Introduction
Beclin l (the mammalian counterpart of the yeast
Atg6 gene) is an essential player in autophagy. Allelic loss or
deficiency of the Beclin 1 gene has been demonstrated in human
breast cancer, ovarian cancer and prostate cancer; in lung cancer,
hepatocellular carcinoma, cervical cancer and lymphoma, the
expression of Beclin 1 is very low/almost undetectable (1–4). A
previous study identified that Beclin 1−/− mice died
early in embryonic development (5). Although Beclin 1+/− mice
were able to survive, the incidence of cancer was much higher in
these animals. In addition, the measured in vivo cell
autophagy activity was markedly decreased, and cells reproduced
faster in Beclin 1-deficient animals. These findings clearly
suggested that there is a close correlation between the inhibition
of autophagy activity and the occurrence of cancer.
Although Beclin 1 has been demonstrated to exert an
important role in cell autophagy during carcinogenesis, its
biological function in lung cancer has yet to be fully elucidated.
A previous study by our laboratory identified that knockdown of
Beclin 1 promoted cell growth and inhibited apoptosis in the A549
lung cancer cell line (6). In the
present study, a Beclin 1 lentiviral expression vector was
constructed, and an A549 cell line was established with a steady
expression of Beclin 1. The effects of Beclin 1 overexpression on
cell invasion and apoptosis, changes in the activities of the
apoptosis-associated caspases-3 and -9, and the expression of
esophageal cancer-related gene 4 (ECRG4) were examined.
Materials and methods
A549 cell culture
Human lung adenocarcinoma cell line A549 was
obtained from the Chinese Center for Type Culture Collection
(Wuhan, China). An A549 cell suspension was added into a centrifuge
tube containing 5 ml RPMI-1640 culture medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and centrifuged at 1,200
× g for 5 min. The supernatant was subsequently discarded, and
fresh RPMI-1640 medium was added. Following the second
centrifugation, cells were resuspended in 10 ml RPMI-1640 culture
medium containing 10% fetal bovine serum (FBS) and cultured
overnight in a 5% CO2 incubator at 37°C. A549 cells at a
density of 5×105 cells/ml were seeded into a 6-well
culture plate (2 ml per well). When cells had grown to 70–80%
confluence, 2 ml of RPMI-1640 medium containing 10% FBS and
different concentrations of G418 solution (Invitrogen; Thermo
Fisher Scientific, Inc.) were added. A G418 concentration (800
μg/ml) that could cause cell death in 6–8 days was selected
as the concentration to be used for subsequent cell selection and
screening experiments.
Infection of A549 cells
A549 cells were divided into three groups: Cells
infected with lentiviral particles packaged with the recombinant
vector, pLenex-Beclin 1; those infected with lentiviral particles
packaged with the empty vector, pLenex; and non-transfected A549
cells. Recombinant lentiviruses were generated from our previously
constructed pLenex-Beclin 1 and pLenex vectors (6). At 24 h prior to virus infection, A549
cells in each group were digested with 0.25% trypsin solution,
seeded in 6-well plates at a density of 1.5×106 cells
per well, and cultured in RPMI-1640 culture medium containing 10%
FBS. After 12 h, when the A549 cells had reached 100% confluence,
the virus suspension was added at a concentration of
1.5×107 international units (IU). Following a further
incubation for 12 h, the plates were washed three times with
phosphate-buffered saline (PBS) and cultured with complete culture
medium. The culture medium was changed every other day. Cell
morphology and growth were observed every day using inverted
microscopy. On day 3 following infection, A549 cells were cultured
with 800 μg/ml G418 (neomycin)-selective culture medium to
select cells that were able to tolerate G418-selective medium and
stably grow in order to establish a stable cell line.
Detection of Beclin 1 and ECRG4
expression by western blot analysis
Non-infected A549 cells and two groups of infected
cells were added into tubes containing RLT buffer. After the cells
had been harvested and lysed, the protein concentration was
determined using a bicinchoninic acid protein assay kit. Samples
were dissolved in loading buffer, boiled for 5 min, and
subsequently loaded onto 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) gels. Protein bands were
subsequently transferred onto a polyvinylidene difluoride membrane.
The membrane was blocked overnight at 4°C in Tris-buffered
saline-Tween 20 (TBST) containing 5% defatted milk, incubated with
a rat anti-human Beclin 1 or ECRG4 primary antibody (dilution,
1:150; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 2
h, washed three times and incubated with horseradish
peroxidase-conjugated rabbit anti-rat secondary antibody (dilution,
1:2,000) for 1 h at room temperature. The bands were visualized
using 3,3′-diaminobenzidine (Pierce Biotechnology, Appleton, WI,
USA).
