Introduction
Mesenchymal stem cells (MSCs) are a promising cell
source for use in tissue regeneration and their potential
therapeutic application has already been investigated in several
clinical trials (1–3). Human MSCs have the ability to
differentiate into different mesodermal cell lineages, including
osteocytes, chondrocytes, adipocytes, hepatocytes and neurons
(4,5). Therefore, MSCs have important
therapeutic potential. Typically, these cells are expanded prior to
their clinical application. However, MSCs have a limited lifespan
in vitro, as do any normal somatic cells, and there is no
precise molecular definition of MSC long-term culture in
vitro. Several studies have demonstrated that long-term culture
of MSCs results in continuous changes to the cells, including
decreased proliferation rate, increased cell size and different
differentiation potentials (6,7).
These problems have hindered the expansion of MSCs for therapeutic
use, causing a major bottleneck in clinical applications. MSCs have
become an attractive therapeutic tool due of their unique
characteristics, including their ability to self-renewal, and ease
of isolation and expansion. MSCs possess a broad spectrum for
regenerative medicine due to their potential to repair tissue and
to differentiate into osteoblasts, chondroblasts, adipocytes and
myoblasts. The transplantation of pluripotent MSCs has been tested
in the treatment of neurological disorders, such as Parkinson's
disease, cerebral infarction, brain injury and spinal cord injury,
bone tissue engineering, cardiovascular diseases, severe liver
damage repair, pulmonary fibrosis and reduction of bone marrow
transplant rejection indicating the potential for porting
applications.
It has been reported that long-term culture of MSCs
causes the cells to undergo replicative senescence with the cell
morphology becoming enlarged and flattened, the development of
prominent nucleoli and cytoplasmic granules, alteration in the
differentiation potential, and shortened telomere length over
progressively increasing passages (8). Additionally, these senescent cells
can be stained by senescence-associated β-galactosidase (SA-β-gal).
It is important, therefore, to fully understand the biological
alterations that occur in these expanded stem cell populations.
Cellular senescence is induced by intrinsic and
extrinsic factors (9,10). With numerous passages, cell
senescence can be triggered by replicative exhaustion, DNA damage
and telomere shortening. Increasing evidence indicates that the
continuous accumulation of intracellular reactive oxygen species
(ROS) is a major initiating factor of replicative senescence
(11,12). Furthermore, extrinsic stresses,
such as oxidative stress, may affect intrinsic factors, and
subsequently lead to DNA damage accumulation and telomere
shortening. In addition to replicative senescence, premature
senescence is another model of in vitro senescence.
Premature senescence is induced by various extrinsic factors,
including hydrogen peroxide, ionizing radiation, high glucose,
D-galactose, high oxygen and old rat serum (13–15).
In addition, human fibroblasts undergo premature senescence when
cultured in 8-methoxypsoralen/ultraviolet A conditions (16). Zhang et al (14) reported that old rat serum induced
the senescence of adult MSCs, and inhibited their proliferation and
survival. Apurinic/apyrimidinic endonuclease 1/redox factor-1
(APE1/Ref-1) is a redox factor for transcription factors that
alters trinucleotide stores, which are vital to energy metabolism,
via regulation of DNA repair processes and transcription factors,
including P53 (17). P21, a
cyclin-dependent kinase inhibitor, is a major transcriptional
target of P53. It was originally identified as a gene that inhibits
DNA synthesis and promotes cell cycle arrest (18). Further analysis demonstrated that
senescence of numerous cell lines was correlated with the
upregulation of P21 and P53 (19).
Thus, establishing an optimized microenvironment for the culture
and expansion of MSCs that preserves their properties and prolongs
their lifespan is imperative (20–23).
Cellular senescence is a complex process and the
sequence of its molecular mechanisms is thus far unknown. Sharpless
and DePinho (24) proposed that
humans grow old due to stem cell aging as a result of mechanisms
designed to suppress the development of cancer. Thus, it may be
important to determine how individuals grow old and how cancer
develops via investigation of the process of stem cell aging. The
current study compares several biological characteristics of human
umbilical cord MSCs (hucMSCs) during long-term in vitro
expansion for passages 4, 11 and 17 (P4, P11 and P17). In addition,
the present study established an H2O2-induced
premature senescence model to examine MSC senescence.
Materials and methods
Isolation and culture of hucMSCs
The experimental protocol was approved by Jiangsu
University ethics committee (Zhenjiang, China). Fresh umbilical
cords were collected in March 2015, immediately after birth from
healthy donors at the First People's Hospital of Zhenjiang
(Zhenjiang, China). The umbilical cords were rinsed twice in
phosphate-buffered saline (PBS) until the cord blood was cleared.
The blood vessels were removed from each cord, then the remaining
tissue was cut into 1-mm3 pieces, and suspended in
low-glucose Dulbecco's modified Eagle's medium (L-DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 1%
penicillin and 1% streptomycin (Beyotime Institute of
Biotechnology, Haimen, China). All cultures were incubated at
37°C with an atmosphere of 5% CO2 in a humidified
chamber. The medium was changed every 3 days after initial plating.
When well-developed colonies of fibroblast-like cells reached
70-80% confluence, the cells were trypsinized with 0.25%
trypsin-EDTA (Thermo Fisher Scientific, Inc.) and passaged into new
culture flasks for further expansion. To establish a cell model of
H2O2-induced MSC premature senescence, early
passage (P4) hucMSCs were treated with H2O2
at different concentrations (0, 200, 400, 600 and 800 μM)
for 2 h. The stimulus was then removed and cells were further
incubated in fresh medium for 48 h.
Flow cytometry
To determine the phenotypes of hucMSCs, fluo-rescein
isothiocyanate (FITC)- or phycoerythrin (PE)-labeled mouse
monoclonal antibodies against human leukocyte antigen (HLA)-DR,
cluster of differentiation (CD)105, CD34, CD29, CD90 and CD44
(1:10; BD Biosciences, Franklin Lakes, NJ, USA; cat. nos. 555811,
560839, 348053, 555443, 555596 and 555479, respectively) were used.
Briefly, at P4, MSCs were trypsinized, washed twice with PBS and
stained with the monoclonal antibodies, according to the
manufacturer's protocol. Mouse monoclonal PE-immunoglobulin G1
(IgG1) and FITC-IgG1 (BD Biosciences; cat. nos. 555574 and 555573,
respectively) were used as isotype controls. The stained cells were
analyzed using the FACSAria flow cytometer(BD Biosciences).
Morphological observation of hucMSCs
The cell surface morphology of hucMSCs was analyzed
using scanning electron microscopy (SEM; Hitachi S-3400N; Hitachi,
Ltd., Tokyo, Japan) at P4, P11 and P17. Cells were seeded in 6-well
plates and the media was removed following 3 days of culture. The
cells were washed with PBS and fixed with 2.5% glutaraldehyde
(Sangon Biotech Co., Ltd., Shanghai, China) for 1 h. The cells were
then rinsed with distilled water and dehydrated with a series of
ethanol gradients starting at 30% and increasing to 50, 70, 80, 90,
95 and 100% (v/v). Subsequently, the cells were air-dried overnight
at room temperature in a fume hood. The cells were gold-coated and
cell morphology was analyzed using SEM.
