Introduction
It is well known that reperfusion strategies,
including primary percutaneous coronary interventions and
thrombolytic therapy, are important therapeutic strategies for
acute ST segment-elevated myocardial infarction (1,2).
However, restoration of the blood flow through the previously
ischemic myocardium can result in reperfusion-associated myocardial
dysfunction and cell death (3,4). To
date, it has been widely accepted that apoptosis serves a pivotal
role in the progress of myocardial ischemia/reperfusion (I/R)
injury (5,6). Therefore, it is important to identify
a target for the development of an effective treatment of
cardiomyocyte apoptosis, and therefore for attenuating myocardial
I/R injury.
Peroxisome proliferator-activated receptor gamma
(PPARG) is a ligand-activated transcription factor of the nuclear
hormone receptor superfamily, which abundant in adipose tissue
(7). PPARs are a family of at
least three nuclear receptors (α, δ, and γ), which regulate genes
involved in lipid metabolism, adipocyte differentiation and
inflammation (8). Previous studies
have indicated that PPARG activation is also pivotal for the
regulation of a variety of pathophysiologic processes within the
cardiovascular system (9,10). In addition, it has been
demonstrated that activation of PPARG attenuates apoptosis induced
by hypoxia/reoxygenation in cardiomyocytes (11) or myocardial I/R injury (12). Activation of Akt by
phosphatidylinositol-3-kinase (PI3K) leads to the phosphorylation
of a variety of downstream targets, including pro-apoptotic
proteins, transcription factors and other protein kinases, thereby
regulating cell survival (13).
Induced myeloid leukemia cell differentiation protein-1 (Mcl-1),
which is upregulated in several human cancers, is an anti-apoptotic
Bcl-2 family protein (14).
Previous studies have indicated that activation of PPARG inhibits
apoptosis in cardiomyocytes, which may be associated with the
activation of Akt (11) and
expression of Mcl-1 (12).
MicroRNAs (miRNAs) are small noncoding RNAs,
comprised of ~22 nucleotides. miRNAs negatively regulate gene
expression via either degrading the target mRNA or by direct
translational inhibition (15).
Several candidate miRNAs (miRs), such as miR-27a (16), miR-27b (17), miR-130a and miR-130b (18,19),
have been reported to be negative regulators for PPARG expression.
In a preliminary study, candidate miRNAs that bind to PPARG were
screened using a bioinformatics algorithm (TargetScan 6.2;
http://www.targetscan.org), according to a
previous study (20). The results
of this screen identified a putative miR-128 binding sequence
(ACUGUGA) in the 3′-untranslated region (3′-UTR) of PPARG mRNA.
Therefore, it was hypothesized that miR-128 inhibition may
attenuate myocardial I/R injury-induced cardiomyocyte apoptosis by
the targeted activation of PPARG.
The present study aimed to confirm that PPARG is a
direct target of miR-128 in the HEK293 cell line and in cultures of
neonatal rat ventricular myocytes (NRVMs). Furthermore, the role of
miR-128 inhibition in preventing cardiomyocyte apoptosis through
its PPARG activation-mediated effect was investigated in a rabbit
myocardial I/R injury model. Myocardial I/R injury was induced by a
protocol of 60 min of left anterior descending coronary artery
(LAD) ischemia followed by 6 h of reperfusion (21). The aim was to shed new light on the
roles of miRNAs, in particular miR-128, in myocardial I/R
injury-induced cardiomyocyte apoptosis.
Materials and methods
HEK293 cell culture and preparation of
primary cultured neonatal cardiomyocytes
A total of 24 neonatal (1–3 day old) male
Sprague-Dawley rats were purchased from the Center for Experimental
Animals, Guangxi Medical University (Nanning, China). The rats were
maintained under a 12-h light/dark cycle at 22±1°C. Food and water
were available ad libitum. NRVMs were isolated from the
rats, as described previously (22). Briefly, following anesthetization
with intraperitoneal ketamine (50 mg/kg; Phoenix Pharmaceuticals,
Inc., St. Joseph, MO, USA), the rats were sacrificed by
decapitation and their hearts were aseptically removed. Ventricle
tissues were dissected, minced and trypsinized at 37°C for 10 min.
Dispersed NRVMs were plated in 24-well plates in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS) and 0.1 mM bromodeoxyuridine for further experiments.
All procedures involving animal use were performed in accordance
with the European Community Guidelines for the Care and Use of
Animals, and the Institutional Ethics Committee for Animal Usage
approved the research protocol. The HEK293 cell line was purchased
from the Cell Resource Center (Shanghai Institutes for Biological
Sciences, China Academy of Sciences, Shanghai, China). HEK293 cells
were cultured in DMEM supplemented with 10% FBS and 100
µg/ml penicillin/streptomycin in a humidified incubator at
37°C with 5% CO2.
Transfection of NRVMs and HEK293 cells
with miRNAs
The miRNAs were designed and chemically synthesized
by Shanghai GenePharma Co., Ltd. (Shanghai, China). The following
sequences were synthesized: Rat (rno)-miR-128 mimics, sense
5′-UCACAGUGA ACCGGUCUCUUU-3′ and antisense
5′-AGAGACCGGUUCACUGUGAUU-3′; rno-miR-128 inhibitor (antagomir-128)
5′-AAAGAGACCGGTTCACTGTGA-3′; and negative control, sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. NRVMs and HEK293 cells were
transfected with the mimics or inhibitors of miR-128 or negative
control RNA at a final concentration of 100 nM using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and OPTI-MEM
reduced serum medium (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Following transfection, the cells
were incubated for 48 and 72 h prior to reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Luciferase reporter assay
The PPARG 3′-UTR containing the miR-128 target
sequence (position 82–88) and mutant sequences (a single-base
mutant in the 3′UTR) were chemically synthesized (GenScript,
Nanjing, China) and cloned into the PGL3 control vector (Promega
Corporation, Madison, WI, USA) downstream of the luciferase gene
using the XbaI site. The resulting plasmids were designated
PPARG 3′-UTR-luci-wild type (WT) and PPARG 3′-UTR-luci-mutant
(MUT). HEK293 cells were transfected with either PPARG
3′-UTR-luci-WT or PPARG 3′-UTR-luci-MUT in 24-well plates using
Lipofectamine 2000 transfection reagent according to the
manufacturer's instructions, and co-transfected with miR-128 mimic,
antagomir-128 or the same concentration of negative control. Each
well was also co-transfected with the pRL-TK plasmid (Promega
Corporation) to determine the transfection efficiency.