Detection of cell apoptosis by flow
cytometry
Infected and non-infected cells were cultured for 48
h. Following trypsin digestion, cells were adjusted to a density of
5×105-1×106 cells/ml and fixed with 75% cold
ethanol overnight. Following washing, cells were fully centrifuged
at 1,200 g, mixed with 100 μl RNA enzyme (1 mg/ml; Promega,
Inc., Madison, WI, USA) and incubated in a water bath at 37°C for
30 min. An aliquot of 10 μg/ml propidium iodide dye solution
was subsequently added. Following an incubation for 30 min in the
dark, PBS was added to dilute the cells. The cell suspension was
filtered through a 300-mesh nylon sieve, and the rate of cell
apoptosis was determined using a flow cytometer.
Detection of active caspase-3 and
caspase-9
A total of ~5×105 cells in each group
were centrifuged at 1,200 rpm for 5 min. The harvested cells were
fixed in an ice-cold cell lysate buffer for 10 min at a density of
2×105 cells/50 μl. The microcentrifuge tubes were
subsequently centrifuged at 15,000 rpm for 5 min at 4°C. The
supernatants were then transferred to another set of ice-cold
microcentrifuges. Samples (50 μl) were pipetted into a
96-well microplate, mixed, and sealed with paraffin wax. Following
a 2 h incubation in the dark, the fluorescence was determined using
a plate reader with an excitation wavelength of 380 nm and an
emission wavelength of 460 nm.
Matrigel invasion assay
The Boyden chamber assay was performed. Matrigel
previously stored at −20°C was kept on ice overnight. With
pre-cooled tips, 100 μl Matrigel was pipetted into 300
μl ice pre-cooled serum-free medium and mixed thoroughly.
After the polycarbonate ester membrane with a pore size of 8
μm was placed onto the Transwell plate between the upper and
lower chambers of the Boyden chamber, the diluted Matrigel was
added in the chamber to cover the entire PCS ester membrane and
placed at 37°C for 30 min to make the Matrigel coagulate. The
digested cells from each group were rinsed three times with PBS to
prepare single cell suspensions (at a density of 1×106
cells/ml) with RPMI-1640 serum-free medium. Each group of cells was
divided into two parts, one for the Trypan blue exclusion
experiment and the other for invasion assay. The Trypan blue
exclusion experiment demonstrated that cell viability was >90%.
Samples (200 μl) of chemokines from the medium were added
into the lower chamber of the Transwell culture plate, and 400
μl of cell suspension from each group was added into the
upper chamber of the Transwell culture plate. Following culture at
37°C in 5% CO2 for 24 h, the cells were gently removed
from the surface of the Matrigel and the polycarbonate ester
membrane with wet cotton swabs. The upper chamber was subsequently
removed, marked, fixed with ice pre-cooled ethanol for 30 min,
stained with hematoxylin for 1 min, dehydrated in graded methanol
(80%, 95%, 100%), and cleared with xylene. The PCS ester base
membrane was carefully cut away from the base of the upper chamber,
placed on glass slides and mounted with neutral resin. The number
of cells attached to the lower surface of the PCS ester membrane
was counted in six randomly chosen high-power (magnification, ×400)
fields to calculate the average number. The experiments were
performed in triplicate, and repeated twice.
