Introduction
Cancer is a major cause of mortality, and colorectal
cancer (CRC) is the third most common cancer in men and women
worldwide, representing a leading cause of cancer-associated
mortality (1). The majority of
human cancer-associated deaths are linked with metastasis.
Metastasis is a complex process, involving the spread of malignant
tumor cells from a primary tumor site to a secondary organ, and
colonization of the distant organ.
The metastasis of CRC results from the interactions
among a number of genetic and environmental factors. Multiple
signal transduction pathways are involved in the process, including
the nuclear factor-κB (NF-κB) (2,3) and
the focal adhesion kinase (FAK) (4) pathways, and so forth. The NF-κB
pathway exerts an important role in the process of tumor invasion
and metastasis. The NF-κB family of transcription factors consists
of five members: p65 (or RelA), RelB, c-Rel, NF-κB 1 (or p105; a
precursor of p50) and NF-κB 2 (or p100; a precursor of p52)
(5–7). To date, two principal pathways for
NF-κB activation have been characterized: A classical and an
alternative pathway (6,8).
NIBP, a novel NF-κB-inducing kinase (NIK) and IκB
kinase β (IKKβ) binding protein, directly interacts with NIK and
IKKβ, and is required for cytokine-induced NF-κB activation. The
overexpression of NIBP increased the expression of IKKβ and NIK
(9,10). The activated IKK complex
phosphorylates IκB molecules to induce their ubiquitination and
degradation, resulting in the translocation of NF-κB dimers
(predominantly p65/p50) to the nucleus and the transcriptional
activation of specific target genes. This pathway is termed the
NF-κB classical pathway. In the NF-κB alternative pathway, NIK
activates IκB kinase α (I K K-α) to induce the processing of p100,
generating the p52 subunit, which leads to nuclear translocation of
RelB/p52 dimers to regulate specific target gene expression. At
present, studies of NIBP have largely concentrated on nerve cells,
and the protein has been studied only infrequently in tumor cells.
A previous study by our laboratory identified that there was a
certain basal expression level of NIBP in colon cancer cells
(11). Therefore, it was
hypothesized that NIBP may affect the invasive and metastatic
abilities of tumor cells by regulating the NF-κB signaling
pathway.
E-cadherin and vimentin are important markers of the
epithelial-to-mesenchymal transition (EMT). EMT is a critical
process by which epithelial cells lose their polarity, and are
converted into the mesenchymal phenotype. The hallmarks of EMT are
a loss of epithelial cell markers, including E-cadherin, and an
increased expression of mesenchymal markers, including vimentin and
fibronectin (12,13). EMT enhances the ability of cancer
cells to migrate to, invade, increase apoptosis in and degrade
cells of the extracellular matrix. It is crucial for a variety of
original and early metastases of tumor cells. In recent years, a
burgeoning body of evidence has identified that EMT exerts an
important role in the invasion and metastasis of colon cancer
(14,15).
Previous studies have shown that, on activation of
the NF-κB pathway, the expression levels of E-cadherin decreased,
whereas that of vimentin increased (16,17).
In the light of these results, it was hypothesized that NIBP may
exert an influence on the expression levels of E-cadherin, CD44 and
vimentin in CRC cells through the NF-κB classical and alternative
pathways. In the present study, the correlation between NIBP,
E-cadherin, CD44, vimentin and tumor progression in patients with
CRC was examined. To investigate this hypothesis, gene transfection
experiments were used to control the expression of NIBP in order to
observe any influence on the expression of E-cadherin, CD44 and
vimentin in colon cancer cells.
Materials and methods
Patients and tissues
Randomly selected paired tissues of CRC and
corresponding normal tissues were obtained from patients (n=114)
who underwent surgical resection at the First Affiliated Hospital
of Guangxi Medical University (Guangxi, China) between March and
October 2013. Written informed consent was obtained from each
patient prior to surgery and the study protocol was approved by the
Institutional Review Board for Human Genetic and Genomic Research
of the First Affiliated Hospital of Guangxi Medical University.
Every sample had undergone an immunohistochemical analysis in a
previous study (11).
Cell culture and lentivirus
infection
HT29 and HCT116 cells were obtained from Shanghai
R&S Biotechnology Co. (Shanghai, China). HT29 is a cell line
that expresses small quantities of the NIBP protein, whereas HCT116
cells express higher levels. All cell lines were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Waltham, MA, USA) with 10% fetal bovine serum (Excell
Bio, Shanghai, China) at 37° C under an atmosphere of 5%
CO2.
Groups of cells were used for the various
experiments as follows: the non-specific control group (29-NC
group) and NIBP upregulated expression group (29-NIBP group) of the
HT29 cell line, and the non-specific control group (116-NC group)
and NIBP downregulated expression group (116-NIBPmir group) of the
HCT116 cell line. The stable NIBP-overexpressing (11) and underexpressing (18) cell lines were transfected and
obtained by fluorescence-activated cell sorting in previous
studies.