Subsequently, firefly and Renilla luciferase activity levels were
measured in cells that were harvested 24 h post-transfection using
the Dual-Luciferase Reporter Assay System (Promega Corporation).
Each transfection was performed in triplicate.
Treatment groups and rabbit myocardial
I/R injury model
A total of 40 healthy, adult male New Zealand white
rabbits (weight, 2.0–2.5 kg; age, 8–12 weeks) were purchased from
the Center for Experimental Animals at the Guangxi Medical
University. Animals were housed at 25±2°C and 60±5% humidity, and
exposed to a 12:12 h light-dark cycle with pellet food and tap
water ad libitum. The 40 rabbits were randomly divided into
five groups (n=8 each). The sham-operated and I/R groups were
administered vehicle only (10% dimethyl-sulfoxide solution). The
GW9662 group received the PPARG antagonist GW9662 (Sigma-Aldrich,
St. Louis, MO, USA) at a daily dose of 0.5 mg/kg body weight by
gastric gavage for three days. The antagomir-128 group received
antagomir-128 at a single dose of 80 mg/kg body weight through ear
marginal vein injection for three days prior to regional ischemia
(23,24). The antagomir-128+GW9662 group was
administered antagomir-128 (a single dose of 80 mg/kg at three days
prior to regional ischemia) and GW9662 (0.5
mg·kg−1·d−1 for three days). All animals were
anesthetized using 20% urethane via an ear marginal vein injection.
Following midline thoracotomy, a 4–0 silk ligature was placed under
the LAD. The ends of the suture were threaded through polyethylene
tubing to form a snare. The ends of the suture were pulled tight
and a hemostat was used to clamp the snare to occlude the coronary
artery. Following 60 min of ischemia, the ligature was untied and
the snare was loosened, allowing the ischemic myocardium to
re-perfuse for 6 h. Sham-operated animals were subjected to all of
the previously described procedures, except ligation of the
LAD.
Tissue sampling
Rabbits received the same treatment as described
above and were subjected to 60 min of ischemia and 6 h of
reperfusion. At 6 h, the coronary artery was re-ligated, and Evans
blue dye (1 mg/kg), which stains non-viable cells, was injected
into the right ventricle to identify the myocardial area at risk.
All animals were anesthetized by injection of 20% urethane, 10%
potassium chloride (10 ml) into the ear marginal vein, resulting in
cessation of the heart beat during diastole. Subsequently,
cardioectomy was performed, and hearts were removed, sliced and
stained with 2,3,5-triphenyl-tetrazolium-chloride (TTC) to
differentiate infarcted (TTC unstained) from viable myocardium (TTC
stained). The border zone was identified as Evans blue unstained
and TTC stained. A 2 mm section of myocardium tissue from the
border zone was fixed in 4% formalin solution and embedded in
paraffin for terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) assays. The border zone was also used for
determination of the level of miR-128 in cardiomyocytes by RT-qPCR.
In addition, the border zone was frozen in liquid nitrogen and
stored at −80°C for western blot analysis. The border zone was cut
into a sample of ~1 mm3 of subendocardial tissue to
examine the ultrastructure of cardiomyocyte apoptosis by electron
microscopy.
RT-qPCR analysis
Total RNA, including miRNA, was extracted from NRVMs
and rabbit cardiac ventricular myocytes using TRIzol reagent
(Invitrogen; Themo Fisher Scientitific, Inc.), according to the
manufacturer's protocol. Following isolation, total RNA was
incubated with RQ1 RNase-free DNase (Promega Corporation) to remove
contaminating DNA. Using the ABI Prism 7300 Sequence Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.), the
mRNA levels of PPARG and miR-128 levels were examined by RT-qPCR,
as reported previously (25). The
commercial kits used in the RT-qPCR were the TaqMan®
Universal PCR Master Mix (cat. no. 4304437; Applied Biosystems;
Thermo Fisher Scientific, Inc.), TaqMan® Reverse
Transcription Reagents (cat. no. N8080234; Applied Biosystems;
Thermo Fisher Scientific, Inc.), TaqMan® MicroRNA Assays
(cat. no. 4427975; Applied Biosystems; Thermo Fisher Scientific,
Inc.) and the TaqMan® MicroRNA Reverse Transcription kit
(cat. no. 4366596; Applied Biosystems; Thermo Fisher Scientific,
Inc.). β-actin or U6 was used as an internal reference. The primer
sequences were as follows: PPARG forward,
5′-GCGACATCGACCAACTGAAC-3′ and reverse, 5′-ACGGAGCGAAACTGACACC-3′;
miR-128 forward, 5′-CGCGCTCACAGTGAACCG-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; β-actin forward,
5′-TGTGATGGTGGGAATGGGTCAGAA-3′ and reverse,
5′-TGTGGTGCCAGATCTTCTCCATGT-3′; and U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′ (Shanghai GenePharma Co., Ltd.). The
PCR cycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec and 60°C for 35 sec. Individual
samples were run in triplicate, and each experiment was repeated a
minimum of three times. Data analyses were performed using the
2−ΔΔCq method for calculating relative gene expression
levels (26).
Western blot analysis
Total protein from NRVMs was extracted with 1%
radioimmuniprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) containing 1 mM
phenylmethanesulfonyl fluoride. The supernatants were collected,
and protein concentration was determined using a Bicinchoninic Acid
Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Proteins (80 µg) were resolved on 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and
transferred to nitrocellulose membranes (Sigma-Aldrich). The
membranes were blocked for 1 h at room temperature with 5% non-fat
milk dissolved in PBS and incubated overnight at 4°C with rabbit
anti-PPARG (1:500; cat. no. sc-7196) and anti-β-actin (1:1,000;
cat. no. sc-130656) polyclonal antibodies (both Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), followed by three 10-min
washes with Tris-buffered saline solution containing 0.05% Tween-20
(TBST) and incubation with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G (1:5,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.). Subsequently, the membrane was washed three
times for 10 min in TBST, and the protein was detected by
chemiluminescence with the Pierce Enhanced Chemiluminescence
Western Blotting Substrate (Pierce Biotechnology, Inc.).
