Introduction
Tetrandrine (TET) is a bis-benzylisoquinoline
alkaloid extracted from the dried roots of Han-Fang-Chi
(Stephania tetrandra S Moore). TET has a variety of
biological activities and is traditionally used to treat patients
with fungal infection, silicosis or hypertension. The antitumor
properties of TET have also been demonstrated (1,2).
Previous studies have suggested that TET reduces the inflammatory
response by inhibiting the production of inflammatory mediators.
In vitro studies have shown that TET inhibits cellular
proliferation and cytokine production in activated monocytes and T
cells (3–5). TET suppresses nuclear factor (NF)-κB
signaling pathways and reduces the production of tumor necrosis
factor-α (TNFα), interleukin (IL)-1β, IL-6 and NO in
lipopolysaccharide (LPS) and amyloid β (Aβ)-activated microglia
(3–5). TET could also affect the
mitogen-activated protein kinases signaling pathways, including
extracellular signal-regulated kinase (ERK) in activated microglial
and mast cells (5,6). Therefore, TET has been suggested to
be a potent anti-inflammatory agent. Macrophages are critical in
the inflammatory response. Upon stimulation, macrophages display an
activated state and produce proinflammatory mediators, such as TNFα
and IL-1β. Macrophage activation has been demonstrated in numerous
inflammatory diseases. Therefore, targeting the proinflammatory
activation of macrophages may have therapeutic potential.
β-glucans are polymers of glucose linked by
β-glycosidic bonds. β-glucans are found in fungal cell walls,
bacteria and plants. In recent years, a number of studies have
shown that β-glucans can activate the immune system (7–9).
β-glucans are recognized by their receptors, dectin-1 and toll-like
receptor-2 in various cells (10,11).
Activation of the downstream pathways results in the induction of
genes that activate the immune system. β-glucans have been shown to
potently activate macrophages through activation of NF-κB (12). For those patients with infective
diseases, β-glucans from fungal cell walls and bacteria may evoke
macrophage activation and trigger the inflammatory response.
Inhibition of β-glucan-mediated macrophage activation may represent
a novel approach for treating inflammatory diseases. However, few
studies have determined the effects of TET on β-glucan-induced
macrophage activation.
In this study, the functional role of TET in
β-glucan-activated macrophages and its possible mechanisms were
investigated. It was demonstrated that pretreatment with TET
inhibited IL-1β and TNF-α production by macrophages. These effects
of TET may be attributed to its inhibitory effect on the NF-κB,
ERK, signal transducer and activator of transcription 3 (STAT3)
pathways during macrophage activation. The present findings
highlight the clinical value of TET for the treatment of
β-glucan-associated inflammatory diseases.
Materials and methods
Materials
β-glucan was purchased from Sigma-Aldrich (St.
Louis, MO, USA). TET was purchased from PuZhen Biology (Shanghai,
China). TET was dissolved in 0.1 M HCl and adjusted to pH 7.3.
Cell culture
Macrophage-like cells (murine RAW264.7 and human
THP-1) were purchased from Cell Bank, Type Culture Collection
Committee, Chinese Academy of Sciences (Shanghai, China). RAW264.7
macrophages were grown in high glucose Dulbecco's modified Eagle's
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% heat-inactivated fetal bovine serum (Thermo Fisher
Scientific, Inc.). THP-1 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS. All the cells were grown at 37°C under a
humidified atmosphere of 5% CO2.
Cell viability assay
Cell viability was determined using an MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay (Thermo Fisher Scientific, Inc.). Briefly, cells (3,000
RAW264.7 cells and 5,000 THP-1 cells) were seeded into 96-well
plates overnight. TET was added at the indicted doses for 24 h (0,
0.2, 0.5 μM, 1, 2, 5 and 10 μM). Then, 10 μl
MTT solution (5 mg/ml) was added to each well and incubated for an
additional 4 h. Absorbance was measured at 570 nm using a
microplate reader (Biotek, Winooski, VT, USA). Optical density was
identified as the relative numbers of viable cells. All experiments
were performed in triplicate.
Cell apoptosis assay
Cell apoptosis was measured by
fluorescence-activated cell sorting (FACS) analyses (FACS Calibur;
BD Biosciences, Franklin Lakes, NJ, USA) using the Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, cells were seeded into 6-well plates overnight, then
treated with various concentrations of TET (0.2, 0.5, 1, 2, 5 and
10 μM) for 24 h. Cells were collected and resuspended in
Annexin V binding buffer, stained with Annexin V-FITC and propidium
iodide (PI), and analyzed by FACS. All samples were examined in
triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol
Reagent (Invitrogen, Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Equal quantities (1 μg) of
RNA was converted into cDAN using HiScript First Strand cDNA
Synthesis kit (Vazyme, Nanjing, China). RT-qPCR was performed using
SYBR Green I Real-time Detection kit (Cwbio, Beijing, China) on a
Bio-Rad CFX96 Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The relative expression was calculated using
the 2−ΔΔCq method. β-actin was used as an internal
control. The sequences of specific primers are listed in Table I. PCR conditions were as follows:
95°C for 5 min, followed by 40 cycles of 94°C for 20 sec and 61°C
for 20 sec. All samples were examined in triplicate.
 | Table ISequences of specific primers for
reverse transcription-quantitative polymerase chain reaction. |
Table I
Sequences of specific primers for
reverse transcription-quantitative polymerase chain reaction.
| mRNA | Primer | Sequences
(5′-3′) | Tm (°C) |
|---|
| β-actin | Forward |
CACGAAACTACCTTCAACTCC | 61 |
| Reverse |
CATACTCCTGCTTGCTGATC | |
| TNFα | Forward |
CCGAGTGACAAGCCTGTAGC | 61 |
| Reverse |
AGGAGGTTGACCTTGGTCTG | |
| IL-1β | Forward |
TACGAATCTCCGACCACCA | 61 |
| Reverse |
GGACCAGACATCACCAAGC | |
Western blotting
Cells were homogenized and lysed in
radioimmunoprecipitation assay (RIPA) buffer supplemented with 1 mM
phenylmethanesulfonyl fluoride (PMSF, Sigma-Aldrich). Equal amounts
of proteins were loaded and separated on a 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gel (Thermo Fisher
Scientific, Inc.) for electrophoresis followed by electro-transfer
to polyvinylidene difluoride membranes (Thermo Fisher Scientific,
Inc.). The membranes were blocked in 5% (w/v) non-fat milk for 1 h
and then incubated with primary antibodies overnight. The
antibodies were obtained from the following sources: Antibodies
against rabbit phosphorylated (p-)ERK (cat. no. 4376; 1:1,000),
rabbit ERK (cat. no. 9102; 1:1,000), rabbit p-STAT3 (cat. no. 9145;
1:1,000), rabbit STAT3 (cat. no. 4904; 1:1,000), rabbit p-p65 (cat.
