Introduction
Various organs that are adversely affected by aging
exhibit structural changes and are also prone to disease. Thus,
disorders of the colon, such as constipation, are more prevalent
with age (1,2). However, the majority of studies
regarding the disruption of colonic motility have been reported in
animals (3). Therefore, it is
unclear whether the increased prevalence of constipation with age
in humans is due to confounding factors or age-associated
abnormalities in the nerves and muscles of the colon.
Neurodegeneration in the enteric nervous system
(ENS) has been shown to occur with age (4). However, compared with the central
nervous system (CNS), little is known about age-associated changes
in the ENS. In the present study, a possible underlying mechanism
of the aging process in the intestinal system was investigated.
In the CNS, Syn has been linked with the regulation
of neuronal plasticity, neurotransmission and presynaptic vesicle
dynamics (5–7). Syn is considered to be a suitable
marker for identifying the dystrophic features in the ENS of the
colon (8). Furthermore, fibril
formation of Syn results in insoluble intracellular aggregates.
These aggregates are involved in synucleinopathies, which occur
predominantly in the elderly human brain (9,10). A
previous report has demonstrated that certain autonomic axons in
the wall of the intestinal tract are immunopositive for wild-type
and phosphorylated Syn (8).
However, due to the limitation in collecting tissue samples from
particularly young and elderly subjects, to the best of our
knowledge, there have been no reports on whether a correlation
exists between the expression of Syn and age-associated colonic
dysfunction.
Syn has been shown to be phosphorylated (11) or nitrated (N) (12) at different residue sites. Previous
studies have identified that an oligomer-promoting effect of serine
129 phosphorylation is key in Syn neurotoxicity and inclusion
formation (13). Furthermore,
studies have shown that N-Syn is prone to oligomerization (14) and promotes dopaminergic loss of
neurons in culture (14,15). Therefore, the present study
hypothesizes that Syn, as well as N-Syn, may participate in
age-associated colonic neurodegeneration. In addition, it was
hypothesized that neurodegeneration of Syn and N-Syn causes
functional movement disorders, which commonly occur in elderly
individuals. In the current study, Syn and its post-translational
modifications (PTMs) were investigated in colonic tissue samples
obtained from individuals of different ages to determine which
types are specific to the colon. Furthermore, the study aimed to
determine whether these PTMs correlate with neurodegeneration
during normal aging. Thus, these results may provide information on
the processes underlying normal aging, which may in turn be
involved in pathological aging.
Materials and methods
Subjects
Following approval by the local research ethics
committee and obtaining written informed consent, biopsies (at full
thickness) were obtained from the normal margin (≥5 cm away from
the tumor) of 33 adult colorectal cancer patients during resection.
The biopsies were collected between September 2009 and February
2010 at Xuanwi Hospital, Capital Medical University (Beijing,
China) from the following three groups: Young individuals (n=12; 9
men, 3 women) mean age, 34.08±5.12 years; middle-aged individuals
(n=10; 7 men, 3 women) mean age, 51.80±3.52 years; and elderly
individuals (n=11; 5 men, 6 women) mean age 75.82 ± 7.70 years. To
avoid regional differences in neuron density, all biopsies were
obtained from the ascending colon. No tumor was present in the
sections examined.
Samples were immediately fixed in 10%
neutral-buffered formalin (OriGene Technologies, Inc., Beijing,
China) for 24 h. For conventional histology, 5-µm thick
paraffin (OriGene Technologies, Inc.) sections were prepared for
hematoxylin and eosin (OriGene Technologies, Inc.) staining and
immunohistochemical studies. The study was approved by the ethics
committee of Xuanwu Hospital, Capital Medical University (Beijing,
China). Written informed consent was obtained from the
patients.
Immunohistochemistry
Immunohistochemistry was performed on the tissue
samples using the following primary antibodies: Mouse monoclonal
anti-Syn 3D5 antibody (1:4,000;cat. no. sc-69977; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal anti-N-Syn
antibody (1:500; cat. no. sc-32279; Santa Cruz Biotechnology,
Inc.), and mouse monoclonal anti-NF antibody (1:500; cat. no.
MAB-0135; Maxim Biotech, Inc., Rockville, MD, USA), as previously
described (16). The Syn antibody
recognizes amino acids 115–121 of human Syn (15,17).
Immunostaining was conducted using a
peroxidase-based visualization kit (UltraSensitiveTM SP IHC kit;
Maxim Biotech, Inc.) according to the manufacturer's instructions
and 3,3′-diaminobenzidine tetrahydrochloride was used as the
chromogen. The slides were counterstained with Mayer's hematoxylin
(OriGene Technologies, Inc.) for 5 sec, dehydrated and mounted. The
slides were dehydrated with sequential ethanol washes of 1 min each
starting with 75%, followed by 80% and finishing with a 100%
ethanol (Sangon Biotech Co., Ltd., Shanghai, China). Next, the
cover slips were mounted on slides with neutral gums (Biogot
Technology Co., Ltd., Shanghai, China). To account for nonspecific
staining, the sections were subjected to peptides (in normal goat
serum; OriGene Technologies, Inc.), which blocked polyclonal
antibody binding, or the sections were incubated without the
primary antibodies.