The expression of phosphorylated Akt (p-Akt),
total-Akt (t-Akt), PPARG and Mcl-1 protein in the border zone of
the myocardium was examined by western blotting. Total protein was
extracted from the border zone using standard procedures described
previously (27). Proteins were
separated by SDS-PAGE (as described for NRVM extracts) and
incubated overnight at 4°C with rabbit anti-Akt (1:500; cat. no.
sc-8312), anti-p-Akt (1:500; cat. no. sc-33437), anti-PPARG
(1:500), anti-Mcl-1 (1:1,000; cat. no. sc-819) and anti-β-actin
(1:1,000) polyclonal antibodies (all Santa Cruz Biotechnology,
Inc.). Proteins were detected and visualized as described for NRVM
extracts. All western blot data were quantified using ImageJ 1.48
software (National Institutes of Health, Bethesda, MD, USA) and
data were normalized to β-actin.
Apoptotic cardiomyocyte ultrastructure
and the TUNEL assay
The ultrastructure of apoptotic cardiomyocytes in
the subendocardial tissue of the border zone of hearts subjected to
the I/R protocol was examined by transmission electron microscopy
(H-500; Hitachi, Tokyo, Japan), as described above. The tissues
were prepared for transmission electron microscopy as described
previously (28).
Myocardial apoptosis in the border zone was
quantified by a TUNEL apoptosis assay kit (Nanjing KeyGen Biotech,
Co., Ltd., Nanjing, China). All procedures were performed according
to the manufacturer's instructions. Cells were defined as apoptotic
if the whole nuclear area of the cell was labeled positively.
TUNEL-positive myocytes were determined by randomly counting 10
fields using an inverted microscope (TE-300; Nikon Corporation,
Tokyo, Japan). The apoptotic index (AI) was then determined as the
number of apoptotic myocytes/total number of myocytes counted ×
100.
Statistical analyses
Data are presented as the mean ± standard deviation.
Statistical differences were determined using one-way analysis of
variance followed by Fisher's Least Significant Difference tests.
P<0.05 was considered to indicate a statistically significant
difference. All analyses were performed using SPSS software,
version 16 (SPSS, Inc., Chicago, IL, USA).
Results
PPARG gene is a target of miR-128
NRVMs were transfected with a miR-128 mimic (100
nM), antagomir-128 (100 nM) or miR-control (100 nM) for 48 h. The
efficiency of transfection was measured by RT-qPCR (Fig. 1A). The miR-128 levels were
significantly higher in the miR-128 mimic group compared with the
levels in the control group (P<0.001). By contrast, the miR-128
levels were significantly lower in the antagomir-128 group compared
with the control and miR-128 mimic groups (P<0.001). PPARG mRNA
and protein expression levels were significantly downregulated in
NRVMs that were transfected with the miR-128 mimic compared with
the control group (P<0.001; Fig.
1B–D). By contrast, antagomir-128 significantly upregulated
PPARG mRNA and protein levels compared with the control and miR-128
mimic groups (P<0.001). Data from the luciferase reporter assay
showed that the downregulation of miR-128 following transfection
with antagomir-128 resulted in a marked increase in luciferase
activity of PPARG 3′-UTR-luci-WT compared with the control group
(P<0.001), without obvious alterations in the luciferase
activity of PPARG 3′-UTR-luci-MUT compared with the control group
(Fig. 1E). By contrast, the
luciferase activity of PPARG 3′-UTR-luci-WT was reduced upon
transfection of miR-128 mimics compared with the control group
(P<0.001; Fig. 1E). The
myocardial miR-128 levels in the I/R and GW9662 groups were higher
compared with the sham-operated group (P<0.001). Furthermore,
the levels of myocardial miR-128 in the antagomir-128 and
antagomir-128+GW9662 groups were significantly lower than in the
sham and I/R groups (P<0.001; Fig.
1F).
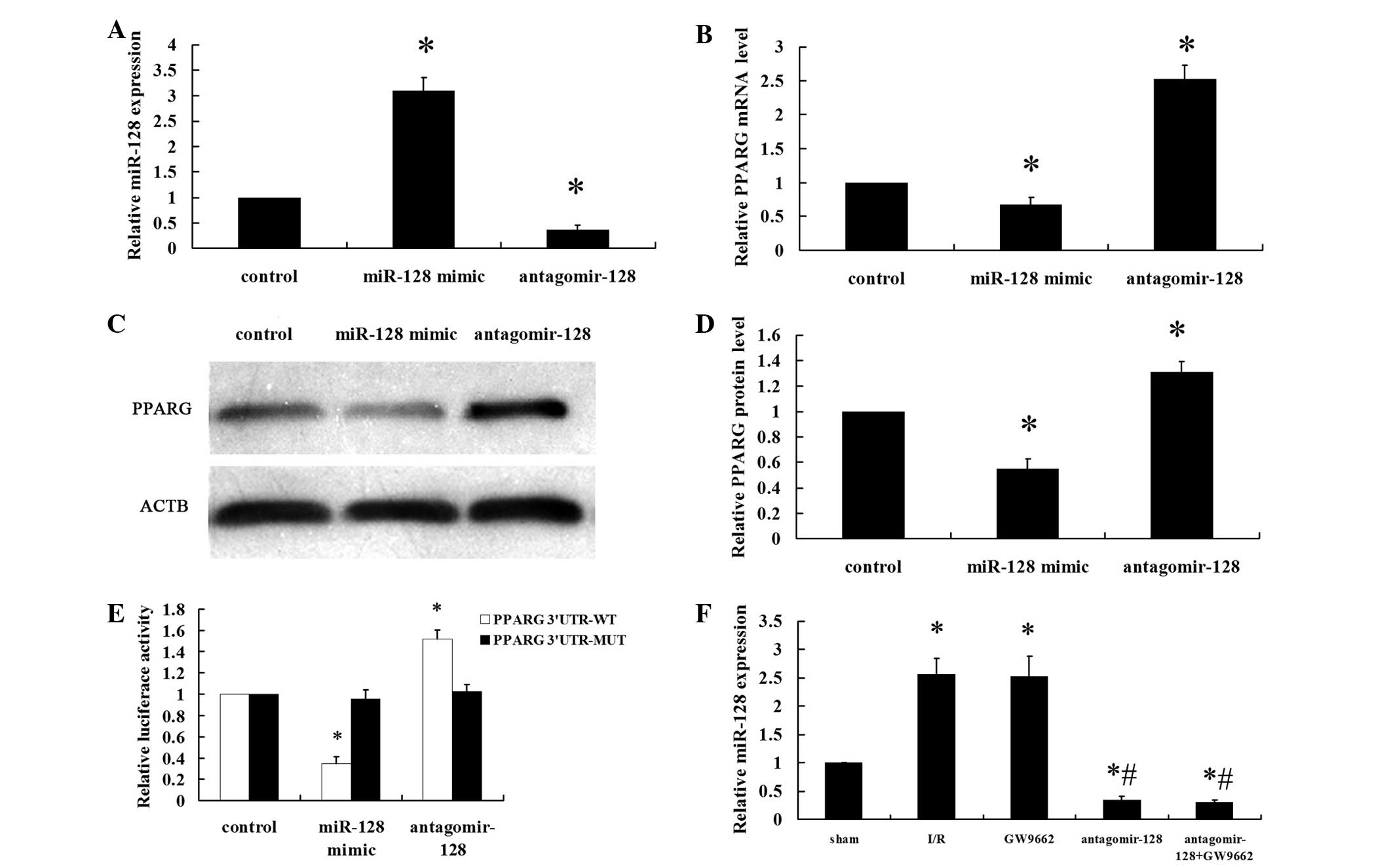 | Figure 1PPARG is a direct target gene of
miR-128. Neonatal rat ventricular myocytes were transfected with a
miR-128 mimic (100 nM), antagomir-128 (100 nM) or miR-control (100
nM) for 48 h. (A) The miR-128 levels were determined by RT-qPCR.