no. 3033; 1:1,000) and rabbit p65 (cat. no. 4764; 1:1,000) were
obtained from Cell Signaling Technology Inc. (Danvers, MA, USA);
mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat.
no. 1A6; 1;1,000), horseradish peroxidase (HRP)-conjugated goat
anti-rabbit (cat. no. BS13278; 1:1,000) and goat anti-mouse (cat.
no. BS50350) antibodies were purchased from Bioworld Technology
(St. Louis Park, MN, USA). After washing with Tris-buffered saline
(Thermo Fisher Scientific, Inc.) with Tween 3 times, the membrane
was incubated with HRP conjugated secondary antibody and developed
with enhanced chemiluminescence substrate (Thermo Fisher
Scientific, Inc.) and imaged using an chemiluminescent system
(LAS4000mini; GE Healthcare Life Sciences, Logan, UT, USA). The
relative integrated intensity for p-p65, p65, p-ERK, ERK, p-STAT3,
STAT3 was normalized to that of GAPDH in the same sample.
Quantification was performed using ImageJ (version 2.0; National
Institutes of Health, Bethesda, MD, USA). All tests were conducted
in triplicate.
Immunofluorescence
Cells seeded on slides were pre-treated with various
concentrations of TET for 2 h, followed by incubation with β-glucan
(100 μg/ml) for 24 h. Cells were washed with PBS twice and
fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1%
Triton X-100 for 10 min, blocked with 5% bovine serum albumin
(BSA), and then incubated with indicated primary antibodies at 4°C
overnight followed by a Cy3-conjugated anti-rabbit secondary
antibody. The cells were then counterstained with DAPI for 5 min,
and the images were acquired with a Nikon eclipse Ti-S microscope
(Nikon, Tokyo, Japan).
Enzyme-linked immunosorbent assay
(ELISA)
The protein levels of TNF-α and IL-1β were measured
using ELISA kits (Bangyi Biotech, Shanghai, China), according to
the manufacturer's protocol. A volume of 100 μl culture
supernatants were added to each well and the absorbance was
measured at 450 nm. ELISA was performed in triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical comparisons between two different treatments were
