Introduction
Myocardial infarction is a serious threat to human
health. In addition to the possibility of sudden cardiac death,
long-time persistent ischemia reduces survival and induces death of
cardiomyocytes, thus leading to heart failure (1). Although the survival of patients with
myocardial infarction is increasing as treatment strategies
improve, the mortality associated with this disease remains
high.
Apelin is synthesized as a 77 amino acid
pre-pro-protein, and is sequentially cleaved into four circulating
active peptides: Apelin-12, apelin-13, apelin-17 and apelin-36.
Apelin is a secreted adipokine involved in the regulation of
cardiovascular functions (2).
Angiotensin II (Ang-II) receptor-like 1 (APJ) is an endogenous
receptor of apelin. APJ is expressed in several cell types,
including endothelial cells, vascular smooth muscle cells and
cardiomyocytes. Apelin regulates the production of nitric oxide
(NO) (3), causes vasodilatation,
reduces ventricular preload and afterload, and increases cardiac
contractility in failing hearts (4). Furthermore, apelin has previously
been demonstrated to exert vasodilatation and positive inotropic
effects (5–8), and is regarded as an important
therapeutic target in heart failure (9).
Apelin was previously reported to have a protective
function during heart failure and ischemia/reperfusion injury
(10–12). Growing evidence has demonstrated
that apelin is important for left ventricular remodeling (4,9).
However, the effects of apelin treatment on myocardial
infarction-induced myocardial fibrosis remain unclear. The present
study investigated the effects of apelin-13 treatment on myocardial
fibrosis.
Materials and methods
Materials
Apelin-13 [(Pyr1)-Apelin-13] was purchased from GL
Biochem (Shanghai) Ltd. (Shanghai, China). Apelin-13 was dissolved
in sterile normal saline to form a 10 mg/ml stock solution.
Animal experiment protocol
Male Sprague-Dawley rats (age, 8-years-old) weighing
180–220 g were obtained from the Experimental Animal Center of
China Medical University (Shenyang, China). Mice were maintained in
a temperature-controlled (20–22°C) environment under a 12 h
light-dark cycle. Rats were divided into three groups (n=6/group),
as follows: Sham; left anterior descending artery (LAD) ligation;
and LAD + apelin-13 groups. Rats in the LAD group underwent a LAD
ligation operation (13,14). Briefly, rats were anesthetized with
10% chloral hydrate (3.5 ml/kg; Sinopharm, Shanghai, China) via
intraperitoneal injection. In a supine position, endotracheal
intubation was performed and the rats were ventilated using a
rodent ventilator (rate, 80 breaths/min; tidal volume, 6–8 ml/kg).
The thoracic cavity was then opened and the heart was exposed. The
LAD was ligated using 7-0 silk. Successful LAD ligation was
confirmed by myocardial blanching and the thoracic cavity was
closed layer-by-layer. Following LAD ligation operation, rats
received an intramuscular injection of 2.5×104 U
penicillin (MOTIAN, Harbin, China). Rats in the sham group received
an equivalent operation, but without ligation. Rats in the LAD +
apelin-13 group received an intraperitoneal injection with
apelin-13 (200 µg/kg/day) for 4 weeks following the LAD
ligation operation. Rats in the sham and LAD groups received an
equal amount of normal saline. After 4 weeks, rats in each group
were anesthetized with 10% chloral hydrate. The levels of left
ventricular systolic pressure (LVSP), left ventricular
end-diastolic pressure (LVEDP), left ventricular maximal rate of
pressure rise (LV+dp/dtmax) and left ventricular maximal
rate of pressure decline (LV−dp/dtmax) were measured.
The rats were anesthetized with 10% chloral hydrate (3.5 ml/kg;
intraperitoneal injection). The blood was harvested and stored at
room temperature for 2–4 h and was centrifuged at 4,000 rpm for 10
min. The supernatant was collected and the serum was obtained.
Animal experiments were performed according to the Guide for the
Care and Use of Laboratory Animals, and were approved by the
Institutional Animal Care and Use Committee at China Medical
University.
Cell culture
The H9C2 rat myoblast cell line was obtained from
Type Culture Collection Center of Chinese Academy of Science
(Shanghai, China). Cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), and maintained in a
humidified atmosphere containing 5% CO2 at 37°C. Prior
to treatment with Ang-II (Cloud-Clone, Wuhan, China), cells were
cultured in serum-free medium overnight. The cells were then
treated with 100 nM Ang-II for 48 h. Apelin-13 (100 nM) was added
to the cell medium 20 min prior to Ang-II treatment. Following
treatment with Ang-II and apelin-13, cells were collected for
western blotting and electrophoretic mobility shift assay
(EMSA).
Histopathology
The hearts of the rats in each group were harvested,
fixed in 4% paraformaldehyde, embedded in paraffin, and cut into
5-µm sections. Subsequently, the sections were subjected to
routine hematoxylin and eosin (HE) and Masson's trichrome staining.
Images were captured using an optical microscope (DP73; Olympus,
Tokyo, Japan), and the ratio of cardiac tissue fibrosis was
analyzed.