(B) RT-qPCR showed that regulation of miR-128 levels affects the
mRNA level of PPARG. (C) Representative western blot image of PPARG
and ACTB protein levels. (D) Quantitative analyses of the
expression of PPARG protein in the western blot analysis. PPARG
values were normalized to ACTB expression levels. (E) Transient
transfection of HEK293 cells with the PPARG 3′-UTR-luci-WT and
PPARG 3′-UTR-luci-MUT constructs, along with the miR-128 mimic,
antagomir-128 or miR-control added to the cultures 24 h following
transfection. Luciferase activities were measured and normalized to
the control luciferase activity. (F) The myocardial miR-128 levels
were determined by RT-qPCR. Values are presented as the mean ±
standard deviation; n=8 for each group. *P<0.05 vs.
the control group or sham group; #P<0.05 vs. the I/R
group. PPARG, peroxisome proliferator-activated receptor gamma;
miR, microRNA; RT-qPCR, reverser transcription-quantitative
polymerase chain reaction; ACTB, β-actin; UTR, untranslated region;
luci, luciferase; WT, wild type; MUT, mutant. |
In vivo miR-128 inhibition increases the
activation of Akt and the expression of PPARG and Mcl-1 protein in
myocardial I/R rabbits
To further evaluate the role of miR-128 in PPARG
signaling in vivo, miR-128 was knocked down via a single ear
marginal vein injection of antagomir-128 (80 mg/kg) in a rabbit
myocardial I/R injury model. Alterations in the protein levels of
PPARG and the downstream signaling molecules Mcl-1, t-Akt and p-Akt
were assessed by western blot analysis (Fig. 2A and B) and densitometry (Fig. 2C–F). There were no significant
differences in t-Akt protein expression between the five
experimental groups (Fig. 2A and
C). However, when examining the phosphorylated, and therefore
activated from, of Akt (p-Akt; Fig. 2A
and D), and the expression of PPARG and Mcl-1 (Fig. 2B, E and F), protein expression in
all treatment groups was higher compared with the sham-operated
group (P<0.001). Furthermore, the levels of p-Akt (Fig. 2A and D) and the expression of PPARG
and Mcl-1 protein (Fig. 2B, E and
F) in the antagomir-128 group were significantly higher than in
the I/R group (P<0.001). By contrast, there were no significant
differences in p-Akt and the expression of PPARG and Mcl-1 protein
between the I/R, GW9662 and antagomir-128+GW9662 groups (Fig. 2D–F).
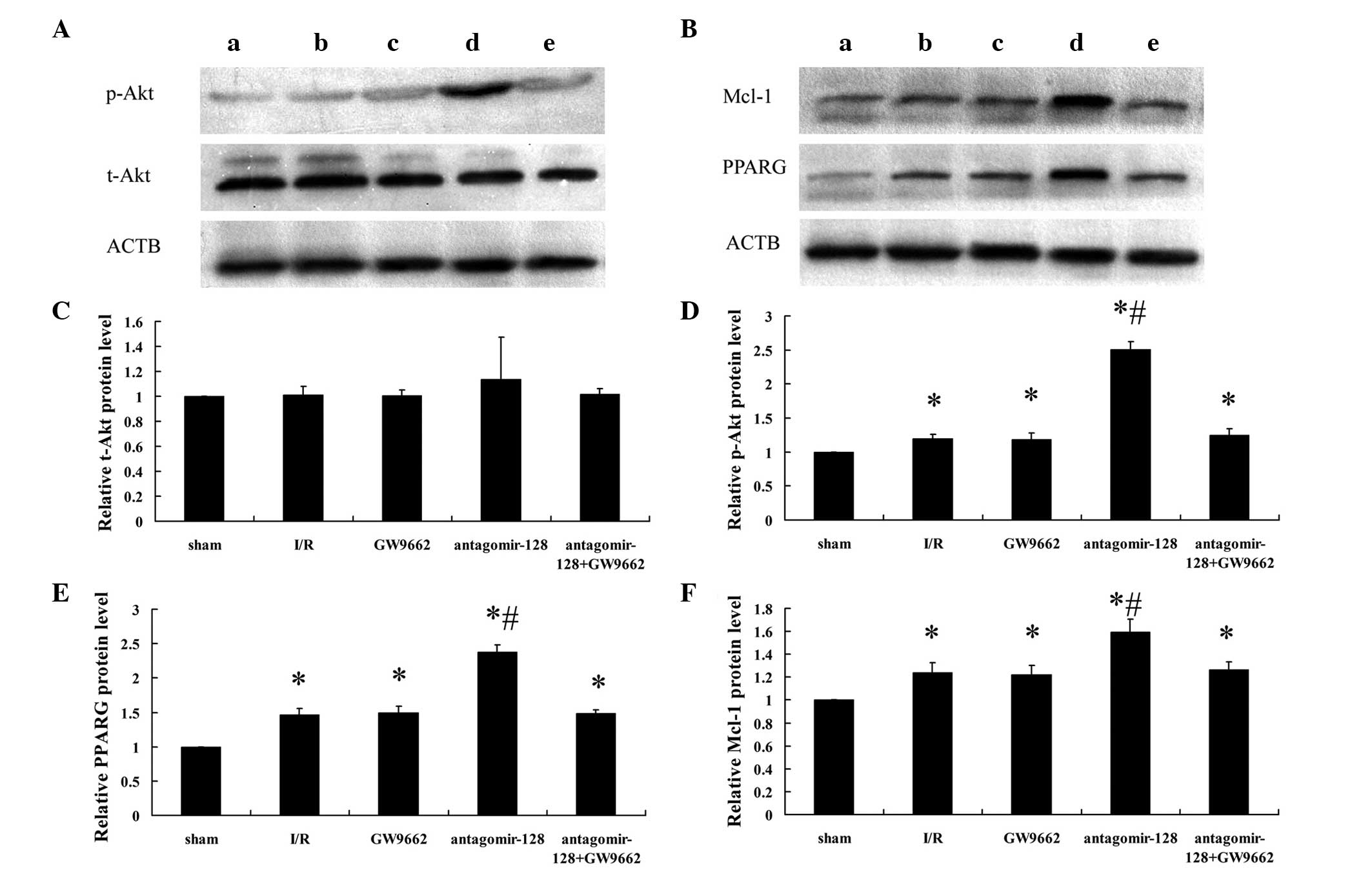 | Figure 2Determination of myocardial p-Akt,
t-Akt, PPARG, Mcl-1 and ACTB protein expression by western blot
analysis. (A) Representative western blot images showing p-Akt and
t-Akt expression in extracts from the border zone of the myocardium
of the (a) sham-operated, (b) myocardial I/R injury, (c) GW9662,
(d) antagomir-128 and (e) antagomir-128+GW9662 group. (B)
Representative western blot images showing PPARG and Mcl-1
expression in extracts from the border zone of the myocardium of
the (a) sham-operated, (b) myocardial I/R injury, (c) GW9662, (d)
antagomir-128 and (e) antagomir-128+GW9662 group. Quantification of
the relative expression levels of (C) t-Akt, (D) p-Akt, (E) PPARG
protein and (F) Mcl-1 protein. The values obtained by densitometric
measurements were normalized to ACTB expression levels. Values are
presented as the mean ± standard deviation; n=8 for each group.