Western blot analysis
Proteins were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel (Beyotime Institute of Biotechnology,
Haimen, China) electrophoresis for 1 h at 100 V, and transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were subsequently blocked with non-fat milk
[5% in Tris-buffered saline with Tween-20; TBST (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China)] buffer at room
temperature for 1 h to block non-specific binding, and were
subsequently incubated overnight with antibodies diluting with WB
Antibody Diluent (Beyotime Institute of Biotechnology) at 4° C.
Subsequently, conjugated secondary antibodies were incubated for 1
h at room temperature. Membranes were scanned and analyzed using an
Odyssey® CLx Infrared Imaging system (LI-COR
Biosciences, Lincoln, NE, USA). Relevant signal intensities were
determined using LI-COR imaging software. Rabbit monoclonal
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1,000; cat.
no. 2118) and rabbit monoclonal anti-E-cadherin (1:1,000; cat. no.
3195) antibodies were obtained from Cell Signaling Technology
(Danvers, MA, USA); rabbit polyclonal anti-vimentin (1:500; cat.
no. 10366-1-AP) was obtained from ProteinTech Group, Inc. (Chicago,
IL, USA), and rabbit polyclonal anti-CD44 (1:500) was obtained from
BIOSS (Beijing, China; cat. no. bs-0521R) and ProteinTech Group,
Inc. (cat. no. 15675 1-AP).
Statistical analysis
Each experiment was performed at least three times.
Differences between groups and controls were assessed using the
Student's t-test and univariate analysis. P<0.0167 (0.05/3) was
considered to indicate a statistically significant value in the
clinical samples. For the in vitro experiments, relative
fold changes in protein expression were calculated following
normalization against GAPDH; in these cases, *P< 0.05
was considered to indicate a statistically significant value.
Results
The association between NIBP and
E-cadherin, CD44, vimentin in clinical samples
In a previous study, every sample had undergone an
immunohistochemical analysis to examine the expression levels of
NIBP and E-cadherin, CD44 and vimentin (Table I) (19). In the present study, these data
were analyzed from another aspect. The pairwise comparisons with
normal colon tissue and non-metastatic colon cancer (Table II), normal colon tissue and
metastatic colon cancer (Table
III), and non-metastatic colon cancer and metastatic colon
cancer (Table IV) are shown.
 | Table INegative and positive cases of NIBP
and E-cadherin, CD44 and vimentin protein in clinical samples
assessed by immunohistochemistry analysis. |
Table I
Negative and positive cases of NIBP
and E-cadherin, CD44 and vimentin protein in clinical samples
assessed by immunohistochemistry analysis.
| Tissue | No. of cases | NIBP
| CD44
| Vimentin
| E-cadherin
|
|---|
| − | + | − | + | − | + | − | + |
|---|
| Normal colon | 50 | 40 | 10 | 37 | 13 | 42 | 8 | 9 | 41 |
| Non-metastatic colon
cancer | 63 | 33 | 30 | 35 | 28 | 40 | 23 | 15 | 48 |
| Metastatic colon
cancer | 51 | 14 | 37 | 17 | 34 | 19 | 32 | 27 | 24 |
 | Table IIExpression of NIBP and CD44, vimentin
and E-cadherin in normal colon tissue and non-metastatic colon
cancer. |
Table II
Expression of NIBP and CD44, vimentin
and E-cadherin in normal colon tissue and non-metastatic colon
cancer.
| Tissue | No. of cases | NIBP
| CD44
| Vimentin
| E-cadherin
|
|---|
| − | + | P | − | + | P | − | + | P | − | + | P |
|---|
| Normal colon | 50 | 40 | 10 | | 37 | 13 | | 42 | 8 | | 9 | 41 | |
| Non-metastatic
colon cancer | 63 | 33 | 30 | 0.002 | 35 | 28 | 0.043 | 40 | 23 | 0.015 | 15 | 48 | 0.453 |
 | Table IIIExpression of NIBP and CD44, vimentin
and E-cadherin in normal colon tissue and metastatic colon
cancer. |
Table III
Expression of NIBP and CD44, vimentin
and E-cadherin in normal colon tissue and metastatic colon
cancer.
| Tissue | No. of cases | NIBP
| CD44
| Vimentin
| E-cadherin
|
|---|
| − | + | P | − | + | P | − | + | P | − | + | P |
|---|
| Normal colon | 50 | 40 | 10 | <0.001 | 37 | 13 | <0.001 | 42 | 8 | <0.001 | 9 | 41 | <0.001 |
| Metastatic colon
cancer | 51 | 14 | 37 | | 17 | 34 | | 19 | 32 | | 27 | 24 | |
 | Table IVExpression of NIBP and CD44, vimentin
and E-cadherin in non-metastatic colon cancer and metastatic colon
cancer. |
Table IV
Expression of NIBP and CD44, vimentin
and E-cadherin in non-metastatic colon cancer and metastatic colon
cancer.