analyzed using Student's t-test. Differences among more than two
groups were tested by one-way analysis of variance, followed by a
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TET does not influence cell viability of
macrophages at optimal concentrations
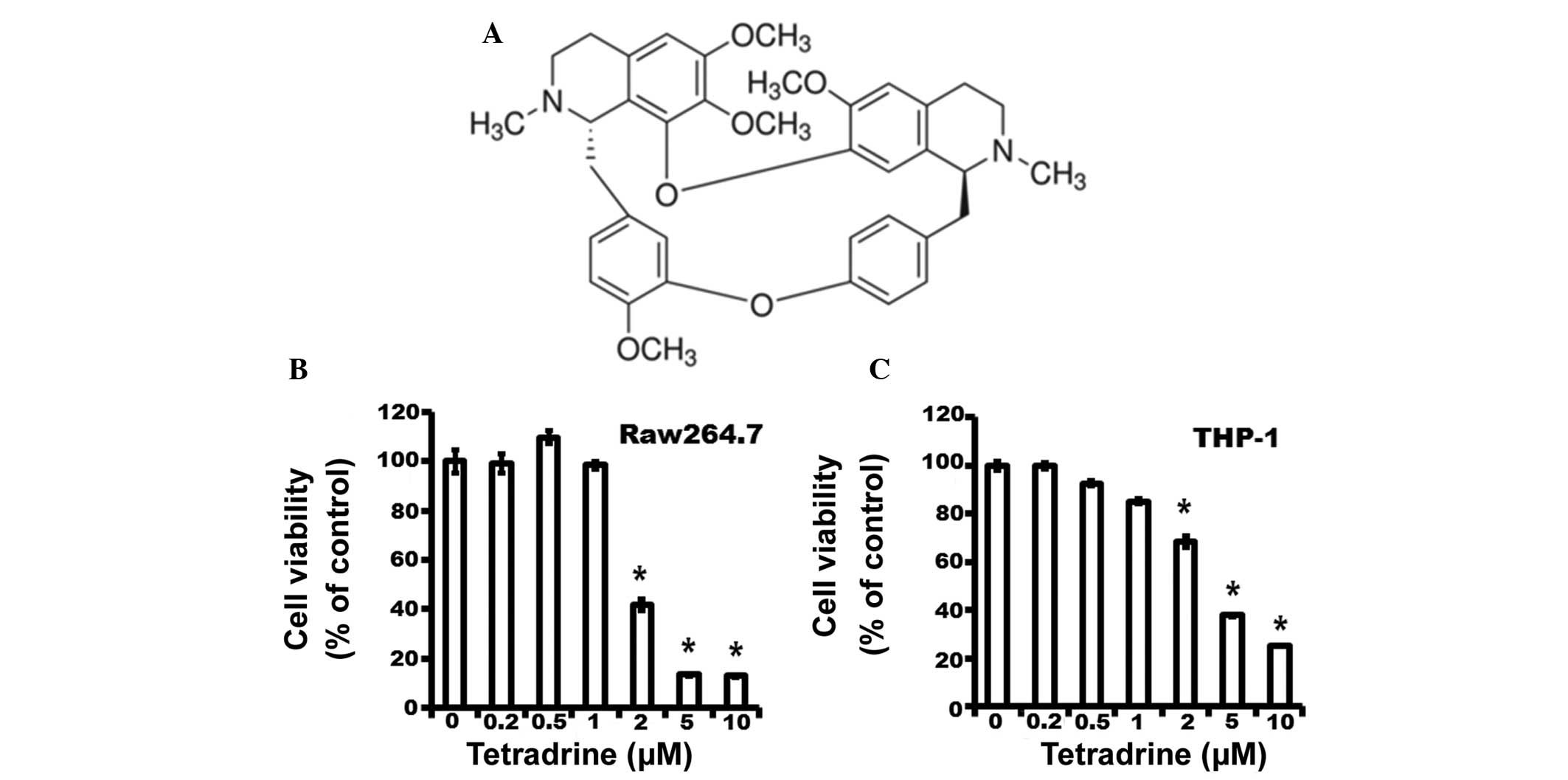
The molecular structure of TET is shown in Fig. 1A. To investigate the cytotoxic
effect of TET on macrophages, Raw264.7 macrophages and THP-1 cells
were treated with TET at various concentrations (ranging from 0 to
10 μM) for 24 h. Cell viability was assessed using an MTT
assay. As shown in Fig. 1B and C,
TET at 0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 μM resulted in
99.15±3.74, 109.70±2.66, 98.40±2.36, 41.86±2.32, 13.39±0.38 and
12.28±0.71% of RAW264.7 macrophages surviving, respectively, and
99.39±1.28, 92.50±1.07, 85.17±1.01, 68.35±2.20, 37.88±0.52 and
25.32±0.24% of THP-1 cells surviving, respectively. TET showed no
cytotoxicity at a relatively low concentration (ranging from 0.2 to
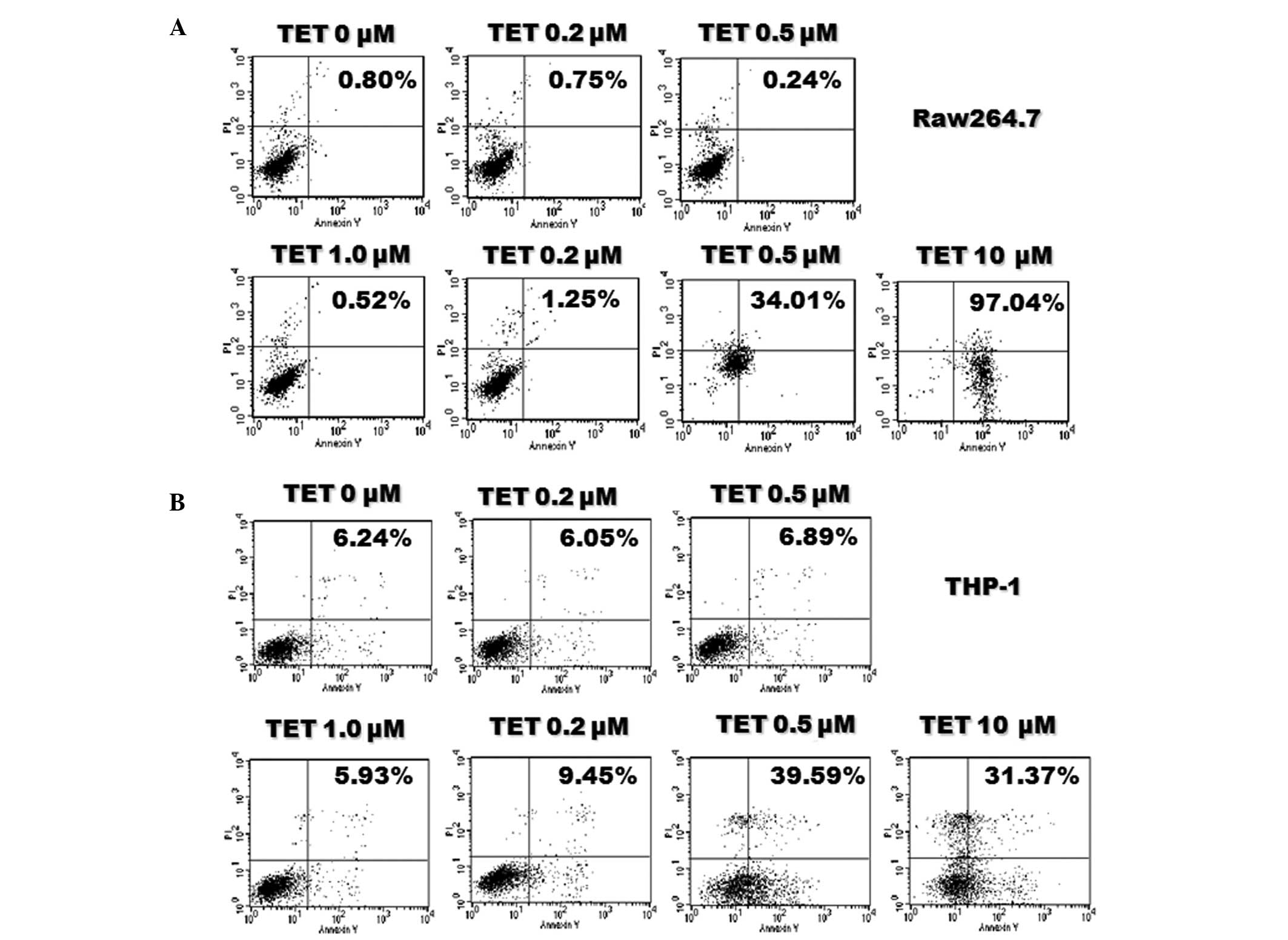
1.0 μM). Consistent with the results of the MTT assay, TET
at a relatively low concentration did not induce significant cell
apoptosis (Fig. 2). Therefore, TET
at a concentration ranging from 0.2 to 1 μM was used in the
following experiments.
β-glucan activates NF-κB, ERK and STAT3
signaling pathways and induces TNFα and IL-1β expression in THP-1
cells
The phosphorylation of p65, which was considered a
marker of NF-κB activation, was markedly increased after treatment
with β-glucan compared with control (Fig. 3A and B). β-glucan also stimulated
the phosphorylation of ERK and STAT3. To investigate the functional
role of β-glucan on macrophages, the expression of TNFα and IL-1β
mRNA was determined using RT-qPCR. As shown in Fig. 3C and D, the treatment with β-glucan
dose-dependently induced the expression of TNFα and IL-1β mRNA in
THP-1 cells. The expression of TNFα and IL-1β proteins was
investigated using ELISA assays. β-glucan treatment was shown to
upregulate the protein levels of TNFα and IL-1β in THP-1 cells
(Fig. 3E and F).
