Introduction
Extracellular matrix (ECM) contributes to the
overall mechanical properties of tissues. ECM is also considered to
modulate disease progression, and current understanding of the
modulatory function of ECM suggests that the stiffness of the ECM
affects tumor cell progression (1). Tumor-associated ECM remodeling is
characterized by increased ECM deposition and the stiffness of the
matrix, which leads to persistent migration and tissue invasion of
cancer cells (2). It is known that
ECM is involved in cell movement, attachment, proliferation and
differentiation, and in orchestrating inflammation (3–7).
Versican is a large ECM proteoglycan, encoded on human chromosome 5
and spanning >90kb. There are globular structures at the
N-terminal (G1 domain) and C-terminal (G3 domain) of the protein
core, similar to the other members of the lectican family (8,9). The
G1 domain is characterized by a hyaluronan binding region. The G3
domain consists of epidermal growth factor-like domains, a
carbohydrate recognition domain and a complement binding domain.
Between G1 and G3 in versican, core proteins are present, where
chondroitin sulfate glycosaminoglycan (GAG) side chains attach
(8). There are at least five
splice variants of versican, termed V0, V1, V2, V3 and V4, which
differ predominantly in the sizes of their core proteins, generated
by alternative splicing of the mRNA encoding the two GAG chain
binding domains. V0 (is silenced by the same siRNA as V1, as it
contains the full length protein encoded by all the exons) and V1
are distributed widely in adult tissues; V1 is the principal
proteoglycan found in pulmonary ECM (10). V2 appears to be expressed only in
the central nervous system (11–14).
The V3 variant is generally expressed at low levels in adult
tissues (14). V4 has been
identified as an additional isoform in breast cancer (15). The G3 domain promotes cell adhesion
through its interaction with β1 integrin and activation of focal
adhesion kinase (16). In
addition, versican binds with CD44 through its GAG chains or G1
hyaluronan binding domain to promote cell proliferation, migration
and adhesion, and enhance the spread of tumors and inflammation
(17–19). Versican binds to hyaluronan to
affect T lymphocyte phenotypes and cytokine secretion (19). Versican also acts as an endogenous
ligand of toll-like receptors (TLRs), generating a rapid
inflammatory response (20).
Several reports have shown that, particularly in tumors, V1 is a
natural ligand of TLR2. By activating TLR2:TLR6 complexes and
inducing tumor necrosis factor (TNF)-α secretion, V1 markedly
enhances tumor metastatic growth (21,22).
Acute lung injury (ALI) is a type of severe
inflammatory disease, which is life-threatening and is
characterized by inflammatory damage of the alveolar capillary
membrane, increased proteinaceous edema and the production of
inflammatory cytokines. LPS, a major constituent of the outer
membranes of Gram-negative bacteria, has been identified as a
pivotal risk factor and prominent stimulus in the pathogenesis of
ALI (23,24). LPS challenge induces neutrophil
infiltrations, triggers acute inflammatory responses and generates
early pathological changes in the lungs. The intratracheal
administration of LPS in experimental animals is a suitable and
reproducible method for investigations involving ALI. The
predominant LPS sensor in the host is the TLR superfamily, of which
TLR2 and TLR4 are the primary sensors of LPS, acting as
transmembrane receptors and signal transduction molecules (25). On binding with LPS, TLRs activate
the nuclear factor (NF)-κB pathway through myeloid differentiation
factor 88, resulting in the production of pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, and IL-6, recruiting neutrophils in the lung. As the most
widely investigated member of the TNF super family, TNF-α is
important in the homeostasis and pathophysiology of ALI. LPS
administration induces high levels of TNF-α, which exacerbates ALI
(26). TNF-α also downregulates
the gene expression of surfactant protein A in lung epithelial
cells via the p38 mitogen-activated protein kinase signal
transduction pathway, resulting in the loss of lung surfactant
(27).
As versican has complex roles in inflammation, and
as V1 is the predominant isoform present in the lungs, the present
study hypothesized that V1 is also involved in the modulation of
lung inflammation. The present study aimed to investigate the
effects of V1 knockdown in ALI. As our previous in vitro
study demonstrated that high levels of V1 are expressed in the
lungs, and increase further following insult by LPS, it is possible
to silence V1 and examine its function in ALI. This may demonstrate
that V1 is one of the pivotal regulators in the inflammation of ALI
and, therefore establish a novel target for the treatment of
ALI.
Materials and methods
Animals
Male specific pathogen-free C57BL/6J mice (age, 6–8
weeks) were obtained from Shanghai Laboratory Animal Center (SLAC
Laboratory Animal Co., Ltd., Shanghai, China). The mice were housed
under a 12 h light/dark cycle and at a constant temperature of
22±2°C with food and water available ad libitum. The mice
were allowed to acclimate to these conditions for 7 days prior to
initiation of the experiment. All experimental procedures in the
present study were approved by the Institutional Ethics Committee
of Fudan University (Shanghai, China) and were performed, according
to the guidelines for experimental animals developed by Fudan
University.
Animal model establishment and sample
collection
The male C57BL/6J mice were randomly divided into a
normal control group (control; n=6), an LPS-stimulated ALI group
(LPS; n=6), a scramble small interfering (si)RNA group (scramble;
n=6), a V1-siRNA group (V1-siRNA; n=6), a scramble siRNA and
LPS-stimulated group (scramble+LPS; n= 6) and a V1-siRNA and
LPS-stimulated group (V1-siRNA+LPS; n=6). On day 1, the mice were
anesthetized with tribromoethanol (1.2%; 20 ml/kg; Sigma-Aldrich,
St. Louis, MO, USA), and then suspended vertically and intubated
with a section of 22G intravenous catheter. The scramble siRNA (5
nmol) and the V1-siRNA modified with cholesterol and methyl
(5′-GCAATTACCACCTCACCTA-3′) dissolved in phosphate-buffered saline
(PBS), obtained from RiboBio Co., Ltd. (Guangzhou, China), were
administered separately in the different treatment groups. On day
3, LPS (1 mg/kg; from Pseudomonas aeruginosa 10;
Sigma-Aldrich) or PBS (50 µl per mouse) were administered
intratracheally, as described above. All the mice were anesthetized
and sacrificed by cervical dislocation on day 4, bronchoalveolar
lavage fluid (BALF) was collected using 1.5 ml PBS divided into two
volumes, each of which was injected and aspirated repeatedly five
times in the trachea through the catheter. Blood samples were also
collected, and the lungs were removed. The lung tissues were fixed,
using 4% paraformaldehyde (1.0×0.8×0.5 cm; Sangon Biotech, Co.,
Ltd., Shanghai, China), for hematoxylin and eosin (H&E;
Jiancheng Bioengineering Institute, Nanjing, China) and
immunohistochemical staining, the rest of the lung tissues were
frozen at −80°C.