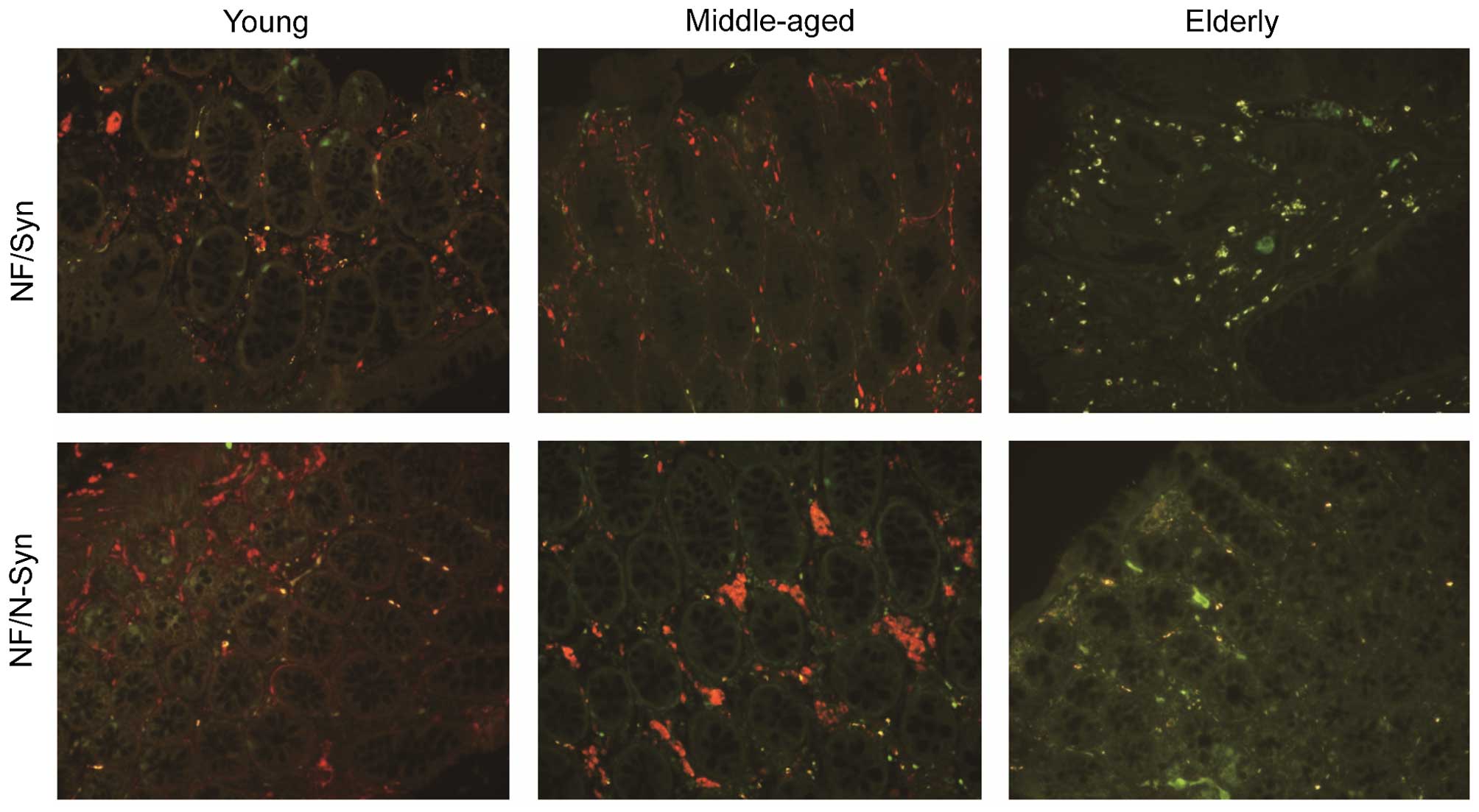
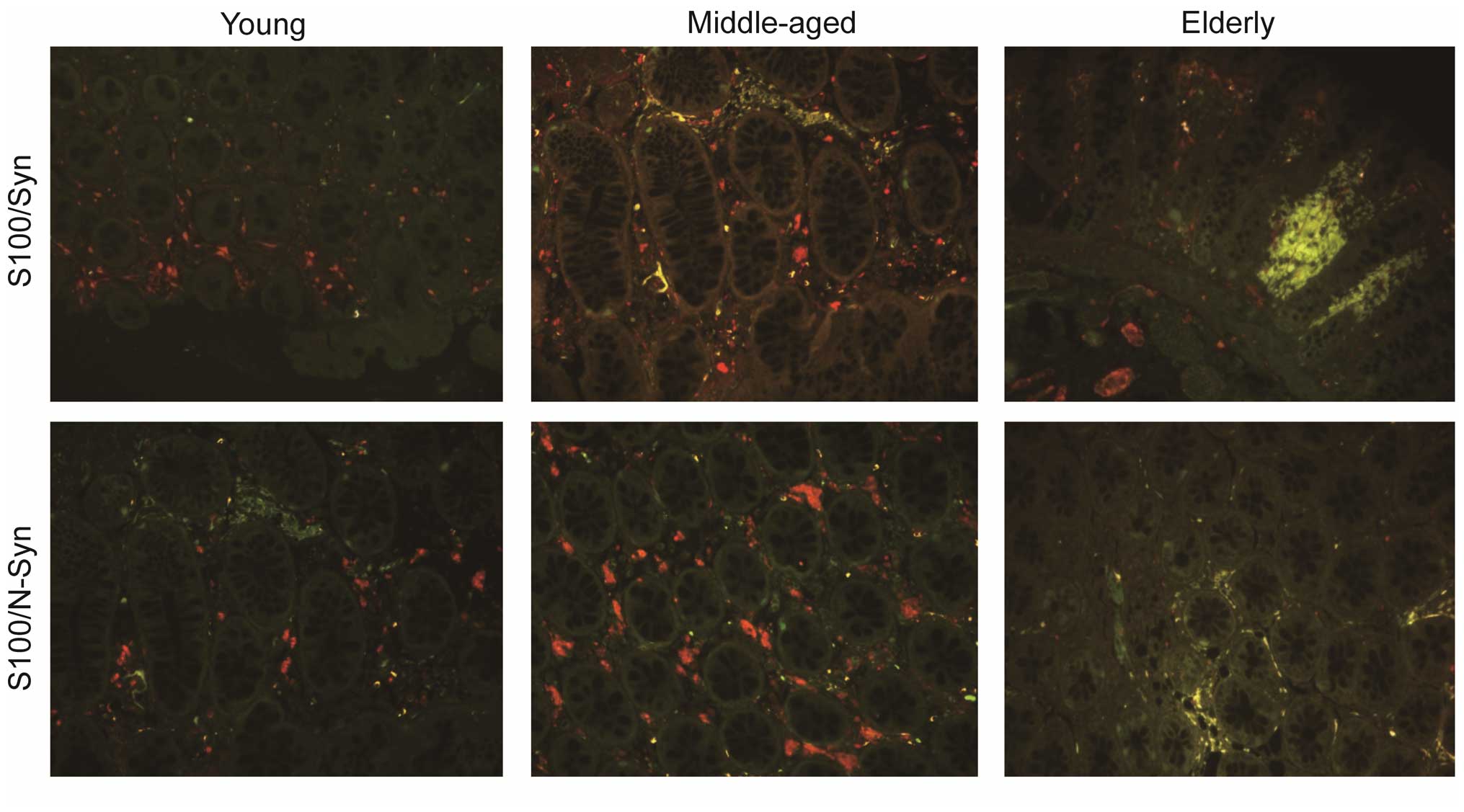
Double immunofluorescence
Double-label immunofluorescence was used to
determine the specific type of Syn present in the cells. Sections
from an elderly individual were incubated with rabbit anti-Syn
polyclonal antibody (15) and
mouse monoclonal anti-NF antibody (1:500), mouse monoclonal
anti-S100 protein antibody (1:500; cat. no. MAB-0697; Maxim
Biotech, Inc.) or mouse monoclonal anti-N-Syn antibody (1:500) at
4°C, overnight.
Sections were then incubated for 2 h (at room
temperature) with biotinylated horse anti-mouse immunoglobulin (Ig)
G (1:500; cat no. PK-4002; ABC kits; Vector Laboratories, Inc.,
Burlingame, CA, USA), followed by Cy3-conjugated goat anti-horse
IgG (1:200; Sigma-Aldrich, St. Louis, MO, USA) and fluorescein
isothiocyanate-conjugated goat anti-rabbit IgG (1:200; cat no.
ZLI-9021; OriGene Technologies, Inc., China).
All staining experiments were performed with the
appropriate positive and negative controls. A series of slides
containing tissues with a previously established positive reaction
with the primary antibodies were used as the positive control. To
account for nonspecific staining, sections incubated without the
primary antibody were used as the negative control. Sections were
analyzed by fluorescence microscopy (Olympus BX51; Olympus
Corporation, Tokyo, Japan). Colocalization of staining was
visualized using image analysis software (Image Pro Plus 5.0; Media
Cybernetics, Inc., Rockville, MD, USA) by applying the Boolean
operator 'AND'. This operator combined individual unmixed images
into a novel image that consisted solely of colocalized
(double-stained) pixels, thus representing only double-stained
cells (yellow). The relative intensities of NF, S-100, Syn, N-Syn
within individual cells were determined by Image Pro Plus software
by measuring the integral optical densities (IOD) of 80–100 cells
that were sampled from each subject randomly (16)
Data analysis
All slides were reviewed blindly by two
pathologists. Immunopositive cells were counted on 10 well-stained,
randomly selected, microscopic fields at x40 magnification (Olympus
BX51) for each region of interest.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The Kruskal-Wallis test by ranks followed by the
Mann-Whitney test was used to rank the Syn-immunoreactivity data.
