Introduction
Inflammation is a series of coordinated defensive
responses against a diverse range of pathogenic insults, which is
driven by the programmed release of inflammatory mediators
(1). The sequential expression of
inflammation-associated genes is tightly regulated at multiple
checkpoints, including various post-translational modifications
(2). Acetylation is an important
post-translational modification of histones, determining the
structure and function of chromatin (3). Acetylation of lysines generally
results in neutralization of the positive charge on histone tails,
weakening the electrostatic interaction between the histone and the
DNA backbone, which has been associated with opened structure of
chromatin, accessibility for transcription factors and eventually,
transcriptional activation (4).
In addition to histones, several non-histone
proteins are targets of acetylation modification and their function
can be modified following acetylation. For example,
mitogen-activated protein kinase (MAPK) phosphatase-1 (MKP-1) can
be acetylated on a lysine residue within its substrate-binding
domain, which may lead to enhanced interaction with p38, thus,
increasing its phosphatase activity and interrupting MAPK signaling
(5). Additionally, nuclear
factor-κB (NF-κB), the pivotal transcription factor that controls
the expression of numerous inflammatory mediators, is also
acetylated at various lysine residues (6). Acetylation modifications have been
previously reported to positively and negatively regulate the
subcellular location, DNA binding affinity and transcriptional
activity of NF-κB (6). Thus,
acetylation modification may markedly alter the function of various
proteins involved in the expression of inflammation-associated
genes (7).
Two groups of enzymes, histone acetyltransferases
(HATs) and histone deacetylases (HDACs), have been previously
demonstrated to maintain the delicate dynamic equilibrium of the
acetylation level of histone lysine residues (8). HDACs have been demonstrated to be
important for the inflammatory response and HDAC inhibitors are
emerging as promising reagents for the treatment of inflammatory
disease (9). However, the
pharmacological effects of HAT inhibitors on the inflammatory
response remain to be fully elucidated. In the present study, the
potential pharmacological effects of garcinol, a commonly used HAT
inhibitor (10,11), on the expression of inflammatory
genes was investigated in vitro using LPS-stimulated murine
RAW264.7 macrophages. Furthermore, garcinol was administrated into
mice with LPS-induced endotoxemia and the effects on the plasma
pro-inflammatory cytokine level, organ inflammation and injury, and
survival were determined. The present study may indicate the
potential modulatory effects of HAT inhibitors on inflammation.
Materials and methods
Reagents
Garcinol and lipopolysaccharide (LPS; from
Escherichia coli;, 055:B5) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Alanine aminotransferase (ALT) and blood urea
nitrogen (BUN) assay kits were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Monoclonal rabbit
anti-mouse p65 (cat. no. 8242) and monoclonal rabbit anti-mouse
acetyl-p65 (Lys310; cat. no. 12629) antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Biotinylated
goat anti-rabbit antibody (cat. no. 14709) was obtained from Pierce
Biotechnology, Inc. (Rockford, IL, USA). The enzyme-linked
immunosorbent assay (ELISA) kits for detecting mouse tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6) were purchased from
NeoBioscience Technology Company (Shenzhen, China).
Animals
Male BALB/c mice (n=112; age, 6–8 weeks; weight,
20–25 g) were obtained from the Experimental Animal Center of
Chongqing Medical University (Chongqing, China). The animals were
fed with a standard laboratory diet and water ad libitum.
They were housed in a specific-pathogen free room at a temperature
of 20–25°C, 50±5% relative humidity under a 12-h light/dark cycle.
All experimental procedures involving animals were approved by the
Animal Care and Use Committee of Chongqing Medical University.
LPS-induced lethal inflammation
Mice were intraperitoneally injected with LPS (20
mg/kg) to induce lethal inflammation. Dimethyl sulfoxide (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) as a
vehicle and garcinol (10 mg/kg, intraperitoneally) were
administrated 0.5 h prior to LPS challenge. The mice were
sacrificed at 18 h (n=8 per group) subsequent to LPS challenge by
anesthesia with pentobarbital (50 mg/kg; Shanghai Xinya
Pharmaceutical Corporation, Shanghai, China). Blood samples and
lung tissues were harvested for further experiments. To determine
the effect of garcinol on the outcome of lethal inflammation,
another group of animals (n=20) were used and the lethality was
evaluated every 6 h for a minimum of 7 days. Surviving mice were
sacrificed on the fourteenth day subsequent to LPS exposure by
pentobarbital anesthesia as above.
Histological analysis
Formalin-fixed (Chengdu KeLong Chemical Co., Ltd.,
Chengdu, China) lung specimens were embedded in paraffin (Chengdu
KeLong Chemical Co., Ltd.) and stained with hematoxylin and eosin
(Nanjing Jiancheng Bioengineering Institute) routinely for
conventional morphological examination under a light microscope
(BX43; Olympus Corporation, Tokyo, Japan).
ALT and BUN detection
To evaluate the degree of liver and renal injuries,
the plasma levels of ALT and BUN were determined with the
corresponding detection kits according to the manufacturer's
instructions.