Assessments of protein concentration and
cell count in the BALF
The recovery of BALF was >85%. The total protein
concentration of the BALF was determined using a bicinchoninic acid
(BCA) protein assay, according to the manufacturer's protocol
(Beyotime Institute of Biotechnology, Haimen, China). For counting
of the cells in the BALF, the BALF was centrifuged at 300 × g for 5
min at 4°C, and the sediment was treated with 200 µl
erythrocyte lysate (Yeasen Biotechnology Co., Ltd., Shanghai,
China) for 10 min, centrifuged at 300 × g for 10 min at 4°C, and
washed with PBS. Subsequently, 200 µl PBS was added to
resuspend the cells, which were then counted under an inverted
microscope (Eclipse TS100-F; Nikon Corporation, Tokyo, Japan).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the lung tissues using
an Ultrapure RNA kit (CW Biotech Co., Ltd., Peking, China),
according to the manufacturer's protocol. Total RNA (500 ng) was
transcribed and cDNA was synthesized according to the
manufacturer's protocol (Shanghai Yeasen Biotechnology Co., Ltd.,
Shanghai, China). Hieff™ qPCR SYBR® Green Master mix
(Shanghai Yeasen Biotechnology Co., Ltd; 10 µl Master Mix,
0.4 µl Forward Primer (10 mM), 0.4 µl Reverse Primer
(10 mM), 1 µl cDNA and 8.2 µl ddH2O) was
used, and qPCR was performed, according to the manufacturer's
protocol, using the Master Cycler ep realplex PCR system
(Eppendorf, Hamburg, Germany). The cycling conditions were as
follows: 95°C for 5 min (pre-denaturation), 95°C for 10 sec, 60°C
for 30 sec (40 cycles), 95°C for 15 sec, 60°C for 60 sec and 95°
for 15 sec (melting). The expression levels of target mRNAs were
normalized to that of GAPDH using the 2−ΔΔCq method
(28). The primer pairs (Sangon
Biotech, Co., Ltd.) used were as follows: V1, forward
5′-GCTGTAAACGTCGATTGAGTG-3′ and reverse 5′-TCTTCACTGCAAGGTTCCTC-3′;
GAPDH, forward 5′-CATGGCCTTCCGTGTTCCTA-3′ and reverse
5′-GCGGCACGTCAGATCCA-3′; TNF-α, forward 5′-CCTGTAGCCCACGTCGTAG-3′
and reverse 5′-GGGAGTAGACAAGGTACAACCC-3′; IL-6, forward
5′-TCCAGTTGCCTTCTTGGGAC-3′ and reverse
5′-GTGTAATTAAGCCTCCGACTTG-3′; TLR2, forward 5′-GCGGACTGTTTCCTTCTG
AC-3′ and reverse 5′-CCAAAGAGCTCGTAGCATCC-3′; and TLR4, forward
5′-CAGCAAAGTCCCTGATGACA-3′ and reverse
5′-AGAGGTGGTGTAAGCCATGC-3′.
Immunohistochemistry
Immunohistochemistry was performed using the
paraffin (Beyotime Institute of Biotechnology)-embedded tissue
sections [size, 4 µm; sliced using a Microtome (5,000 smz)
from Campden Instruments Ltd., Loughborough, England] mounted on
glass slides. The slides were deparaffinized and rehydrated.
Antigens were retrieved using 10 mM citrate solution (Beyotime
Institute of Biotechnology) and a microwave oven on high heat for 3
min, and a medium heat for 12 min, and then blocked using 10%
bovine serum albumin (Beyotime Institute of Biotechnology) for 30
min at room temperature. The slides were then immunostained using
the following primary antibodies at 4°C overnight: Monoclonal mouse
anti-human versican (#MABT161; 1:100; EMD Millipore, Billerica, MA,
USA), polyclonal rabbit anti-human phosphorylated (p)-P65
[(Ser536); #11014; 1:150; Signalway Antibody LLC,
College Park, MD, USA], polyclonal rabbit anti-human TLR2 (#BA1716)
and TLR4 (#BA1717) (1:100; Wuhan Boster Biological Technology Co.,
Ltd., Wuhan, China). This was followed by incubation with
horseradish peroxidase(HRP)-conjugated goat anti-mouse and rabbit
secondary antibody (#CK500605A; GeneTech, Shanghai, China) for 1 h
at room temperature. Diaminobenzidine (GeneTech) reactions were
performed, and the slides were reacted with hematoxylin for 30 sec,
washed with running water, dehydrated in a graded series of ethanol
and xylene (Sangon Biotech, Co., Ltd.), then mounted with a
coverslip. The immunoreactivity was observed and images were
captured under an inverted microscope (Eclipse TS100-F).
Western blot analysis
Total protein was extracted from the lung tissues
using cold lysis buffer (Beyotime Institute of Biotechnology), and
concentrations were measured using a BCA protein assay. The samples
(30 µg/sample) were separated by 10% SDS-PAGE (Beyotime
Institute of Biotechnology) and transferred onto polyvinylidene
fluoride membranes (EMD Millipore). The membranes were blocked with
skimmed milk, and incubated overnight at 4°C with the following
primary antibodies: Anti-TLR2, TLR4 and GAPDH (monoclonal; 1:1,000;
Wuhan Boster Biological Technology Co., Ltd.). The membranes were
incubated with HRP-conjugated secondary antibodies (1:1,000;
Beyotime Institute of Biotechnology). The immunoreactive signals
were visualized using an enhanced chemiluminescence detection
system (Beyotime Institute of Biotechnology).
Enzyme-linked immunosorbent assay
(ELISA)
The lung tissues were homogenized with saline and
centrifuged at 12,000 × g for 20 min at 4°C, and the resulting
supernatants were used as homogenates, with the other samples being
BALF and plasma. For the ELISA analysis, IL-6 and TNF-α kits
(BioLegend, San Diego, CA, USA) were used. The levels of TNF-α and
IL-6 were determined according to the manufacturer's protocol.