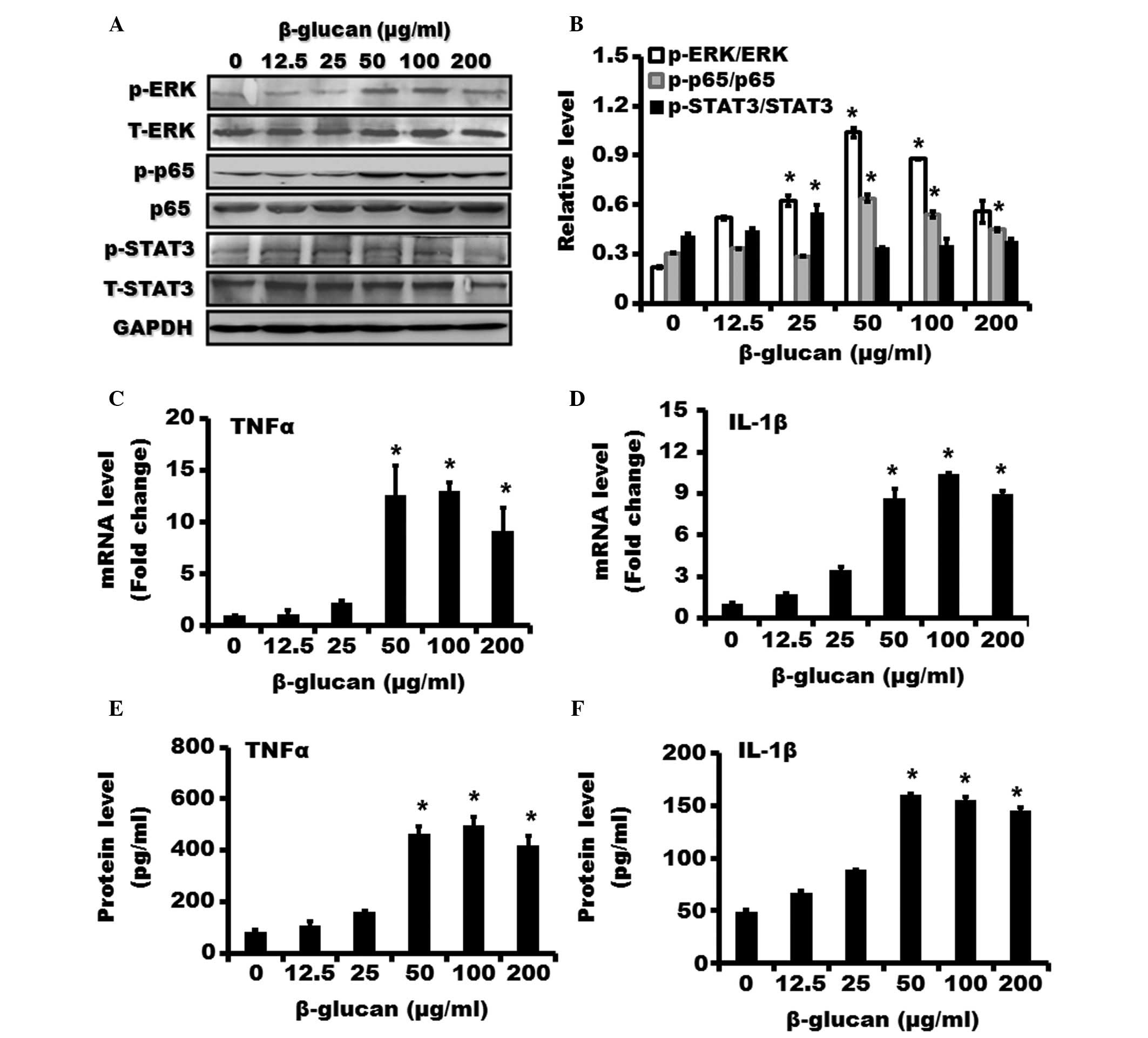 | Figure 3Effect of β-glucan on NF-κB, ERK and
STAT3 pathways and expression of IL-1β and TNFα in THP-1 cells.
THP-1 cells were treated with various concentrations of β-glucan
(0, 12.5, 25, 50, 100 and 200 μg/ml) for 24 h. (A) The
expression of phosphorylated and total forms of p65, ERK and STAT3
proteins were examined using western blotting. (B) The relative
expression of p-p65/p65, p-ERK/ERK and p-STAT3/STAT3 was shown. The
mRNA expression of (C) TNFα and (D) IL-1β in THP-1 cells treated
with β-glucan at various concentrations for 24 h was examined by
using revere transcription-quantitative polymerase chain reaction.
The protein levels of (E) TNFα and (F) IL-1β in THP-1 cells treated
with β-glucan at various concentrations for 24 h were examined by
an enzyme-linked immunosorbent assay. *P<0.05,
compared with control. ERK, extracellular signal-regulated kinase;
NF-κB, nuclear factor-κB; STAT3, signal transducer and activator of
transcription 3; TNFα, tumor necrosis factor-α; IL, interleukin;
p-, phosphorylated; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; T-, total. |
TET suppresses β-glucan-induced
activation of NF-κB, ERK and STAT3 signaling pathways and
attenuates the expression of TNFα and IL-1β in β-glucan-stimulated
THP-1 cells
The effect of TET on NF-κB, ERK and STAT3 activities
in β-glucan-treated THP-1 cells was then investigated. It was
demonstrated that TET pretreatment significantly inhibited the
induced expression of p-p65, p-ERK and p-STAT3 by β-glucan
treatment in a dose-dependent manner (Fig. 4A and B). β-glucan-induced
upregulation of TNFα and IL-1β mRNA levels in THP-1 cells was
inhibited by TET pretreatment (Fig. 4C
and D). In addition, the increase in TNFα and IL-1β protein
expression in THP-1 cells by β-glucan was also reversed by TET
pretreatment (Fig. 4E and F).