Western blot analysis
The hearts of the rats in each group were harvested
and homogenized in radioimmunoprecipitation (RIPA) lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) with 1%
phenylmethanesulfonyl fluoride (PMSF; Beyotime Institute of
Biotechnology). H9C2 cells were also lysed in RIPA lysis buffer
with 1% PMSF. Protein concentration was determined using a
bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts of protein (40 µg) were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (either 8, 10 or 13% gels). The separated proteins
were then transferred to polyvinylidene fluoride (PVDF; EMD
Millipore, Billerica, MA, USA) membranes. The membranes were
blocked with 5% skimmed milk and were incubated with the
corresponding primary antibodies. The antibodies used were as
follows: Rabbit anti-transforming growth factor-β (TGF-β) (1:200;
cat. no. sc-146 Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
rabbit anti-IκB [nuclear factor (NF)-κB inhibitor] (1:500; cat. no.
bs-1287R), rabbit anti-phosphorylated-IκB (p-IκB; 1:500; cat. no.
bs5515R), rabbit anti-connective tissue growth factor (CTGF; 1:500;
cat. no. bs-0743R), all from BIOSS (Beijing, China), rabbit
anti-collagen-I (Col-I; 1:400; cat. no. BA0325), rabbit anti-matrix
metalloproteinase-2 (MMP-2; 1:400; cat. no. BA0569), MMP-9 (1:400;
cat. no. BA2202) all from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China) and mouse anti-β-actin (1:1,000; cat. no. sc-47778
Santa Cruz Biotechnology, Inc.) at 4°C overnight. Following washing
with Tris-buffered saline-0.05% Tween 20, the membranes were
incubated with either goat anti-rabbit (cat. no. A0208) or goat
anti-mouse (cat. no. A0214) horseradish peroxidase (HRP)-conjugated
secondary antibodies (1:5,000, Beyotime Institute of Biotechnology)
at 37°C for 45 min. Subsequently, the membranes were visualized
using an enhanced chemiluminescence (ECL) detection system and
analyzed with Gel-Pro-Analyzer 4.5 software (Media Cybernetics,
Inc., Rockville, MD, USA). The protein expression levels were
normalized to β-actin.
Enzyme-linked immunosorbent assay
(ELISA)
The concentration of Ang-II in the hearts and serum
was measured by ELISA. Hearts of rats in each group were harvested
and homogenized. The concentration of total protein in the samples
was determined using the BCA protein assay kit, and the
concentration of Ang-II in the heart tissues and serum was detected
using an ELISA kit for Angiotensin II (USCN Life Science, Inc.,
Wuhan, China), according to the manufacturer's protocol.
EMSA
NF-κB activity was detected by EMSA. Following
treatment, the rat hearts and the H9C2 cells from each group were
harvested. The nucleoproteins were extracted using a nucleoprotein
and cytoplasmic protein extraction kit (Beyotime Institute of
Biotechnology), and EMSA was performed using an NF-κB EMSA kit
(Viagene Biotech, Inc., Tampa, FL, USA), according to the
manufacturer's protocols. Briefly, equal amounts of nucleoprotein
from each group were incubated with a biotin-labeled NF-κB probe at
room temperature for 20 min, and were then separated on an EMSA
gel. The separated protein was transferred to PVDF membranes and
cross-linked under a UV transilluminator (EUV002; Beyotime
Institute of Biotechnology). Following incubation with HRP-labeled
streptavidin, the signal was detected with an ECL detection
system.
Statistical analysis
Each experiment was performed three times. The