To image the cell nuclei, cells were cultured at a
comparable density on coverslips in 6-well plates. They were washed
with PBS, fixed for 15 min with 4% formaldehyde (Sangon Biotech
Co., Ltd.) and washed with PBS 3 times. DNA was visualized with
4′6-diamidino-2-phenylindole (1 mg/ml) (Beyotime Institute of
Biotechnology) by fluorescence microscopy (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Cell proliferation assay
Assessment of the proliferative ability of hucMSCs
was performed using a
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT; AMRESCO LLC, Solon, OH, USA) assay at P4, P11 and P17. The
cells were seeded in 96-well plates at a density of 1,000 cells per
well. At days 1, 2, 3, 4, 5, 6 and 7, MTT (20 μl) was added
to each well for 4 h. When the reaction was terminated, the medium
was discarded and 150 μl dimethylsulfoxide (Sigma-Aldrich,
St. Louis, MO, USA) was added to each well. Following uniform
oscillation for 10 min to fully dissolve the purple formazan
crystals, the absorbance values were determined at 490 nm with a
spectrophotometer (FLX800; BioTek Instruments, Inc., Winooski, VT,
USA).
SA-β-gal staining
SA-β-gal activity was analyzed in different passages
(P4, P11 and P17) of hucMSCs using a SA-β-gal staining kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. In brief, the cells were cultured to
comparable densities on coverslips in 24-well plates and washed
with PBS, fixed for 15 min with 4% formaldehyde and washed with
PBS. Subsequently, the cells were incubated overnight at
37°C in a CO2-free chamber with freshly prepared
SA-β-gal stain solution. SA-β-gal-positive cells exhibited a blue
color; the number of positive cells was counted for every 200 cells
in randomly selected fields of view using light microscopy (TE300;
Nikon Corporation, Tokyo, Japan).
Cell cycle analysis
Cells at P4, P11 and P17 were collected, washed
twice with PBS and stained with propidium iodide (Sigma-Aldrich)
for 30 min in dark conditions. The stained cells were analyzed by
flow cytometry (FACSAria; BD Biosciences).
Generation of conditioned media (CM) for
hucMSC migration assay
To prepare CM, 8×04 hucMSCs of P4, P11
and P17 were plated on 6-well culture plates with 10% FBS L-DMEM
and allowed to adhere overnight at 37°C with 5%
CO2 atmosphere. The following day, the media was
removed, the cells were washed twice with PBS and then re-incubated
with 1.5 ml serum-free culture media. After 12 h, the CM was
collected, centrifuged for 5 min at 447 × g to remove cell debris
and passed through a 0.45-μm filter (Sigma-Aldrich). CM
aliquots were frozen at -20°C until analysis (not exceeding
2 weeks).
P4 MSCs (4×104) in 200 μl
serum-free L-DMEM were plated in the upper chambers and 600
μl undiluted CM with 10% FBS was added to the lower
chambers. After 10 h of incubation, the cells were fixed with 4%
formaldehyde for 30 min. The cells that remained on the membrane of
the upper chamber were removed with cotton swabs and migrating
cells were stained with crystal violet (Sigma-Aldrich). Four
low-power fields (×100) were randomly selected in each chamber to
observe the cells and the number of stained migrated cells on each
image was counted.
ROS detection
To detect the accumulation of intracellular ROS in
hucMSCs, a ROS assay kit (Beyotime Institute of Biotechnology) was
used. Following culture to different passages (P4, P11 and P17),
the cells were washed 3 times in serum-free medium and incubated in
a final concentration of 10 mM dihydrodichlorofluorescein diacetate
(H2DCFDA) at 37°C for 25 min. The media was then removed and
cells were washed 3 times with serum-free medium. The cells were
observed using a fluorescence microscope (TE300; Nikon
Corporation).
To quantify the ROS level, the H2DCFDA fluorescence
intensity of the cells was detected by flow cytometry. Briefly,
5×104 cells were collected and resuspended in a final
concentration of 10 mM H2DCFDA with serum-free medium. After 25 min
incubation at 37°C, cells were washed with serum-free medium
3 times and resuspended in PBS, then placed on ice for immediate
detection using a FACScan flow cytometer (BD Biosciences) with
excitation at 488 nm and emission at 525 nm.
HucMSC differentiation assays
HucMSCs at P4, P11 and P17 were cultured in a medium
containing either osteogenic (50 mM ascorbate-phosphate, 10 mM
β-glycerophosphate and 0.1 mM dexamethasone) or adipogenic (1 mM
dexamethasone, 10 mM insulin, 0.5 mM isobutyl-methylxanthine and
200 mM indomethacin) reagents (Sigma-Aldrich) at 5% CO2
at 37°. After 2 weeks of culturing, osteogenic
differentiation was assessed by the examination of neutrophil
alkaline phosphatase with Alizarin Red dye (Sigma-Aldrich). After 3
weeks of culturing, adipogenic differentiation was detected via
intracellular lipid vesicles; the cells were stained with Oil Red-O
(Cyagen, Guangzhou, China) to detect lipids using an inverted
microscope (TE300; Nikon Corporation).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Reverse transcription was conducted
using Moloney murine leukemia virus reverse transcriptase (Promega,
USA) and the obtained cDNA was subjected to PCR. PCR was performed
using 1 μg of cDNA sample with 0.3 U of Taq polymerase
(CinnaGen Co., Tehran, Iran), 200 μM dNTPs, 10 pM of each
primer, reaction buffer and MgCl2 (Takara Bio, Inc., Otsu, Japan)
in a 25-μl volume. PCR amplification was performed for 35
cycles using an ABI 2720 thermal cycler (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The cycling conditions were: 94°C
for 30 sec, 60°C (primer) for 30 sec, 72°C for 30 sec
and a final extension at 72°C for 10 min. The PCR products
were separated on a 1.5% agarose gel, stained with ethidium bromide
(Thermo Fisher Scientific, Inc.) and visualized under UV light
using the GeneGenius bio-imaging system (Syngene, MD, USA). Primers
were as follows: APE1/Ref-1, F 5′-GCTTCGAGCCTG GATTAAGA-3′ and R
5′-TCATCGCCTATGCCGTAAGA-3′; P21, F 5′-CTACCTCAGGCAGCTCAAG-3′ and R,
5′-AGCCTCTACTGCCACCATC-3′; P53, F 5′-TCTGTGACTTGCACGTACTC-3′ and R
5′-TGTAGTGGATGGTGGTACAG-3′; β-actin, F 5′-CACGAAACTACCTTCAACTC-3′
and R 5′-CATACTCCTGCTTGCTGATC-3′. The primers were produced by
Shanghai Bio-Engineering Company (Shanghai, China).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using GraphPad Prism
software (version 5; GraphPad Software, Inc., La Jolla, CA, USA).