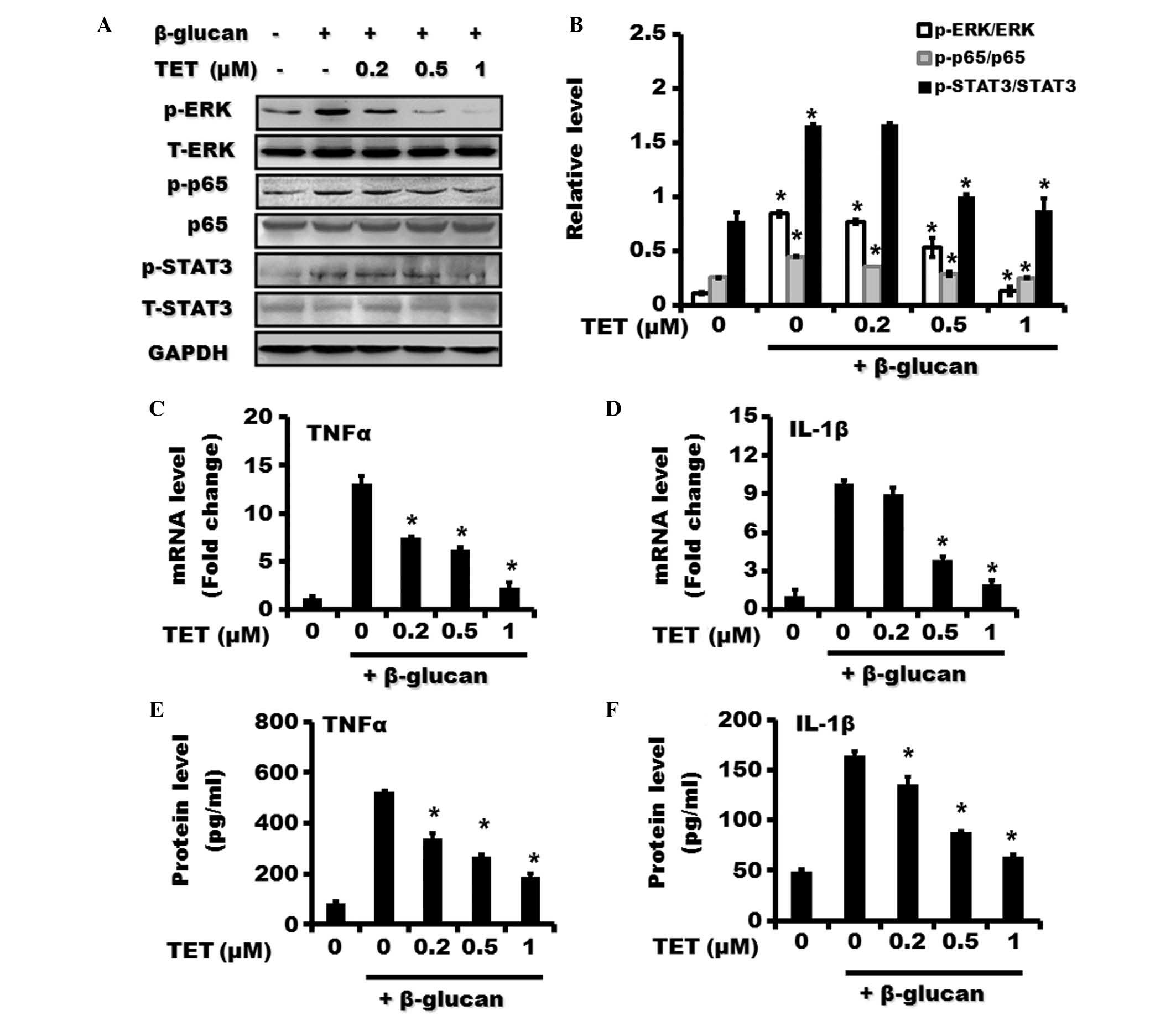 | Figure 4Effect of TET on β-glucan-induced
activation of NF-κB, ERK and STAT3 pathways and upregulation of
TNFα and IL-1β in THP-1 cells. THP-1 cells were pretreated with
various concentrations of TET (0, 0.2, 0.5 and 1 μM) for 2 h
followed by treatment with β-glucan (100 μg/ml) for 24 h.
(A) The expression of phosphorylated and total forms of p65, ERK
and STAT3 proteins were examined by western blotting. (B) The
relative expression of p-p65/p65, p-ERK/ERK and p-STAT3/STAT3 is
shown. The expression of (C) TNFα and (D) IL-1β in THP-1 cells was
examined by using reverse transcription-quantitative polymerase
chain reaction. The expression of (E) TNFα and (F) IL-1β proteins
in THP-1 cells was examined using an enzyme-linked immunosorbent
assay. *P<0.05, compared with control. TET,
tetrandrine; ERK, extracellular signal-regulated kinase; NF-κB,
nuclear factor-κB; STAT3, signal transducer and activator of
transcription 3; TNFα, tumor necrosis factor-α; IL, interleukin;
p-, phosphorylated; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; T-, total. |
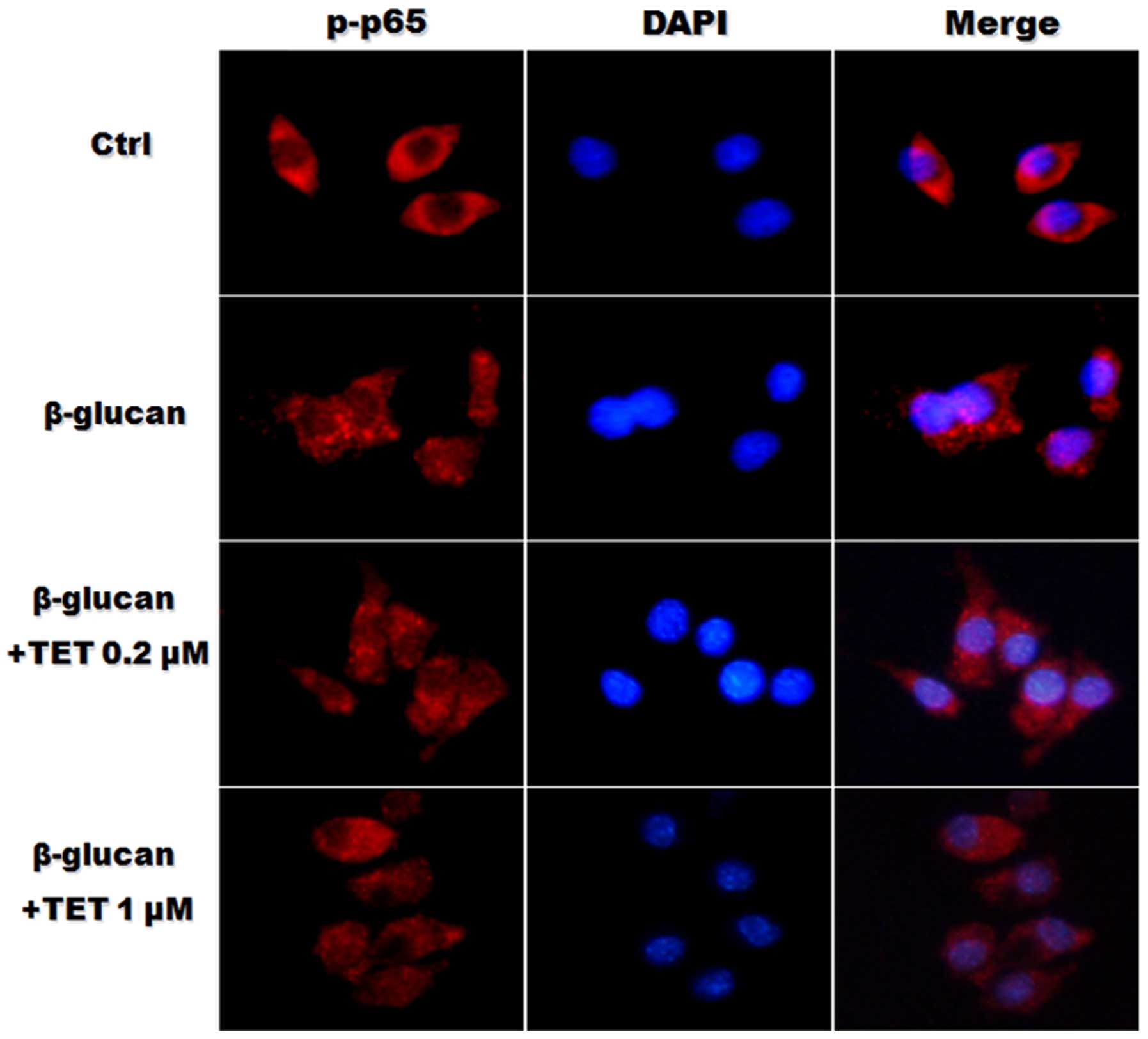
TET inhibits the nuclear translocation of
NF-κB in β-glucan-stimulated RAW264.7 macrophages
The increased nuclear translocation of NF-κB is an
indicator of activation. To further demonstrate that β-glucan
stimulates the activation of NF-κB, the distribution of NF-κB was
detected in murine RAW264.7 macrophages using immunofluorescent
staining. As shown in Fig. 5,
NF-κB p65 protein was predominantly localized in the cytosol of
RAW264.7 macrophages. β-glucan stimulation significantly increased
the nuclear translocation of NF-κB protein. However, pretreatment
with TET reduced the nuclear translocation of NF-κB in
β-glucan-stimulated RAW264.7 macrophages.