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was
used for data analysis. Data are expressed as the mean ± standard
division. Comparisons among three or more experimental groups were
performed using one-way analysis of variance. Significant
differences between groups were analyzed using Student's
t-test or a Mann-Whitney U test, when appropriate. P<0.05
was considered to indicate a statistically significant
difference.
Results
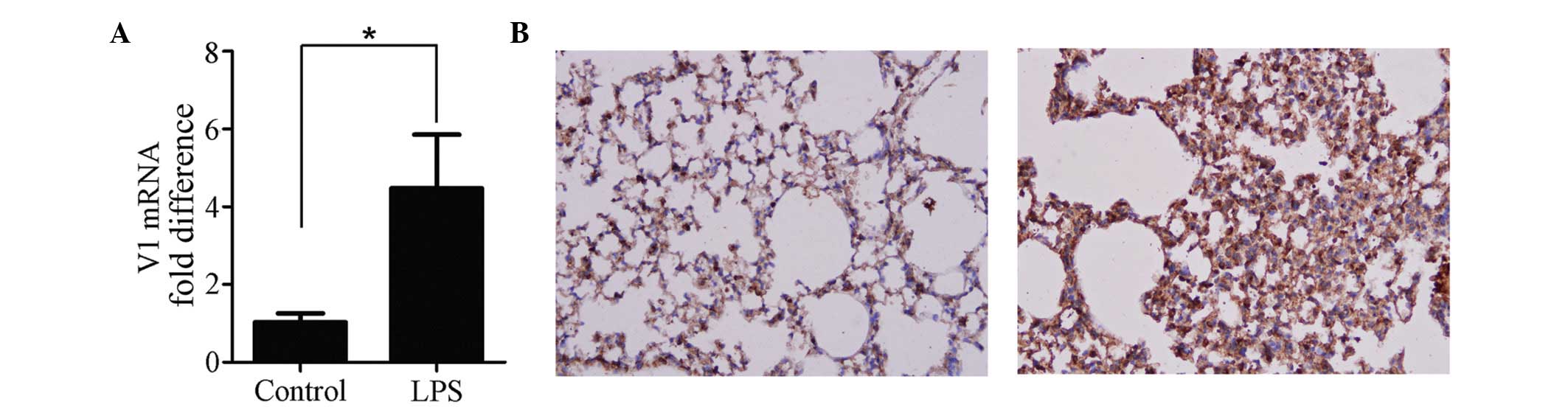
Expression of V1 increases in the ALI
mouse model
The expression of V1 was increased in the ALI mouse
model. The mRNA (Fig. 1A) and
protein (Fig. 1B) expression
levels of V1 were altered by LPS. The mRNA expression of V1 was
increased >4-fold, and the immunohistochemical analyses of the
lung tissues confirmed that a higher level of V1 was expressed
following ALI (Fig. 1B).
Lung injury is aggravated by
V1-siRNA
In order to investigate the role of V1 in ALI,
V1-siRNA was administered in the lungs to inhibit V1. The levels of
transcription decreased in the V1-siRNA group by >50%, compared
with the control group, whereas no significant differences were
observed between the control and scramble siRNA groups (Fig. 2A). The changes in the protein
expression levels of V1 were in accordance with those of mRNA,
which were confirmed using immunohistochemical staining (Fig. 2B–D). Injury to the mouse lung
tissues was also investigated, as described previously (29) in the H&E-stained sections. The
lung injury score in the V1-siRNA+LPS group was significantly
higher, compared with those in the control, LPS, scramble+LPS
groups (Fig. 2E–H). Fig. 2I shows histograms of the scores.
Cell count and total protein concentration in the BALF were also
detected. Consistent with the lung injury scores, the cell count
and protein concentration were higher in the V1+LPS group (Fig. 2J and K).
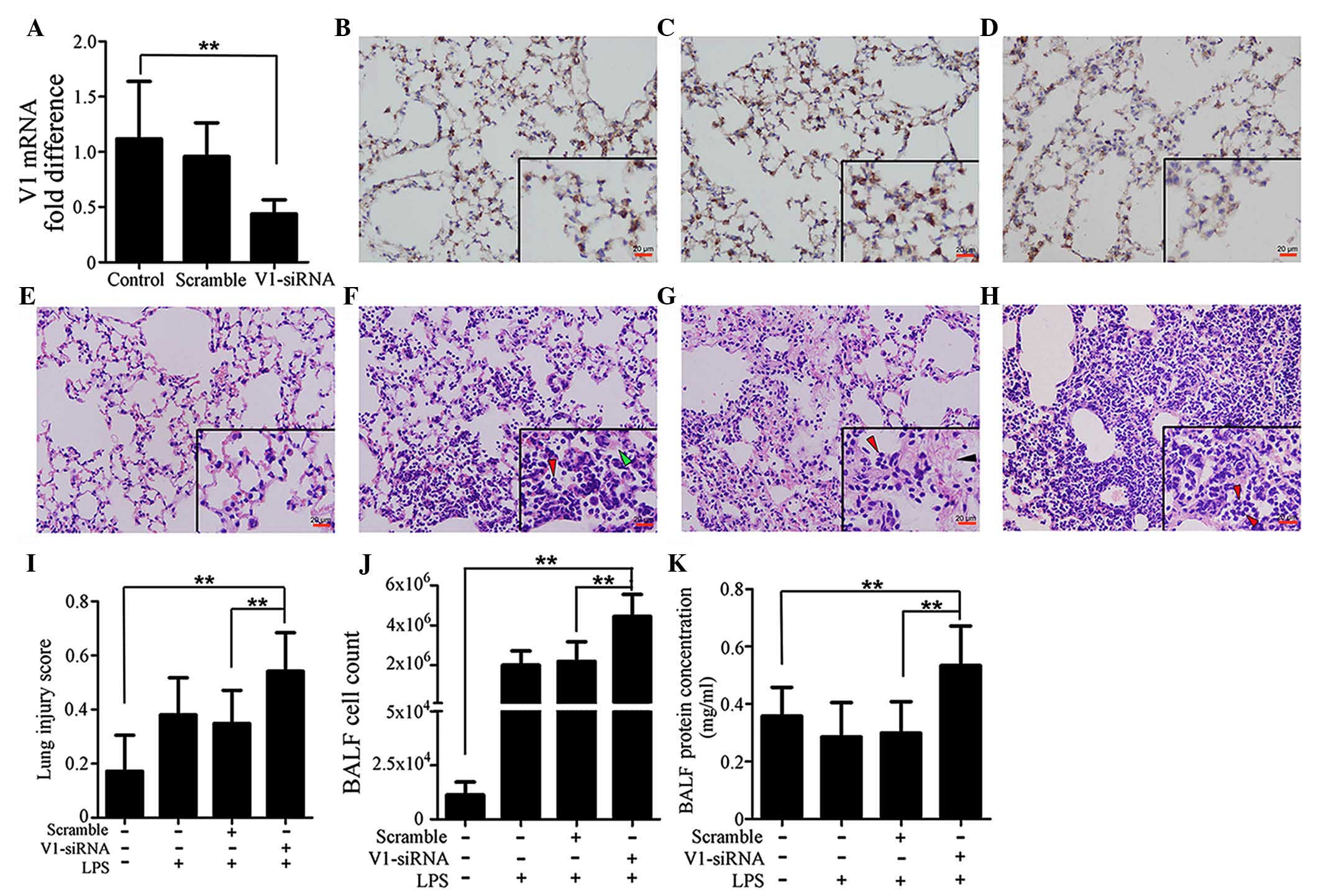 | Figure 2Specific knockdown of V1 aggravates
immune reactions in the mouse lung. (A) mRNA expression of V1 was
decreased by siRNA. No significant differences were found between
the normal and scramble siRNA-treated groups. Immunohistochemical
assays (magnification, ×200; scale bar in vignettes=20 µm)
showed that the V1 expression intensity was consistent in the (B)
control and (C) scramble siRNA groups, but weaker in the (D)
V1-siRNA group. Representative images of H&E-stained sections
(magnification, ×200; scale bars in vignettes=20 µm) in the
(E) lungs of the control group were normal, with clear bronchial
and alveolar structures. (F) At 24 h post-LPS administration (1
mg/kg; intratracheally), typical pathological changes were
observed, with patchy neutrophil infiltration (red arrows) and
liquid entering the alveolar cavity (green arrows). (G) In the
scramble siRNA pre-treated mouse, the LPS-stimulated pathological