| Tissue | No. of cases | NIBP
| CD44
| Vimentin
| E-cadherin
|
|---|
| − | + | P | − | + | P | − | + | P | − | + | P |
|---|
| Metastatic colon
cancer | 51 | 14 | 37 | 0.007 | 17 | 34 | 0.018 | 19 | 32 | 0.005 | 27 | 24 | 0.001 |
| Non-metastatic
colon cancer | 63 | 33 | 30 | | 35 | 28 | | 40 | 23 | | 15 | 48 | |
These results demonstrated that the levels of NIBP,
CD44 and vimentin significantly increased, and that of E-cadherin
significantly decreased, in metastatic colon cancer compared with
normal colon tissue and non-metastatic colon cancer, indicating
that NIBP, E-cadherin, CD44 and vimentin are possibly associated
with metastasis in colon cancer. However, it remained to be
elucidated whether NIBP could impact on the expression levels of
E-cadherin, CD44 and vimentin.
The influence of different expression
levels of NIBP on expression of E-cadherin, CD44 and vimentin in
CRC cell lines
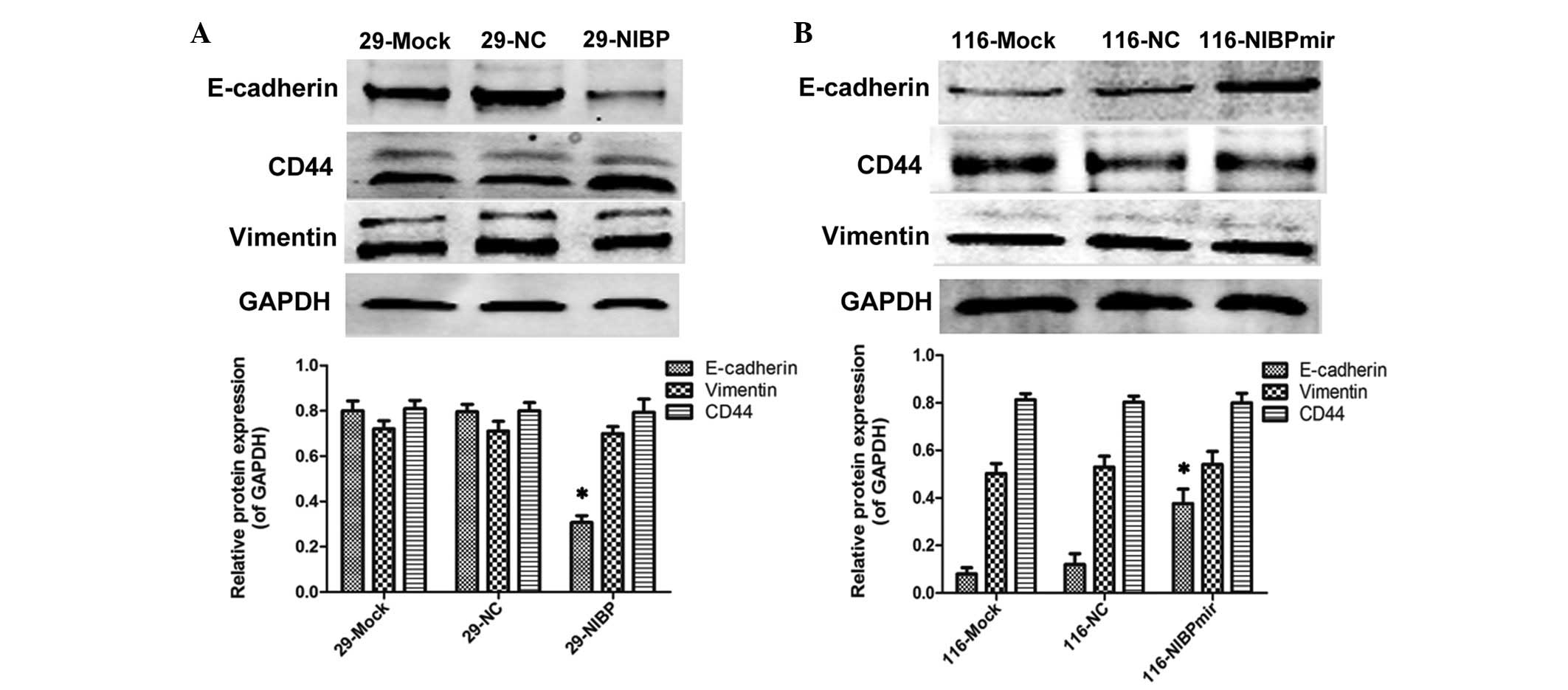
In an in vitro experiment, western blotting
was used to assess the expression of CD44, vimentin and E-cadherin
protein in the HT29 blank control group (29-mock group), the 29-NC
and 29-NIBP groups (Fig. 1A). The
identical experiment was performed with the HCT116 blank control
group (116-mock group) and the 116-NC and 116-NIBPmir groups
(Fig. 1B).