Detection of pro-inflammatory
cytokines
The concentrations of TNF-α and IL-6 in plasma
samples or cell culture supernatants were determined using the
corresponding ELISA kits according to the manufacturer's
instructions. Briefly, samples or standards were pipetted into a
microplate pre-coated with a monoclonal antibody specific for mouse
TNF-α/IL-6. The TNF-α/IL-6 present in the samples was bound by the
pre-coated antibody. Following a washing step, an enzyme-linked
polyclonal antibody specific for mouse TNF-α/IL-6 was added to the
wells. Following a wash to remove any unbound antibody-enzyme
reagent, a substrate solution was added to the wells. The enzyme
reaction generated a blue product that turned yellow when the stop
solution was added. The intensity of the color was measured at 450
nm (Varioskan LUX; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and the sample values were calculated according to the
standard curve.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA levels of TNF-α and IL-6 in lung tissue
were determined by RT-qPCR. Briefly, total RNA was isolated from
lung samples using TRIzol reagent (Takara Biotechnology Co., Ltd.,
Dalian, China). DNase I (Takara Biotechnology Co., Ltd.) was used
for genomic DNA digestion prior to RT-qPCR. First-strand
complementary DNA (cDNA) was synthesized using oligo-dT primers
(Takara Biotechnology Co., Ltd.) and the M-MLV reverse
transcriptase (Takara Biotechnology Co., Ltd.)in PrimeScript II
buffer (Takara Biotechnology Co., Ltd.). qPCR was performed using
SYBR green PCR Master mix (Takara Biotechnology Co., Ltd.) with the
following cycling conditions (n=35) on a CFX Connect (Bio-Rad
Laboratories, Inc., Hercules, CA, USA): Denaturation, 95°C for 10
sec; annealing, 58°C for 20 sec; and elongation, 72°C for 20 sec.
The mRNA levels of TNF-α and IL-6 were normalized to the levels of
β-actin and quantified using the ΔCT-method (12). The primers used are presented in
Table I.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Target gene | Forward primer | Reverse primer |
|---|
| Tumor necrosis
factor-α |
5-CCAGGTTCTCTTCAAGGGACAA-3 |
5-ACGGCAGAGAGGAGGTTGACT-3 |
| Interleukin-6 |
5-AGTTGCCTTCTTGGGACTGATG-3 |
5-TCTCATTTCCACGATTTCCCAG-3 |
| β-actin |
5-CTGAGAGGGAAATCGTGCGT-3 |
5-CCACAGGATTCCATACCCAAGA-3 |
Western blot analysis
Total protein lysates from frozen liver samples were
prepared according to the method described by Cell Lysis Buffer for
Western and IP (Beyotime Institute of Biotechnology, Haimen,
China). The total protein concentration was determined using a
Pierce BCA Protein assay kit (Thermo Fisher Scientific, Inc.).
Protein extracts (40 μg) were fractionated on 10%
polyacrylamide-sodium dodecyl sulfate gel (Beyotime Institute of
Biotechnology) at 100 V for 90 min, and then transferred to a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). The
membrane was blocked with 5% (w/v) non-fat milk in Tris-buffered
saline (Beijing Dingguo Changsheng Biotechnology Co., Ltd.)
containing 0.05% Tween-20 (Beijing Dingguo Changsheng Biotechnology
Co., Ltd.), and then the membrane was incubated with the primary
antibodies (all diluted 1:1,000) overnight at 4°C, followed by
incubation with the secondary antibody (dilution, 1:5,000).
Antibody binding was visualized with an enhanced chemiluminescence
system (Pierce ECL Western Blotting Substrate; Thermo Fisher
Scientific, Inc.) and short exposure of the membrane to X-ray films
(Kodak, Rochester, NY, USA). The results were visualized using
ChemiDoc Touch Imaging system (Bio-Rad Laboratories, Inc.).
Cell culture and morphological
observation
RAW264.7 cells obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China)
were cultured at 37°C in a 5% CO2 atmosphere humidified
incubator, and maintained in RPMI 1640 culture medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, Utah) containing 10%
heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). RAW264.7 cells were incubated with vehicle or
garcinol (10 μmol/l) in the presence or absence of LPS (100
μg/l). The cells and the supernatants were harvested at 18 h
subsequent to LPS challenge for further experiments.
Statistical analysis
All data from the experiments are expressed as the
mean ± standard deviation. Statistical significance was determined
by Student's t-test for comparisons of two groups. Multi-group
comparisons were performed using one-way analysis of variance for
multiple comparisons among means, with Tukey's post-hoc test.