results are presented as the mean ± standard deviation. Differences
between groups were analyzed using one-way analysis of variance and
Bonferroni's multiple comparison test. Statistical analyses were
performed using GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA) P<0.05 was considered to indicate a
statistically significant difference.
Results
Apelin-13 relieves myocardial
infarction-induced left ventricular dysfunction
Left ventricular function was examined following LAD
ligation and apelin-13 treatment. As presented in Table I, following LAD ligation, LVSP
(P=0.0018), LV+dp/dtmax (P<0.0001) and
LV−dp/dtmax (P=0.0020) were significantly decreased, and
LVEDP was significantly increased (P=0.0015) compared with the sham
group. However, compared with the LAD group, following treatment
with apelin-13, these changes were reversed. These results indicate
that apelin-13 may relieve myocardial infarction-induced left
ventricular dysfunction.
 | Table IPhysiological parameters. |
Table I
Physiological parameters.
| Parameter | Sham | LAD | LAD + Apelin-13 |
|---|
| LVSP (mmHg) | 129.17±16.38 | 96.50±10.97a | 117.67±11.29b |
| LVEDP (mmHg) | 6.43±2.29 | 12.38±2.08a | 7.45±2.60c |
| LV+dp/dt
(mmHg/s) | 5904.67±868.48 |
2722.83±1116.19d |
4468.50±762.94b |
| LV−dp/dt
(mmHg/s) | −5230.83±852.97 |
−2747.33±701.85a |
−4336.83±1351.82b |
Apelin-13 attenuates myocardial
infarction-induced myocardial fibrosis
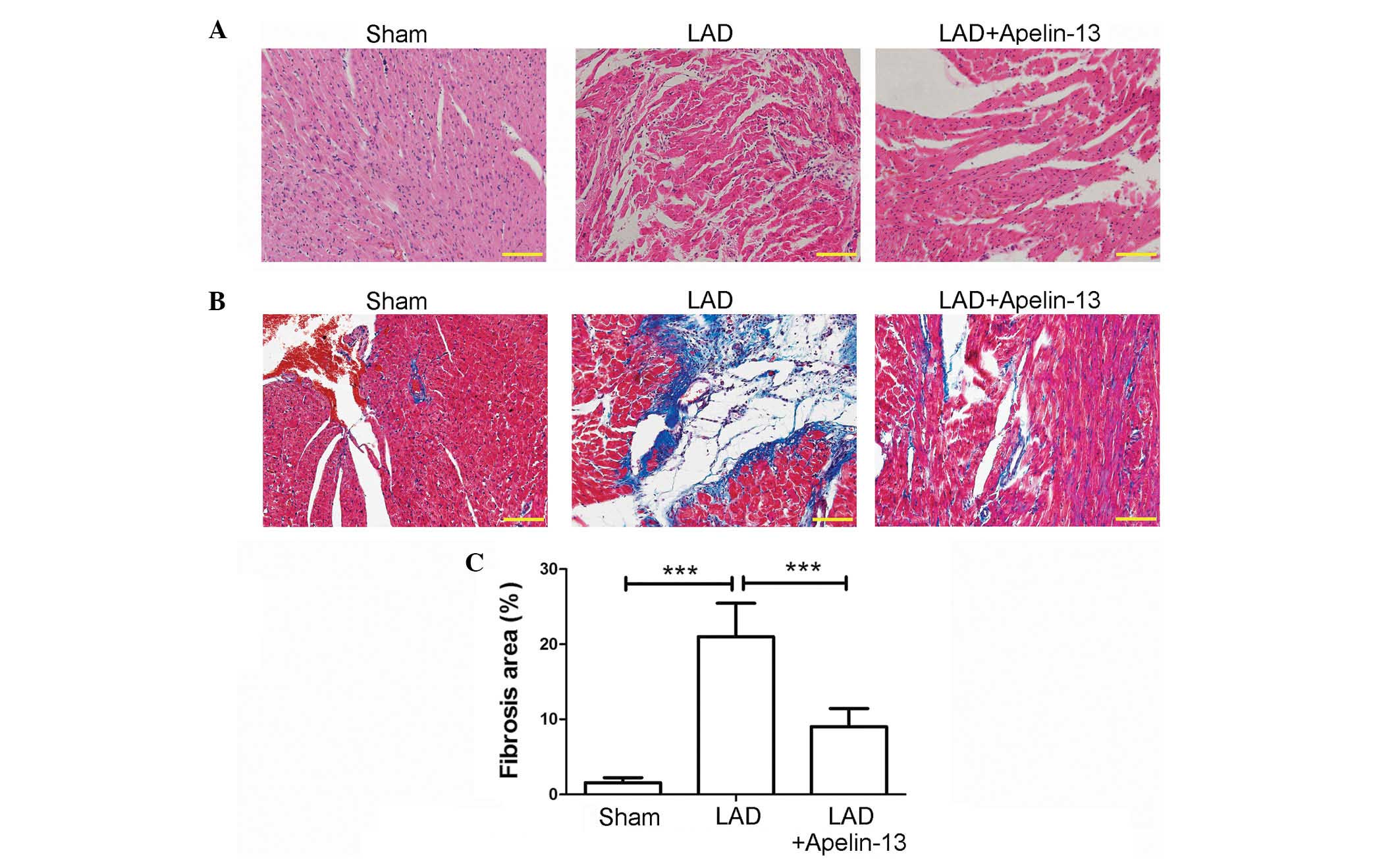
HE staining was used to evaluate histopathological
changes. As demonstrated in Fig.
1A, hearts in the sham group exhibited normal cardiomyocyte
structure, with a clear texture and veins, and plump cytoplasm
(less gaps and spaces between myofilaments). However, hearts in the
LAD group exhibited disordered structure. The myofilaments were
rougher with wave-like changes and the boundary of textures and
veins were unclear. Following treatment with apelin-13 (the LAD +
apelin-13 group), the above histopathological changes were
attenuated compared with the LAD group.
Cardiac fibrosis in each group was evaluated by
Masson's trichrome staining. As demonstrated in Fig. 1B, hearts of rats in the LAD group
exhibited numerous collagenous fibers (blue) compared with the sham
group. However, following treatment with apelin-13, the percentage
of fibrosis was significantly decreased compared with the LAD group
(P<0.0001; Fig. 1B and C).
These results suggest that apelin-13 may attenuate myocardial
infarction-induced fibrosis.
An imbalance between extracellular matrix (ECM) and
matrix-degrading enzymes is the predominant cause of myocardial
fibrosis. TGF-β and CTGF are important factors that promote the
synthesis of collagen and myocardial fibrosis. Col-I is an
important component of the ECM. MMP-2 and MMP-9 are important for
degradation of the ECM. These factors are closely associated with
myocardial fibrosis and were detected by western blotting in the
present study (Fig. 2A). Results
of western blot analysis demonstrated that in the hearts of rats
from the LAD group, the protein expression levels of TGF-β, CTGF,
Col-I, MMP-2 and MMP-9 were significantly increased compared with
the sham group (P=0.0002, P<0.0001, P=0.0007, P=0.0005 and
P=0.0004, respectively; Fig. 2B).
However, following treatment with apelin-13, these changes were
reversed. The results of western blotting and histopathological
analysis suggest that apelin-13 may attenuate myocardial
infarction-induced myocardial fibrosis.