The results revealed that the upregulation of NIBP
decreased the levels of E-cadherin, whereas downregulating the
expression of NIBP increased E-cadherin. A previous study
demonstrated that the activated NF-κB classical pathway decreased
E-cadherin expression (16,20,21).
The data in the present study also confirmed that NIBP fulfilled
the role of activator in the NF-κB classical pathway (9). On the other hand, no significant
differences were observed in the levels of CD44 and vimentin
according to the different expression levels of NIBP in the present
study (Fig. 1A and B). A previous
study demonstrated that activation of the NF-κB alternative pathway
led to an induction of the upregulation of CD44, and EMT
contributed to an increased cancer cell invasiveness (22).
Furthermore, other studies revealed that CD44
upregulated the expression of vimentin, whereas knockdown of CD44
decreased the expression of vimentin (23–25).
Therefore, the results in the present study suggested that the
effects of NIBP are exerted predominantly through the regulation of
the NF-κB classical pathway, rather than the alternative pathway,
when the NF-κB pathway was not subjected to any interventions.
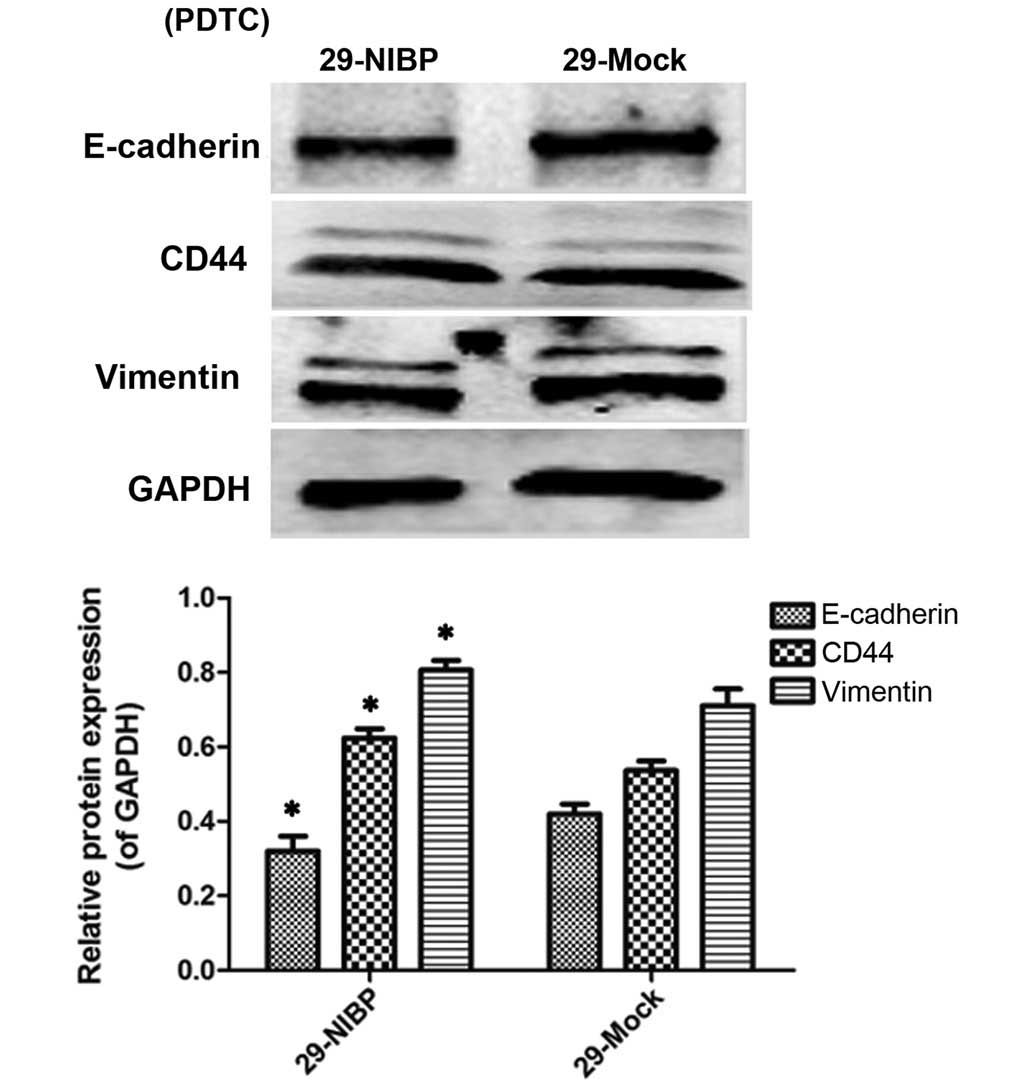
Effects of PDTC on the expression levels
of CD44, vimentin and E-cadherin in the NF-κB pathway in HT29
cells
On treating the 29-mock and 29-NIBP groups with PDTC
(Fig. 2), an NF-κB classical
pathway inhibitor, the expression levels of CD44 and vimentin
tended to increase in 29-NIBP group, whereas that of E-cadherin
decreased.