Survival statistics were analyzed with a Kaplan-Meier curve and
log-rank test. Statistical analysis was conducted with SPSS 12.0
(SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
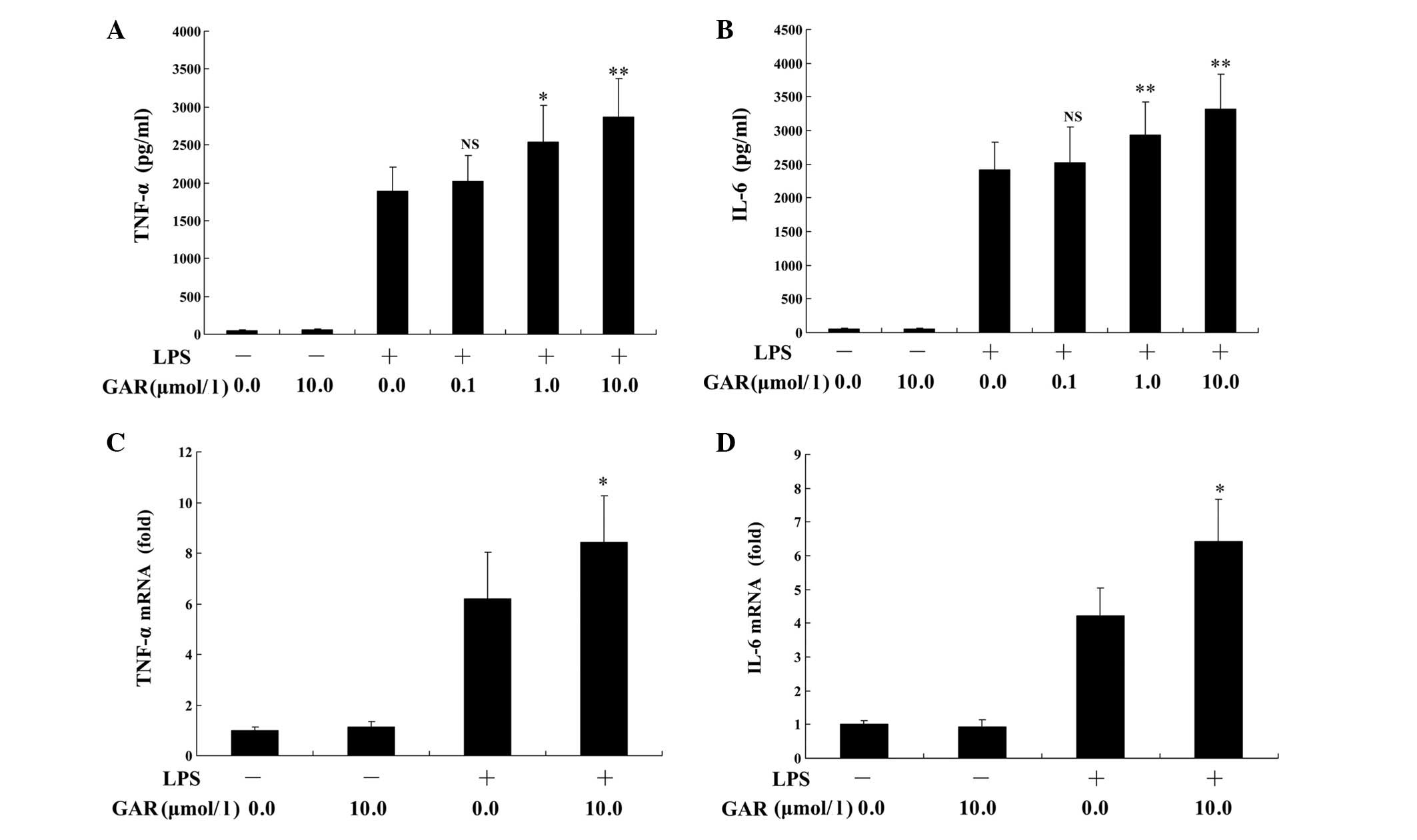
Garcinol enhances LPS-induced
inflammation in vitro
HATs catalyze the acetylation of histones and
promote transcriptional activation (13), thus, inhibition of HATs by garcinol
may result in the suppressed transcription of genes. However,
unexpectedly, the present study demonstrated that garcinol (0.1–10
μmol/l) dose-dependently upregulated LPS-induced production
of TNF-α and IL-6 proteins in RAW264.7 macrophages compared with
LPS treatment alone (Fig. 1A and
B). A similar effect was observed on the mRNA levels of TNF-α
and IL-6 following garcinol treatment, which were significantly
increased compared with LPS treatment alone (P<0.05; Fig. 1C and D).