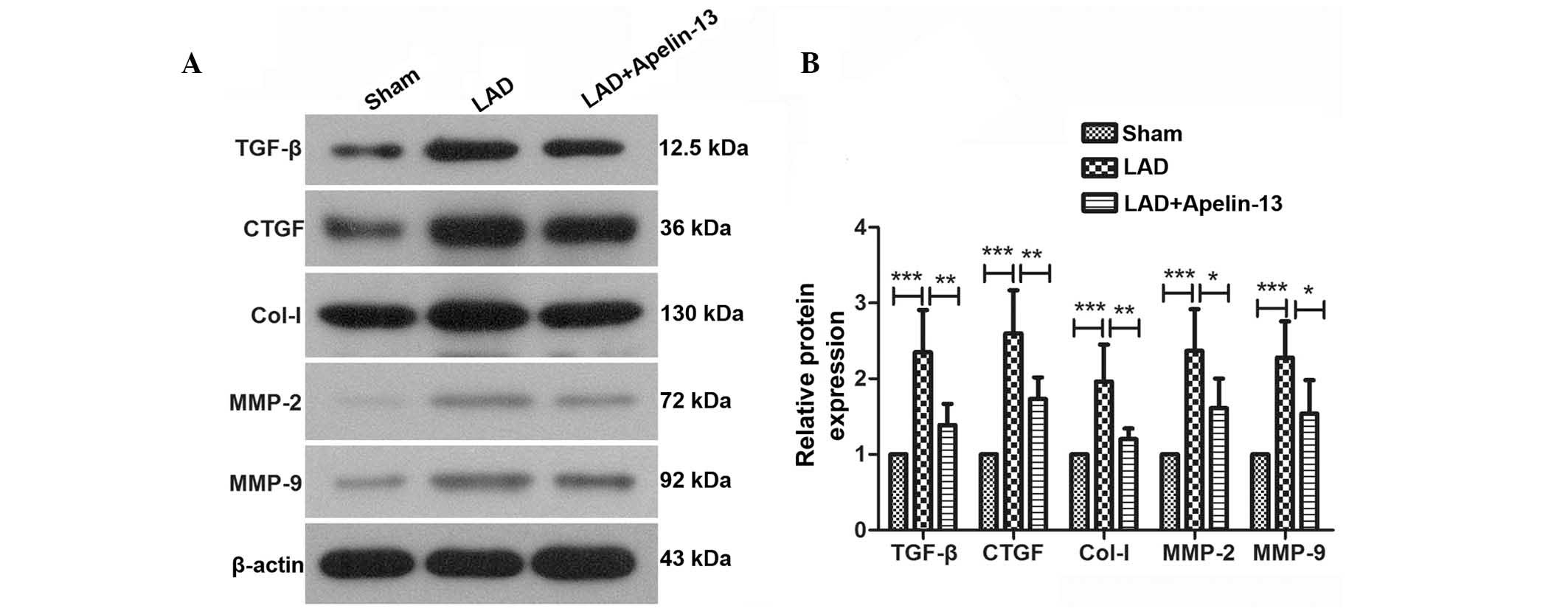 | Figure 2Apelin-13 reduces the protein levels
of fibrosis markers in rats with myocardial infarction. (A) Protein
expression levels of TGF-β, CTGF, Col-I, MMP-2 and MMP-9 were
detected by western blot analysis using β-actin as an internal
reference. (B) Relative protein expression levels were calculated
by densitometric analysis. Typical results are presented. Results
are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001, comparisons indicated by brackets. LAD,
left anterior descending ligation; TGF-β, transforming growth
factor-β; CTGF, connective tissue growth factor; Col-1, collagen-1;
MMP, matrix metalloproteinase. |
Apelin-13 reduces Ang-II levels and
inhibits the activation of NF-κB in rats with myocardial
infarction
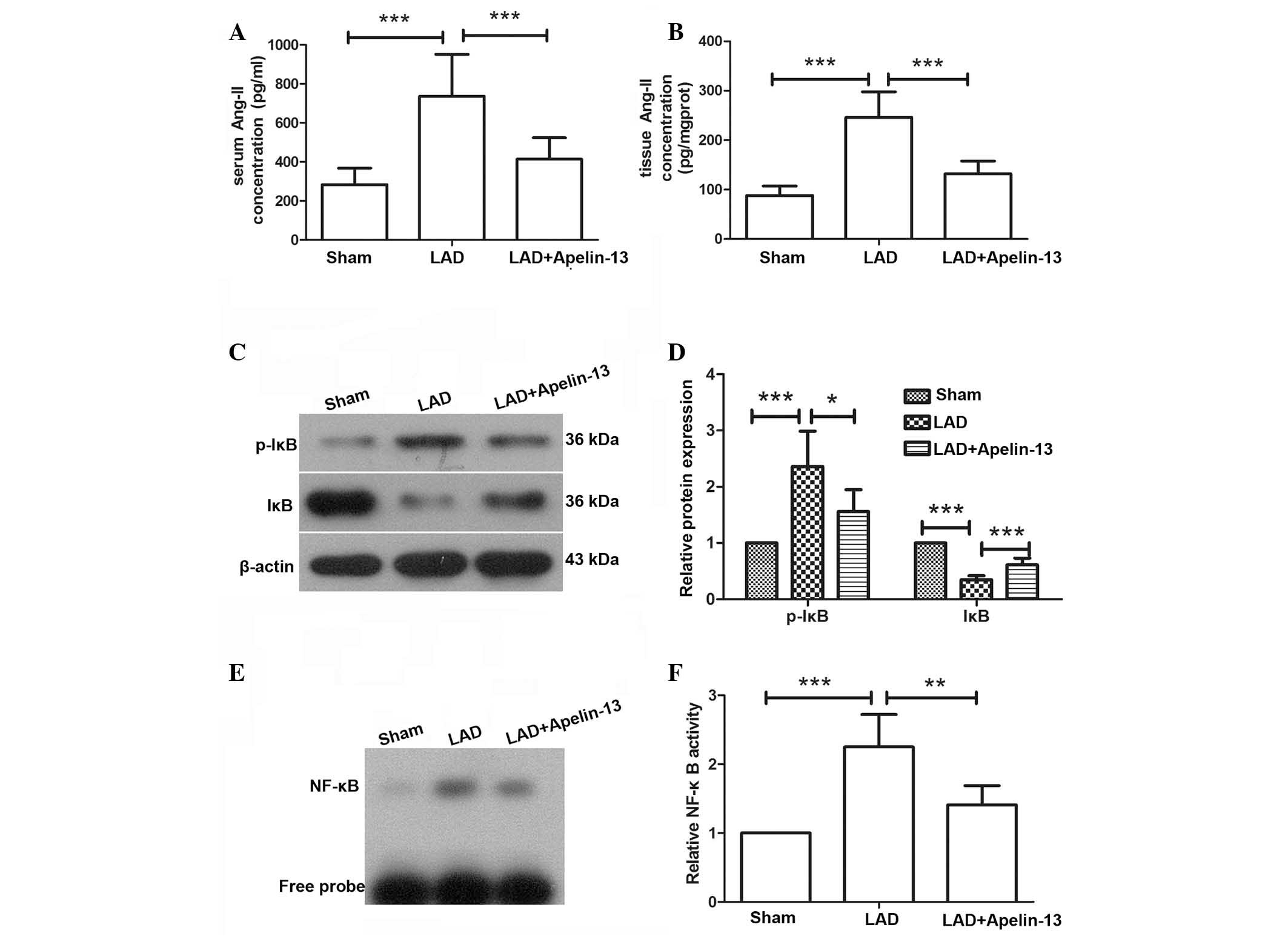
Ang-II was previously demonstrated to be associated
with myocardial fibrosis. The concentration of Ang-II in the serum
and heart tissues was measured by ELISA. In the hearts and serum of
rats in the LAD group, the concentration of Ang-II was increased
compared with rats in the sham group. However, following treatment
with apelin-13, the elevated levels of Ang-II were decreased
compared with the LAD group (P=0.0001 and P=0.0003, respectively;
Fig. 3A and B). These results
suggest that apelin-13 may reduce the increased Ang-II levels
induced by myocardial infarction.