TET suppresses the activation of NF-κB,
ERK and STAT3 signaling pathways in β-glucan-stimulated RAW264.7
macrophages
To further confirm the inhibitory role of TET in
macrophage activation, murine RAW264.7 macrophages with β-glucan
were treated in the presence or absence of TET. As shown in
Fig. 6, treatment with β-glucan
also induced the activation of NF-κB, ERK and STAT3 signaling
pathways in RAW264.7 macrophages. However, TET pretreatment
significantly suppressed the activation of NF-κB, ERK and STAT3
signaling pathways by β-glucan.
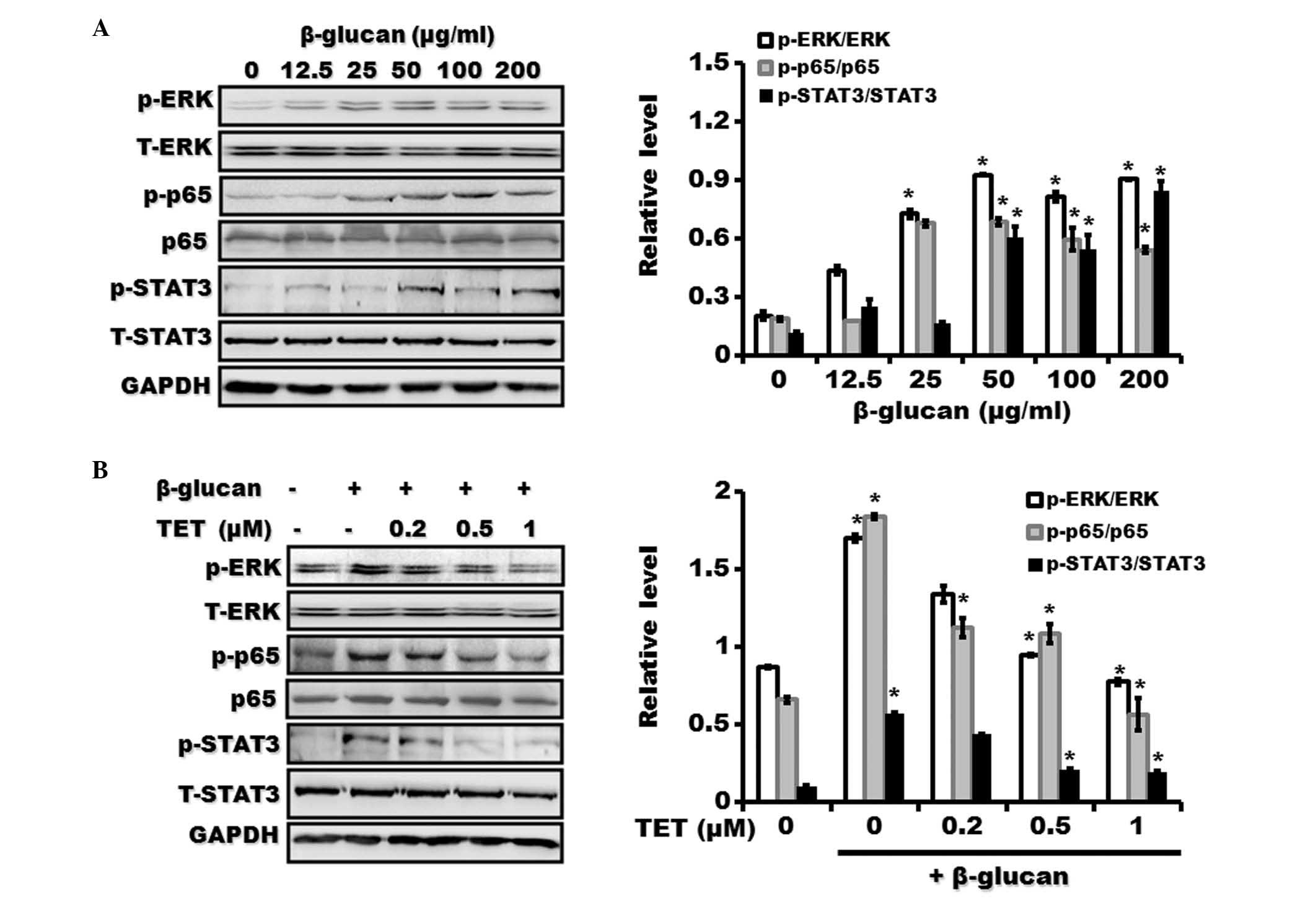 | Figure 6Effect of TET on β-glucan-induced
activation of NF-κB, ERK and STAT3 pathways in Raw264.7
macrophages. (A) Raw264.7 macrophages were pretreated with various
concentrations of β-glucan (0, 12.5, 25, 50, 100 and 200
μg/ml) for 24 h. The expression of phosphorylated and total
forms of p65, ERK and STAT3 proteins were examined using western
blotting. (B) Raw264.7 macrophages were pretreated with various
concentrations of TET (0, 0.2, 0.5 and 1 μM) for 2 h
followed by treatment with β-glucan (100 μg/ml) for 24 h.