One-way analysis of variance followed by the Student-Newman-Keuls
test was used for the comparisons of NF-positive neurons. Pearson
correlations/Spearman's rank order correlation was used to evaluate
the correlations between the neuronal counts and
Syn-immunoreactivity cell numbers. Data were analyzed with SPSS
20.0 software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Age-associated neurodegeneration
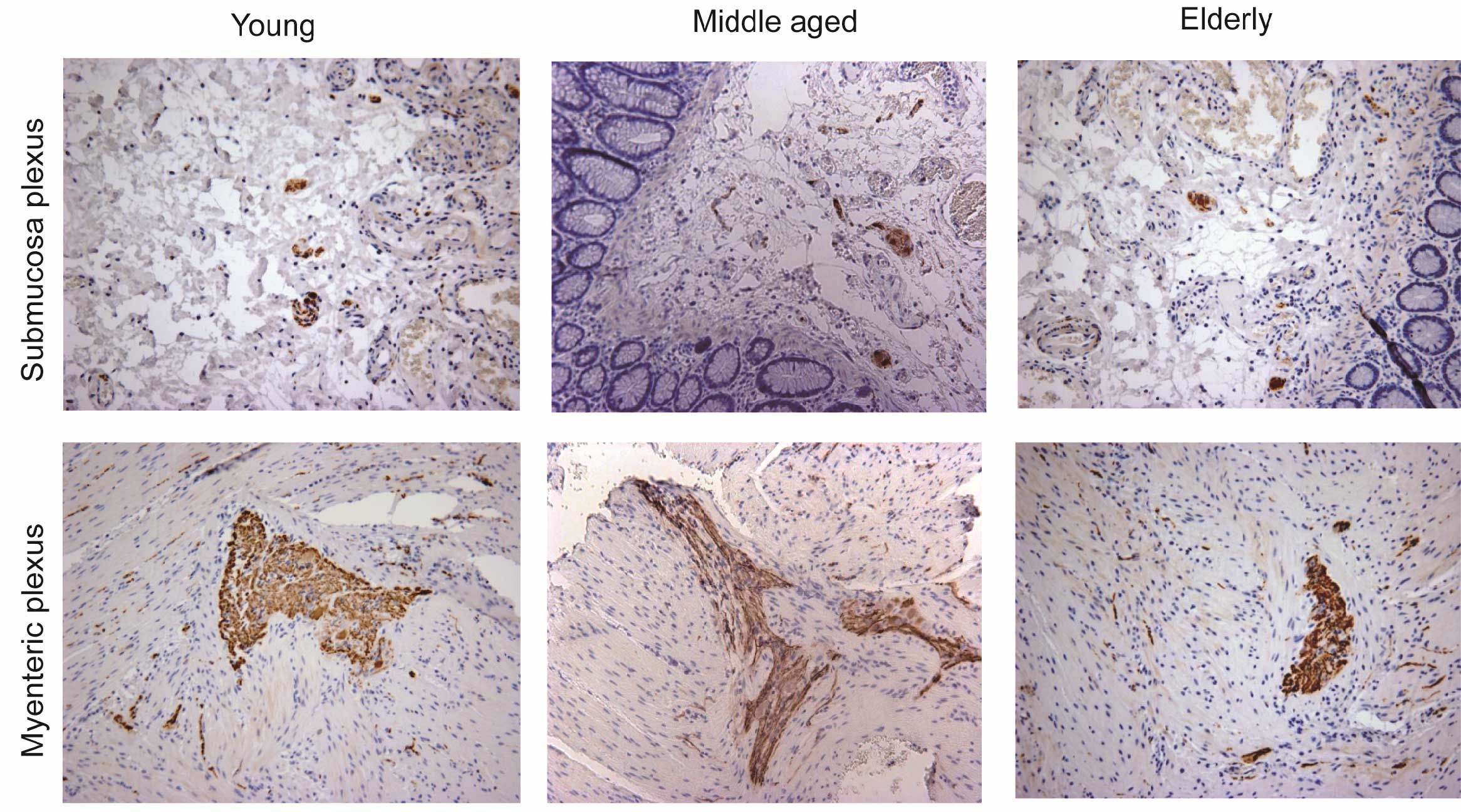
In young individuals, the number of NF-positive
neurons in the submucosal plexus was 4.63±0.82. These neurons were
significantly decreased in elderly individuals (2.96±1.77;
P<0.01). A loss of 36.07% of enteric neurons in the submucosal
plexus was observed in individuals >65 years when compared with
the young individuals (Fig. 1, top
panel).
In the myenteric plexus, the number of NF-positive
neurons in young individuals was 11.82±3.10. These neurons were
significantly reduced in the elderly group (4.96±1.59; P<0.01)
compared with the young and middle-aged groups (10.98±2.92). The
number of NF-positive neurons in the elderly group was reduced by
58.04% when compared with the young group (Fig. 1, bottom panel).
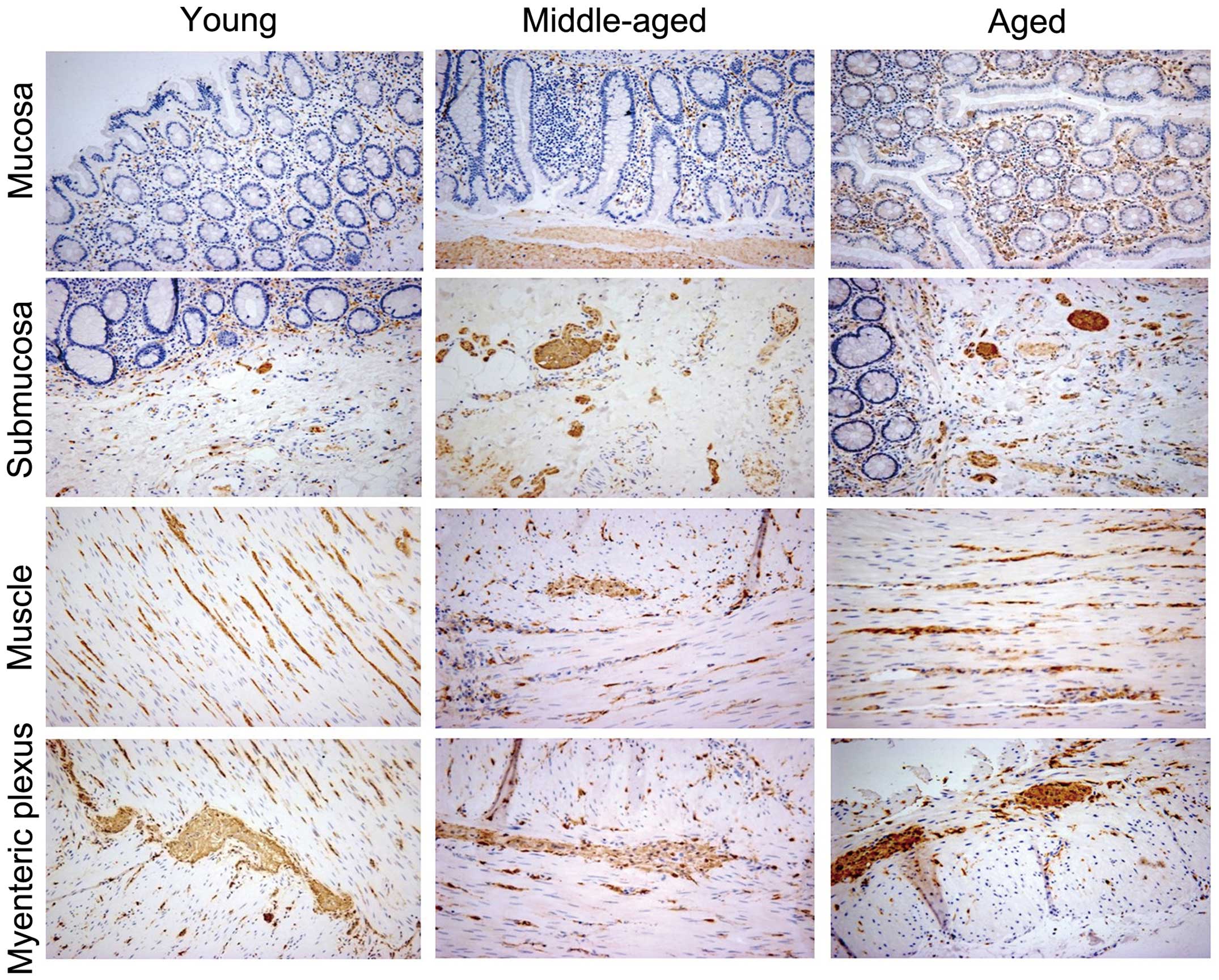
Age-associated accumulation of Syn
In the young, middle-aged, and elderly groups,
immunoreactivity for Syn was observed in all five layers: The
mucosa, submucosa, inner circular muscle, myenteric plexus, and
outer longitudinal muscle (Fig.
2). As the expression profile in the inner circular muscle and
outer longitudinal muscle was similar, these two groups were
combined and the cell number was calculated. Double-staining
indicated that the positively-stained cells were neurons (Fig. 3) or glial cells (Fig. 4). Syn was identified in the
nucleus, cytoplasm and axons (Fig.
3). In young individuals, axons immunoreactive for Syn were
typically smooth in appearance (Fig.
3). However, in the elderly group, axons were densely
distributed with a swollen appearance along their length (Fig. 3). Furthermore, Syn-positive
terminals surrounded the colonic glands and neurons in the ganglia.
The number of Syn-positive cells was low in the young (30.50±5.99)
and middle-aged (51.00±10.22) groups; however, was significantly
greater (120.36±31.10; P<0.05) in the elderly individuals
(Fig. 2). These immunoreactive