Statistical analysis
Statistical analyses were performed using SPSS v.16
software (SPSS, Inc., Chicago, IL, USA). Numerical data were
expressed as the mean ± standard deviation. Analysis of variance
was used to compare means of multiple groups, and the t-test was
used for comparison of means between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction of the Beclin 1 lentivirus
expression vector and steady infection
The Beclin 1 lentivirus expression vector was
initially constructed, and the A549 cell line with Beclin 1
lentivirus steady infection was successfully established. After 2
weeks of G418 selection, anti-G418 resistant cells survived and
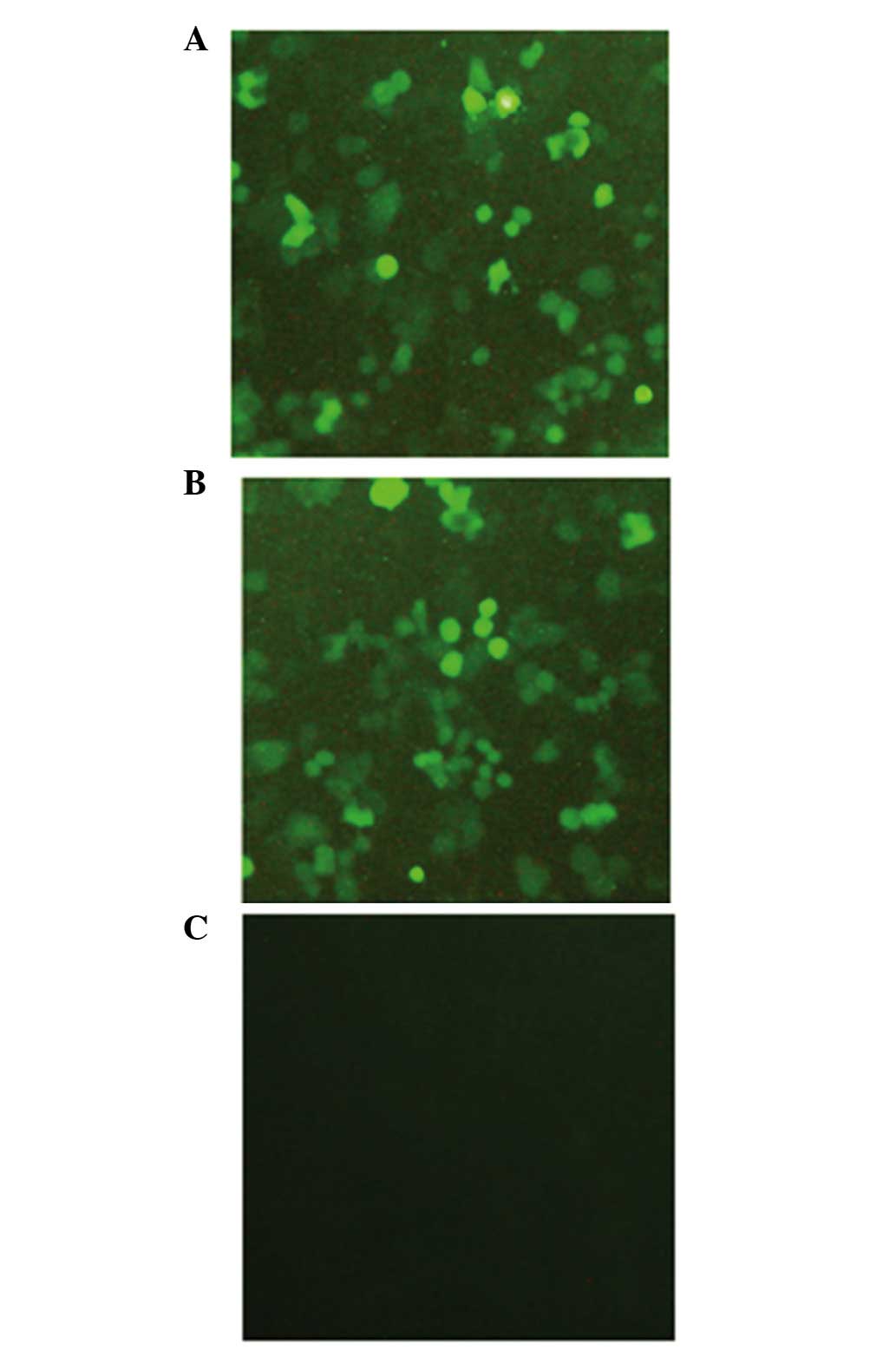
formed a stable clone. As shown in Fig
1, green fluorescence was observed under an inverted
fluorescence microscope in the two lentivirus-infected groups 48 h
following lentivirus infection, and the infection efficiencies were
>90%. Following 2 weeks of selection with G418, all cells
exhibited green fluorescence, indicating that the lentiviral
vector-mediated expression of Beclin 1 in the A549 cells had been
successful and efficient. In contrast, no green fluorescence was
observed in non-transfected A549 cells. In order to further
determine whether the recombinant lentiviral vector had been
successfully transduced into A549 cells and the Beclin 1 gene was
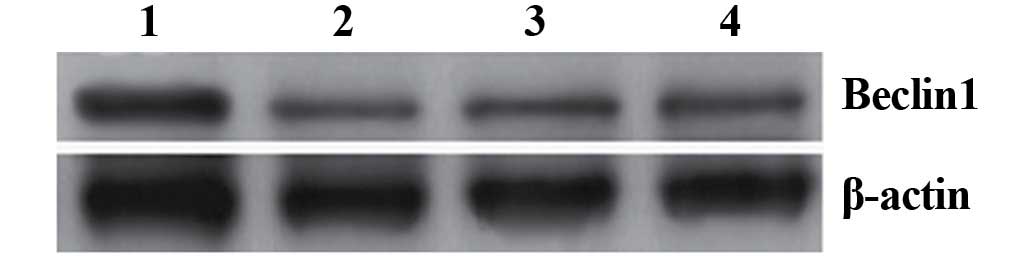
translated into the corresponding protein, western blot analysis
was performed. The results of the western blotting revealed that an
~60 kDa band was observed in all three groups of cells, and the
density of this band was higher in cells infected with
pLenex-Beclin 1-packaged lentiviral particles compared with those
infected with pLenex-packaged lentiviral particles or
non-transfected A549 cells, indicating that the expression of
Beclin 1 protein in cells infected with pLenex-Beclin 1-packaged
lentiviral particles was highly efficient (Fig. 2). This finding suggested that
infection of A549 cells with a lentiviral vector carrying the
Beclin 1 gene could induce the overexpression of Beclin 1, and that
these infected A549 cells were able to be used as model cells for
subsequent functional experiments.