All experiments were replicated 3 times. Analysis of variance was
used to analyze variance among all groups, and Student's t-test was
performed to compare experimental and relative control groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphology and cell surface
characteristics of hucMSCs in primary culture
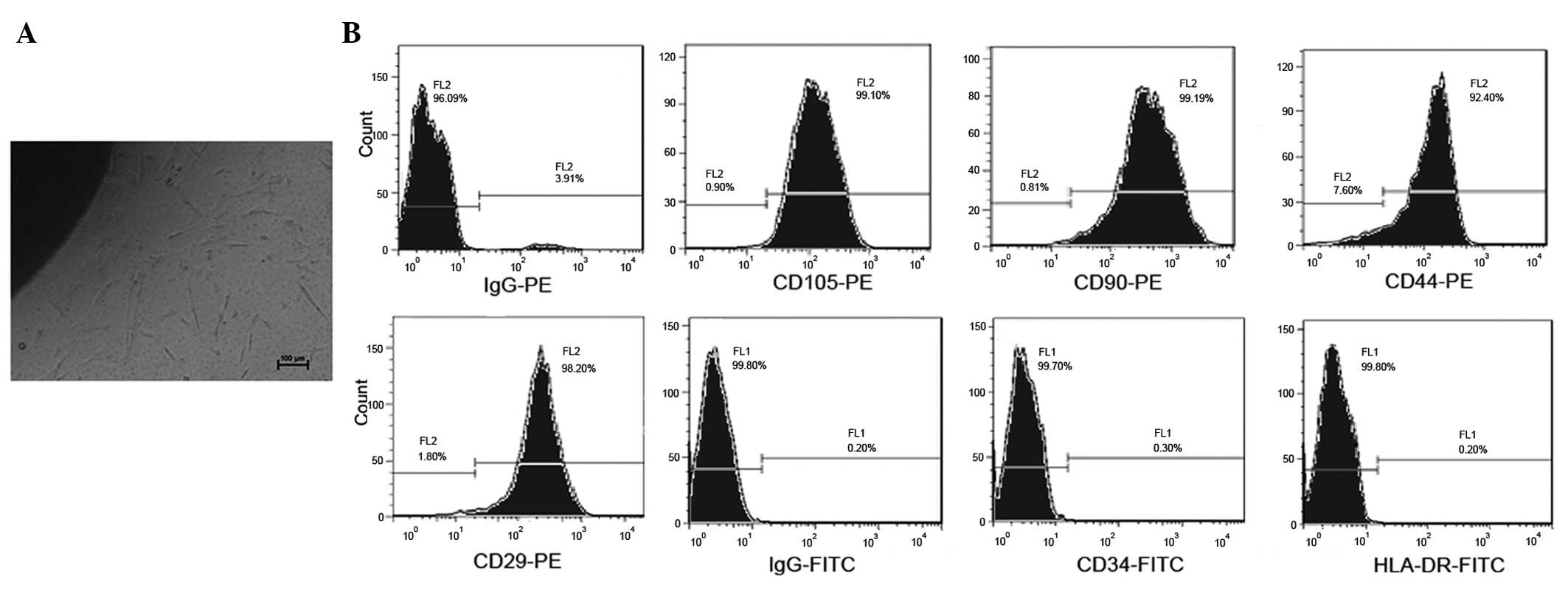
Following the initial 7 days of primary culture,
hucMSCs adhered to the plastic surface of the culture flasks and
presented as a small population of single cells with spindle-like
shape. On day 10 after initial plating, the cells exhibited long
spindle-shaped fibroblastic cells, they had began to form colonies
and were confluent (Fig. 1A). At
P4, hucMSCs were positively stained with CD29, CD90, CD44 and
CD105, but were negative for the hematopoietic lineage markers CD34
and HLA-DR, as measured by fluorescence-activated cell sorting
(FACS) analysis (Fig. 1B).
Changes in hucMSCs during long-term in
vitro culture
HucMSCs from the same donors were characterized by
their morphology, growth, SA-β-gal activity and differentiation
capacity. The present study observed morphological changes during
long-term culture of hucMSCs by analyzing the cell membrane and
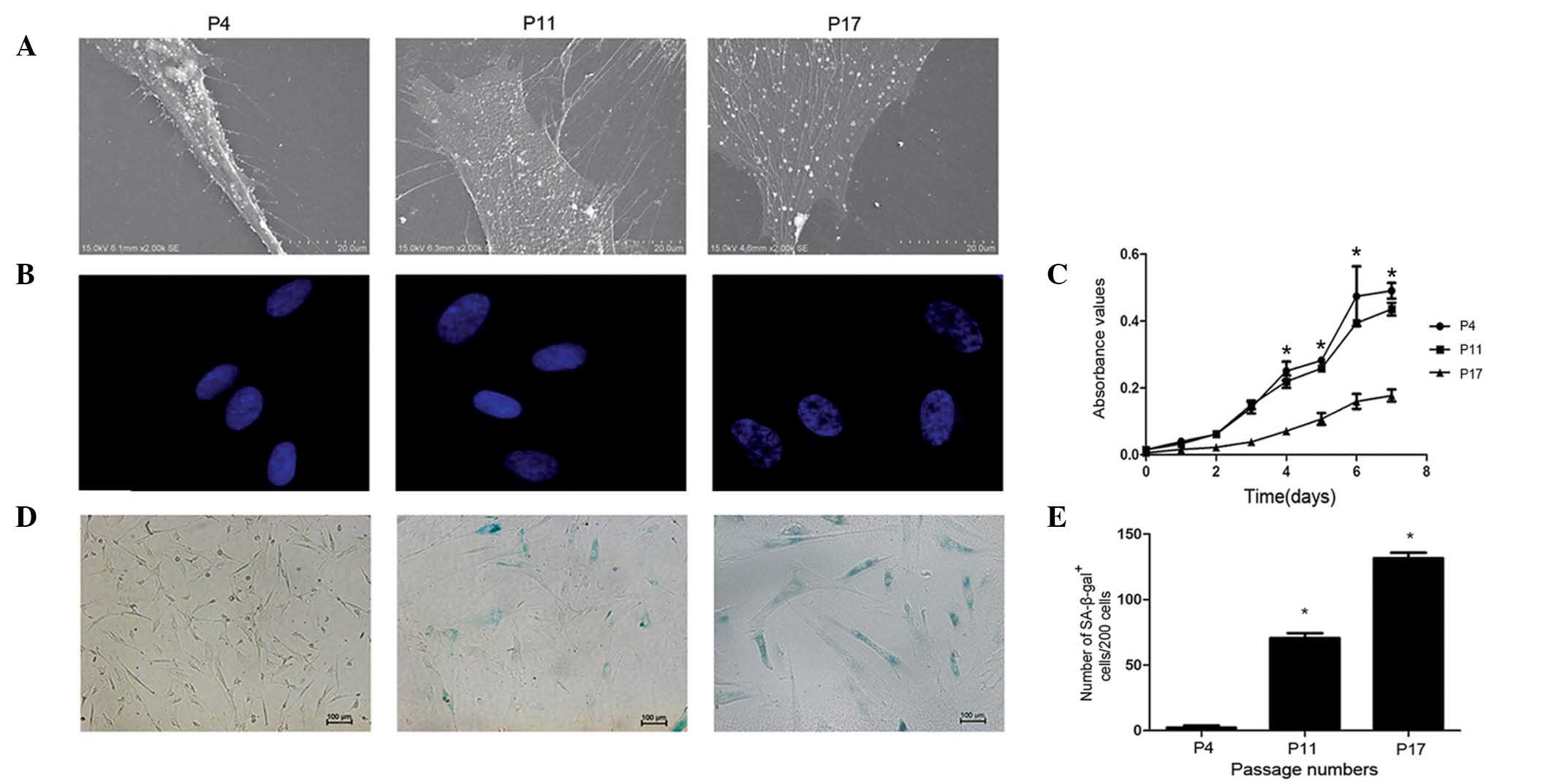
nucleus. The ultrastructure of the cell membrane was observed under
SEM (Fig. 2A). Cells at P4 were
spindle-like, plump, stereoscopic and exhibited a large population
of uniformly distributed vimineous microvilli. However, at the
middle and late phases of the culture (P11 and P17, respectively),
hucMSCs became flatter, broader and had numerous large pseudopods,
often with multiple secondary bifurcations. Furthermore, the number
and length of microvilli was decreased during the late phases,
particularly at P17. The current study also used immunofluorescence
to observe changes to the cell nuclei (Fig. 2B). At P4, the chromatin
distribution in the nuclei was homogeneous and the nucleus size was
uniform. Compared with P4, the nuclei at P11 and P17 were swollen,
and chromatin was localized into a small area termed the
senescence-associated heterochromatic foci (SAHF). It was concluded
that both the nuclei and the cell membranes of hucMSCs had
undergone changes during the long-term in vitro culture.