cells were regionally distributed, with 12.01, 10.13, 56.89, and
20.91% located in the mucosa, submucosa, muscle and myenteric
plexus, respectively. The Spearman's rank order correlation
analysis indicated a negative correlation between the number of
submucosa neurons and wild-type Syn in the mucosa (r=−0.761) and
submucosa (r=−0.713). A negative correlation was also identified
between the number of myenteric plexus neurons and wild-type Syn in
the muscle layer (r=−0.742) and the myenteric plexus (r=−0.641).
Quantitative intensity measurements indicated that the IOD of Syn
intensity was significantly different between the three age groups
(P<0.05). It was higher in the elderly group compared with the
young group, in the mucosa and in the submucosa (Table I).
 | Table IIODs for each of the groups. |
Table I
IODs for each of the groups.
| Region | IODs
|
|---|
| Young, n=12 | Middle-aged,
n=10 | Elderly, n=11 |
|---|
| NF | | | |
| Mucosa | 510.08±6.30 | 510.70±7.77 | 494.45±12.21 |
| Submucosa | 1169.67±11.46 | 1060.10±24.30 | 653.64±29.86a,b |
| S100 | | | |
| Mucosa | 1542.08±33.60 | 1548.30±42.67 | 1658±48.67 |
| Submucosa | 1569.92±30.95 | 1615.00±43.67 | 1687.63±51.34 |
| Syn | | | |
| Mucosa | 951.92±45.46 | 1101.10±16.00 |
1265.45±10.96a,b |
| Submucosa | 842.83±42.18 |
1023.10±21.42a |
1083.45±19.67a |
| N-Syn | | | |
| Mucosa | 212.08±39.32 | 396.00±14.16 |
762.73±19.62a,b |
| Submucosa | 79.67±15.06 | 129.70±3.78 |
216.45±13.37a,b |
| Syn/NF | | | |
| Mucosa | 300.92±12.44 | 312.30±7.70 |
342.45±10.65a |
| Submucosa | 114.75±2.83 | 120.70±2.51 | 127.91±1.77a |
| Syn/S100 | | | |
| Mucosa | 481.08±33.15 | 658.90±23.33 |
874.63±18.36a,b |
| Submucosa | 331.58±35.38 | 386.70±21.61 | 402.36±26.25 |
| N-Syn/NF | | | |
| Mucosa | 22.00±1.34 | 27.80±1.25 | 41.45±3.19a,b |
| Submucosa | 20.42±1.34 | 23.40±1.04 | 25.18±1.34a |
| N-Syn/S100 | | | |
| Mucosa | 279.42±22.39 | 349.70±15.24 |
712.18±16.72a,b |
| Submucosa | 152.25±14.17 | 169.90±11.91 |
205.73±10.02a |
Age-associated accumulation of N-Syn
Immunoreactivity was not present in the majority of
the young individuals. Weak immunoreactivity for N-Syn was apparent
in the muscle layer and myenteric layer in individuals aged >50
years (Figs. 3 and 4). Its presence then increased from young
to middle-aged to elderly. N-Syn-positive cells were only present
in the cytoplasm (Figs. 3 and
4).