changes were almost the same, with neutrophil infiltration (red
arrows) and deposition of fibrin strands (black arrows). (H) In the
V1-siRNA+LPS group, there was increased neutrophil infiltration in
alveolar cavities (red arrows), and reduced clarity of bronchial
and alveolar structures, compared with the control ALI group. (I)
Lung injury scores were assessed, according to the H&E-stained
sections. Higher scores were observed in the V1 knockdown group,
which confirmed the existence of an aggravated inflammation in the
V1 knockdown group. (J) Cell count and (K) total protein
concentrations in the BALF also confirmed that V1 inhibition in the
mouse led to a more severe reaction. Data are presented as the mean
± standard deviation (**P<0.01). siRNA, small
interfering RNA; ALI, acute lung injury; LPS, lipopolysaccharide;
V1, versican V1; BALF, bronchoalveolar lavage fluid. |
V1 knockdown-associated inflammatory
aggravation is associated with activation of NF-κB and deregulated
release of TNF-α and IL-6
Although levels remained stable prior to LPS
stimulation in the control, scramble and V1-siRNA groups (Fig. 3A–C), marked NF-κB signaling pathway
activation was induced by LPS in the V1-siRNA group, compared with
the control and scramble siRNA groups (Fig. 3D–F). Several cytokines were
detected in the present study (data not shown), TNF-α was found to
be negatively correlated with V1. The mRNA expression of TNF-α was
increased by LPS, however, V1 knockdown exhibited more marked
transcriptional activity, compared with the other treatment groups
(Fig. 3G). The protein levels of
TNF-α also increased significantly in the lung tissue homogenates,
BALF and plasma (Fig. 3H–J). The
expression of IL-6 increased, but the significant difference
between the LPS and V1-siRNA+LPS groups was only observed in the
plasma samples (Fig. 3G–J).
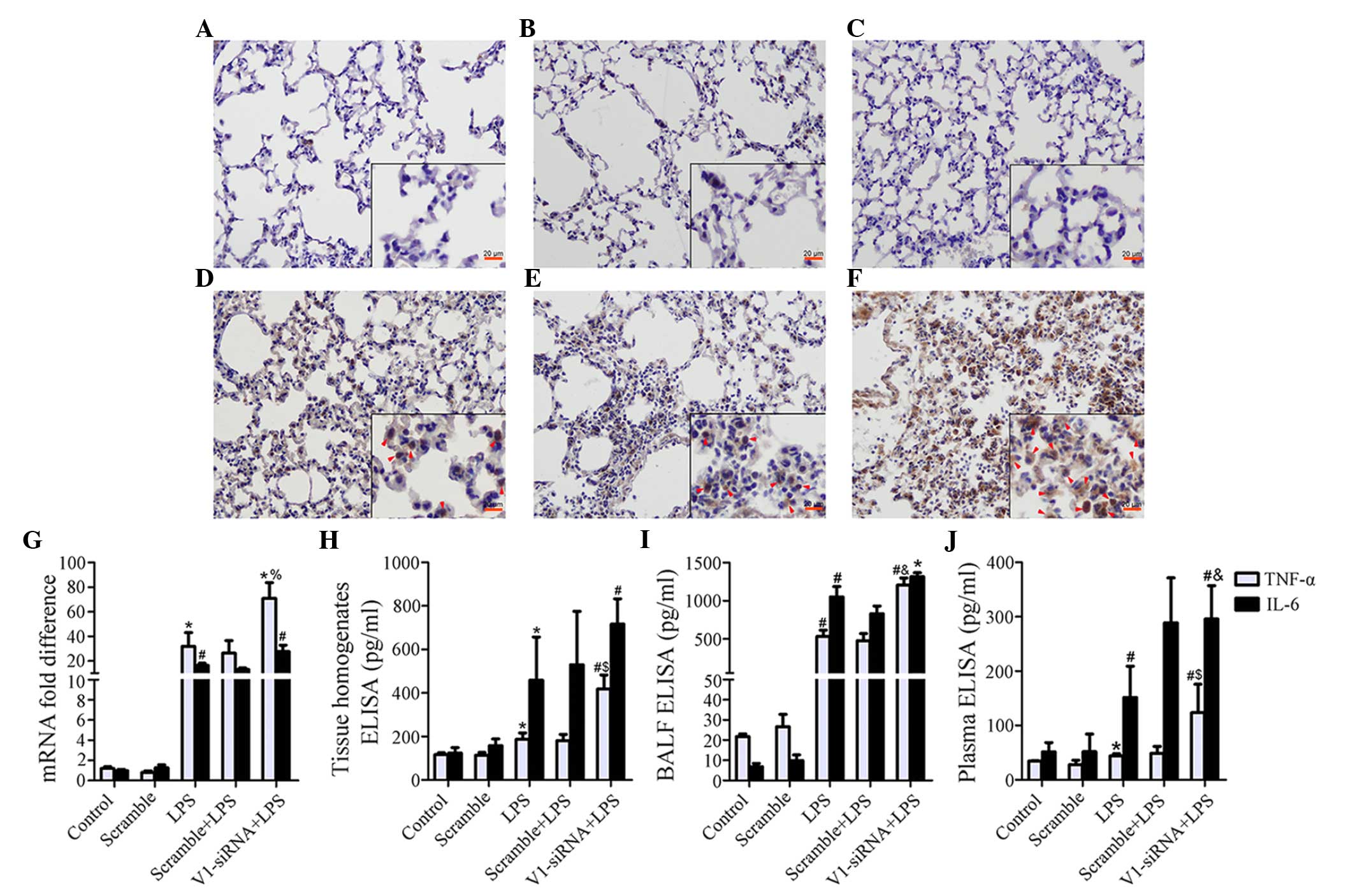 | Figure 3Increased severity of inflammatory
reaction is mediated by NF-κB and TNF-α. (A–C) The immunostaining
of NF-κB subunit P65 is demonstrated in the control, scramble and
V1-siRNA groups (magnification, ×200; scale bar in vignettes=20
mm). P65 remained stable prior to LPS administration. (D–F) The
immunostaining of P65 was demonstrated in the LPS, scramble+LPS and
V1-siRNA+LPS groups (magnification, ×200; scale bar in vignette=20
mm). P65 was phosphorylated following LPS insult, immunostaining
was more marked in the (F) V1-siRNA+LPS group, compared with the
(D) LPS and (E) scramble+LPS groups (red arrows show positive
staining). (G) mRNA levels of TNF-α were increased in all ALI
groups, but were higher in the V1-siRNA+LPS group. (H–J) ELISA
indicated TNF-α as a mediator of the severe inflammatory reaction,
as TNF-α was the only molecule to show a consistent increasing
trend in the (H) tissue homogenates, (I) BALF and (J) plasma. TNF-α