*P<0.05 vs. the sham-operated group;
#P<0.05 vs. the I/R group. p-Akt, phosphorylated Akt;
t-Akt, total Akt; PPARG, peroxisome proliferator-activated receptor
gamma; Mcl-1, myeloid leukemia cell differentiation protein-1;
ACTB, β-actin; I/R, ischemia/reperfusion. |
In vivo miR-128 inhibition protects
against myocardial I/R-induced cardiomyocyte apoptosis
Subsequently, it was examined whether miR-128
inhibition was able to reduce cardiomyocyte apoptosis in
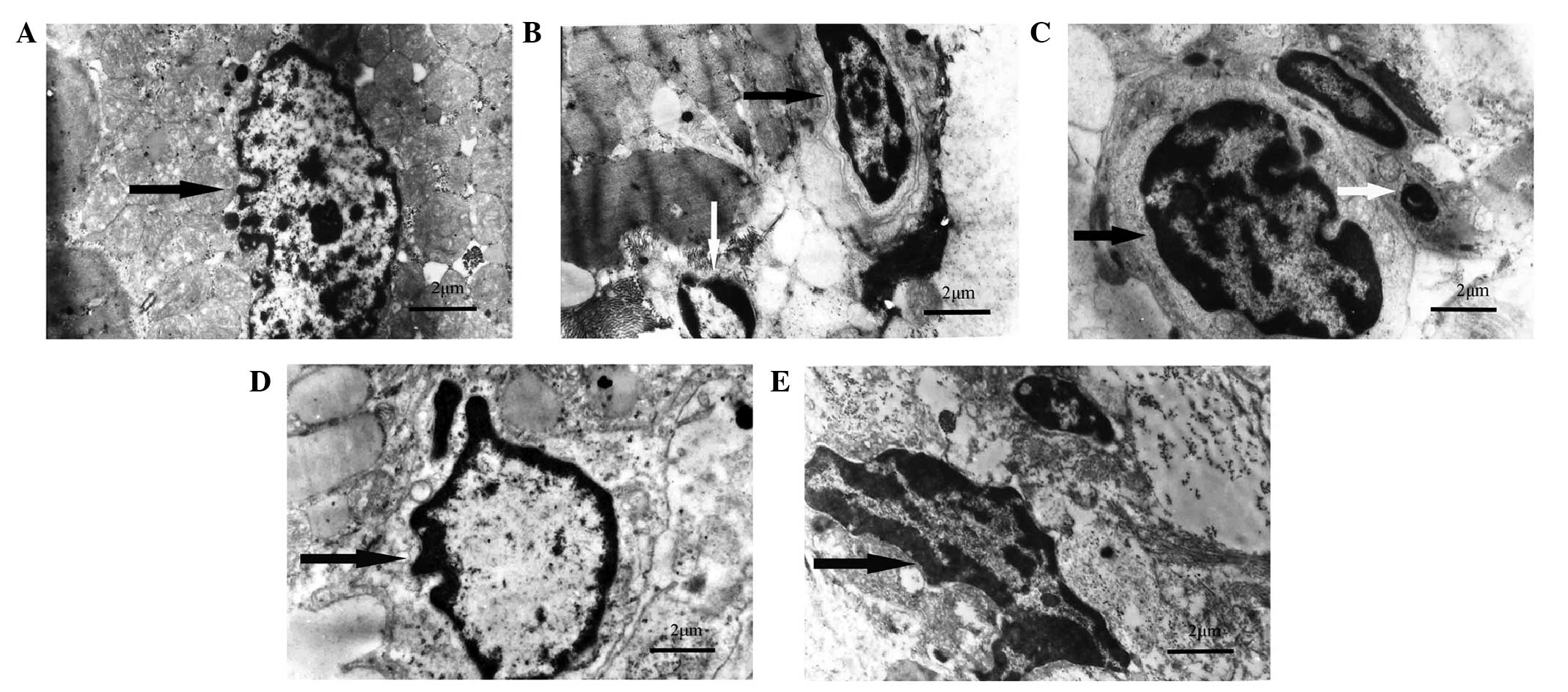
vivo. Ultramicroscopic examination using transmission electron
microscopy showed intact nucleoli, homogeneous chromatin and
visible microvilli in the sham-operated group (Fig. 3A). In the I/R, GW9662, and
antagomir-128+GW9662 groups, morphological features typical for
apoptotic cell death, including pyknotic nuclei, heterochromatic
clumping, peripheral condensation and margination of nuclear
chromatin, were observed. The pyknotic nucleus assumed the
appearance of a half-moon or crescent shape and the formation of
apoptotic bodies was observed (Fig.