Beclin 1 overexpression reduces the
invasion of A549 cells
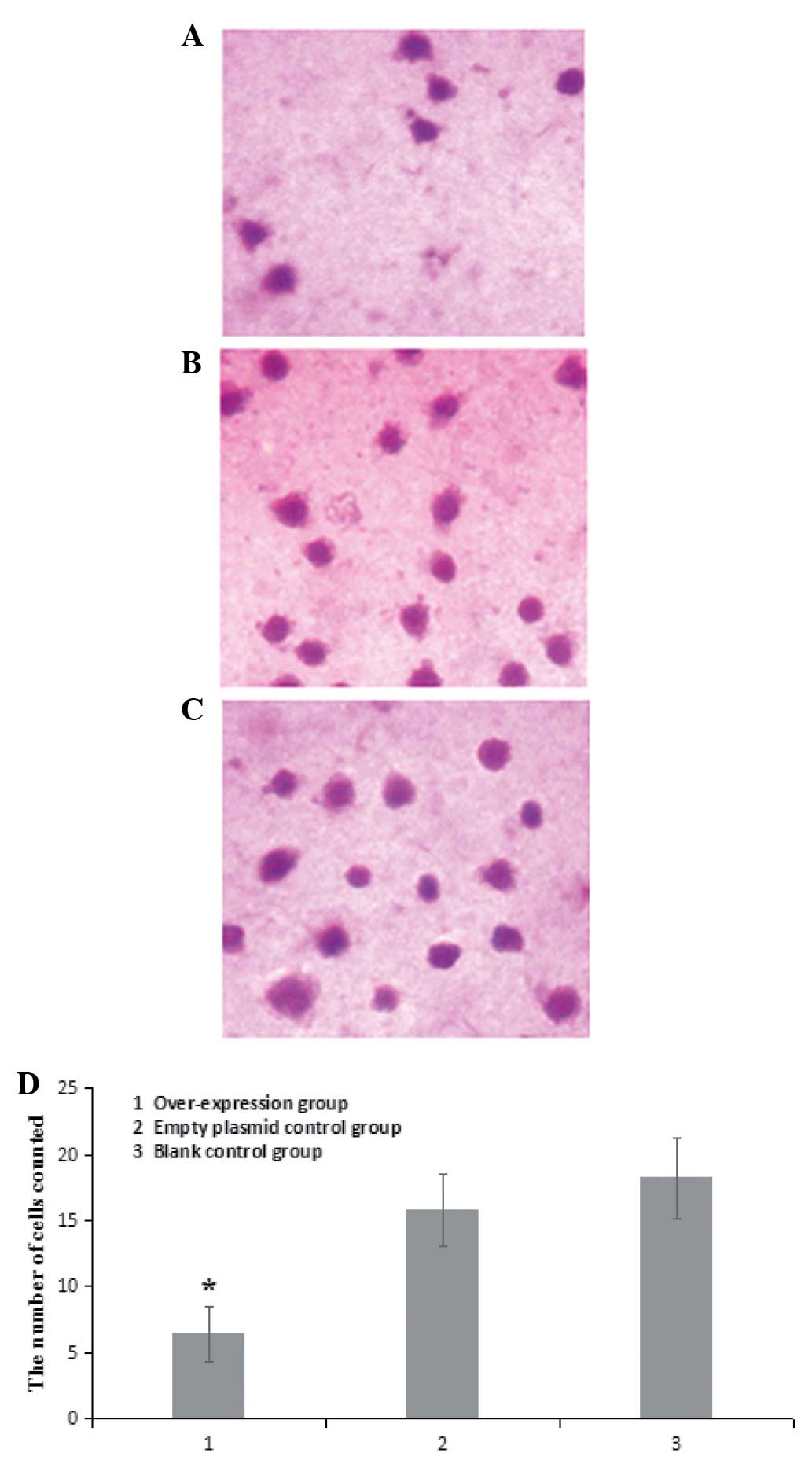
No significant differences were identified in terms
of the average number of cells passing through the Transwell
membrane between non-transfected A549 cells and A549 cells infected
with pLenex-packaged lentiviral particles (15.8±2.7 vs. 18.2±3.1;
P>0.05). However, the average number of cells passing through
the Transwell membrane was significantly lower in A549 cells
infected with pLenex-Beclin 1-packaged lentiviral particles
(6.4±2.1) compared with non-transfected A549 cells and A549 cells
infected with pLenex-packaged lentiviral particles (P<0.05)
(Fig. 3), suggesting that the
overexpression of Beclin 1 reduced the invasive ability of the A549
cells.