was higher in the V1-siRNA+LPS group in all samples, IL-6 increased
without statistical significance. Data are presented as the mean ±
standard deviation. *P<0.05, vs. control group;
#P<0.01, vs. control group; %P<0.05,
vs. LPS group; $P=0.01, vs. LPS group;
&P<0.01, vs. LPS group. NF-κB, nuclear factor-κB;
TNF-α, tumor necrosis factor-α, IL-6, interleukin-6; siRNA, small
interfering RNA; ALI, acute lung injury; LPS, lipopolysaccharide;
V1, versican V1; BALF, bronchoalveolar lavage fluid; ELISA,
enzyme-linked immunosorbent assay. |
V1 knockdown induces the expression of
TLR2, but not TLR4
The expression of TLR2 was increased in the V1
knockdown group, however, no notable changes were observed in the
control and scramble siRNA groups (Fig. 4A–C). No significant differences
were found in the expression levels of TLR4 among the groups
(Fig. 4D–F). The mRNA expression
of TLR2, but not TLR4, increased in the V1 knockdown group, which
was consistent with the changes observed in the mRNA and protein
expression levels (Fig. 4G and
I).
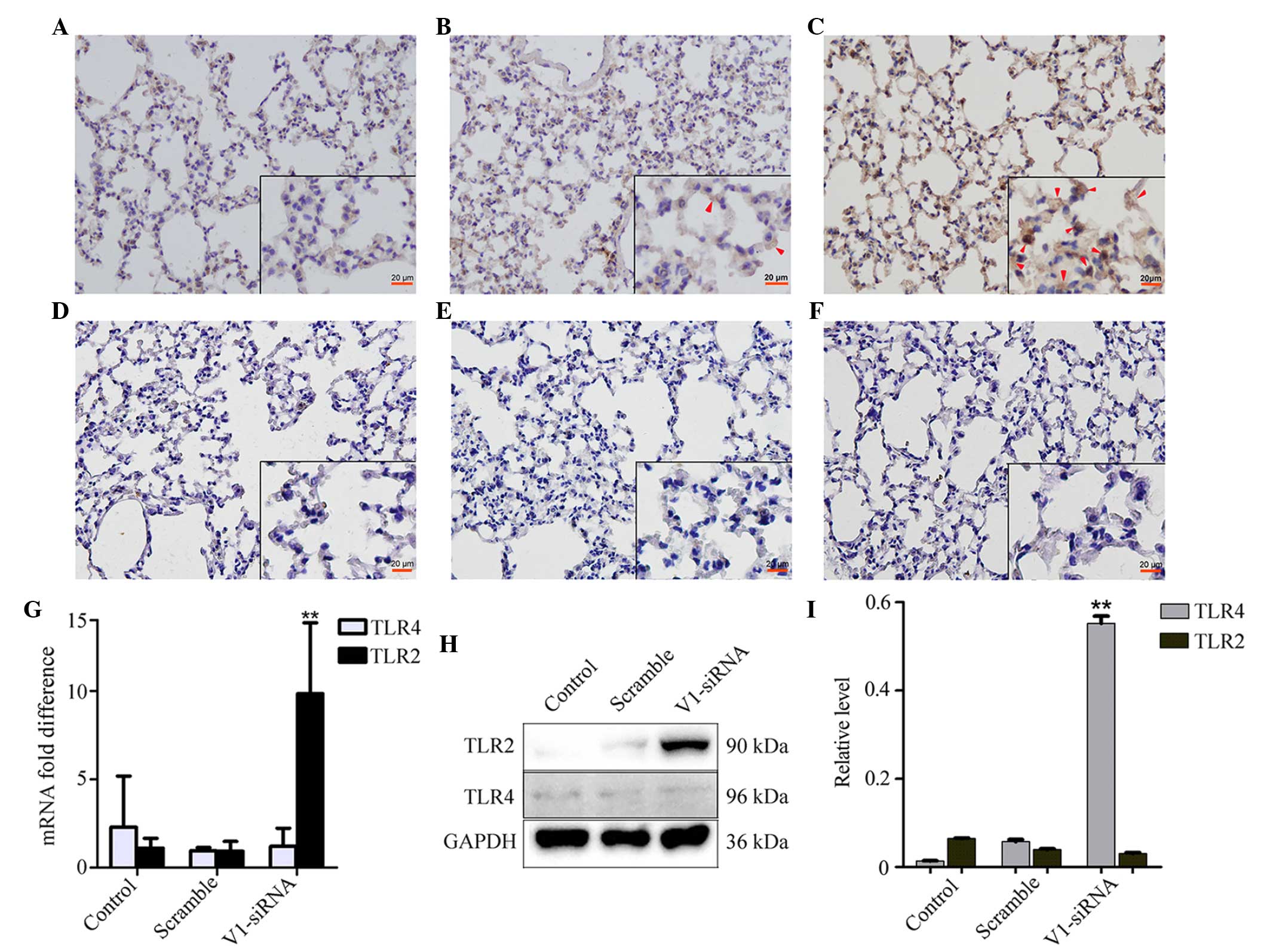 | Figure 4Changes in the expression levels of
TLR2 and TLR4 in the different groups. (A–C) Levels of TLR2
remained stable in the (A) control and (B) scramble groups, but
were altered in the V1-siRNA group. Red arrows show positive
staining. (Magnification, ×200; scale bar in vignettes=20
µm). The levels of TLR4 altered marginally in the (D)
control, (E) scramble and (F) V1-siRNA groups. The (G) mRNA and (H)
protein levels of TLR2 and TLR4 showed the same changes as those
observed in the immunostaining. (I) Relative protein level
calculated as the target grey value/GAPDH grey value from the
western blot. **P<0.01, vs. control. NF-κB, nuclear
factor-κB; TLR, toll-like receptor; siRNA, small interfering RNA;
ALI, acute lung injury; LPS, lipopolysaccharide; V1, versican
V1. |
Discussion
Previous reports have indicated that secreted
proteoglycans act as signaling molecules; in various inflammatory
diseases and in response to LPS, activated macrophages synthesize
and secrete versican (30–33) and, according to the results of the
present study, LPS induces V1 secretion in the lungs. It appeared
that V1 increase was not only confined to macrophages, but spreads
all over the lungs. The present study found that LPS also induced
the expression of V1 in lung fibroblasts and A549 cells in
vitro (data not shown), which supports the ubiquitous
LPS-associated expression of V1. The TGF-β1 signaling pathway is
associated with the synthesis of versican in lung fibroblasts
(34). The versican promoter
contains a typical TATA box, together with other 5′-flanking
elements, allowing for the regulation of versican in different
situations, such as cancer, inflammation, and growth and
development. V1 is also modulated by micro (mi)RNAs, and myocardin