3B, C and E). In the antagomir-128 group, the peripheral
chromatin condensation and aggregation of nuclear chromatin in
dense masses was attenuated, while the phenomenon of crescent
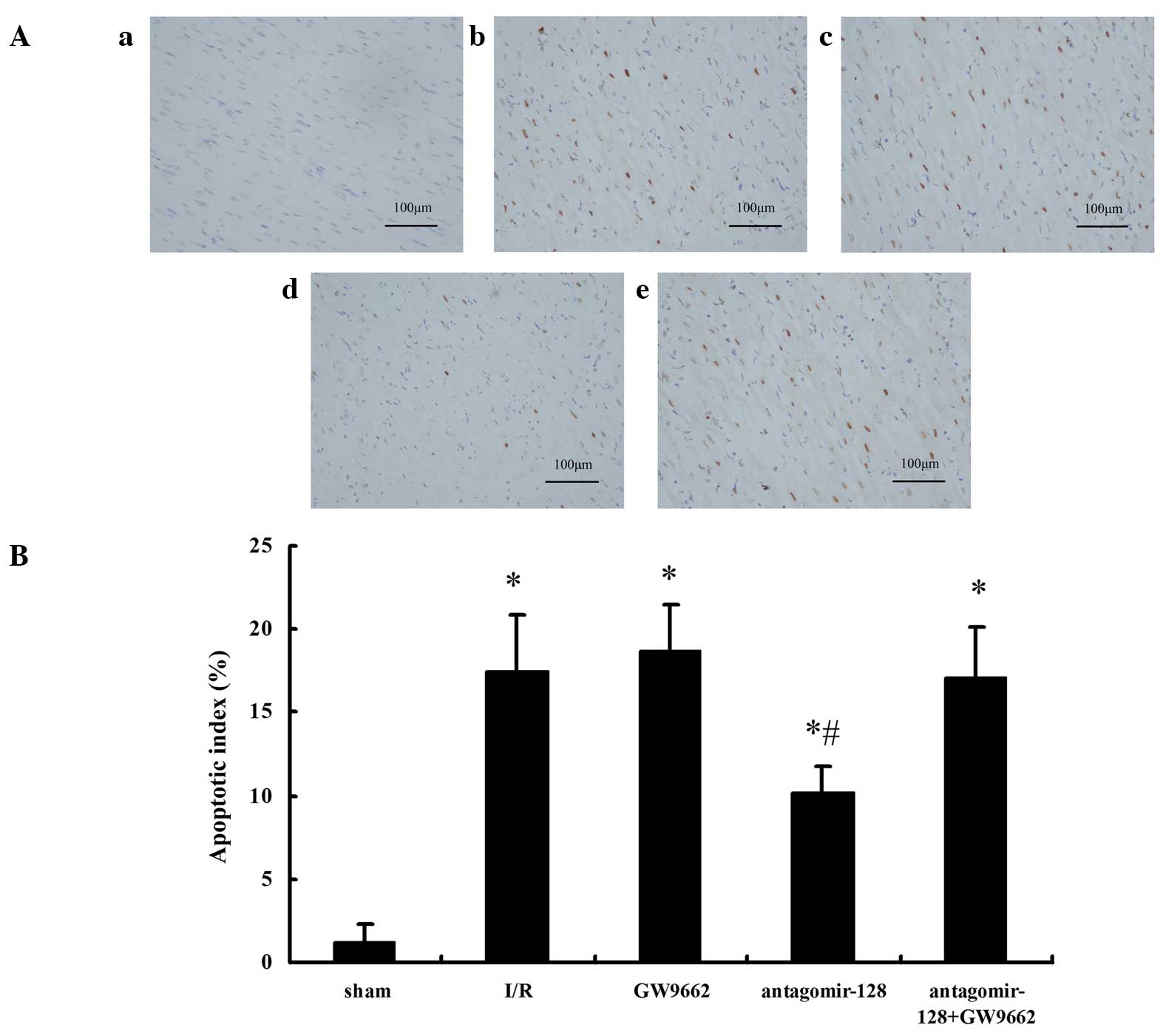
formation and apoptotic bodies was not observed (Fig. 3D). Furthermore, TUNEL assays
indicated that the AI in the I/R, GW9662, antagomir-128, and
antagomir-128+GW9662 groups were higher when compared with the
sham-operated group (P<0.001; Fig.
4A and B). The AI in the antagomir-128 group was significantly
lower than that in the I/R group (P<0.001). There was no
significant difference in AI between the I/R, GW9662 and
antagomir-128+GW9662 groups (Fig.
4B).
Discussion
The main findings of the current study are that
PPARG mRNA and protein expression levels are downregulated in NRVMs
transfected with miR-128 mimics compared with the control group. By
contrast, the miR-128 inhibitor markedly upregulates PPARG mRNA and
protein levels. Second, PPARG is a direct miR-128 target, as
indicated by the luciferase reporter assays using PPARG 3′-UTRs
containing the putative miR-128 binding site and a mutant version.
Finally, an in vivo myocardial I/R injury model in rabbits
demonstrated that the intravenous injection of antagomir-128
increases the levels of p-Akt, Mcl-1 and PPARG in the myocardium
compared with controls and results in a significant reduction in
apoptosis. This effect can be blocked by the PPARG inhibitor,
GW9662. These results show for the first time, to the best of our
knowledge, an anti-apoptotic effect of miR-128 inhibition in
myocardial I/R injury, which is mediated through its effect on
PPARG protein levels and the activation of signaling cascades
downstream of PPARG, including activated Akt and the anti-apoptotic
Bcl-2 family protein Mcl-1.
miRNAs are endogenous regulators of gene expression.
Considering that cardiomyocyte apoptosis is a key cellular event in
ischemic hearts that depends on gene expression (6), it is reasonable to hypothesize that
miRNAs may be involved in I/R-induced cardiac apoptosis. It should
be noted that several studies have demonstrated that miRNAs protect
against cardiomyocyte apoptosis. For example, Li et al
(29) reported that miR-145
protects cardiomyocytes against hydrogen peroxide-induced apoptosis
through targeting the mitochondrial apoptotic pathway. Wang et
al (30) reported that
transfection of a lentivirus expressing miR-146a attenuated
I/R-induced myocardial apoptosis and caspase-3/7 and -8 activities.
Ye et al (12) reported
that antagomirs against miR-29a or -29c significantly reduced
myocardial infarct size and apoptosis in hearts subjected to IR
injury.