Beclin 1 overexpression promotes
apoptosis of A549 cells
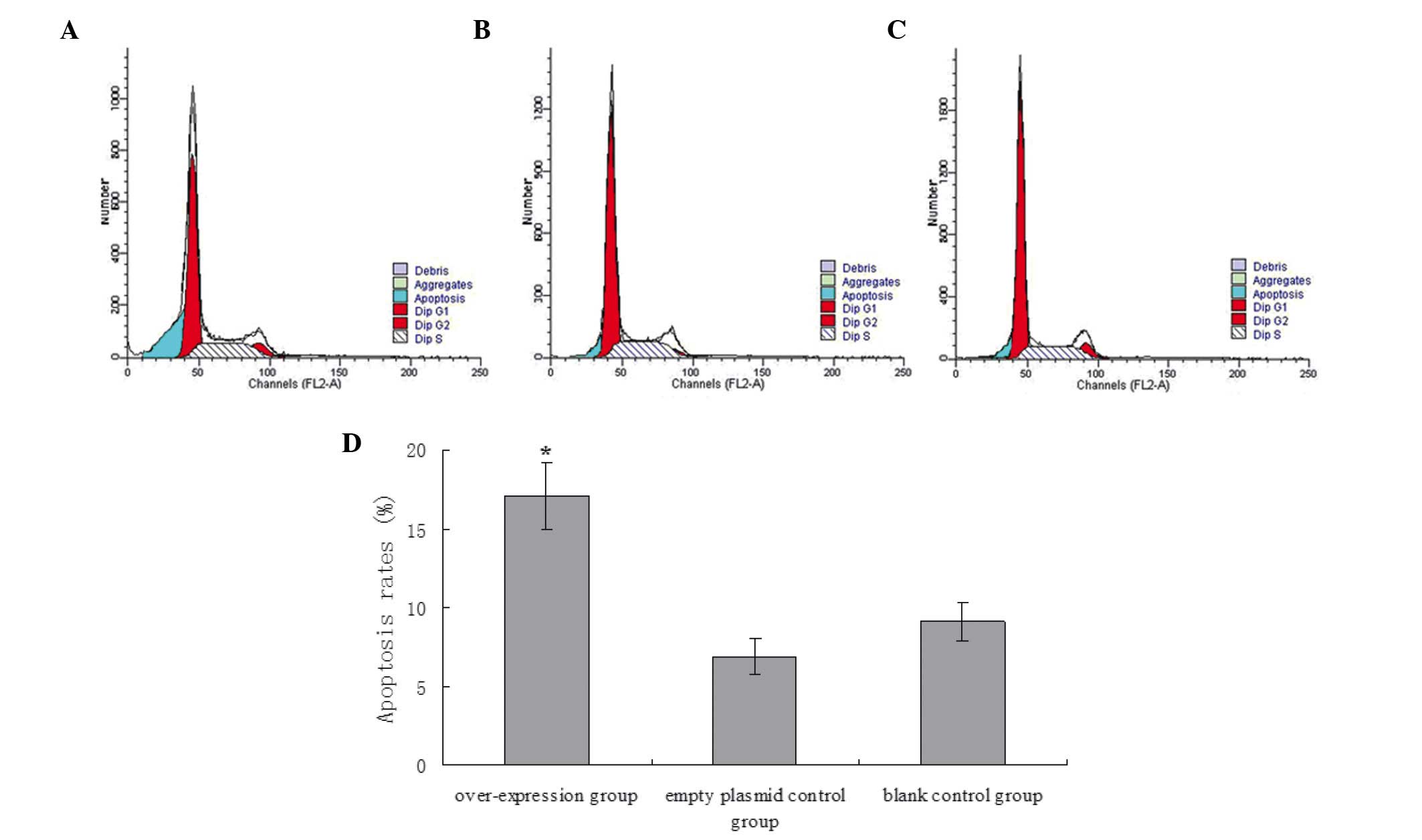
As determined from the flow cytometric analysis, no
statistical differences were identified in the apoptotic rate
between non-transfected A549 cells and A549 cells infected with
pLenex-packaged lentiviral particles (9.12±1.21% vs. 6.87±1.17%;
P>0.05). However, the apoptotic rate was significantly higher in
A549 cells infected with pLenex-Beclin 1-packaged lentiviral
particles (17.12±2.12%) compared with non-transfected A549 cells
and A549 cells infected with pLenex-packaged lentiviral particles
(P<0.05), indicating that the overexpression of Beclin 1
promoted apoptosis of the A549 cells (Fig. 4).