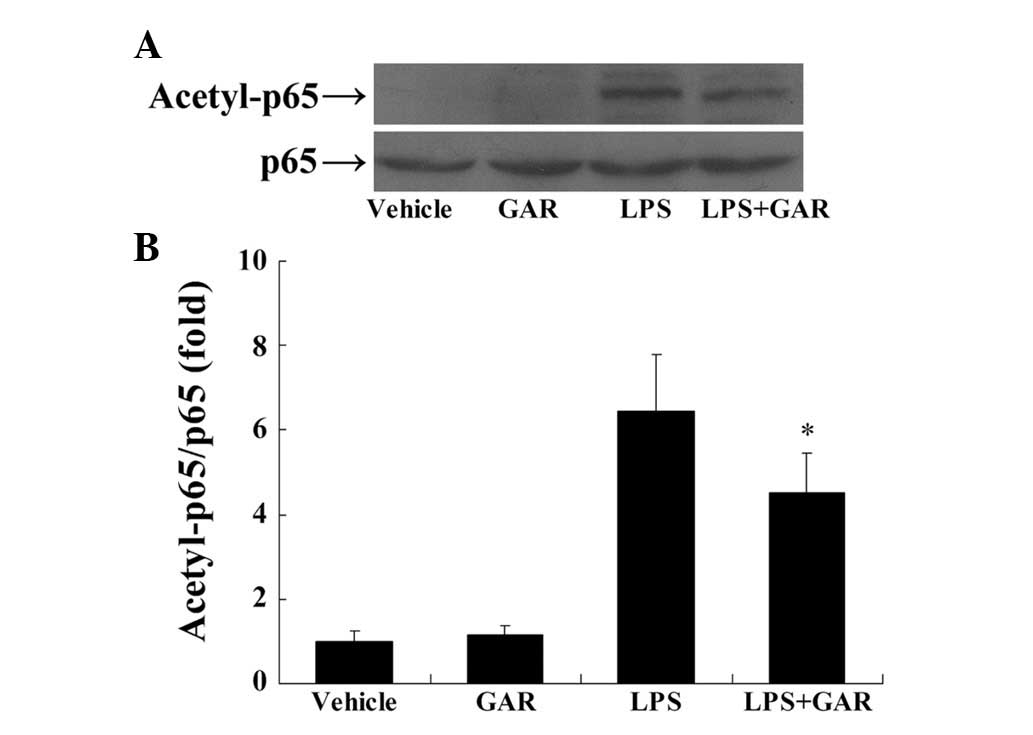
Garcinol suppresses LPS-induced
acetylation of NF-κB
NF-κB is a pivotal transcription factor in
inflammation and its activity is enhanced by acetylation at Lys310
in its p65 subunit (14). The
present study demonstrated that LPS markedly stimulated the
acetylation of p65 at Lys310, suggesting that acetylation of p65
may be involved in LPS-induced inflammation. By contrast, treatment
with garcinol significantly attenuated LPS-induced p65 acetylation
compared with LPS treatment only (Fig.
2), which may be caused by the inhibitory activity of garcinol
on HATs.
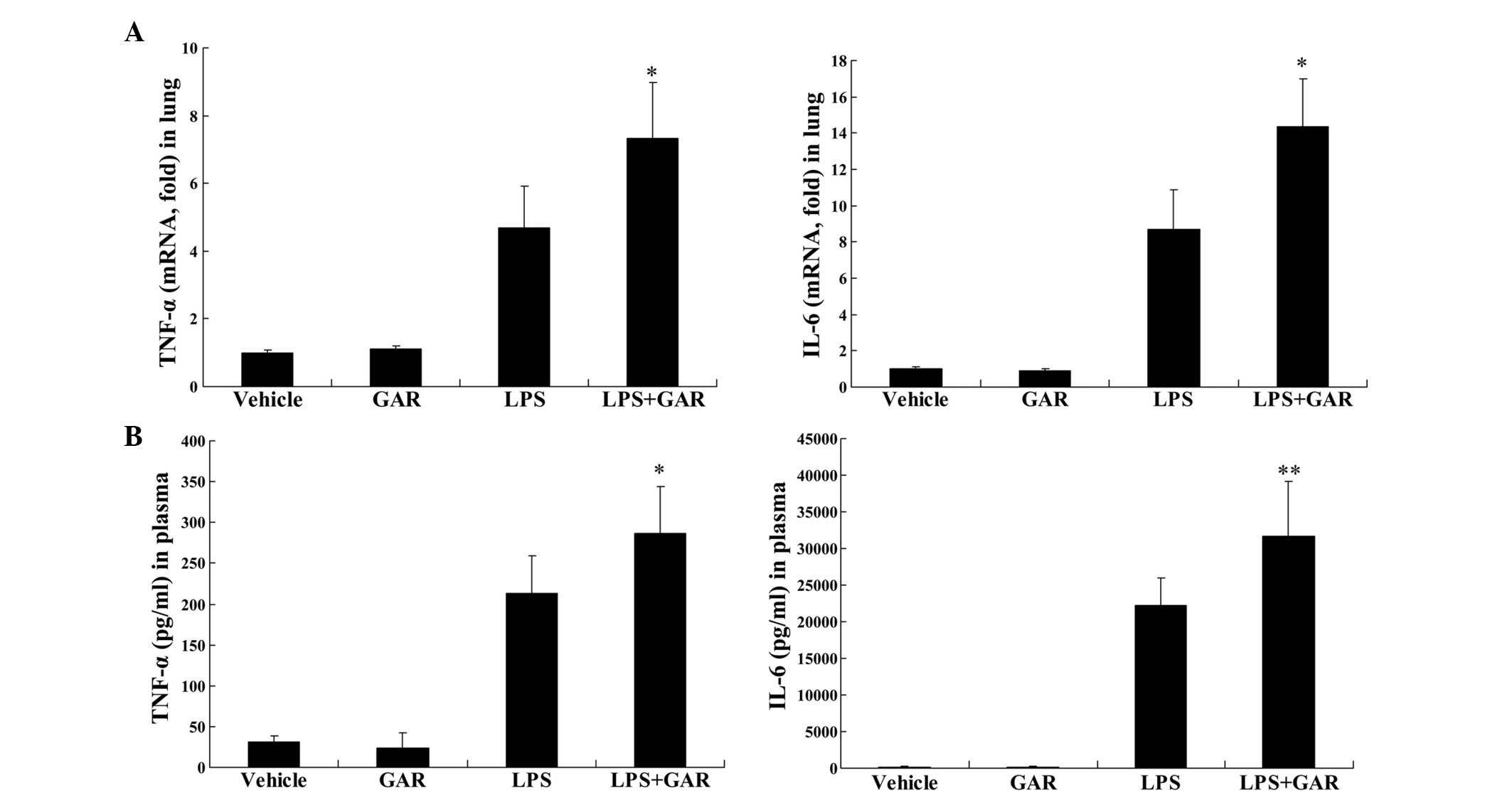
Garcinol enhances LPS-induced production
of pro-inflammatory cytokines in mice
In LPS-challenged mice, the present study
demonstrated that treatment with garcinol significantly increased
LPS-induced upregulation of TNF-α mRNA in lung tissue (P<0.05;
Fig. 3A) and TNF-α protein in
plasma (P<0.05; Fig. 3B)
compared with LPS treatment only. Similarly, the mRNA (P<0.05;
Fig. 3A) and protein (P<0.01;
Fig. 3B) levels of IL-6 in
LPS-insulted mice were significantly increased following garcinol
treatment compared with LPS treatment alone. These data suggest
that the HAT inhibitor, garcinol, may enhance LPS-induced
inflammation in vivo.