Subsequently, the current study analyzed the growth
characteristics of hucMSCs during long-term in vitro
culture. The proliferation rate of hucMSCs was measured at P4, P11
and P17 by MTT assay (Fig. 2C).
The hucMSC proliferation rate in the early and middle phases
exhibited an S-shaped growth curve, with a minor decrease in
proliferation rate in the middle phase compared with the early
phase. However, when reaching P17, the hucMSC proliferation rate
was significantly decreased compared with the early phase cells
(P<0.05). The shape of long-term growth curves differed
considerably between passages, with almost a straight line
exhibited during the late phase of hucMSC culture.
Additionally, the current study detected SA-β-gal
activity at the 3 passages (Fig.
2D). The number of senescent cells were increased during the
middle and late phase, compared with the early phase (P<0.05;
Fig. 2E). The results of the
current study indicate that hucMSCs undergo replicative senescence
during long-term in vitro culture.
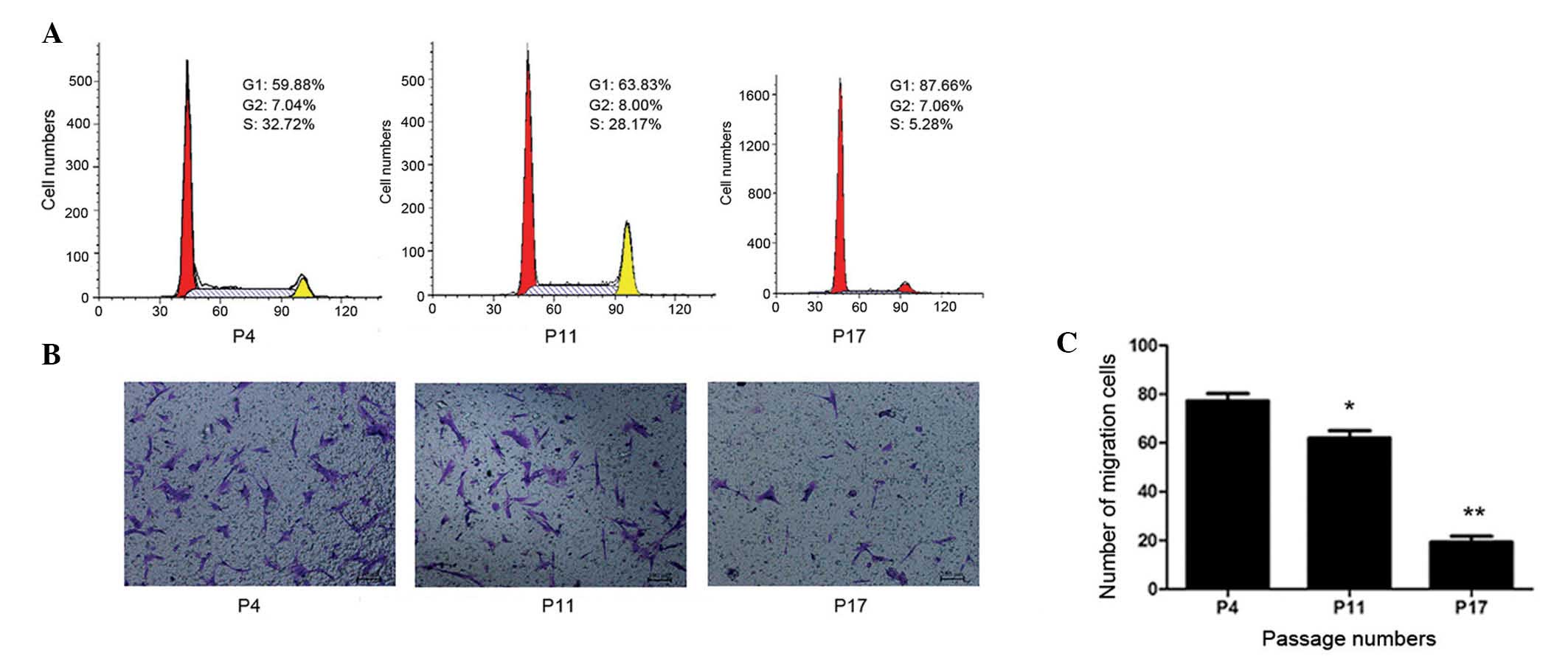
Considering the functional implications of changes
to growth characteristics during multiple passages, the present
study analyzed the cell cycle status of hucMSCs. The cell cycle
distribution at different passages was determined by FACS analysis.
Single-variable histograms of DNA provided data measuring the
percentages of cells in the G0/G1, S and G2/M phases of the cell
cycle. As presented in Fig. 3A,
hucMSCs demonstrated a progressive increase in the frequency of
cells in G0/G1 phase during long-term in vitro culture. Cell
cycle analysis of P17 revealed an obvious increase in the number of
cells in the G0/G1 phase and a reduction of S-phase cells compared
with P4 and P11. The current study did not observe the development
of polyploidy in hucMSCs throughout the long-term culture.
CM was collected from P4, P11 and P17 cells, and
used as the medium in the lower chamber during a cell migration
assay. In the P4 and P11 CM groups, obvious migration of cells was
observed, whereas few migrated cells were observed in the P17 CM
group. Cell counting demonstrated that the migration of cells in
the P17 CM group (19±3.4 cells/100 field) was significantly reduced
compared with the P4 and P11 groups (77±3.9 and 62±4.3 cells/field,
respectively; P<0.01; Fig. 3B and
C). The results indicated that senescent hucMSCs may secrete
toxic factors into the microenvironment that inhibit hucMSC
migration and are harmful to neighboring cells.