The protein expression and phosphorylation levels of
IκB, which indicates activation of NF-κB signaling, were analyzed
by western blotting. The western blot analysis demonstrated that
the protein levels of IκB were decreased and the phosphorylation
levels of IκB were increased in the LAD group compared with the
control group (P<0.0009); however, following treatment with
apelin-13 the levels were significantly reversed compared with the
LAD group (P<0.0360 and P<0.0008, respectively; Fig. 3C and D). These results indicate
that the NF-κB signaling pathway was activated by LAD ligation and
inhibited after apelin-13 treatment. To further measure the
activation of NF-κB, an EMSA was carried out. As demonstrated in
Fig. 3E, in the LAD group, the
level of NF-κB bound to the NF-κB probe, which indicates the
activity of NF-κB, was significantly increased compared with the
sham group (P=0.0001). However, following treatment with apelin-13,
the level of NF-κB bound to the NF-κB probe was decreased compared
with the LAD group (P=0.0037), thus suggesting that NF-κB activity
was inhibited by apelin-13 (Fig. 3E
and F).
Apelin-13 inhibits the activation of
NF-κB signaling induced by Ang-II in vitro
To further verify the mechanism underlying the
function of apelin-13, in vitro experiments were performed.
The protein levels of TGF-β, CTGF, Col-I, MMP-2 and MMP-9 were
evaluated by western blot analysis. Western blotting results
demonstrated that the protein expression levels of TGF-β
(P=0.0015), CTGF (P=0.0018), Col-I (P=0.002), MMP-2 (P=0.0003) and
MMP-9 (P=0.001) were significantly increased by Ang-II treatment,
which was consistent with the results of western blot in
vivo. However, the elevated protein levels of TGF-β, CTGF,
Col-I, MMP-2 and MMP-9 were significantly decreased following
apelin-13 treatment compared with the Ang-II treatment group
(Fig. 4).
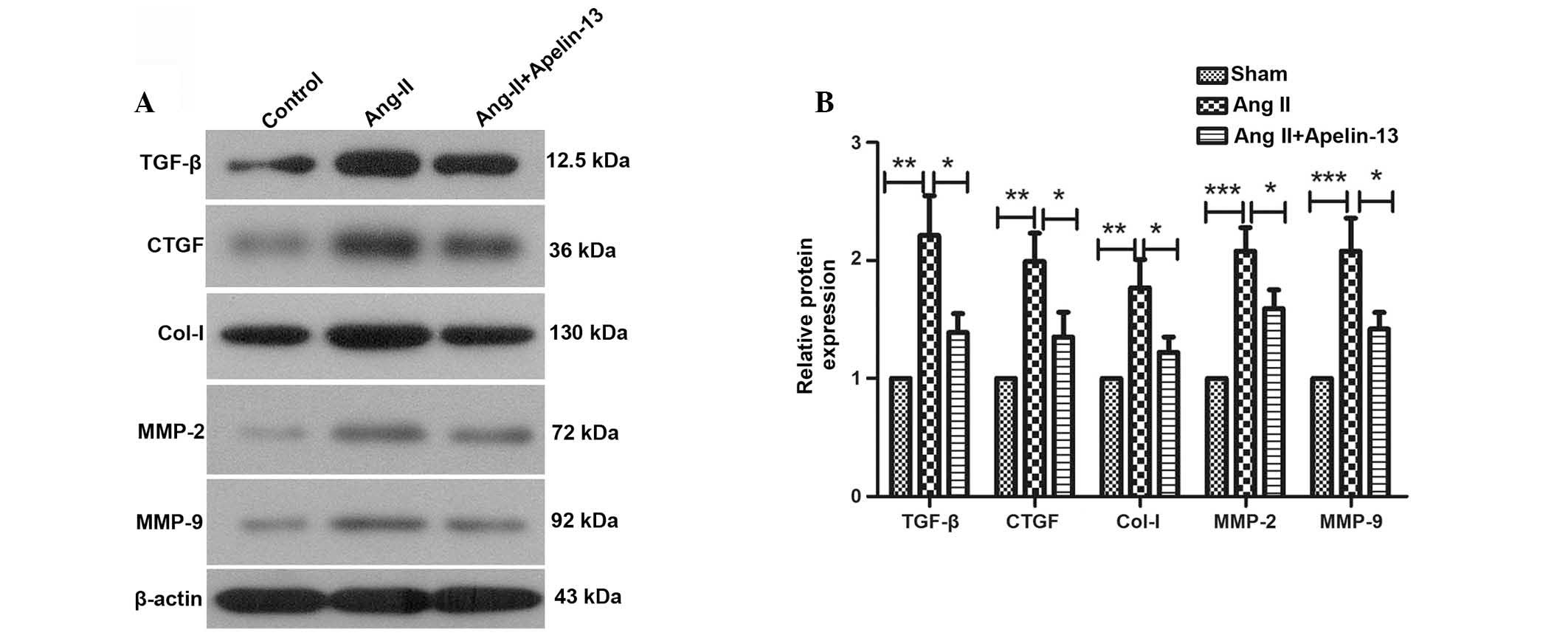 | Figure 4Apelin-13 reduces the protein levels
of fibrosis markers induced by Ang-II. (A) Protein expression
levels of TGF-β, CTGF, Col-I, MMP-2 and MMP-9 were detected by
western blotting using β-actin as an internal reference and (B) the
relative protein levels were calculated. Typical results are
presented. Results are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001, comparisons indicated by brackets.