The rate of nitration of Syn in elderly subjects was
significantly higher (36%) when compared with that of the young
individuals (5%; P<0.05). In 28 out of the 33 subjects (all
ages) the submucosa was identified to be the most affected region.
This was followed by the mucosa, with 26 individuals. The other
regions were found to be less affected. In the affected
individuals, 48.37% of immunoreactive cells were distributed within
the mucosa and 19.07% within the submucosa, while 19.81% was
located in the muscle and 12.75% in the myenteric plexus N-Syn
immunoreactive intensity was increased in the elderly group, in the
mucosa and in the submucosa. In young and middle-aged individuals,
there was a significant increase in the IOD of N-Syn in the elderly
individuals (P<0.05) (Table
I).
The spearman's rank order correlation analysis
indicated a negative correlation between the number of submucosa
neurons and N-Syn in the mucosa (r=−0.816) and in the submucosa
(r=−0.832). A negative correlation was also identified between the
number of myenteric plexus neurons and N-Syn in the muscle layer
(r=−0.601) and in the myenteric plexus (r=−0.611).
Discussion
Colonic function has been shown to significantly
deteriorate in individuals aged ≥65 years (18). In this age group, the internal anal
sphincter pressure and pelvic muscle strength is reduced. Changes
in rectal sensitivity and anal function have also been observed in
this group (19).
Neurodegeneration of the ENS may be the key to these functional
changes that are observed with advancing age. Studies have
demonstrated that deficits in intestinal function are linked with
age-associated loss of enteric neurons in rats (20), guinea pigs (21) and humans (22). Previous reports indicated that
age-associated cell loss in the myenteric plexus occurs exclusively
in the cholinergic (i.e. excitatory) enteric neurons, but not in
nitrergic (i.e. inhibitory) neurons (20). However, the majority of studies
have not investigated the underlying mechanisms of neuronal
vulnerability with increasing age. The present study has
demonstrated age-associated loss of submucosal and myenteric
neurons in the ascending colon. In addition, the expression pattern
of wild-type Syn and its PTMs were investigated to elucidate the
pathogenesis of neurodegeneration.
In the present study, the expression of wild-type
Syn was found to be different when compared with its PTM,
nitration. Wild-type Syn-positive cells were observed in all five
layers of the colonic tissue sample. Whereas, N-Syn was found in
only the mucosa and submucosa in the majority of the young
patients, and its presence in the muscle and myenteric plexus did
not occur until middle age. Wild-type Syn was present in the
nucleus, cytoplasm and axons, while N-Syn was only present in the
cytoplasm. In the cells that were Syn-positive the wildtype form
was more prevalent than N-Syn. Immunofluorescence double-staining
revealed that N-Syn was only expressed in cells with wild-type
Syn.
Previous studies have shown that Syn increased in
the gastrointestinal (GI) tract of patients with Parkinson's
disease (8,23). These upregulated forms have also
been shown in elderly rats (24).
However, to the best of our knowledge, they have not been studied
in the human colon of elderly humans. Although N-Syn has been
thoroughly investigated in the CNS (15,25),
it has not yet been investigated in the GI tract. In the present
study, it was demonstrated that a small quantity of Syn precedes
nitration. N-Syn initially appeared in individuals that were their
late thirties or older. Although the cells that were N-Syn-positive
were limited in number, they were found to be significantly
different between the age groups. The elderly subjects exhibited
the highest frequency of nitration. This result was consistent with
that of a previous study in which age-associated hyper-inflammatory
responses enhanced Syn nitration in the rat brain (26).
The present results indicated that Syn and N-Syn
were significantly greater in the elderly individuals than in the
younger individuals (Table I).
Furthermore, the results suggest that during the aging process,
nitration of Syn initially occurs in the mucosa and submucosa, and
subsequently in the muscle and myenteric plexus. The common
characteristic of the various PTM forms of Syn in the mucosa was a
predominant distribution in the surface layer. This layer is in
closer contact with environmental toxins in the colon lumina.
Little or no Syn was observed in the deep mucosa. In the elderly
group, the staining density intensified, and axons were swollen,
particularly in the wild-type Syn. The dendrites became shorter,
thicker and twisted. A previous study identified that pathological
accumulation of Syn in neurons may hinder axonal-stromal transport
due to disturbances in nerve conduction (27). Furthermore, previous reports
suggest that nerve terminal degeneration may precede degeneration
of ganglia neurons or even cell death during these pathological
processes (28). Studies have
shown a significant age-associated loss of submucosal intrinsic
sensory neurons in the rat distal colon (2,3).
Another report has suggested that a reduced input to enteric
microcircuits may result in reduced motility (29).
Environmental-mediated induction of metabolized
proteins and reactive oxygen species have been shown to be involved
in neurodegeneration (30). Based
on the results of the current study, regarding the temporal and
spatial patterns of Syn in colonic tissue, it was proposed that
age-associated infiltration of Syn and its PTM forms through the
damaged mucosal barrier of the tract allows their access to axons.