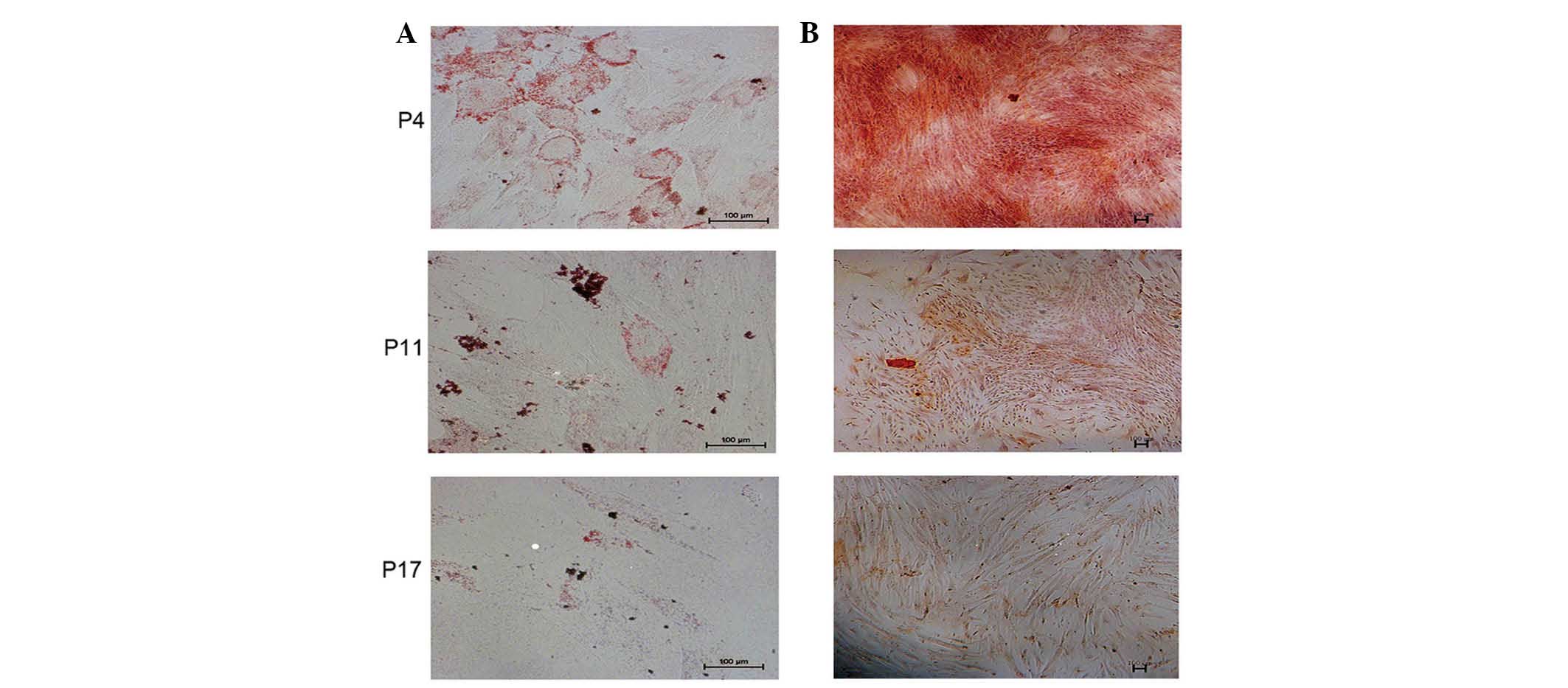
Long-term culturing of hucMSCs impairs
the capacity to differentiate in vitro
In order to observe the differentiation potential of
hucMSCs at P4, P11 and P17, the cells were induced to differentiate
into adipocytes or osteocytes, as measured by positive staining of
Oil Red-O (Fig. 4A) and Alizarin
Red (Fig. 4B), respectively. Based
on visual assessment of the extent of Oil Red O-positive lipid
inclusions, it was determined that the adipogenic differentiation
capacity of hucMSCs was decreased at P11 and P17 compared with at
P4. Similarly, the capability of osteogenic differentiation of
hucMSCs was decreased at P11 and P17 compared with at P4.
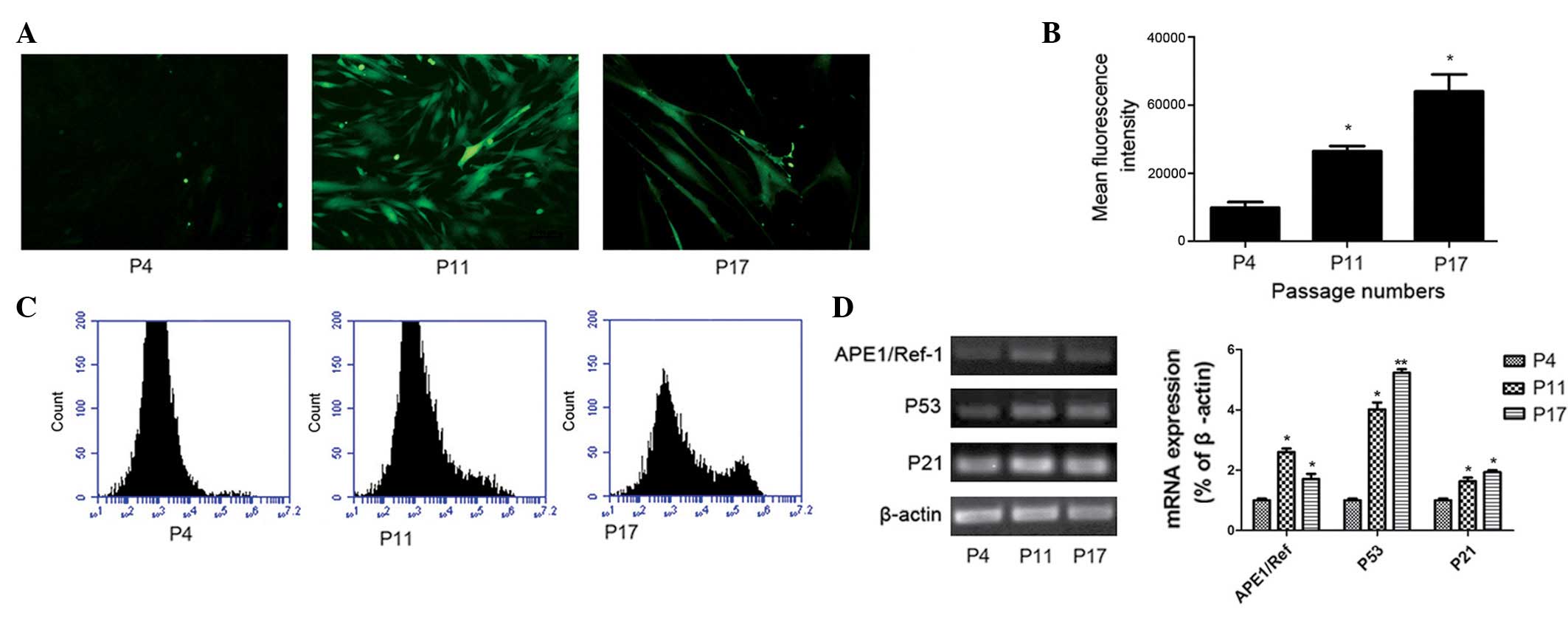
Increased levels of intracellular ROS and
senescence markers during the long-term culture of hucMSCs
To investigate the levels of intracellular ROS
during long-term culture, a fluorescence protocol using H2DCFDA was
performed on P4, P11 and P17 hucMSCs. As presented in Fig. 5A, the green fluorescence was more
intense in P11 and P17 hucMSCs compared with P4 hucMSCs. This
suggests that hucMSCs exhibit low intracellular ROS levels during
the early phases; but the levels increase in the middle and late
phases. To quantify the ROS levels, fluorescence intensity was
detected by flow cytometry (Fig. 5B
and C). The mean fluorescence intensity (MFI) of P11 and P17
were increased compared with at P4. The MFI was increased by 3-fold
in P17 hucMSCs and by 2-fold in P11 hucMSCs compared with P4
hucMSCs. Thus, hucMSCs exhibited higher intracellular ROS levels at
later passages during the long-term culture (P<0.05; Fig. 5C).