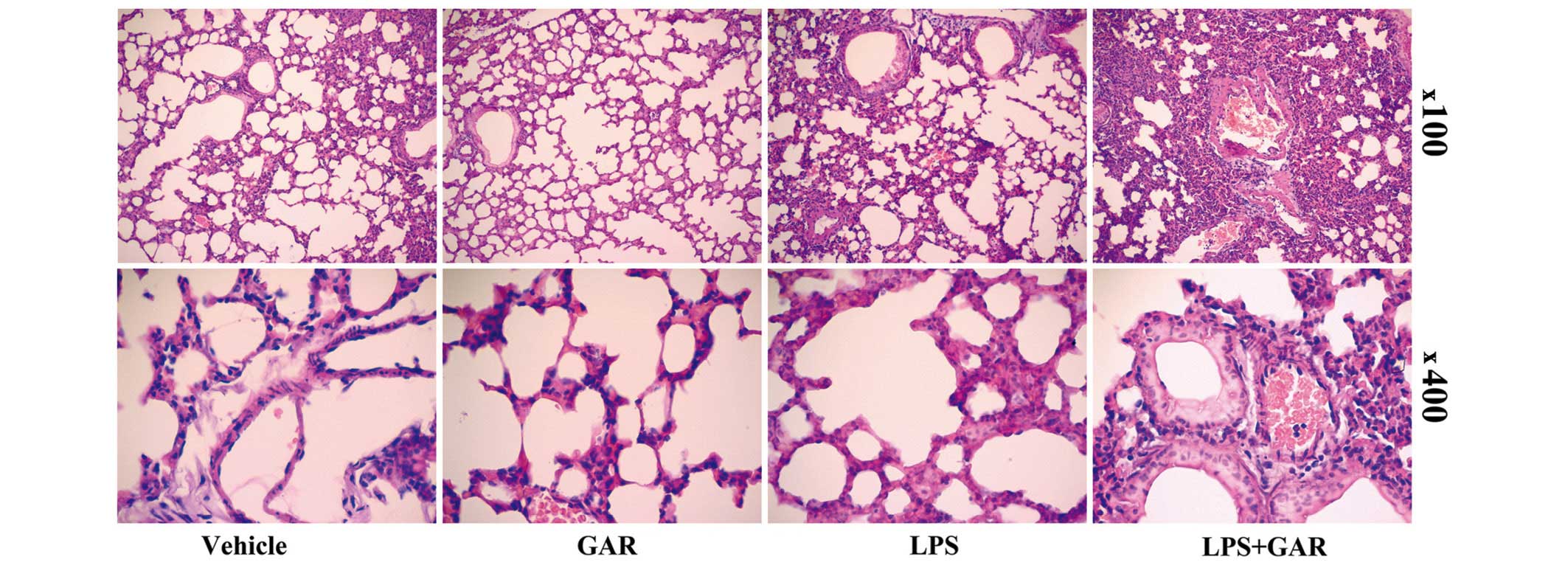
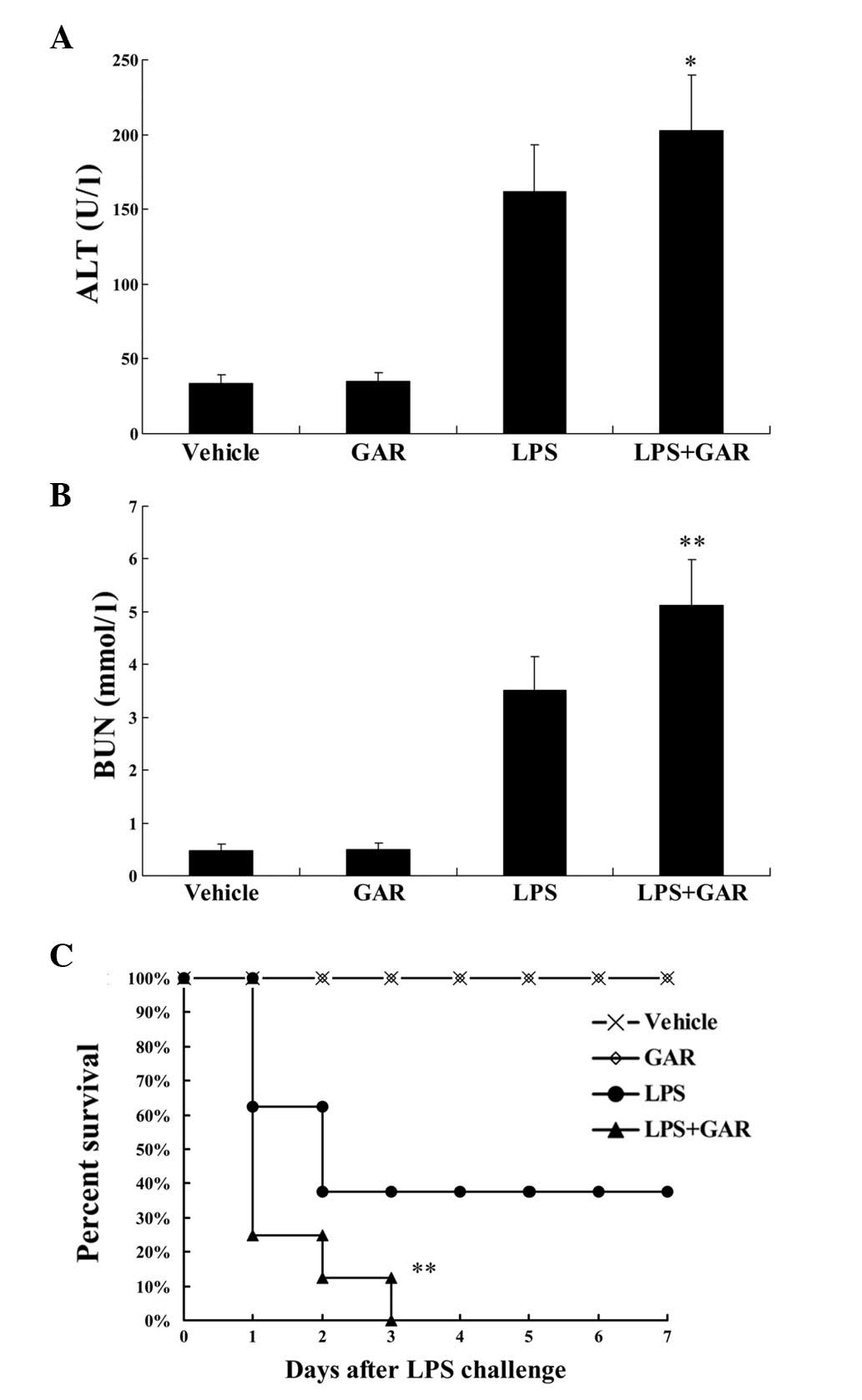
Garcinol increased LPS-induced
multi-organ injury and death in mice
Increased production of pro-inflammatory cytokines
can result in more severe tissue damage. Consistent with the
upregulated expression of TNF-α and IL-6 (Fig. 3), histopathological examination
indicated that, compared with LPS treatment, the LPS-induced
swelling of the alveolar walls and infiltration of leukocytes were
further exacerbated by garcinol treatment (Fig. 4). Additionally, the plasma levels
of ALT and BUN in LPS-exposed mice, used as markers for evaluating
the lesions of the liver and kidneys, respectively, were
significantly increased in the garcinol treated group compared with
the LPS group (P<0.05 and P<0.01, respectively; Fig. 5A and B). To determine the final
outcome of LPS-induced systemic inflammation, the difference in
survival rate between LPS and LPS + garcinol-treated animals was
compared. As demonstrated in Fig.
5C, compared with LPS treatment only, garcinol significantly
shortened the survival time and increased the mortality of
LPS-insulted mice (P<0.01).
Discussion
Garcinol, a polyisoprenylated benzophenone
derivative of Garcinia indica fruit rind, was previously
identified as a potent HAT inhibitor (15,16).
HATs catalyze the acetylation of histones, which is associated with
opened chromatin structure and transcriptional activation (17,18).
Thus, inhibition of HATs by garcinol may result in suppressed
transcription of genes. It was previously reported that garcinol
inhibits the expression of inducible nitric oxide synthase and
cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages, and
TNF-α-stimulated 3T3-L1 adipocytes (17,19).
Additionally, a previous study demonstrated that treatment with
garcinol markedly inhibited cigarette smoke extract-stimulated
COX-2 expression in human tracheal smooth muscle cells (19). Thus, garcinol appears to exhibit
certain anti-inflammatory activities.