A previous study indicated that PPARG activation
attenuates apoptosis induced by myocardial I/R injury (12). By using computational approaches
(TargetScan), the current study predicted a putative miR-128
binding sequence in the PPARG mRNA. To validate that PPARG is a
miR-128 target, it was demonstrated that transfection with miR-128
mimics or inhibitors affects PPARG mRNA and protein levels in
NRVMs. Subsequently, it was verified that PPARG is a direct miR-128
target using luciferase reporter assays in HEK293 cells. These
results indicate that miR-128 directly modulates PPARG expression
by binding to the 3′-UTR of PPARG and, thus, confirm that PPARG is
a novel target of miR-128s.
To evaluate the biological role of miR-128 in PPARG
signaling in vivo, miR-128 expression was knocked down via a
single ear marginal vein injection of antagomir-128 in a rabbit
myocardial I/R injury model. Activation of the PI3K/Akt pathway has
been previously reported to prevent cardiomyocyte apoptosis and
protect the heart from myocardial I/R injury (31,32).
Endothelial nitric oxide synthase is activated by Akt, which leads
to nitric oxide production (13,33).
Furthermore, the activation of the PI3K/Akt pathway can mediate
survival signals through the upregulation of members of the Bcl-2
family of anti-apoptotic proteins (34). For instance, a critical role of the
Bcl-2 family member Mcl-1 in cardiomyocytes is to prevent the
induction of cell death (35,36).
Previous studies have suggested that the activation of PPARG
inhibits apoptosis in cardiomyocytes by increasing phosphorylation
of Akt (11) and protein levels of
Mcl-1 (12). In the present study,
histological examination of the myocardium in the myocardial I/R
injury model revealed that miR-128 inhibition was able to reduce
cardiomyocyte apoptosis in vivo. Furthermore, the
administration of antagomir-128 was observed to significantly
increase the levels of p-Akt, Mcl-1 and PPARG in the myocardium,
suggesting that miR-128 inhibition activates the PPARG signaling
pathway in vivo. In response to the miR-128-modulated
increase in PPARG protein, Akt was phosphorylated and Mcl-1 protein
levels were increased, in turn attenuating I/R-induced
cardiomyocyte apoptosis. Furthermore, antagomir-128 together with
the PPARG inhibitor GW9662 significantly reduced p-Akt and Mcl-1
levels by antagonizing PPARG activation, thus resulting in
significant reduction in the miR-128 inhibition-induced protective
effects on cardiomyocyte apoptosis. These data show that activated
PPARG is necessary for the anti-apoptotic effects in the myocardial
I/R injury model.
These data indicate that miR-128 inhibition may be a
promising intervention for the management of apoptosis induced by
myocardial I/R injury. Although the current studies were performed
in an animal model and the experimental results cannot be
extrapolated directly to humans, these results may provide a
starting point for further preclinical studies to investigate
whether targeting miR-128 is a valid therapeutic approach for
cardioprotection following myocardial ischemia.
However, some limitations of the present study
should be considered. First, the miR-128 mimics and inhibitors were
derived from the rat sequence, while the in vivo
investigations used adult rabbit hearts. However, computational
miRNA target prediction analysis indicates that the miR-128
fragment 5′-UCACAGU-3′ pairs well with the fragment 5′-ACUGUGA-3′
of the PPARG 3′ UTR, which is a highly conserved in mammals (e.g.
rat, human, chimpanzee, rhesus, bushbaby, treeshrew, mouse and
rabbit). Therefore, the difference in animal species for miR-128
mimics and inhibitors are unlikely to reduce the validity of the
data interpretations. Second, additional immuno-stainings for
anti-apoptotic proteins, apoptosis detection by flow cytometry and
cardiac functional data, including echocardiography, will enable
confirmation of the current finding in future studies.
Taken together, the results from the current study
suggest that miR-128 inhibition has a protective effect against
cardiomyocyte apoptosis during myocardial I/R, through the targeted
activation of PPARG.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560067) and the
Youth Science Foundation of Guangxi Medical University (grant no.
GXMUYSF2014028).
References
|
1
|
Canadian Cardiovascular Society American
Academy of Family Physicians American College of Cardiology;
American Heart Association; Antman EM, Hand M, Armstrong PW, Bates
ER, Green LA, Halasyamani LK, et al: 2007 focused update of the
ACC/AHA 2004 guidelines for the management of patients with
ST-elevation myocardial infarction: A report of the American
college of cardiology/American heart association task force on
practice guidelines. J Am Coll Cardiol. 51:210–247. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stenestrand U, Lindbäck J and Wallentin L:
RIKS-HIA Registry: Long-term outcome of primary percutaneous
coronary intervention vs. prehospital and in-hospital thrombolysis
for patients with ST-elevation myocardial infarction. JAMA.
296:1749–1756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buja LM: Myocardial ischemia and
reperfusion injury. Cardiovasc Pathol. 14:170–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takemura G, Nakagawa M, Kanamori H,
Minatoguchi S and Fujiwara H: Benefits of reperfusion beyond
infarct size limitation. Cardiovasc Res. 83:269–276. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baines CP: How and when do myocytes die
during ischemia and reperfusion: The late phase. J Cardiovasc
Pharmacol Ther. 16:239–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eefting F, Rensing B, Wigman J, Pannekoek
WJ, Liu WM, Cramer MJ, Lips DJ and Doevendans PA: Role of apoptosis
in reperfusion injury. Cardiovasc Res. 61:414–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jozkowicz A, Dulak J, Piatkowska E, Placha
W and Dembinska-Kiec A: Ligands of peroxisome
proliferator-activated receptor-gamma increase the generation of
vascular endothelial growth factor in vascular smooth muscle cells
and in macrophages. Acta Biochim Pol. 47:1147–1157. 2000.
|
|
8
|
Mersmann J, Tran N, Zacharowski PA,
Grotemeyer D and Zacharowski K: Rosiglitazone is cardioprotective
in a murine model of myocardial I/R. Shock. 30:64–68.