represses V1 through the induction of miR-143 in smooth muscle
cells (35).
Activation of the NF-κB pathway was more marked
following V1 knockdown, which may be the predominant reason for the
deregulated TNF-α and IL-6 release, and aggravated immune
reactions. NF-κB signaling-induced TNF-α synthesis is now widely
accepted, in which the κB subunit binds to the 5′-untranslated
region of TNF-α to promote gene transcription (36). The present study suggested two
possible reasons for alterations in TNF-α levels, one of which is
the direct release from local affected cells, as V1-siRNA and LPS
can be taken up by epithelial cells, macrophages and fibroblasts,
which are all sources of TNF-α. The second is that, in LPS-insulted
lung fibroblasts, V1 knockdown increases the production of IL-6 and
IL-8 (data not shown), and these cytokines in vivo may
stimulate macrophages to release TNF-α in a paracine manner. The
present study examined TNF-α, IL-6, IL-8 and myeloperoxidase (data
not shown), however, only TNF-α increased significantly in all
samples.
The activation of NF-κB occurs via signaling through
the TLR superfamily and its adaptors, and the pro-inflammatory
function of proteoglycans is always associated with TLR2 and TLR4
(20). Therefore, the present
study examined the levels of TLR2 and TLR4 in the lung sections. Of
note, the level of TLR2 increased markedly, unlike TLR4. Versican
derived from Lewis lung carcinoma cells has been shown to interact
with TLR2 and its co-receptors, TLR6 and CD14, on macrophages,
inducing the secretion of TNF-α and IL-6, indicating the
association between the tumor and its pro-metastatic
microenvironment (20–22,37).
Versican also binds with TLR2 on ovarian tumor cells to stimulate
tumor cell proliferation (38).
Although the present study is the first, to the best of our
knowledge, to show that V1 knockdown can induce the expression of
TLR2, it is understandable as, being a natural ligand of TLR2, a
decreases in versican may lead to an increase in TLR2 in response.
However, the exact mechanism requires further investigation. The
TLR2 promoter contains binding motifs of several transcription
factors; a series of 5′-truncated TLR2 promoter-luciferase
constructs are required for further investigation of the reasons
for TLR2 synthesis. The canonical LPS sensor is TLR4, however, the
present study found minimal changes in TLR4 in the lungs, thus not
providing an explanation for the severe reaction in the
V1-knockdown mice. The upregulation of TLR2 may, at least
partially, provide an explanation. The present study hypothesized
that, as proteoglycan binds with TLR2 on cells, as a type of
comparative competitor of other pathogen-derived ligands, a
decrease in proteoglycan may increase the release of receptors for
LPS on the cell surface and result in a more severe reaction.