Ang-II, angiotensin II; TGF-β, transforming growth factor-β; CTGF,
connective tissue growth factor; Col-1, collagen-1; MMP, matrix
metalloproteinase. |
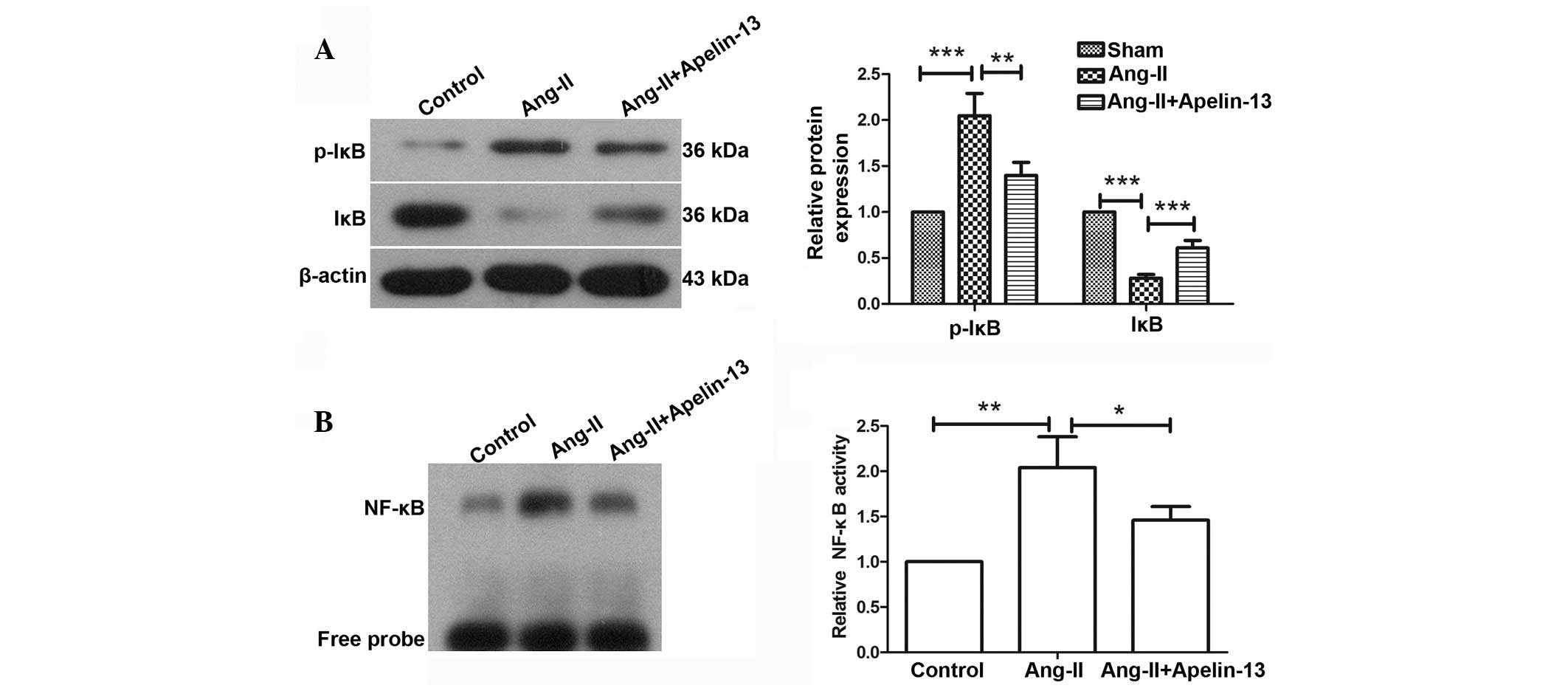
Furthermore, the activation of NF-κB signaling was
detected by western blot analysis and EMSA. The western blotting
results demonstrated that following Ang-II treatment, the
phosphorylation levels of IκB were significantly increased and the
protein expression levels were significantly decreased compared
with the sham group (P=0.0006). However, compared with the Ang-II
group, treatment with apelin-13 attenuated these changes (Fig. 5A and B). EMSA results demonstrated
that compared with the sham group, the levels of NF-κB bound to the
NF-κB probe were significantly increased following Ang-II treatment
(P=0.0031); however, compared with the Ang-II group the levels were
significantly reduced following treatment with apelin-13 (P=0.0486;
Fig. 5B). These results indicate
that the NF-κB signaling pathway was activated by Ang-II, and this
effect was inhibited by apelin-13 treatment. The results of the
present study suggest that apelin-13 may attenuate myocardial
infarction-induced myocardial fibrosis via the regulation of NF-κB
signaling.
Discussion
The present study investigated the effects of
apelin-13 on myocardial infarction-induced myocardial fibrosis. The
study demonstrated that apelin-13 was able to relieve myocardial
fibrosis, and reduce Ang-II levels in heart tissues and serum.