As NIBP is considered to act as the ʻbridgeʼ of the
NF-κB classical and alternative pathways (9), it was hypothesized that NIBP may
predominantly activate the NF-κB alternative pathway following the
complete inhibition of the classical pathway. Therefore, on
treatment of the cells with PDTC, the expression levels of CD44 and
vimentin were revealed to be increased in the 29-NIBP group
compared with the 29-mock group.
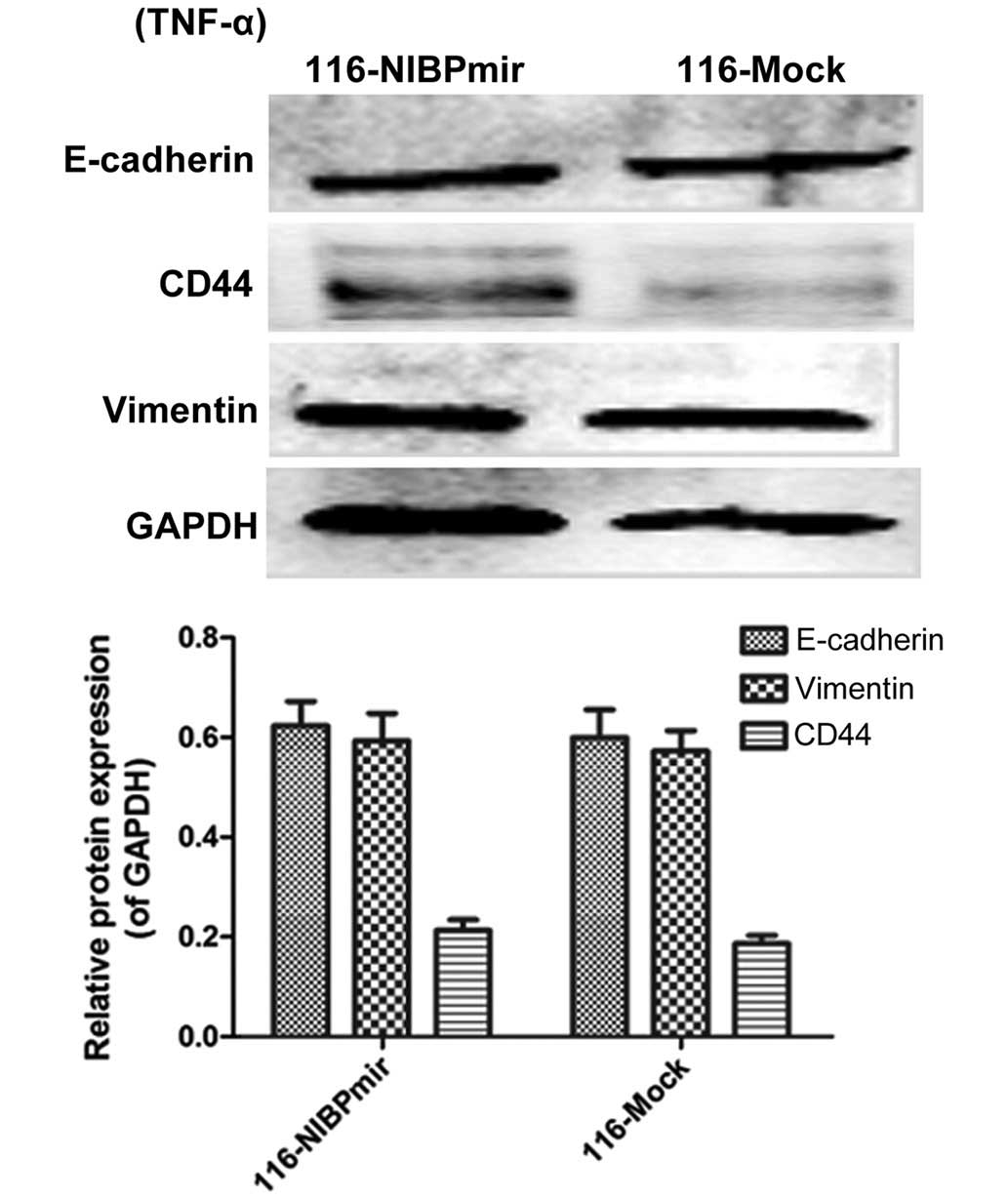
Downregulation of the expression of NIBP
disturbs TNF-α-induced NF-κB activation in HCT116 cells
The 116-mock and 116-NIBPmir groups were treated
with TNF-α (Fig. 3), an NF-κB
classical pathway activator, revealing that the expression levels
of E-cadherin, CD44 and vimentin were similar between the two
groups. Since downregulation of the expression of NIBP increased
E-cadherin levels in the HCT116 cell line without treatment with
TNF-α (Fig. 1B), this suggested
that TNF-α could impact on the NF-κB classical pathway, but was not
able to influence the NF-κB alternative pathway when NIBP was
silenced in cells, revealing that NIBP acts as the bridge between
the NF-κB classical and alternative pathways.