To further investigate the association between
senescence and oxidative stress, the current study analyzed the
mRNA expression of P53, P21 and APE1/Ref-1 in different phases of
hucMSC culture. In agreement with a previous study (19), telomeric foci containing multiple
DNA damage response factors were assembled in a subset of senescent
cells and signaled through ATM to p53, upregulating p21 and causing
G1 phase arrest. As demonstrated in Fig. 5D, compared with the early passages,
P21, P53 and APE1/Ref-1 mRNA levels were significantly increased in
the middle and late phases.
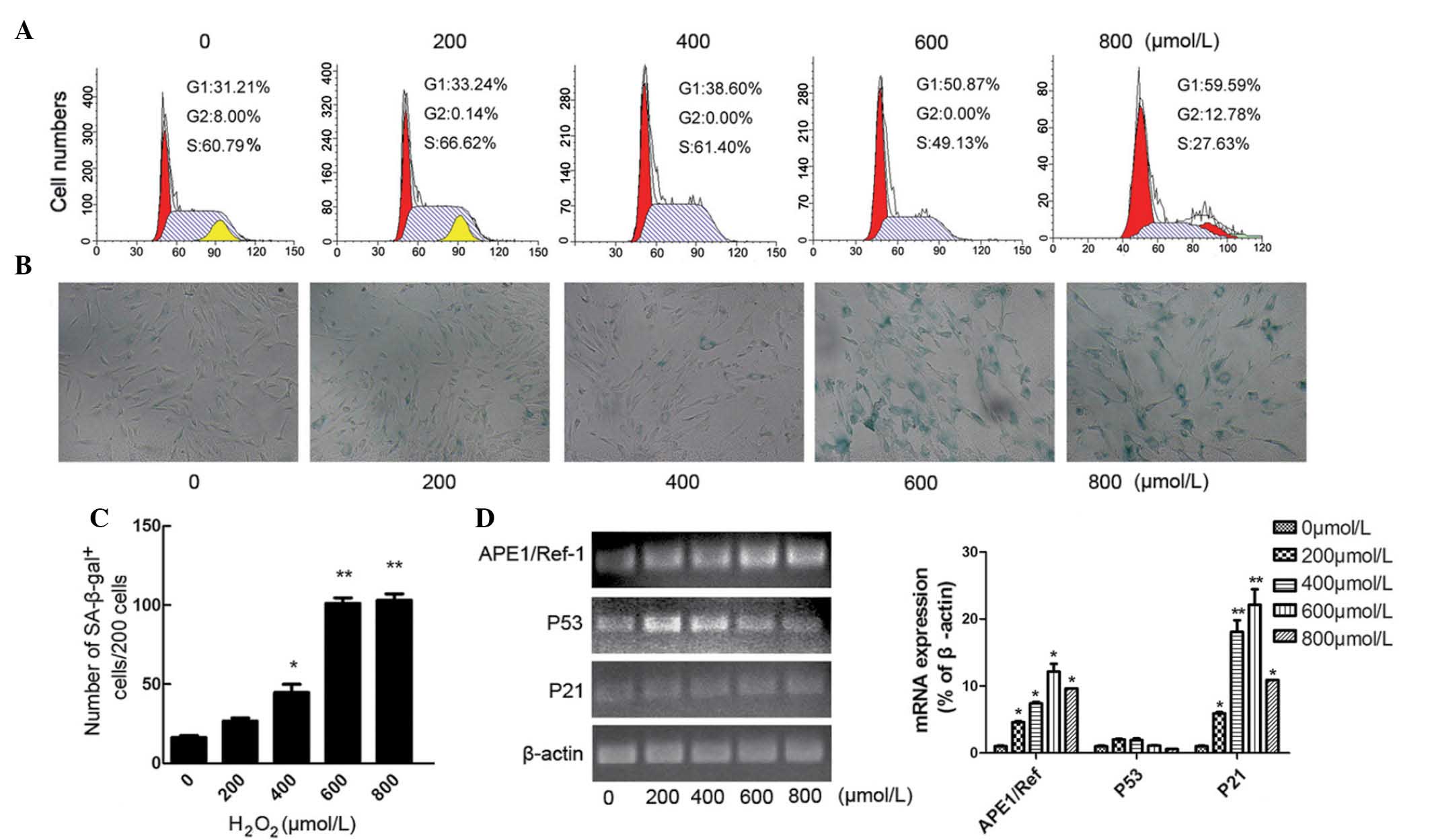
Establishing a cell model of
H2O2-induced MSC premature senescence
Early passage hucMSCs were treated with
H2O2 at different concentrations (0, 200,
400, 600 and 800 μM) for 2 h. The stimulus was then removed
and cells were further incubated in fresh medium for 48 h. The
effect of H2O2 treatment on the cell cycle in
P4 hucMSCs was analyzed (Fig. 6A).
FACS analysis demonstrated that, with increasing concentrations of
H2O2, the number of hucMSCs in the G0/G1
fraction progressively increased, and treatment with 600 μM
H2O2 significantly promoted cell cycle arrest
in the G0/G1 phase (Fig. 6;
P=0.036).