Further mechanistic analyses demonstrated that the cardioprotective
effects of apelin-13 may be mediated via inhibition of NF-κB
signaling.
Apelin was previously reported to protect against
isoproterenol-induced myocardial injury (15), and exhibited cardioprotective
activity in hearts undergoing ischemia and reperfusion (11). Apelin exhibits cardioprotective
effects and the present study demonstrated that treatment with
apelin-13 was able to attenuate myocardial fibrosis. A previous
study also demonstrated that apelin can limit infarct size
(16), and the loss of apelin has
been reported to exacerbate myocardial infarction-associated
adverse remodeling (17).
Myocardial infarction also causes myocardial ischemia. Apelin
enhances the production of NO, dilates vessels and improves blood
supply, thus attenuating myocardial damage (18). Furthermore, apelin-13 can reduce
oxidative injury induced by myocardial ischemia (18) and exerts anti-apoptotic effects.
Apelin activates phosphatidylinositol 3-kinase (PI3K)/v-akt
murine thymoma viral oncogene homolog 1 (AKT) signaling, increases
the expression of B-cell lymphoma-2 (Bcl-2), and inhibits the
expression of Bcl-2-associated X protein, thus reducing cell
apoptosis (19,20), which may also contribute to the
cardioprotective effects of apelin-13.
Cardiac fibrosis is characterized by an imbalance
between ECM proteins and matrix-degrading enzymes, and an excessive
accumulation of ECM. In the present study, the protein expression
levels of CTGF, Col-I, MMP-2 and MMP-9 were elevated following LAD
ligation or Ang-II treatment, but were reduced by apelin-13
treatment. These results provide further evidence indicating that
apelin-13 attenuates myocardial infarction-induced myocardial
fibrosis. A similar effect was also demonstrated on the expression
pattern of TGF-β, a protein that is closely associated with cell
transformation. TGF-β is a crucial mediator of cardiac fibroblast
activation and differentiation into myofibroblasts, which produce a
large amount of collagen (21).
The activation of cardiac fibroblasts and their differentiation
into myofibroblasts are key events in the progression of cardiac
fibrosis. Pretreatment with apelin has previously been reported to
reduce the expression of TGF-β-mediated myofibroblast markers and
collagen production (22).
Ang-II is an important factor involved in cardiac
fibrosis. Ang-II contributes to cardiovascular injury via the
regulation of inflammation and oxidative stress, stimulation of
smooth muscle cells growth (23)
and promotion of ECM formation (24). In the present study, the levels of
Ang-II in heart tissue and serum decreased following apelin-13
treatment, thus indicating that apelin-13 exerts a protective
effect. Siddiquee et al (25) also reported that apelin protects
against Ang-II-induced cardiovascular fibrosis in vivo,
which is consistent with the results of the present study.
Furthermore, apelin-13 was previously reported to promote the
synthesis of NO (3). By contrast
with the effects of Ang-II, NO induces vasodilatation and lowers
blood pressure.
Apelin was previously reported to perform a
protective effect against ischemia-reperfusion injury via the
regulation of the PI3K/AKT, extracellular signal-regulated kinase
and mitogen-activated protein kinase signaling pathways (26–28).
NF-κB is a multifunctional nuclear factor associated with cell
growth and inflammatory responses. The present study demonstrated
that apelin-13 inhibits the activation of NF-κB signaling. This
indicates that apelin-13 may exert its cardio-protective effect
through the regulation of NF-κB, however further investigation is
required to confirm this hypothesis.
In conclusion, myocardial infarction-induced
myocardial ischemia leads to the death of cardiomyocytes and
fibrotic lesions. The expression and secretion of apelin was
previously reported to be increased in ischemic myocardium
(29), and the present study
demonstrated that apelin exerted a cardioprotective effect. The
current study demonstrated that treatment with apelin-13 may
attenuate myocardial infarction-induced myocardial fibrosis, and
this cardioprotective effect may be mediated via regulation of
NF-κB signaling. The present study provides a theoretical basis for
further exploration into apelin, and provides information regarding
the clinical application of apelin-13.
Acknowledgments
The present study was supported by a grant from the
Special Foundation for Science and Technology Innovation of
Shenyang City (grant no. F13-220-9-42).
References
|
1
|
Rosamond W, Flegal K, Friday G, Furie K,
Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, et al
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart- disease and stroke statistics -
2007 update: A report from the American Heart Association
Statistics Committee and Stroke Statistics Subcommittee.
Circulation. 115:e69–e171. 2007. View Article : Google Scholar
|
|
2
|
Quazi R, Palaniswamy C and Frishman WH:
The emerging role of apelin in cardiovascular disease and health.
Cardiol Rev. 17:283–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH,
Zhao J, Yu F, Duan XH, Tang CS and Qi YF: Apelin activates
L-arginine/nitric oxide synthase/nitric oxide pathway in rat
aortas. Peptides. 28:2023–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japp AG and Newby DE: The apelin-APJ
system in heart failure: Pathophysiologic relevance and therapeutic
potential. Biochem Pharmacol. 75:1882–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tatemoto K, Takayama K, Zou MX, Kumaki I,
Zhang W, Kumano K and Fujimiya M: The novel peptide apelin lowers
blood pressure via a nitric oxide-dependent mechanism. Regul Pept.
99:87–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashley EA, Powers J, Chen M, Kundu R,
Finsterbach T, Caffarelli A, Deng A, Eichhorn J, Mahajan R, Agrawal
R, et al: The endogenous peptide apelin potently improves cardiac
contractility and reduces cardiac loading in vivo. Cardiovasc Res.