However, as far as we know, certain types of proteoglycans interact
with TLR2/4 and a second TLR, which is not involved in pathogen
sensing, thereby exacerbating the host response to microbial
invasion (20). Prior to LPS
insult, this complex may exist on the cell surface, and increase
further following TLR2 increase, acting as a latent threat to cell
homeostasis. Following the synchronous upregulation of TLR2 and LPS
stimulation, a marked inflammatory reaction occurs, however,
further investigations are required to elucidate the consequences
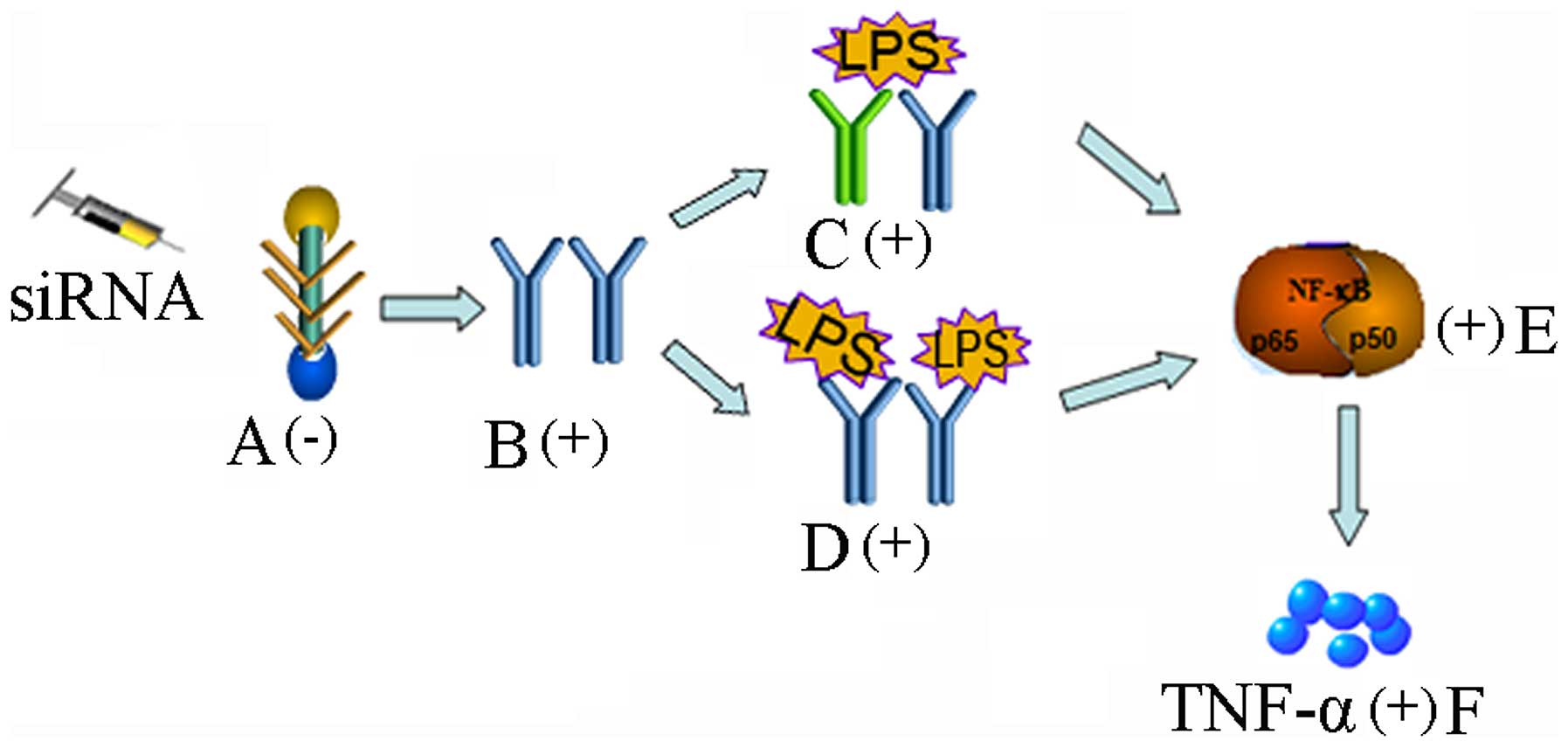
of LPS/versican and TLR interactions on TLR signaling (Fig. 5).
Inhibiting the expression of versican in cancer
cells inhibits the inflammation associated with these tumors
(21,22,39).
However, the mouse ALI model is a non-cancerous model, and the
mechanisms of ALI are complicated. Due to activated NF-κB and
increased TNF-α, the total immune reaction is more marked. The
present study demonstrated that the same condition existed in the
V1 knockdown fibroblasts in vitro (data not shown). The
anti-inflammatory effect of proteoglycan has also been confirmed in
human uterine cervical fibroblasts, in which primary cervical
fibroblasts were treated with proteoglycan and LPS concomitantly,
and significant decreases in IL-6 and IL-8 were observed (40).
The present study provided novel evidence of V1
modulating inflammation in the lungs and indicated its potential
regulatory role in V1-TLR2 interactions. Further investigations are
required to investigate the mechanisms involved in the upregulation
of TLR2, and the effects of LPS/versican and TLR interactions on
TLR signaling.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470231).
References
|
1
|
Seewaldt V: ECM stiffness paves the way
for tumor cells. Nat Med. 20:332–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spill F, Reynolds DS, Kamm RD and Zaman
MH: Impact of the physical microenvironment on tumor progression
and metastasis. Curr Opin Biotechnol. 40:41–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zamir E and Geiger B: Molecular complexity
and dynamics of cell-matrix adhesions. J Cell Sci. 114:3583–3590.
2001.PubMed/NCBI
|
|
4
|
Ieda M, Tsuchihashi T, Ivey KN, Ross RS,
Hong TT, Shaw RM and Srivastava D: Cardiac fibroblasts regulate
myocardial proliferation through beta1 integrin signaling. Dev
Cell. 16:233–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanekar S, Borg TK, Terracio L and Carver
W: Modulation of heart fibroblast migration and collagen gel
contraction by IGF-I. Cell Adhes Commun. 7:513–523. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valiente-Alandi I, Schafer AE and Blaxall
BC: Extracellular matrix-mediated cellular communication in the
heart. J Mol Cell Cardiol. 91:228–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee-Sayer SS, Dong Y, Arif AA, Olsson M,
Brown KL and Johnson P: The where, when, how, and why of hyaluronan
binding by immune cells. Front Immunol. 6:1502015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zimmermann DR and Ruoslahti E: Multiple
domains of the large fibroblast proteoglycan, versican. EMBO J.
8:2975–2981. 1989.PubMed/NCBI
|
|
9
|
Andersson-Sjöland A, Hallgren O,
Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK,
Rydell-Törmänen K, Bjermer L, Malmström A, Karlsson JC and
Westergren-Thorsson G: Versican in inflammation and tissue
remodeling: the impact on lung disorders. Glycobiology. 25:243–251.
2015. View Article : Google Scholar :
|
|
10
|
Sampson PM, Boyd RB, Pietra GG and Fishman
AP: Glycosaminoglycan biosynthesis in the isolated perfused rat
lung. J Appl Physiol Respir Environ Exerc Physiol. 57:1648–1654.