However, the present study unexpectedly demonstrated
that garcinol promotes LPS-induced TNF-α and IL-6 production in
vivo and in vitro. These effects were accompanied by
enhanced organ damage and a reduced survival rate in LPS-challenged
mice. Notably, it was previously reported that garcinol treatment
did not alter the levels of TNF-α and IL-1β in LPS-stimulated
leukocytes (20). Thus, it appears
that garcinol may exhibit diverse effects on the expression of
inflammatory genes and only suppresses inflammation under certain
circumstances.
In contrast to HATs, HDACs catalyze the removal of
the acetyl group from histones, which typically results in a
condensed chromatin structure and transcriptional silencing
(21). However, increasing
evidence suggests that pharmacological inhibition of HDACs may
suppress the expression of inflammatory genes and attenuate tissue
injury in inflamed organs (4,22,23).
The observations of the present study were consistent with the
previously reported anti-inflammatory actions of HDAC inhibitors,
as garcinol HAT inhibitor exhibited the opposite effects on
inflammation.
Although acetylation modification was originally
observed in histones, and the enzymes controlling this modification
were termed HATs and HDACs, it is now widely accepted that
HATs/HDACs also catalyze the acetylation/deacetylation of certain
non-histone proteins (6). In
addition to histones, NF-κB, a pivotal inflammation-associated
transcription factor (24), can be
acetylated at multiple lysine residues. It was previously reported
that acetylation of the p65 NF-κB subunit at Lys310 or Lys221
increases the transcriptional activity or DNA binding affinity of
NF-κB (25,26), whereas, acetylation at Lys122 and
Lys123 facilitated the relocation of NF-κB from the nucleus
(27). Therefore, acetylation of
p65 at discrete sites has distinct regulatory action on the
functions of NF-κB.
Other acetylation targets are also associated with
the regulation of inflammation. High mobility group box 1 (HMGB1)
is a chromatin protein that is secreted by activated macrophages as
an inflammatory mediator (28,29).
Acetylation of HMGB1 promotes its translocation into the cytosol
and facilitates its secretion (30,31).
Additionally, acetylation of MKP-1, a negative regulator of the
MAPK pathway, increases its phosphatase activity and interrupts the
inflammatory signals transduced via the MAPK pathway (4,32).
Thus, acetylation modifications have positive and negative
regulatory effects on inflammatory gene expression. The outcomes of
HDAC or HAT inhibition in inflammation may be a result of the
combined effects on these multiple targets.
In conclusion, the present study demonstrated that
garcinol HAT inhibitor enhanced LPS-induced inflammation in
vitro and in vivo. The observations of the current
study, together with the anti-inflammatory properties of HDAC
inhibitors, suggest that inhibition of deacetylation modulation may
be correlated with suppressed inflammatory responses and alleviated
tissue injury, however, the detailed molecular mechanisms
underlying the pro-inflammatory effects of HAT inhibitors and the
anti-inflammatory effects of HDAC inhibitors require further
investigation. Thus, HDAC inhibitors, but not HAT inhibitors, may
exert therapeutic effects in inflammatory disorders.
Acknowledgments
The current study was supported by grants from the
Health and Family Planning Commission of Chongqing (grant no.
2012-2-035), the Natural Science Foundation of Chongqing (grant no.
cstc2012jjA10041), the Hubei Provincial Department of education
(grant no. D20144301) and the National Natural Science Foundation
of China (grant no. 81370179).
References
|
1
|
Kotas ME and Medzhitov R: Homeostasis,
inflammation, and disease susceptibility. Cell. 160:816–827. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ehrentraut SF and Colgan SP: Implications
of protein post-translational modifications in IBD. Inflamm Bowel
Dis. 18:1378–1388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choudhary C, Weinert BT, Nishida Y, Verdin
E and Mann M: The growing landscape of lysine acetylation links
metabolism and cell signalling. Nat Rev Mol Cell Biol. 15:536–50.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong Y, Du R, Zhu X, Yin S, Wang J, Cui
H, Cao W and Lowenstein CJ: Histone deacetylase isoforms regulate
innate immune responses by deacetylating mitogen-activated protein
kinase phosphatase-1. J Leukoc Biol. 95:651–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chi H and Flavell RA: Acetylation of MKP-1
and the control of inflammation. Sci Signal. 1:pe442008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spange S, Wagner T, Heinzel T and Krämer
OH: Acetylation of non-histone proteins modulates cellular
signalling at multiple levels. Int J Biochem Cell Biol. 41:185–198.
2009. View Article : Google Scholar
|
|
7
|
Hu X, Yu Y, Eugene Chin Y and Xia Q: The
role of acetylation in TLR4-mediated innate immune responses.
Immunol Cell Biol. 91:611–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gräff J and Tsai LH: Histone acetylation:
Molecular mnemonics on the chromatin. Nat Rev Neurosci. 14:97–111.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Royce SG and Karagiannis TC: Histone
deacetylases and their inhibitors: New implications for asthma and
chronic respiratory conditions. Curr Opin Allergy Clin Immunol.