Discussion
A major challenge during cancer therapy is
metastasis. However, the underlying mechanisms have not been
entirely elucidated. Metastasis is one of the fundamental
characteristics of malignant tumors, as well as the predominant
cause of treatment failure for most types of carcinoma (26,27).
EMT is the important biological process, which enables malignant
tumor epithelial cells to acquire their migrational and invasive
abilities. Furthermore, increasing the expression of vimentin, and
decreasing the expression of E-cadherin, are its predominant
features (12,28,29).
The present study revealed that the expression of vimentin
increased significantly, and that of E-cadherin decreased
significantly, in colon cancer tissues. It also revealed that
E-cadherin and vimentin are closely linked with invasion and
metastasis in colon cancer. The results obtained are consistent
with those of previously published studies (30,31).
NIBP acts as the ʻbridgeʼ of NF-κB classical and
alternative pathways, and NIBP is involved in regulating the
classical and alternative pathways. The present study demonstrated
showed that there were plentiful quantities of NIBP in metastatic
and non-metastatic colon cancer tissues compared with normal
tissue, suggesting that NIBP may be involved in the occurrence and
development of colon cancer. Therefore, it was surmised that NIBP
possibly exerts an important role in impacting on the expression of
E-cadherin and vimentin via the NF-κB classical and alternative
pathways in colon cancer cells.
A previous study has shown that the levels of
phosphorylated p65 increased on activation of the NF-κB classical
pathway (32). Subsequently, the
phosphorylated p65 was transported into the nucleus to regulate
gene transcription, promoting the expression of snail (33,34),
one of the important transcription factors associated with EMT,
thereby suppressing the expression of E-cadherin. Furthermore,
activation of the NF-κB alternative pathway led to an increase in
the expression of CD44, a stem-cell-surface molecule (35). Vimentin expression decreased when
the expression of CD44 was knocked down (25). In order to further confirm that
NIBP has a role as an activator of the NF-κB classical pathway
(9), HT29 cells were transfected
to upregulate NIBP, which demonstrated that the expression of
E-cadherin was markedly decreased, in accordance with the
anticipated result. However, it was interesting to note that the
expression levels of CD44 and vimentin were not significantly
altered, demonstrating that NIBP predominantly regulated the NF-κB
classical pathway, and exerted almost no influence on the
alternative pathway in the absence of drugs to completely inhibit
the classic pathway.
Furthermore, the cells were treated with PDTC to
completely inhibit the NF-κB classical pathway. This revealed that
the expression levels of CD44 and vimentin in the 29-NIBP group
were also higher compared with the 29-mock group, suggesting that
NIBP may be able to activate the alternative pathway when the
classical pathway is blocked. Furthermore, the expression levels of
E-cadherin in the 29-NIBP group were revealed to be lower compared
with the 29-mock group, indicating that PDTC had failed to
completely inhibit the NF-κB classical pathway when NIBP was
upregulated in cells. These results, in part, may explain why
certain previously published studies have shown that
chemotherapeutic drugs, such as bortezomib and MLN120B, which
predominantly suppress IKKβ, one of the key factors of the NF-κB
classical pathway, were also able to induce activation of NF-κB
p65, leading to a poor curative response for patients (36,37).
To further reveal the importance of NIBP in the
NF-κB pathway, NIBP was knocked down in HCT116 cells, the highly
aggressive colon cancer cell line. These results demonstrated that
the expression of E-cadherin was increased, providing further
evidence in support of the premise that NIBP predominantly
regulates the NF-κB classical pathway in the absence of drugs which
would block it, and that knocking down NIBP led to an inhibition of
the classical pathway. Treating the cells with TNF-α, to induce the
NF-κB classical pathway, revealed a paralleled expression of
E-cadherin, CD44 and vimentin in the 116-NIBPmir and 116-mock
groups. These results demonstrated that TNF-α was able to impact on
the NF-κB classical pathway, although it was not able to influence
the NF-κB alternative pathway when NIBP was silenced in cells,
revealing that NIBP acts as the ʻbridgeʼ between the NF-κB
classical and alternative pathways.
In conclusion, the findings of the present study
have revealed that upregulating the activity of NIBP leads to a
regulation of the expression of E-cadherin, CD44 and vimentin in
CRC via the NF-κB classical and alternative pathways. These
findings have provided a possible rationale for the development of
NIBP inhibitors, which may form part of the therapeutic regimen for
patients with CRC in future.