This effect may be due to the marked neuronal innervation in the
mucosa (31). Reactive oxygen
species have been shown to facilitate phosphorylation (32,33)
and nitration (34), stabilize Syn
protofibrils and delay their fibrillar formation (35). The accumulation of Syn may reduce
mitochondrial Complex I activity, thus interfering with its
function (36), it may also lead
to further damage from increased oxidative stress and free
radicals, thereby causing death of neurons (37). Thus, the environmental toxins may
specifically affect those neurons that innervate the mucosa or
submucosa, as the terminals of these neurons are located in the
vicinity of the compromised epithelium. In addition, Syn may be
continuously transmitted from one neural system to another via
endocytosis (38); this effect may
result in the accumulation of Syn, thereby causing
neurodegeneration. In the elderly population, if the threshold of
neuronal death in the colon is reached, colonic dysmotility and
decreased secretory activity may lead to the clinical response of
constipation. A previous study has indicated that the level of
accumulated Syn during constipation is markedly higher when
compared with that of a control group (25).
Additionally, the current results suggest that
neuropathies in the ENS may occur in healthy adults. Furthermore,
Syn and its aggregated PTM forms in the ENS, including its
peripheral projections, may also be involved in these pathological
processes.
In conclusion, the present findings may facilitate
with understanding the etiology of aging-mediated dysmotility. The
current study demonstrates the accumulation of Syn and N-Syn in the
colonic tissue during aging, this may be linked to colonic neuronal
degeneration during normal aging, which may cause functional
deficits. Further research is warranted to identify whether the
expression of Syn and its nitrated form are involved in the
pathogenesis of aging-associated disorders, including slow transit
constipation or inflammatory bowel disease.
Acknowledgments
The present study was supported by grants, awarded
to Professor Dian-Gang Liu, from the National Natural Science
Foundation of China (grant no. 81170408), the China Postdoctoral
Science Special Foundation (grant no. 2013T60386), and the China
Postdoctoral Science Foundation (grant no. 2012M510094).
References
|
1
|
Norton C: Constipation in older patients:
Effects on quality of life. Br J Nurs. 15:188–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wattchow D, Brookes S, Murphy E, Carbone
S, De Fontgalland D and Costa M: Regional variation in the
neurochemical coding of the myenteric plexus of the human colon and
changes in patients with slow transit constipation.
Neurogastroenterol Motil. 20:1298–1305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Phillips RJ, Pairitz JC and Powley TL:
Age-related neuronal loss in the submucosal plexus of the colon of
Fischer 344 rats. Neurobiol Aging. 28:1124–1137. 2007. View Article : Google Scholar
|
|
4
|
Phillips RJ and Powley TL: Innervation of
the gastrointestinal tract: Patterns of aging. Auton Neurosci.
136:1–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellani S, Sousa VL, Ronzitti G, Valtorta
F, Meldolesi J and Chieregatti E: The regulation of synaptic
function by alpha-synuclein. Commun Integr Biol. 3:106–109. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sousa VL, Bellani S, Giannandrea M, Yousuf
M, Valtorta F, Meldolesi J and Chieregatti E: {alpha}-synuclein and
its A30P mutant affect actin cytoskeletal structure and dynamics.
Mol Biol Cell. 20:3725–3739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adamczyk A, Solecka J and Strosznajder JB:
Expression of alpha-synuclein in different brain parts of adult and
aged rats. J Physiol Pharmacol. 56:29–37. 2005.PubMed/NCBI
|
|
8
|
Beach TG, Adler CH, Sue LI, Vedders L, Lue
L, White lii C, Akiyama H, Caviness JN, Shill HA, Sabbagh MN and
Walker DG: Arizona Parkinson's Disease Consortium: Multi-organ
distribution of phosphorylated alpha-synuclein histopathology in
subjects with Lewy body disorders. Acta Neuropathol. 119:689–702.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wenning GK and Jellinger KA: The role of
alpha-synuclein and tau in neurodegenerative movement disorders.
Curr Opin Neurol. 18:357–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phillips RJ, Walter GC, Wilder SL,
Baronowsky EA and Powley TL: Alpha-synuclein-immunopositive
myenteric neurons and vagal preganglionic terminals: Autonomic
pathway implicated in Parkinson's disease? Neuroscience.
153:733–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujiwara H, Hasegawa M, Dohmae N,
Kawashima A, Masliah E, Goldberg M, Shen J, Takio K and Iwatsubo T:
Alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat
Cell Biol. 4:160–164. 2002.PubMed/NCBI
|
|
12
|
Giasson BI, Duda JE, Murray IV, Chen Q,
Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ and Lee VM:
Oxidative damage linked to neurodegeneration by selective
alpha-synuclein nitration in synucleinopathy lesions. Science.
290:985–989. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen LI and Feany MB: Alpha-synuclein
phosphorylation controls neurotoxicity and inclusion formation in a
drosophila model of parkinson disease. Nat Neurosci. 8:657–663.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamin G, Uversky VN and Fink AL: Nitration
inhibits fibrillation of human alpha-synuclein in vitro by
formation of soluble oligomers. FEBS Lett. 542:147–152. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu Z, Xu X, Xiang Z, Zhou J, Zhang Z, Hu C
and He C: Nitrated alpha-synuclein induces the loss of dopaminergic
neurons in the substantia nigra of rats. PLoS One. 5:e99562010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xuan Q, Xu SL, Lu DH, Yu S, Zhou M, Uéda
K, Cui YQ, Zhang BY and Chan P: Increased expression of α-synuclein
in aged human brain associated with neuromelanin accumulation. J
Neural Transm (Vienna). 118:1575–1583. 2011. View Article : Google Scholar
|
|
17
|
Yu S, Li X, Liu G, Han J, Zhang C, Li Y,
Xu S, Liu C, Gao Y, Yang H, et al: Extensive nuclear localization
of alpha-synuclein in normal rat brain neurons revealed by a novel
monoclonal antibody. Neuroscience. 145:539–555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madsen JL and Graff J: Effects of ageing
on gastrointestinal motor function. Age Ageing. 33:154–159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McHugh SM and Diamant NE: Effect of age,
gender and parity on anal canal pressures. Contribution of impaired
anal sphincter function to fecal incontinence. Dig Dis Sci.