14:44–48. 2014. View Article : Google Scholar
|
|
10
|
Maddox SA, Watts CS, Doyère V and Schafe
GE: A naturally-occurring histone acetyltransferase inhibitor
derived from Garcinia indica impairs newly acquired and reactivated
fear memories. PLoS One. 8:e544632013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye X, Yuan L, Zhang L, Zhao J, Zhang CM
and Deng HY: Garcinol, an acetyltransferase inhibitor, suppresses
proliferation of breast cancer cell line MCF-7 promoted by
17β-estradiol. Asian Pac J Cancer Prev. 15:5001–5007. 2014.
View Article : Google Scholar
|
|
12
|
Schefe JH, Lehmann KE, Buschmann IR, Unger
T and Funke-Kaiser H: Quantitative real-time RT-PCR data analysis:
Current concepts and the novel 'gene expression's CT difference'
formula. J Mol Med (Berl). 84:901–910. 2006. View Article : Google Scholar
|
|
13
|
Gong F and Miller KM: Mammalian DNA
repair: HATs and HDACs make their mark through histone acetylation.
Mutat Res. 750:23–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghizzoni M, Haisma HJ, Maarsingh H and
Dekker FJ: Histone acetyltransferases are crucial regulators in
NF-κB mediated inflammation. Drug Discov Today. 16:504–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semwal RB, Semwal DK, Vermaak I and
Viljoen A: A comprehensive scientific overview of Garcinia
cambogia. Fitoterapia. 102:134–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Padhye S, Ahmad A, Oswal N and Sarkar FH:
Emerging role of Garcinol, the antioxidant chalcone from Garcinia
indica Choisy and its synthetic analogs. J Hematol Oncol. 2:382009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao CH, Sang S, Liang YC, Ho CT and Lin
JK: Suppression of inducible nitric oxide synthase and
cyclooxygenase-2 in down-regulating nuclear factor-kappa B pathway
by Garcinol. Mol Carcinog. 41:140–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang CM, Lee IT, Lin CC, Yang YL, Luo SF,
Kou YR and Hsiao LD: Cigarette smoke extract induces COX-2
expression via a PKCalpha/c-Src/EGFR, PDGFR/PI3K/Akt/NF-kappaB
pathway and p300 in tracheal smooth muscle cells. Am J Physiol Lung
Cell Mol Physiol. 297:L892–L902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu CL, Lin YJ, Ho CT and Yen GC: The
inhibitory effect of pterostilbene on inflammatory responses during
the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J
Agric Food Chem. 61:602–610. 2013. View Article : Google Scholar
|
|
20
|
Sailhamer EA, Li Y, Smith EJ, Shuja F,
Shults C, Liu B, Soupir C, deMoya M, Velmahos G and Alam HB:
Acetylation: A novel method for modulation of the immune response
following trauma/hemorrhage and inflammatory second hit in animals
and humans. Surgery. 144:204–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seto E and Yoshida M: Erasers of histone
acetylation: The histone deacetylase enzymes. Cold Spring Harb
Perspect Biol. 6:a0187132014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cantley MD and Haynes DR: Epigenetic
regulation of inflammation: progressing from broad acting histone
deacetylase (HDAC) inhibitors to targeting specific HDACs.
Inflammopharmacology. 21:301–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen HY, Li L and Fu ZJ: Histone
deacetylase inhibitors trichostatin A and suberoylanilide
hydroxamic acid attenuate ventilator-induced lung injury.
Pharmazie. 69:55–59. 2014.PubMed/NCBI
|
|
24
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
25
|
Rothgiesser KM, Erener S, Waibel S,
Lüscher B and Hottiger MO: SIRT2 regulates NF-κB dependent gene
expression through deacetylation of p65 Lys310. J Cell Sci.
123:4251–4258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y and Qiu J: AMP-activated protein
kinase suppresses endothelial cell inflammation through
phosphorylation of transcriptional coactivator p300. Arterioscler
Thromb Vasc Biol. 31:2897–2908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen LF and Greene WC: Shaping the nuclear
action of NF-kappaB. Nat Rev Mol Cell Biol. 5:392–401. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SA, Kwak MS, Kim S and Shin JS: The
role of high mobility group box 1 in innate immunity. Yonsei Med J.
55:1165–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Erlandsson Harris H and Andersson U:
Mini-review: The nuclear protein HMGB1 as a proinflammatory
mediator. Eur J Immunol. 34:1503–1512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andersson U, Antoine DJ and Tracey KJ: The
functions of HMGB1 depend on molecular localization and
post-translational modifications. J Intern Med. 276:420–424. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dhupar R, Klune JR, Evankovich J, Cardinal
J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR and Tsung A:
Interferon regulatory factor 1 mediates acetylation and release of
high mobility group box 1 from hepatocytes during murine liver
ischemia-reperfusion injury. Shock. 35:293–301. 2011. View Article : Google Scholar
|
|
32
|
Chuang YF, Yang HY, Ko TL, Hsu YF, Sheu
JR, Ou G and Hsu MJ: Valproic acid suppresses
lipopolysaccharide-induced cyclooxygenase-2 expression via MKP-1 in
murine brain micro-vascular endothelial cells. Biochem Pharmacol.
88:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|