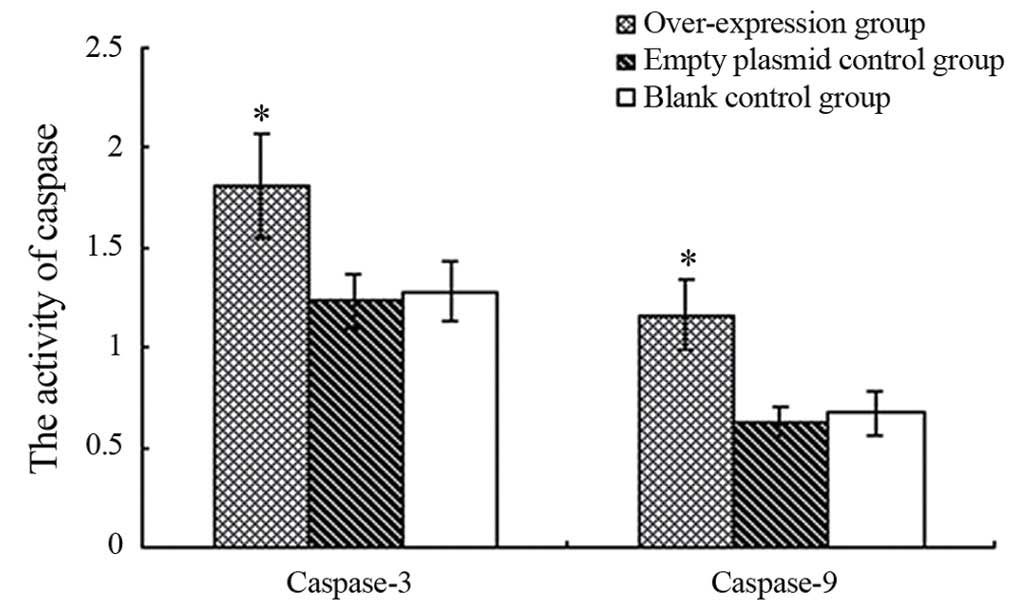
Beclin 1 overexpression increases the
activity of caspase-3 and caspase-9 in A549 cells
No significant differences were identified in the
activities of caspases-3 and caspase-9 between non-transfected A549
cells and A549 cells infected with pLenex-packaged lentiviral
particles (1.28±0.15 vs. 1.23±0.14, and 0.67±0.11 vs. 0.63±0.08,
respectively; P>0.05 for both). However, the activities of
caspase-3 and caspase-9 were significantly higher in A549 cells
infected with pLenex-Beclin 1-packaged lentiviral particles
(1.81±0.26 vs. 1.16±0.18) compared with the non-transfected A549
cells and A549 cells infected with pLenex-packaged lentiviral
particles (P<0.05 for both) (Fig.
5), suggesting that the overexpression of Beclin 1 increases
the activities of caspase-3 and caspase-9.