The expression of phosphorylated and total forms of p65, ERK and
STAT3 proteins was examined by using western blotting.
*P<0.05, compared with control. TET, tetrandrine;
ERK, extracellular signal-regulated kinase; STAT3, signal
transducer and activator of transcription 3; p-, phosphorylated;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
β-glucan is a heterogeneous group of glucose
polymers found in the cell walls of fungi, bacteria and plants.
β-glucan from fungi and bacteria activates immune cells, such as
macrophages, to produce a wide spectrum of proinflammatory
mediators, which cause cell damage and tissue injury.
β-glucan-induced production of proinflammatory cytokines has been
considered to be critical in the pathogenesis of inflammatory
diseases. In this study, it was demonstrated that TET, a compound
isolated from a Chinese herb, attenuated β-glucan-induced
inflammatory responses in murine and human macrophages without
exhibiting cytotoxic effects. Given the broad biological activities
of TET, the findings may provide a novel opportunity for
β-glucan-associated inflammatory disease treatment.
Macrophage activation has been implicated in
numerous inflammatory diseases. In vitro and in vivo
studies have shown that β-glucan is able to stimulate the
functional activation of macrophages. In this study, the effects of
TET, a major pharmacologically-active compound of Chinese herb
Stephania tetrandra S Moore on macrophage activation induced
by β-glucan was investigated. Murine- and human-derived macrophages
pretreated with TET were stimulated by β-glucan in vitro.
Data showed that TET inhibited the expression of inflammatory
mediators including TNF-α and IL-1β in β-glucan-stimulated
macrophages. The suppressive roles of TET may be attributed to its
inhibitory effect on the NF-κB pathway during macrophage
activation. TET has been previously suggested to exhibit
immunosuppressive properties in vitro and in vivo. To
the best of our knowledge, the present study demonstrated for the
first time that TET showed significant suppressive effects on
β-glucan-induced macrophage activation. Furthermore, this study has
also shown that the inhibitory effect of TET is not due to its
direct cytotoxicity since the doses of used in this study did not
affect the viability of macrophages.
TNFα and IL-1β are produced at the early stages of
inflammation. The levels of TNF-α and IL-1β are elevated in the
majority of inflammatory diseases and are involved in the pathology
of inflammation. The elevated production of TNF-α and IL-1β leads
to local inflammation and tissue damage. In this study, it was
demonstrated that β-glucan induced the expression of TNF-α and
IL-1β in a concentration-dependent manner in macrophages. TET
pretreatment could suppress the induction of TNF-α and IL-1β,
suggesting that TET is a potent inhibitor for β-glucan-induced
expression of inflammatory mediators in macrophages.
NF-κB is an important transcriptional factor for
regulating the expression of inflammatory mediators. NF-κB is key
in the release of proinflammatory cytokines. NF-κB is retained in
the cytosol by inhibitory proteins in resting cells. Once
stimulated, NF-κB translocates from the cytosol to the nucleus
where it binds to the regulatory element in the promoter region of
its target genes (such as TNFα and IL-1β). In the present study, it
was demonstrated that β-glucan treatment significantly increased
the expression of phosphorylated p65, which is a subunit of NF-κB
transcription factor and is considered to be a marker of NF-κB
activation, and significantly upregulated the expression of TNFα
and IL-1β. In previous studies, TET has been found to inhibit NF-κB
activation and nuclear translocation in several cell and animal
models (6,13,14).
Studies have demonstrated that the inhibitory effect of TET on
NF-κB activation can be attributed to its ability to prevent the
degradation of IκBα (a cytoplasmic inhibitor of NF-κB) and inhibit
nuclear translocation of p65 (15). In this study, it was demonstrated
that TET exerted its inhibitory effect on β-glucan-induced
macrophage activation by inhibiting the nuclear translocation of
p65, leading to the inactivation of its downstream target genes. In
addition, the ERK and STAT3 signaling pathways have been shown to
be critical in regulating the release of proinflammatory cytokines.
In this study it was demonstrated that the phosphorylation of ERK
and STAT3 was markedly inhibited by TET. ERK and STAT3 signaling
pathways have been suggested to control the activation of NF-κB in
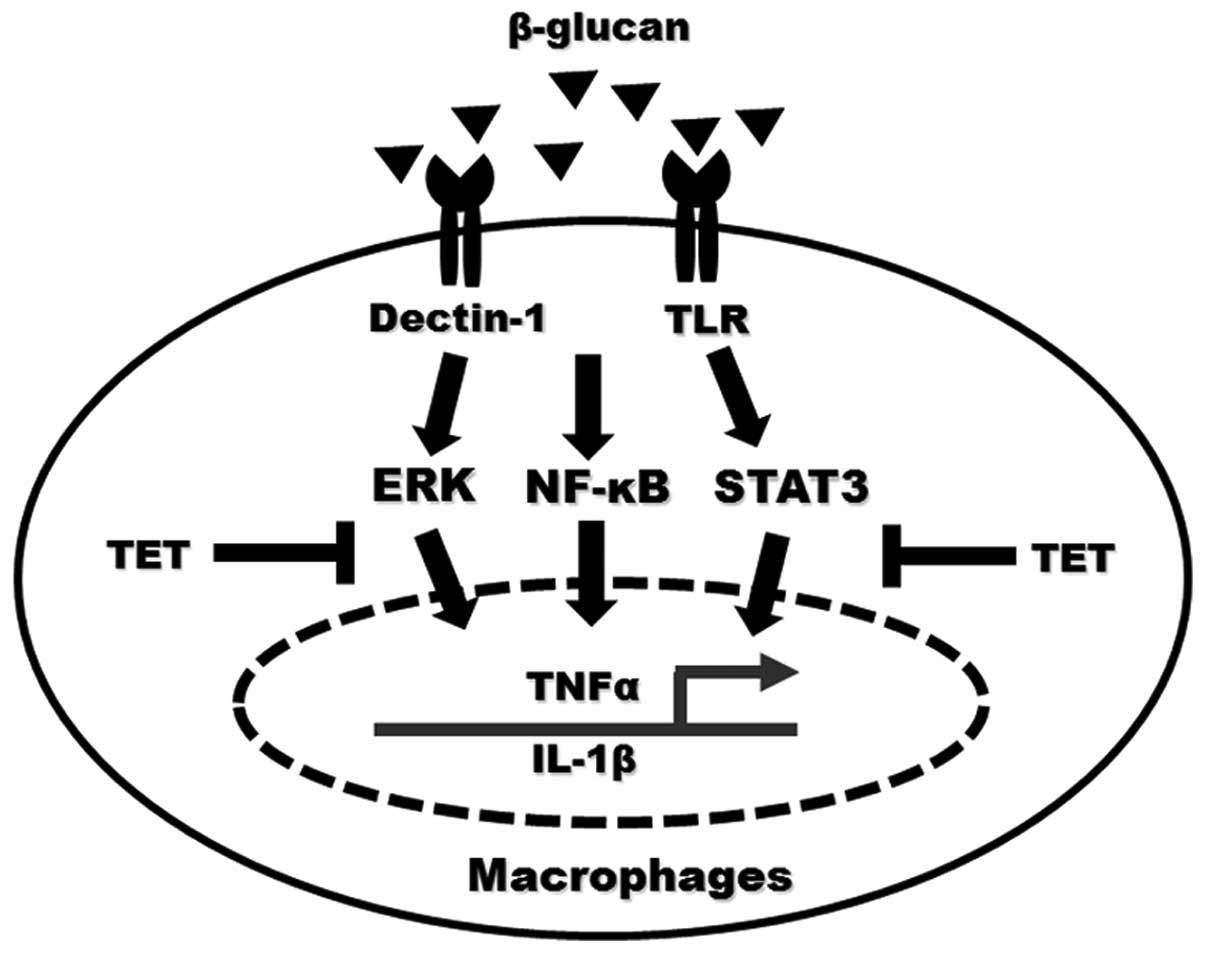
activated cells. Therefore, these results indicate that
β-glucan-induced activation of NF-κB, ERK and STAT3 cooperatively
regulate inflammatory cytokine expression in macrophages, and that
TET exerts inhibitory roles by suppressing NF-κB, ERK and STAT3
activation (Fig. 7).