65:73–82. 2005. View Article : Google Scholar
|
|
7
|
Berry MF, Pirolli TJ, Jayasankar V,
Burdick J, Morine KJ, Gardner TJ and Woo YJ: Apelin has in vivo
inotropic effects on normal and failing hearts. Circulation. 110(11
Suppl 1): II187–II193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szokodi I, Tavi P, Földes G,
Voutilainen-Myllylä S, Ilves M, Tokola H, Pikkarainen S, Piuhola J,
Rysä J, Tóth M and Ruskoaho H: Apelin, the novel endogenous ligand
of the orphan receptor APJ, regulates cardiac contractility. Circ
Res. 91:434–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chandrasekaran B, Dar O and McDonagh T:
The role of apelin in cardiovascular function and heart failure.
Eur J Heart Fail. 10:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng XJ, Zhang LK, Wang HX, Lu LQ, Ma LQ
and Tang CS: Apelin protects heart against ischemia/reperfusion
injury in rat. Peptides. 30:1144–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao J, Zhu W, Li Y, Xin P, Li J, Liu M, Li
J, Redington AN and Wei M: Apelin-13 protects the heart against
ischemia-reperfusion injury through inhibition of ER-dependent
apoptotic pathways in a time-dependent fashion. Am J Physiol Heart
Circ Physiol. 301:H1471–H1486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barnes G, Japp AG and Newby DE:
Translational promise of the apelin - APJ system. Heart.
96:1011–1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patten RD, Aronovitz MJ, Deras-Mejia L,
Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME and Konstam MA:
Ventricular remodeling in a mouse model of myocardial infarction.
Am J Physiol. 274:H1812–H1820. 1998.PubMed/NCBI
|
|
14
|
Ahn D, Cheng L, Moon C, Spurgeon H,
Lakatta EG and Talan MI: Induction of myocardial infarcts of a
predictable size and location by branch pattern
probability-assisted coronary ligation in C57BL/6 mice. Am J
Physiol Heart Circ Physiol. 286:H1201–H1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia YX, Pan CS, Zhang J, Geng B, Zhao J,
Gerns H, Yang J, Chang JK, Tang CS and Qi YF: Apelin protects
myocardial injury induced by isoproterenol in rats. Regul Pept.
133:147–154. 2006. View Article : Google Scholar
|
|
16
|
Rastaldo R, Cappello S, Folino A, Berta
GN, Sprio AE, Losano G, Samaja M and Pagliaro P: Apelin-13 limits
infarct size and improves cardiac postischemic mechanical recovery
only if given after ischemia. Am J Physiol Heart Circ Physiol.
300:H2308–H2315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, McKinnie SM, Patel VB, Haddad G,
Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, et al:
Loss of Apelin exacerbates myocardial infarction adverse remodeling
and ischemia-reperfusion injury: Therapeutic potential of synthetic
Apelin analogues. J Am Heart Assoc. 2:e0002492013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azizi Y, Faghihi M, Imani A, Roghani M and
Nazari A: Post-infarct treatment with [Pyr1]-apelin-13 reduces
myocardial damage through reduction of oxidative injury and nitric
oxide enhancement in the rat model of myocardial infarction.
Peptides. 46:76–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui RR, Mao DA, Yi L, Wang C, Zhang XX,
Xie H, Wu XP, Liao XB, Zhou H, Meng JC, et al: Apelin suppresses
apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt
signaling pathways. Amino Acids. 39:1193–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eskew RT Jr, Stromeyer CF III, Picotte CJ
and Kronauer RE: Detection uncertainty and the facilitation of
chromatic detection by luminance contours. J Opt Soc Am A.
8:394–403. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pchejetski D, Foussal C, Alfarano C,
Lairez O, Calise D, Guilbeau-Frugier C, Schaak S, Seguelas MH,
Wanecq E, Valet P, et al: Apelin prevents cardiac fibroblast
activation and collagen production through inhibition of
sphingosine kinase 1. Eur Heart J. 33:2360–2369. 2012. View Article : Google Scholar
|
|
23
|
Griendling KK, Tsuda T, Berk BC and
Alexander RW: Angiotensin II stimulation of vascular smooth muscle
cells. Secondary signalling mechanisms. Am J Hypertens. 2:659–665.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kagami S, Border WA, Miller DE and Noble
NA: Angiotensin II stimulates extracellular matrix protein
synthesis through induction of transforming growth factor-beta
expression in rat glomerular mesangial cells. J Clin Invest.
93:2431–2437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siddiquee K, Hampton J, Khan S, Zadory D,
Gleaves L, Vaughan DE and Smith LH: Apelin protects against
angiotensin II-induced cardiovascular fibrosis and decreases
plasminogen activator inhibitor type-1 production. J Hypertens.
29:724–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kleinz MJ and Baxter GF: Apelin reduces
myocardial reperfusion injury independently of PI3K/Akt and P70S6
kinase. Regul Pept. 146:271–277. 2008. View Article : Google Scholar
|
|
27
|
Simpkin JC, Yellon DM, Davidson SM, Lim
SY, Wynne AM and Smith CC: Apelin-13 and apelin-36 exhibit direct
cardioprotective activity against ischemia-reperfusion injury.
Basic Res Cardiol. 102:518–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Zhang X, Cui H, Zhang C, Zhu C and
Li L: Apelin-13 protects the brain against ischemia/reperfusion
injury through activating PI3K/Akt and ERK1/2 signaling pathways.
Neurosci Lett. 568:44–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Atluri P, Morine KJ, Liao GP, Panlilio CM,
Berry MF, Hsu VM, Hiesinger W, Cohen JE and Joseph Woo Y: Ischemic
heart failure enhances endogenous myocardial apelin and APJ
receptor expression. Cell Mol Biol Lett. 12:127–138. 2007.
View Article : Google Scholar
|