1984.PubMed/NCBI
|
|
11
|
Wu YJ, La Pierre DP, Wu J, Yee AJ and Yang
BB: The interaction of versican with its binding partners. Cell
Res. 15:483–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dours-Zimmermann MT, Maurer K, Rauch U,
Stoffel W, Fässler R and Zimmermann DR: Versican V2 assembles the
extracellular matrix surrounding the nodes of ranvier in the CNS. J
Neurosci. 29:7731–7742. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang ZW, Zhang JP, Zhou TT, Feng WH and
Jiao BH: Does the expression of versican isoforms contribute to the
pathogenesis of neurodegenerative diseases? Arch Med Res.
42:258–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cattaruzza S, Schiappacassi M,
Ljungberg-Rose A, Spessotto P, Perissinotto D, Mörgelin M, Mucignat
MT, Colombatti A and Perris R: Distribution of PG-M/versican
variants in human tissues and de novo expression of isoform V3 upon
endothelial cell activation, migration, and neoangiogenesis in
vitro. J Biol Chem. 277:47626–47635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kischel P, Waltregny D, Dumont B, Turtoi
A, Greffe Y, Kirsch S, De Pauw E and Castronovo V: Versican
overexpression in human breast cancer lesions: Known and new
isoforms for stromal tumor targeting. Int J Cancer. 126:640–650.
2010. View Article : Google Scholar
|
|
16
|
Wu Y, Chen L, Zheng PS and Yang BB: beta
1-Integrin-mediated glioma cell adhesion and free radical-induced
apoptosis are regulated by binding to a C-terminal domain of
PG-M/versican. J Biol Chem. 277:12294–12301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang BL, Zhang Y, Cao L and Yang BB: Cell
adhesion and proliferation mediated through the G1 domain of
versican. J Cell Biochem. 72:210–220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ang LC, Zhang Y, Cao L, Yang BL, Young B,
Kiani C, Lee V, Allan K and Yang BB: Versican enhances locomotion
of astrocytoma cells and reduces cell adhesion through its G1
domain. J Neuropathol Exp Neurol. 58:597–605. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wight TN, Kang I and Merrilees MJ:
Versican and the control of inflammation. Matrix Biol. 35:152–161.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frey H, Schroeder N, Manon-Jensen T, Iozzo
RV and Schaefer L: Biological interplay between proteoglycans and
their innate immune receptors in inflammation. FEBS J.
280:2165–2179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim S, Takahashi H, Lin WW, Descargues P,
Grivennikov S, Kim Y, Luo JL and Karin M: Carcinoma-produced
factors activate myeloid cells through TLR2 to stimulate
metastasis. Nature. 457:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang W, Xu GL, Jia WD, Ma JL, Li JS, Ge
YS, Ren WH, Yu JH and Liu WB: Ligation of TLR2 by versican: A link
between inflammation and metastasis. Arch Med Res. 40:321–323.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang HM, Bodenstein M and Markstaller K:
Overview of the pathology of three widely used animal models of
acute lung injury. Eur Surg Res. 40:305–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induce acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Netea MG, van Deuren M, Kullberg BJ,
Cavaillon JM and Van der Meer JW: Does the shape of lipid A
determine the interaction of LPS with Toll-like receptors? Trends
Immunol. 23:135–139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Togbe D, Schnyder-Candrian S, Schnyder B,
Doz E, Noulin N, Janot L, Secher T, Gasse P, Lima C, Coelho FR, et
al: Toll-like receptor and tumor necrosis factor dependent
endotoxin-induced acute lung injury. Int J Exp Pathol. 88:387–391.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNF alpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar
|
|
28
|
Fink L, Seeger W, Ermert L, Hänze J, Stahl
U, Grimminger F, Kummer W and Bohle RM: Real-time quantitative
RT-PCR after laser-assisted cell picking. Nat Med. 4:1329–1333.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matute-Bello G, Downey G, Moore BB,
Groshong SD, Matthay MA, Slutsky AS and Kuebler WM; Acute Lung
Injury in Animals Study Group: An official American thoracic
society workshop report: Features and measurements of experimental
acute lung injury in animals. Am J Respir Cell Mol Biol.
44:725–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toeda K, Nakamura K, Hirohata S, Hatipoqlu
OF, Demircan K, Yamawaki H, Oqawa H, Kusachi S, Shiratori Y and
Ninomiya Y: Versican is induced in infiltrating monocytes in
myocardial infarction. Mol Cell Biochem. 280:47–56. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asplund A, Fridén V, Stillemark-Billton P,
Camejo G and Bondjers G: Macrophages exposed to hypoxia secrete
proteoglycans for which LDL has higher affinity. Atherosclerosis.
215:77–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gill S, Wight TN and Frevert CW:
Proteoglycans: Key regulators of pulmonary inflammation and the
innate immune response to lung infection. Anat Rec (Hoboken).
293:968–981. 2010. View
Article : Google Scholar
|
|
33
|
Chang MY, Tanino Y, Vidova V, Kinsella MG,
Chan CK, Johnson PY, Wight TN and Frevert CW: Reprint of: A rapid
increase in macrophage-derived versican and hyaluronan in
infectious lung disease. Matrix Biol. 35:162–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Westergren-Thorsson G, Antonsson P,
Malmström A, Heinegård D and Oldberg A: The synthesis of a family
of structurally related proteoglycans in fibroblasts is differently
regulated by TFG-beta. Matrix. 11:177–183. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Hu G and Zhou J: Repression of
versican expression by microRNA-143. J Biol Chem. 285:23241–23250.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raabe T, Bukrinsky M and Currie RA:
Relative contribution of transcription and translation to the
induction of tumor necrosis factor-alpha by lipopolysaccharide. J
Biol Chem. 273:974–980. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Z, Miao L and Wang L: Inflammation
amplification by versican: The first mediator. Int J Mol Sci.
13:6873–6882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang
F, Wu KY and Wan HY: Tumor-produced versican V1 enhances
hCAP18/LL-37 expression in macrophages through activation of TLR2
and vitamin D3 signaling to promote ovarian cancer progression in
vitro. PLoS One. 8:e566162013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Said N, Sanchez-Carbayo M, Smith SC and
Theodorescu D: RhoGDI2 suppresses lung metastasis in mice by
reducing tumor versican expression and macrophage infiltration. J
Clin Invest. 122:1503–1518. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fukuyama A, Tanaka K, Kakizaki I, Kasai K,
Chiba M, Nakamura T and Mizunuma H: Anti-inflammatory effect of
proteoglycan and progesterone on human uterine cervical
fibroblasts. Life Sci. 90:484–488. 2012. View Article : Google Scholar : PubMed/NCBI
|