In conclusion, the results suggest that TET
suppresses β-glucan-induced macrophage activation and reduces the
release of inflammatory mediators. TET exerts the suppressive
effects through the inhibition of NF-κB, ERK and STAT3 pathways.
These findings, together with those of the previous studies,
suggest the potential use of TET in the treatment of
β-glucan-associated inflammatory diseases.
Acknowledgments
This study was supported by the Technology Support
Program of Zhenjiang (grant no. SH2013062).
References
|
1
|
Qin R, Shen H, Cao Y, Fang Y, Li H, Chen Q
and Xu W: Tetrandrine induces mitochondria-mediated apoptosis in
human gastric cancer BGC-823 cells. PLoS One. 8:e764862013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu W, Su M, Xie F, Ai J, Ren Y, Zhang J,
Guan R, He W, Gong Y and Guo Y: Tetrandrine blocks autophagic flux
and induces apoptosis via energetic impairment in cancer cells.
Cell Death Dis. 5:e11232014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He FQ, Qiu BY, Li TK, Xie Q, Cui de J,
Huang XL and Gan HT: Tetrandrine suppresses amyloid-β-induced
inflammatory cytokines by inhibiting NF-κB pathway in murine BV2
microglial cells. Int Immunopharmacol. 11:1220–1225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue Y, Wang Y, Feng DC, Xiao BG and Xu LY:
Tetrandrine suppresses lipopolysaccharide-induced microglial
activation by inhibiting NF-kappaB pathway. Acta Pharmacol Sin.
29:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dang Y, Xu Y, Wu W, Li W, Sun Y, Yang J,
Zhu Y and Zhang C: Tetrandrine suppresses
lipopolysaccharide-induced microglial activation by inhibiting
NF-κB and ERK signaling pathways in BV2 cells. PLoS One.
9:e1025222014. View Article : Google Scholar
|
|
6
|
Kang OH, An HJ, Kim SB, Mun SH, Seo YS,
Joung DK, Choi JG, Shin DW and Kwon DY: Tetrandrine suppresses
pro-inflammatory mediators in PMA plus A23187-induced HMC-1 cells.
Int J Mol Med. 33:1335–1340. 2014.PubMed/NCBI
|
|
7
|
Chan GC, Chan WK and Sze DM: The effects
of beta-glucan on human immune and cancer cells. J Hematol Oncol.
2:252009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Wang J, Wang W, Liu C, Sun S, Gu J,
Wang X, Boraschi D, Huang Y and Qu D: Immunomodulatory activity of
a novel, synthetic beta-glucan (β-glu6) in murine macrophages and
human peripheral blood mononuclear cells. PLoS One. 8:e803992013.
View Article : Google Scholar
|
|
9
|
Karumuthil-Melethil S, Gudi R, Johnson BM,
Perez N and Vasu C: Fungal β-glucan, a dectin-1 ligand, promotes
protection from type 1 diabetes by inducing regulatory innate
immune response. J Immunol. 193:3308–3321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown GD, Taylor PR, Reid DM, Willment JA,
Williams DL, Martinez-Pomares L, Wong SY and Gordon S: Dectin-1 is
a major beta-glucan receptor on macrophages. J Exp Med.
196:407–412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yadav M and Schorey JS: The beta-glucan
receptor dectin-1 functions together with TLR2 to mediate
macrophage activation by mycobacteria. Blood. 108:3168–3175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang ZQ, Lee JS, Gebru E, Hong JH, Jung
HK, Jo WS and Park SC: Mechanism of macrophage activation induced
by beta-glucan produced from Paenibacillus polymyxa JB115. Biochem
Biophys Res Commun. 391:1358–1362. 2010. View Article : Google Scholar
|
|
13
|
Zhao H, Luo F, Li H, Zhang L, Yi Y and Wan
J: Antinociceptive effect of tetrandrine on LPS-induced
hyperalgesia via the inhibition of IKKβ phosphorylation and the
COX-2/PGE2 pathway in mice. PLoS One. 9:e945862014.
View Article : Google Scholar
|
|
14
|
Wang QS, Cui YL, Gao LN, Guo Y, Li RX and
Zhang XZ: Reduction of the pro-inf lammator y response by
tetrandrine-loading poly (L-lactic acid) films in vitro and in
vivo. J Biomed Mater Res A. 102:4098–4107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin ST, Wang Y, Xue Y, Feng DC, Xu Y and
Xu LY: Tetrandrine suppresses LPS-induced astrocyte activation via
modulating IKKs-IkappaBalpha-NF-kappaB signaling pathway. Mol Cell
Biochem. 315:41–49. 2008. View Article : Google Scholar : PubMed/NCBI
|