Following H2O2 treatment, the
number of apoptotic cells did not markedly increase, however, the
hucMSCs became larger and flatter. Staining of hucMSCs with
SA-β-gal indicated that hucMSCs were subjected to senescence
following H2O2 stimulation (Fig. 6B). In the five
H2O2-treated groups, the proportion of
premature senescence cells, as measured by SA-β-gal staining,
increased progressively with increasing concentrations of
H2O2 (8.17, 13.33, 22.33, 52.17 and 50%;
Fig. 6C). This indicates that
H2O2 promotes senescence of early passage
hucMSCs in a concentration-dependent manner. However, the dose of
800 μM H2O2 exhibited a slight
cytotoxic effect, with viable cells exhibiting poor adherence when
washed with PBS. Hence, we propose that a dose of 600 μM
H2O2 is suitable to establish a model of
aging in hucMSCs. Additionally, treatment of P4 hucMSCs with 600
μM H2O2 caused them to become larger
and flatter, and the cells appeared to be morphologically
indistinguishable from replicative senescent cells (P11 or
P17).
The expression levels of P53, P21 and APE1/Ref-1 are
upregulated in replicative senescence and premature senescence. P53
(P>0.05) and P21 (P<0.05) mRNA expression levels were also
increased following H2O2 treatment (Fig. 6D). However, at the higher
concentrations of H2O2 (600 and 800
μM), the expression level of P53 was reduced. The current
study interpreted that excessive stimulation of oxidation may
decrease the activities of P53 or even inactivate it. Additionally,
exposure to H2O2 for 2 h resulted in
dose-dependent increased expression levels of APE1/Ref-1 and P21
mRNA in the premature senescence hucMSCs (P<0.05; Fig. 6D). This indicates that oxidative
stress is important in regulating MSC senescence.
Discussion
Human MSCs have been isolated from various tissues
and are a potential stem cell source for use in regenerative
medicine. Typically, stem cell numbers are limited and it is
necessary to expand their populations in vitro prior to
clinical use. In order to examine the characteristics and safety of
long-term cultured hucMSCs, the current study evaluated the effects
of long-term culture on hucMSC proliferation, phenotype,
differentiation, intracellular ROS levels, cell cycle status and
senescence-associated gene expression levels. In addition, with the
aim to highlight the mechanisms that may cause oxidative stress and
promote senescence, the present study established a cell model of
H2O2-induced hucMSC premature senescence to
analyze the associations between changes in the microenvi-ronment
and the process of aging (15,25).
The present results indicate that hucMSCs undergo
replicative senescence during long-term culture in vitro, as
demonstrated by the altered cell proliferation curve, peculiar cell
morphology, cell cycle arrest in G0/G1 and increased SA-β-gal
activity, which are known markers of senescence. The current study
observed that senescence occurred following a cumulative number of
passages, ranging between P4 and P17. Additionally, hucMSCs
expanded continuously for ≤30 days and maintained the normal
spindle shape under the culture conditions, without demonstrating
increased SA-β-gal activity. Consistent with these findings, SAHF
were observed in the late phases of hucMSC culture. SAHF is a
specific heterochromatic structure accumulating in the nucleus in
the form of punctate foci (26,27).
By contrast, the chromatin distribution during the early phases of
culture was homogeneous. The middle phase may be an interim period
of cellular senescence.
Numerous studies have reported the differentiation
of MSCs upon replicative senescence (28-31),
however, the underlying regulatory mechanism is still
controversial. Wagner et al (30) demonstrated that the propensity for
osteogenic differentiation of MSCs increased and adipogenic
differentiation potential decreased during in vitro
senescence. Kim et al (28)
reported that the adipogenic and osteogenic differentiation
capacity of MSCs were decreased at >30 population doublings.
Oxidative stress is one of the major factors that
accelerates cell senescence in vivo and in vitro
(32,33). The current study observed that the
generation of ROS in hucMSCs increased during long-term in
vitro culture. The environment in which cellular senescence
evolved is replete with extrinsic hazards and the pace of
senescence may be affected by the culture conditions (10,34).
H2O2 treatment of early phase hucMSCs
provides a useful experimental model to analyze the mechanisms of
senescence-associated changes (35–37).
The results of the current study demonstrate that exogenous stress
or oxidative damage cause changes in gene expression levels,
contributing to certain changes observed in replicative senescence.
It was also observed that the accumulation of intracellular ROS in
senescent hucMSCs was accompanied by the upregulation of P21 and
P53 mRNA levels. APE1/Ref-1 is able to activate transcription
factors associated with the cellular response to various stresses
against oxidative DNA damage (38). Similarly, in the present study, the
mRNA expression levels of APE1/Ref-1 were elevated in senescent
hucMSCs. These results indicate that the P53/P21 pathway may be the
primary mediator of hucMSCs aging, and APE1/Ref-1 may be important
in the processes of replicative and premature senescence.
In conclusion, the data indicate that long-term
in vitro culture and extrinsic control, particularly
oxidative stress pressure, are crucial in the regulation of stem
cell aging. Thus, the quality of MSCs preparations should be
carefully controlled prior to clinical application. Further
research will be necessary to understand the mechanisms that
regulate the replicative senescence of stem cells and how to ensure
they remain in the early, normal phase during long-term
culture.
Acknowledgments
This work was supported by the National Natural
Science Foundation of China (grant no. 30840053), the Natural
Science Foundation of Jiangsu Province (grant no. BK2008232) and
the Foundation of the Jiangsu University for Senior Talented
Investigator (grant no. 11JDG0089).
References
|
1
|
Forostyak S, Jendelova P and Sykova E: The
role of mesenchymal stromal cells in spinal cord injury,
regenerative medicine and possible clinical applications.
Biochimie. 95:2257–2270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen
L, Wang M, Zhou Y, Zhu W, Li W and Xu W: Exosomes derived from
human umbilical cord mesenchymal stem cells alleviate liver
fibrosis. Stem Cells Dev. 22:845–854. 2013. View Article : Google Scholar :
|
|
3
|
Zhang Y, Cai W, Huang Q, Gu Y, Shi Y,
Huang J, Zhao F, Liu Q, Wei X, Jin M, et al: Mesenchymal stem cells
alleviate bacteria-induced liver injury in mice by inducing
regulatory dendritic cells. Hepatology. 59:671–682. 2014.
View Article : Google Scholar
|
|
4
|
Woodbury D, Reynolds K and Black IB: Adult
bone marrow stromal stem cells express germline, ectodermal,
endodermal, and mesodermal genes prior to neurogenesis. J Neurosci
Res. 69:908–917. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choudhery MS, Badowski M, Muise A and
Harris DT: Comparison of human mesenchymal stem cells derived from
adipose and cord tissue. Cytotherapy. 15:330–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mamidi MK, Nathan KG, Singh G, Thrichelvam
ST, Mohd Yusof NA, Fakharuzi NA, Zakaria Z, Bhonde R, Das AK and
Majumdar AS: Comparative cellular and molecular analyses of pooled
bone marrow multipotent mesenchymal stromal cells during continuous
passaging and after successive cryopreser-vation. J Cell Biochem.
113:3153–3164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Izadpanah R, Kaushal D, Kriedt C, Tsien F,
Patel B, Dufour J and Bunnell BA: Long-term in vitro expansion
alters the biology of adult mesenchymal stem cells. Cancer Res.
68:4229–4238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wagner W, Ho AD and Zenke M: Different
facets of aging in human mesenchymal stem cells. Tissue Eng Part B
Rev. 16:445–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Courtois-Cox S, Jones SL and Cichowski K:
Many roads lead to oncogene-induced senescence. Oncogene.
