim SJ and So I: The role of transient receptor potential
channel blockers in human gastric cancer cell viability. Can J
Physiol Pharmacol. 90:175–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Y, Selvaraj S, Varma A, Derry S,
Sahmoun AE and Singh BB: Increase in serum Ca2+/Mg2+ ratio promotes
proliferation of prostate cancer cells by activating TRPM7
channels. J Biol Chem. 288:255–263. 2013. View Article : Google Scholar :
|
|
34
|
Tanni SE, Pelegrino NR, Angeleli AY,
Correa C and Godoy I: Smoking status and tumor necrosis
factor-alpha mediated systemic inflammation in COPD patients. J
Inflamm (Lond). 7:292010. View Article : Google Scholar
|
|
35
|
Chiang CH, Chuang CH and Liu SL:
Transforming growth factor-β1 and tumor necrosis factor-α are
associated with clinical severity and airflow limitation of COPD in
an additive manner. Lung. 192:95–102. 2014. View Article : Google Scholar
|
|
36
|
Churg A, Dai J, Tai H, Xie C and Wright
JL: Tumor necrosis factor-alpha is central to acute cigarette
smoke-induced inflammation and connective tissue breakdown. Am J
Respir Crit Care Med. 166:849–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Churg A, Wang RD, Tai H, Wang X, Xie C and
Wright JL: Tumor necrosis factor-alpha drives 70% of cigarette
smoke-induced emphysema in the mouse. Am J Respir Crit Care Med.
170:492–498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McKay S, Hirst SJ, Haas MB, de Jongste JC,
Hoogsteden HC, Saxena PR and Sharma HS: Tumor necrosis factor-alpha
enhances mRNA expression and secretion of interleukin-6 in cultured
human airway smooth muscle cells. Am J Respir Cell Mol Biol.
23:103–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tliba O, Tliba S, Da Huang C, Hoffman RK,
DeLong P, Panettieri RA Jr and Amrani Y: Tumor necrosis factor
alpha modulates airway smooth muscle function via the autocrine
action of interferon beta. J Biol Chem. 278:50615–50623. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yee NS, Kazi AA and Yee RK: Cellular and
developmental biology of TRPM7 channel-kinase: Implicated roles in
cancer. Cells. 3:751–77. 2014. View Article : Google Scholar : PubMed/NCBI
|