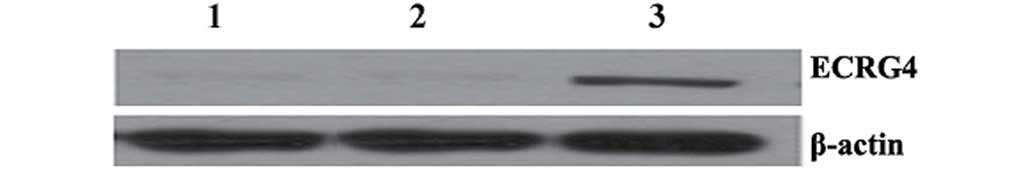
Beclin 1 overexpression increases ECRG4
expression in A549 cells
The results of the western blot analysis showed that
no significant differences were identified in the expression of
ECRG4 between non-transfected A549 cells and A549 cells infected
with pLenex-packaged lentiviral particles. However, the expression
of ECRG4 was significantly higher in A549 cells infected with
pLenex-Beclin 1-packaged lentiviral particles compared with
non-transfected A549 cells and A549 cells infected with
pLenex-packaged lentiviral particles (P<0.05 for both) (Fig. 6), suggesting that the
overexpression of Beclin 1 increases the expression of ECRG4 in
A549 cells.
Discussion
Autophagy is a form of programmed cell death that
has an important role in biological growth and development,
self-renewal, as well as disease development and tumor formation
(7). A previous study has
indicated that abnormal regulation of autophagy is directly
associated with tumor formation (8). It is well known that Beclin 1 is a
key regulator of autophagy. In the present study, non-small cell
lung cancer A549 cells were successfully infected with a previously
constructed recombinant lentiviral vector carrying the Beclin 1
gene. The overexpression of the autophagy-associated gene, Beclin
1, was revealed to markedly decrease invasion, promote apoptosis,
and increase the activities of caspases-3 and caspase-9 and the
expression of ECRG4 in A549 cells.
Invasion and metastasis are the most significant
characteristics of cancer cells. The Matrigel invasion assay is a
commonly used in vitro method for studying tumor cell
invasion. In the present study, the average number of cells passing
through the Transwell membrane was significantly lower in A549
cells infected with pLenex-Beclin 1-packaged lentiviral particles
compared with the two control groups (P<0.01 for both). These
results indicated that the overexpression of Beclin 1 reduced the
invasive ability of the A549 cells. Therefore, Beclin 1 may have a
putative tumor-suppressing function in lung cancer. In the present
study, the overexpression of Beclin 1 was also shown to promote the
apoptosis of A549 cells. This result demonstrated that the
apoptotic rate of Beclin 1-overexpressing cells was significantly
higher compared with those of control cells (P<0.01 for both).
Previous studies indicated that, although autophagy is
morphologically different from apoptosis, it may occur prior to
and/or co-exist with apoptosis, and may be stimulated or induced by
apoptosis, depending on the responses of cells to intracellular or
extracellular stimuli (9,10). Inhibition of cell apoptosis and
autophagy may lead to the escape of tumor cells from normal
clearance mechanisms of the body, resulting in the formation of
malignant tumors.
Although there are significant differences between
autophagy and apoptosis in biochemical pathways, their functions
may be linked. Autophagy does not depend on the involvement of
caspases, and its most notable feature is the presence of
autophagosomes, which are eventually removed via the lysosomal
system (11). Caspases are a group
of apoptotic cysteine proteases, the majority of which exist as
non-active caspase, zymogen forms. The caspase family members are
intracellular proteins that are essential for the implementation of
apoptosis. Their substrates include the caspases themselves, and
several other factors. Through the hydrolysis of substrates,
caspases transduce the apoptotic signal, or they themselves
directly act as the effectors for apoptosis, promoting cytoskeletal
degradation and DNA fragmentation (12). In the caspase family of proteases,
caspases-3 and -9 are key molecules in the intrinsic apoptotic
pathway. Caspase-9 is able to act through the nuclear lamina to
cause cell structural damage and apoptosis (13,14).
Furthermore, ECRG4 is a candidate tumor suppressor in a variety of
tumor types (15–18). It has been previously shown that
ECRG4 is involved in apoptosis and cell autophagy (19,20).
In the present study, we identified that the overexpression of
Beclin 1 increased the activities of caspase-3 and caspase-9 and
the expression of ECRG4 in A549 lung cancer cells. These findings
will provide novel insights into the role of Beclin 1 in the
regulation of autophagy and apoptosis, and the interaction between
autophagy and apoptosis in lung adenocarcinoma. However, the
detailed molecular mechanisms have yet to be fully elucidated.
In conclusion, the overexpression of Beclin 1
promoted apoptosis and decreased invasion by upregulating the
expression of ECRG4 in A549 lung adenocarcinoma cells. Therefore,
the selection of Beclin 1 as a target for gene therapy represents a
more effective method for the treatment of lung cancer.