2008.PubMed/NCBI
|
|
9
|
Goyal S, Arora S, Bhatt TK, Das P, Sharma
A, Kumari S and Arya DS: Modulation of PPAR-gamma by telmisartan
protects the heart against myocardial infarction in experimental
diabetes. Chem Biol Interact. 185:271–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yasuda S, Kobayashi H, Iwasa M, Kawamura
I, Sumi S, Narentuoya B, Yamaki T, Ushikoshi H, Nishigaki K,
Nagashima K, et al: Antidiabetic drug pioglitazone protects the
heart via activation of PPAR-gamma receptors, PI3-kinase, Akt and
eNOS pathway in a rabbit model of myocardial infarction. Am J
Physiol Heart Circ Physiol. 296:H1558–H1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kilter H, Werner M, Roggia C, Reil JC,
Schäfers HJ, Kintscher U and Böhm M: The PPAR-gamma agonist
rosiglitazone facilitates Akt rephosphorylation and inhibits
apoptosis in cardiomyocytes during hypoxia/reoxygenation. Diabetes
Obes Metab. 11:1060–1067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye Y, Hu Z, Lin Y, Zhang C and Perez-Polo
JR: Downregulation of microRNA-29 by antisense inhibitors and a
PPAR-gamma agonist protects against myocardial
ischaemia-reperfusion injury. Cardiovasc Res. 87:535–544. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ou HC, Lee WJ, Lee SD, Huang CY, Chiu TH,
Tsai KL, Hsu WC and Sheu WH: Ellagic acid protects endothelial
cells from oxidized low-density lipoprotein-induced apoptosis by
modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol.
248:134–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hullinger TG, Montgomery RL, Seto AG,
Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C,
Latimer PA, et al: Inhibition of miR-15 protects against cardiac
ischemic injury. Circ Res. 110:71–81. 2012. View Article : Google Scholar :
|
|
16
|
Lin Q, Gao Z, Alarcon RM, Ye J and Yun Z:
A role of miR-27 in the regulation of adipogenesis. FEBS J.
276:2348–2358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Song Y, Zhang Y, Xiao H, Sun Q,
Hou N, Guo S, Wang Y, Fan K, Zhan D, et al: Cardiomyocyte
overexpression of miR-27b induces cardiac hypertrophy and
dysfunction in mice. Cell Res. 22:516–527. 2012. View Article : Google Scholar :
|
|
18
|
Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim
MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, et
al: miR-130 suppresses adipogenesis by inhibiting peroxisome
proliferator-activated receptor gamma expression. Mol Cell Biol.
31:626–638. 2011. View Article : Google Scholar
|
|
19
|
Pan S, Yang X, Jia Y, Li R and Zhao R:
Microvesicle-shuttled miR-130b reduces fat deposition in recipient
primary cultured porcine adipocytes by inhibiting PPAR-g
expression. J Cell Physiol. 229:631–639. 2014. View Article : Google Scholar
|
|
20
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng XC, Li XS and Wen H: Telmisartan
protects against microvascular dysfunction during myocardial
ischemia/reperfusion injury by activation of peroxisome
proliferator-activated receptor γ. BMC Cardiovasc Disord.
13:392013. View Article : Google Scholar
|
|
22
|
Maass AH and Buvoli M: Cardiomyocyte
preparation, culture and gene transfer. Methods Mol Biol.
366:321–330. 2007. View Article : Google Scholar
|
|
23
|
Krützfeldt J, Rajewsky N, Braich R, Rajeev
KG, Tuschl T, Manoharan M and Stoffel M: Silencing of microRNAs in
vivo with 'antagomirs'. Nature. 438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren XP, Wu J, Wang X, Sartor MA, Qian J,
Jones K, Nicolaou P, Pritchard TJ and Fan GC: MicroRNA-320 is
involved in the regulation of cardiac ischemia/reperfusion injury
by targeting heat-shock protein 20. Circulation. 119:2357–2366.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herrera BM, Lockstone HE, Taylor JM, Wills
QF, Kaisaki PJ, Barrett A, Camps C, Fernandez C, Ragoussis J,
Gauguier D, et al: MicroRNA-125a is over-expressed in insulin
target tissues in a spontaneous rat model of Type 2 diabetes. BMC
Med Genomics. 2:542009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan H, Li X, Pan Z, Zhang L, Cai B, Zhang
Y, Xu C, Chu W, Qiao G, Li B, et al: Tanshinone IIA protects
against sudden cardiac death induced by lethal arrhythmias via
repression of microRNA-1. Br J Pharmacol. 158:1227–1235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong JQ, Zhang W, Gao H, Li Y, Zhong M,
Li D, Zhang C and Zhang Y: Changes in connexin 43,
metalloproteinase and tissue inhibitor of metalloproteinase during
tachycardia-induced cardiomyopathy in dogs. Eur J Heart Fail.
9:23–29. 2007. View Article : Google Scholar
|
|
29
|
Li R, Yan G, Li Q, Sun H, Hu Y, Sun J and
Xu B: MicroRNA-145 protects cardiomyocytes against hydrogen
peroxide (H2O2)-induced apoptosis through
targeting the mitochondria apoptotic pathway. PLoS One.
7:e449072012. View Article : Google Scholar
|
|
30
|
Wang X, Ha T, Liu L, Zou J, Zhang X,
Kalbfleisch J, Gao X, Williams D and Li C: Increased expression of
microRNA-146a decreases myocardial ischaemia/reperfusion injury.
Cardiovasc Res. 97:432–442. 2013. View Article : Google Scholar :
|
|
31
|
Liu H, Guo X, Chu Y and Lu S: Heart
protective effects and mechanism of quercetin preconditioning on
anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene.
545:149–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren-an Q, Juan L, Chuyuan L, Wenjuan F,
Chunyan H, Xuemei Y, Lin H and Hong N: Study of the protective
mechanisms of compound Danshen tablet (Fufang Danshen Pian) against
myocardial ischemia/reperfusion injury via the Akt-eNOS signaling
pathway in rats. J Ethnopharmacol. 156:190–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fulton D, Gratton JP, McCabe TJ, Fontana
J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A and Sessa WC:
Regulation of endothelium-derived nitric oxide production by the
protein kinase Akt. Nature. 399:597–601. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Limaye V, Li X, Hahn C, Xia P, Berndt MC,
Vadas MA and Gamble JR: Sphingosine kinase-1 enhances endothelial
cell survival through a PECAM-1-dependent activation of PI-3K/Akt
and regulation of Bcl-2 family members. Blood. 105:3169–3177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thomas RL, Roberts DJ, Kubli DA, Lee Y,
Quinsay MN, Owens JB, Fischer KM, Sussman MA, Miyamoto S and
Gustafsson ÅB: Loss of MCL-1 leads to impaired autophagy and rapid
development of heart failure. Genes Dev. 27:1365–1377. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Bathina M, Lynch J, Koss B,
Calabrese C, Frase S, Schuetz JD, Rehg JE and Opferman JT: Deletion
of MCL-1 causes lethal cardiac failure and mitochondrial
dysfunction. Genes Dev. 27:1351–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|