27:2801–2809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chakkalakal JV, Jones KM, Basson MA and
Brack AS: The aged niche disrupts muscle stem cell quiescence.
Natrue. 490:355–360. 2012. View Article : Google Scholar
|
|
11
|
Brandl A, Meyer M, Bechmann V, Nerlich M
and Angele P: Oxidative stress induces senescence in human
mesenchymal stem cells. Exp Cell Res. 317:1541–1547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandler H and Peters G: Stressing the
cell cycle in senescence and aging. Curr Opin Cell Biol.
25:765–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YJ, Hwang SH, Lee SY, Shin KK, Cho HH,
Bae YC and Jung JS: miR-486-5p induces replicative senescence of
human adipose tissue-derived mesenchymal stem cells and its
expression is controlled by high glucose. Stem Cells Dev.
21:1749–1760. 2012. View Article : Google Scholar
|
|
14
|
Zhang DY, Wang HJ and Tan YZ:
Wnt/β-catenin signaling induces the aging of mesenchymal stem cells
through the DNA damage response and the p53/p21 pathway. PLoS One.
6:e213972011. View Article : Google Scholar
|
|
15
|
Ho PJ, Yen ML, Tang BC, Chen CT and Yen
BL: H2O2 accumulation mediates differentiation capacity alteration,
but not proliferative decline, in senescent human fetal mesenchymal
stem cells. Antioxid Redox Signal. 18:1895–1905. 2013. View Article : Google Scholar :
|
|
16
|
Zhou BR, Xu Y, Wu D, Permatasari F, Gao YY
and Luo D: Ginsenoside Rg1 protects human fibroblasts against
psoralen- and UVA-induced premature senescence through a telomeric
mechanism. Arch Dermatol Res. 304:223–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thakur S, Sarkar B, Cholia RP, Gautam N,
Dhiman M and Mantha AK: APE1/Ref-1 as an emerging therapeutic
target for various human diseases: Phytochemical modulation of its
functions. Exp Mol Med. 46:e1062014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ju Z, Choudhury AR and Rudolph KL: A dual
role of p21 in stem cell aging. Ann N Y Acad Sci. 100:333–344.
2007. View Article : Google Scholar
|
|
19
|
Herbig U, Jobling WA, Chen BP, Chen DJ and
Sedivy JM: Telomere shortening triggers senescence of human cells
through a pathway involving ATM, p53, and p21(CIP1), but not
p16(INK4a). MOL CELL. 14:501–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawasaki H, Guan J and Tamama K: Hydrogen
gas treatment prolongs replicative lifespan of bone marrow
multipotential stromal cells in vitro while preserving
differentiation and paracrine potentials. Biochem Biophys Res
Commun. 397:608–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao H, Chen G, Liu J, Ti D, Zhao Y, Xu S,
Fu X and Han W: Culturing on Wharton's jelly extract delays
mesenchymal stem cell senescence through p53 and p16INK4a/pRb
pathways. PLoS One. 8:e583142013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin TM, Tsai JL, Lin SD, Lai CS and Chang
CC: Accelerated growth and prolonged lifespan of adipose
tissue-derived human mesenchymal stem cells in a medium using
reduced calcium and antioxidants. Stem Cells Dev. 14:92–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharpless NE and DePinho RA: How stem
cells age and why this makes us grow old. Nat Rev Mol Cell Biol.
8:703–713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang TT, Zeng GC, Li XC and Zeng HP: In
vitro studies on the antioxidant and protective effect of
2-substituted -8-hydroxy-quinoline derivatives against
H2O induced oxidative stress in BMSCs. Chem Biol Drugs
Des. 75:214–222. 2010. View Article : Google Scholar
|
|
26
|
Schellenberg A, Lin Q, Schuler H, Schüler
H, Koch CM, Joussen S, Denecke B, Walenda G, Pallua N, Suschek CV,
Zenke M and Wagner W: Replicative senescence of mesen-chymal stem
cells causes DNA-methylation changes which correlate with
repressive histone marks. Aging (Albany NY). 3:873–888. 2011.
View Article : Google Scholar
|
|
27
|
Zhang R, Poustovoitov MV, Ye X, Santos HA,
Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA,
Dunbrack RL, et al: Formation of MacroH2A-containing
senescence-associated heterochromatin foci and senescence driven by
ASF1a and HIRA. Dev Cell. 8:19–30. 2005. View Article : Google Scholar
|
|
28
|
Kim J, Kang JW, Park JH, Choi Y, Choi KS,
Park KD, Baek DH, Seong SK, Min HK and Kim HS: Biological
characterization of long-term cultured human mesenchymal stem
cells. Arch Pharm Res. 32:117–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Liu C, Xie Z, Song P, Zhao RC, Guo
L, Liu Z and Wu Y: Epigenetic dysregulation in mesenchymal stem
cell aging and spontaneous differentiation. Plos One. 6:e205262011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wagner W, Horn P, Castoldi M, Diehlmann A,
Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V and Ho
AD: Replicative senescence of mesenchymal stem cells: a continuous
and organized process. PLoS One. 3:e22132008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moerman EJ, Teng K, Lipschitz DA and
Lecka-Czernik B: Aging activates adipogenic and suppresses
osteogenic programs in mesenchymal marrow stroma/stem cells: The
role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling
pathways. Aging Cell. 3:379–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Edwards M, Rassin DK, Izumi T, Mitra S and
Perez-Polo JR: APE/Ref-1 responses to oxidative stress in aged
rats. J Neurosci Res. 54:635–638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanaka T, Halicka HD, Huang X, Traganos F
and Darzynkiewicz Z: Constitutive histone H2AX phosphory-lation and
ATM activation, the reporters of DNA damage by endogenous oxidants.
Cell Cycle. 5:1940–1945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wagner W, Horn P, Bork S and Ho AD: Aging
of hematopoietic stem cells is regulated by the stem cell niche.
Exp Gerontol. 43:974–980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yagi H, Tan J and Tuan RS: Polyphenols
suppress hydrogen peroxide-induced oxidative stress in human
bone-marrow derived mesenchymal stem cells. J Cell Biochem.
114:1163–1173. 2013. View Article : Google Scholar
|
|
36
|
Seo SK, Yang W, Park YM, Lee WT, Park KA
and Lee JE: Overexpression of human arginine decarboxylase rescues
human mesenchymal stem cells against H O cell survival protein
activation. J Korean Med Sci. 28:366–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang FW, Wang Z, Zhang YM, Du ZX, Zhang
XL, Liu Q, Guo YJ, Li XG and Hao AJ: Protective effect of melatonin
on bone marrow mesenchymal stem cells against hydrogen
peroxide-induced apoptosis in vitro. J Cell Biochem. 114:2346–2355.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fritz G, Grösch S, Tomicic M and Kaina B:
APE/Ref-1 and the mammalian response to genotoxic stress.
Toxicology. 193:67–78. 2003. View Article : Google Scholar : PubMed/NCBI
|