Acknowledgments
This study was supported by the Chinese National
Natural Science Foundation (no. U1304817) and the Zhengzhou City
Science Research Project (no. 141PPTGHG298)
References
|
1
|
Shen Y, Liang LZ, Hong MH, Xiong Y, Wei M
and Zhu XF: Expression and clinical significance of
microtubule-associated protein 1 light chain 3 (LC3) and Beclin 1
in epithelial ovarian cancer. Ai Zheng. 27:595–599. 2008.In
Chinese. PubMed/NCBI
|
|
2
|
Liang XH, Kleeman LK, Jiang HH, Gordon G,
Goldman JE, Berry G, Herman B and Levine B: Protection against
fatal Sindbis virus encephalitis by Beclin, a novel
Bcl-2-interacting protein. J Virol. 72:8586–8596. 1998.PubMed/NCBI
|
|
3
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embronic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15282. 2003. View Article : Google Scholar
|
|
6
|
Wang W, Fan H, Zhou Y, Duan P, Zhao G and
Wu G: Knockdown of autophagy-related gene BECLIN1 promotes cell
growth and inhibits apoptosis in the A549 human lung cancer cell
line. Mol Med Rep. 7:1501–1505. 2013.PubMed/NCBI
|
|
7
|
Lockshin RA and Zakeri Z:
Caspase-independent cell deaths. Curr Opin Cell Biol. 14:727–733.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia L, Dourmashkin LR, Allen PD, Gray AB,
Newland AC and Kelsey SM: Inhibition of autophagy abrogates tumour
necrosis factor alpha induced apoptosis in human T-lymphoblastic
leukaemic cells. Br J Haematol. 98:673–685. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
González-Polo RA, Boya P, Pauleau AL,
Jalil A, Larochette N, Souquère S, Eskelinen EL, Pierron G, Saftig
P and Kroemer G: The apoptosis/autophagy paradox: Autophagic
vacuolization before apoptotic death. J Cell Sci. 118:3091–3102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tóth S, Nagy K, Pálfia Z and Réz G:
Cellular autophagic capacity changes during azaserine-induced
tumour progression in the rat pancreas. Up-regulation in all
premalignant stages and down-regulation with loss of cycloheximide
sensitivity of segregation along with malignant transformation.
Cell Tissue Res. 309:409–416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson CR and Jarvis WD: Caspase-9
regulation: An update. Apoptosis. 9:423–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar
|
|
15
|
Li LW, Yu XY, Yang Y, Zhang CP, Guo LP and
Lu SH: Expression of esophageal cancer related gene 4 (ECRG4), a
novel tumor suppressor gene, in esophageal cancer and its
inhibitory effect on the tumor growth in vitro and in vivo. Int J
Cancer. 125:1505–1513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Zhang C, Li X, Lu S and Zhou Y: The
candidate tumor suppressor gene ECRG4 inhibits cancer cells
migration and invasion in esophageal carcinoma. J Exp Clin Cancer
Res. 29:1332010. View Article : Google Scholar : PubMed/NCBI
Retraction. J Exp Clin Cancer Res.
30:192011. View Article : Google Scholar
|
|
17
|
Li LW, Li YY, Li XY, Zhang CP, Zhou Y and
Lu SH: A novel tumor suppressor gene ECRG4 interacts directly with
TMPRSS11A (ECRG1) to inhibit cancer cell growth in esophageal
carcinoma. BMC Cancer. 11:522011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baird A, Lee J, Podvin S, Kurabi A, Dang
X, Coimbra R, Costantini T, Bansal V and Eliceiri BP: Esophageal
cancer-related gene 4 at the interface of injury, inflammation,
infection, and malignancy. Gastrointest Cancer. 2014:131–142. 2014.
View Article : Google Scholar
|
|
19
|
You Y, Yang W, Qin X, Wang F, Li H, Lin C,
Li W, Gu C, Zhang Y and Ran Y: ECRG4 acts as a tumor suppressor and
as a determinant of chemotherapy resistance in human nasopharyngeal
carcinoma. Cell Oncol (Dordr). 38:205–214. 2015. View Article : Google Scholar
|
|
20
|
Matsuzaki J, Torigoe T, Hirohashi Y,
Kamiguchi K, Tamura Y, Tsukahara T, Kubo T, Takahashi A, Nakazawa
E, Saka E, et al: ECRG4 is a negative regulator of
caspase-8-mediated apoptosis in human T-leukemia cells.
Carcinogenesis. 33:996–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|