32:726–736. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Phillips RJ, Kieffer EJ and Powley TL:
Aging of the myenteric plexus: Neuronal loss is specific to
cholinergic neurons. Auton Neurosci. 106:69–83. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wade PR: Aging and neural control of the
GI tractI. Age-related changes in the enteric nervous system. Am J
Physiol Gastrointest Liver Physiol. 283:G489–G495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bernard CE, Gibbons SJ, Gomez Pinilla PJ,
Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ,
Larson DW, et al: Effect of age on the enteric nervous system of
the human colon. Neurogastroenterol Motil. 21:7462009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lebouvier T, Chaumette T, Damier P, Coron
E, Touchefeu Y, Vrignaud S, Naveilhan P, Galmiche JP, Bruley des
Varannes S, Derkinderen P and Neunlist M: Pathological lesions in
colonic biopsies during Parkinson's disease. Gut. 57:1741–1743.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Phillips RJ, Walter GC, Ringer BE, Higgs
KM and Powley TL: Alpha-synuclein immunopositive aggregates in the
myenteric plexus of the aging Fischer 344 rat. Exp Neurol.
220:109–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lebouvier T, Neunlist M, Burley des
Varannes S, Coron E, Drouard A, N'Guyen JM, Chaumette T, Tasselli
M, Paillusson S, Flamand M, et al: Colonic biopsies to assess the
neuropathology of parkinson's disease and its relationship with
symptoms. PLoS One. 5:e127282010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi DY, Zhang J and Bing G: Aging
enhances the neuroinflammatory response and alpha-synuclein
nitration in rats. Neurobiol Aging. 31:1649–1653. 2010. View Article : Google Scholar
|
|
27
|
Siebert H, Kahle PJ, Kramer ML, Isik T,
Schlüter OM, Schulz-Schaeffer WJ and Brück W: Over-expression of
alpha-synuclein in the nervous system enhances axonal degeneration
after peripheral nerve lesion in a transgenic mouse strain. J
Neurochem. 114:1007–1018. 2010.PubMed/NCBI
|
|
28
|
Orimo S, Uchihara T, Nakamura A, Mori F,
Kakita A, Wakabayashi K and Takahashi H: Axonal alpha-synuclein
aggregates herald centripetal degeneration of cardiac sympathetic
nerve in parkinson's disease. Brain. 131:642–650. 2008. View Article : Google Scholar
|
|
29
|
Wattchow D, Brookes S, Murphy E, Carbone
S, De Fontgalland D and Costa M: Regional variation in the
neurochemical coding of the myenteric plexus of the human colon and
changes in patients with slow transit constipation.
Neurogastroenterol Motil. 20:1298–1305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Migliore L and Coppedè F:
Environmental-induced oxidative stress in neurodegenerative
disorders and aging. Mutat Res. 674:73–84. 2009. View Article : Google Scholar
|
|
31
|
Miwa H, Kubo T, Suzuki A and Kondo T:
Intragastric proteasome inhibition induces
alpha-synuclein-immunopositive aggregations in neurons in the
dorsal motor nucleus of the vagus in rats. Neurosci Lett.
401:146–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi M, Ko LW, Kulathingal J, Jiang
P, Sevlever D and Yen SH: Oxidative stress-induced phosphorylation,
degradation and aggregation of alpha-synuclein are linked to
upregulated CK2 and cathepsin D. Eur J Neurosci. 26:863–874. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCormack AL, Mak SK, Shenasa M, Langston
WJ, Forno LS and Di Monte DA: Pathological modifications of
alpha-synuclein in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine
(MPTP)-treated squirrel monkeys. J Neuropath Exp Neur. 67:793–802.
2008. View Article : Google Scholar
|
|
34
|
Gao HM, Kotzbauer PT, Uryu K, Leight S,
Trojanowski JQ and Lee VM: Neuroinflammation and
oxidation/nitration of alpha-synuclein linked to dopaminergic
neurodegeneration. J Neurosci. 28:7687–7698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hodara R, Norris EH, Giasson BI,
Mishizen-Eberz AJ, Lynch DR, Lee VM and Ischiropoulos H: Functional
consequences of alpha-synuclein tyrosine nitration: Diminished
binding to lipid vesicles and increased fibril formation. J Biol
Chem. 279:47746–47753. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Devi L, Raghavendran V, Prabhu BM,
Avadhani NG and Anandatheerthavarada HK: Mitochondrial import and
accumulation of alpha-synuclein impair complex I in human
dopaminergic neuronal cultures and Parkinson disease brain. J Biol
Chem. 283:9089–9100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu LJ, Sagara Y, Arroyo A, Rockenstein E,
Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M and Masliah E:
Alpha-synuclein promotes mitochondrial deficit and oxidative
stress. Am J Pathol. 157:401–410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Desplats P, Lee HJ, Bae EJ, Patrick C,
Rockenstein E, Crews L, Spencer B, Masliah E and Lee SJ: Inclusion
formation and neuronal cell death through neuron-to-neuron
transmission of alpha-synuclein. Proc Natl Acad Sci USA.
106:13010–13015. 2009. View Article : Google Scholar : PubMed/NCBI
|