Acknowledgments
This work was supported in part by grants from the
National Natural Science Foundation of China (nos. 81260365 and
81460380), the Nature Science Foundation of Guangxi (no.
2013GXNSFAA019159) and the Traditional Chinese Medicine Ethnic
Medicine Self-financing scientific research subject of Guangxi (no.
GZZC14-57). Those responsible for funding this study had no
involvement in study design, data collection or analysis, the
decision to publish, or preparation of the manuscript.
References
|
1
|
Cancer Facts & Figures 2015. American
Cancer Society; 2015
|
|
2
|
Wang F, Yang JL, Yu KK, Xu M, Xu YZ, Chen
L, Lu YM, Fang HS, Wang XY, Hu ZQ, et al: Activation of the NF-κB
pathway as a mechanism of alcohol enhanced progression and
metastasis of human hepatocellular carcinoma. Mol Cancer.
14:102015. View Article : Google Scholar
|
|
3
|
Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D,
Wu Y, Yin J, Xie X, Shen J, et al: Downregulation of MCT1 inhibits
tumor growth, metastasis and enhances chemotherapeutic efficacy in
osteosarcoma through regulation of the NF-κB pathway. Cancer Lett.
342:150–158. 2014. View Article : Google Scholar
|
|
4
|
Zhang LL, Liu J, Lei S, Zhang J, Zhou W
and Yu HG: PTEN inhibits the invasion and metastasis of gastric
cancer via down-regulation of FAK expression. Cell Signal.
26:1011–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perkins ND: Oncogenes, tumor suppressors
and p52 NF-kappaB. Oncogene. 22:7553–7556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patke A, Mecklenbräuker I and Tarakhovsky
A: Survival signaling in resting B cells. Curr Opin Immunol.
16:251–255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demchenko YN, Glebov OK, Zingone A, Keats
JJ, Bergsagel PL and Kuehl WM: Classical and/or alternative
NF-kappaB pathway activation in multiple myeloma. Blood.
115:3541–3552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu WH, Pendergast JS, Mo XM, Brambilla R,
Bracchi-Ricard V, Li F, Walters WM, Blits B, He L, Schaal SM and
Bethea JR: NIBP, a novel NIK and IKK(beta)-binding protein that
enhances NF-(kappa)B activation. J Biol Chem. 280:29233–29241.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Bitner D, Pontes Filho AA, Li F,
Liu S, Wang H, Yang F, Adhikari S, Gordon J, Srinivasan S and Hu W:
Expression and function of NIK- and IKK2-binding protein (NIBP) in
mouse enteric nervous system. Neurogastroenterol Motil. 26:77–97.
2014. View Article : Google Scholar :
|
|
11
|
Qin M and Liu S, Li A, Xu C, Tan L, Huang
J and Liu S: NIK- and IKKbeta-binding protein promotes colon cancer
metastasis by activating the classical NF-kappaB pathway and MMPs.
Tumour Biol. Nov 23–2015.Epub ahead of print.
|
|
12
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995;
discussion 5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gujral TS, Chan M, Peshkin L, Sorger PK,
Kirschner MW and MacBeath G: A noncanonical Frizzled2 pathway
regulates epithelial-mesenchymal transition and metastasis. Cell.
159:844–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong W, Sun D, Wang Q and Suo J: Sorcin
enhances metastasis and promotes epithelial-to-mesenchymal
transition of colorectal cancer. Cell Biochem Biophys. 2015 Jan
8;Epub ahead of print. View Article : Google Scholar
|
|
16
|
Wang X, Wang H, Li G, Song Y, Wang S, Zhu
F, Guo C, Zhang L and Shi Y: Activated macrophages down-regulate
expression of E-cadherin in hepatocellular carcinoma cells via
NF-kappaB/Slug pathway. Tumour Biol. 35:8893–8901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng ZX, Wang DW, Liu T, Liu WX, Xia WB,
Xu J, Zhang YH, Qu YK, Guo LQ, Ding L, et al: Effects of the HIF-1α
and NF-κB loop on epithelial-mesenchymal transition and
chemoresistance induced by hypoxia in pancreatic cancer cells.
Oncol Rep. 31:1891–1898. 2014.PubMed/NCBI
|
|
18
|
Li SY: NIBP expression in colorectal
cancer tissues and its impact on colon cancer cell proliferation
(unpublished PhD thesis). Guangxi Medical University; 2015
|
|
19
|
Tan L: The research of NIBP protein
expression and its relationship with the transformation of
epithelial-mesenchymal in colorectal cancer tissue (unpublished PhD
thesis). Guangxi Medical University; 2015
|
|
20
|
Zheng L, Fu Y, Zhuang L, Gai R, Ma J, Lou
J, Zhu H, He Q and Yang B: Simultaneous NF-κB inhibition and
E-cadherin upregulation mediate mutually synergistic anticancer
activity of celastrol and SAHA in vitro and in vivo. Int J Cancer.
135:1721–1732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strippoli R, Benedicto I, Foronda M,
Perez-Lozano ML, Sánchez-Perales S, López-Cabrera M and Del Pozo
MÁ: p38 maintains E-cadherin expression by modulating
TAK1-NF-kappaB during epithelial-to-mesenchymal transition. J Cell
Sci. 123:4321–4331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Yamada O, Kida S, Matsushita Y,
Yamaoka S, Chagan-Yasutan H and Hattori T: Identification of CD44
as a downstream target of noncanonical NF-κB pathway activated by
human T-cell leukemia virus type 1-encoded Tax protein. Virology.
413:244–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Päll T, Pink A, Kasak L, Turkina M,
Anderson W, Valkna A and Kogerman P: Soluble CD44 interacts with
intermediate filament protein vimentin on endothelial cell surface.
PLoS One. 6:e293052011. View Article : Google Scholar
|
|
24
|
Steinmetz NF, Maurer J, Sheng H, Bensussan
A, Maricic I, Kumar V and Braciak TA: Two domains of vimentin are
expressed on the surface of lymph node, bone and brain metastatic
prostate cancer lines along with the putative stem cell marker
proteins CD44 and CD133. Cancers (Basel). 3:2870–2885. 2011.
View Article : Google Scholar
|
|
25
|
Mashita N, Yamada S, Nakayama G, Tanaka C,
Iwata N, Kanda M, Kobayashi D, Fujii T, Sugimoto H, Koike M, et al:
Epithelial to mesenchymal transition might be induced via CD44
isoform switching in colorectal cancer. J Surg Oncol. 110:745–751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki Y, Yamaguchi T, Matsumoto H, Nakano
D, Honda G, Shinoura N, Karasawa K and Takahashi K: Prognostic
factors and treatment effects in patients with curatively resected
brain metastasis from colorectal cancer. Dis Colon Rectum.
57:56–63. 2014. View Article : Google Scholar
|
|
27
|
Wein A, Emmert M, Merkel S, Harich HD,
Siebler J, Thiemann R, Lamberti C, Göttler B, Fries S, Kiani A, et
al: Palliative treatment of colorectal cancer with secondary
metastasis resection in Germany-impact of the multidis-ciplinary
treatment approach on prognosis and cost: The Northern Bavaria
IVOPAK I Project. Oncology. 88:103–121. 2015.
|
|
28
|
Chaw SY, Majeed AA, Dalley AJ, Chan A,
Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai X, Zhu H, Wang W, Zhang S, Zhang Y
and Mao G: Abnormal expression of EMT-related proteins, S100A4,
vimentin and E-cadherin, is correlated with clinicopathological
features and prognosis in HCC. Med Oncol. 31:9702014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Akiyoshi S, Eguchi H, Sudo T, et
al: Paired related homoeobox 1, a new EMT inducer, is involved in
metastasis and poor prognosis in colorectal cancer. Br J Cancer.
109:307–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Shao L, Creighton CJ, Zhang Y,
Xin L, Ittmann M and Wang J: Function of phosphorylation of NF-kB
p65 ser536 in prostate cancer oncogenesis. Oncotarget. 6:6281–6294.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu H, Shen Y, Hong J, Xia Q, Zhou F and
Liu X: The contribution of TGF-β in epithelial-mesenchymal
transition (EMT): Down-regulation of E-cadherin via snail.
Neoplasma. 62:1–15. 2015. View Article : Google Scholar
|
|
34
|
Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L,
Jiang H, Ren J, Cai J and Li Q: Resveratrol suppresses
epithelial-to-mesenchymal transition in colorectal cancer through
TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin
expression. BMC Cancer. 15:972015. View Article : Google Scholar
|
|
35
|
Du L, Rao G, Wang H, Li B, Tian W, Cui J,
He L, Laffin B, Tian X, Hao C, et al: CD44-positive cancer stem
cells expressing cellular prion protein contribute to metastatic
capacity in colorectal cancer. Cancer Res. 73:2682–2694. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li C, Chen S, Yue P, Deng X, Lonial S,
Khuri FR and Sun SY: Proteasome inhibitor PS-341 (bortezomib)
induces calpain-dependent IkappaB(alpha) degradation. J Biol Chem.
285:16096–16104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hideshima T, Chauhan D, Kiziltepe T, Ikeda
H, Okawa Y, Podar K, Raje N, Protopopov A, Munshi NC, Richardson
PG, et al: Biologic sequelae of IkappaB kinase (IKK) inhibition in
multiple myeloma: Therapeutic implications. Blood. 113:5228–5236.
2009. View Article : Google Scholar : PubMed/NCBI
|