Introduction
Alzheimer's disease (AD) is a chronic
neurodegenerative disease, which accounts for the majority of
dementia cases (1). Common
symptoms of AD include short-term memory loss, disorientation, loss
of motivation, mood swings and behavioral issues (1,2).
Patients with AD often withdraw from family and society as their
condition declines (3,4). The cause of AD is not fully
understood (1), and researchers
have hypothesized that ~70% of the risk is due to genetic factors
(4), followed by other factors,
including head injuries, depression or hypertension (4–7).
Patients with AD rely on caregivers for assistance, and in certain
cases they may pose a burden to them (6). In developed countries, AD is
considered to be one of the most financially challenging diseases
(8–11). The underlying mechanisms of AD are
not fully known and the majority of available drug therapies are
based on the cholinergic hypothesis (12). The cholinergic hypothesis proposes
that AD results from reduced synthesis of the neurotransmitter
acetylcholine (ACh) (12).
Houghton et al (13)
indicated that inhibiting the enzyme acetylcholinesterase (AChE),
which breaks down ACh, is a promising strategy for treating
patients with AD. There are alternative hypotheses, such as the
amyloid (14) and tau (15) hypotheses; however, AChE is a
favorable enzyme target for numerous researchers. Furthermore, AChE
has garnered more attention regarding its screening potential, and
the identification of AChE novel inhibitors from various natural
products, including plants and herbal extracts.
The enzyme AChE is a hydrolase of the
carboxylesterase family, which hydrolyzes the neurotransmitter ACh
(16,17). It is primarily present in the
neuromuscular junctions and cholinergic brain synapses, assisting
in the termination of synaptic transmissions. It belongs to the
carboxylesterase family of enzymes (18,19).
During neurotransmission, ACh is released from the nerve into the
synaptic cleft where it binds to ACh receptors on the post-synaptic
membrane, relaying the signal from the nerve (20). AChE is located on the post-synaptic
membrane and terminates the signal transmission by hydrolyzing Ach
(21). The liberated choline is
then taken up by the pre-synaptic nerve and ACh is synthesized by
combining with acetyl-CoA through the action of choline
acetyltransferase (22).
The present study performed an inhibitory assay to
determine the inhibitory activity of certain plant extracts against
human AChE. In addition, computational research employing virtual
screening of the compounds present in the plant extracts and AChE
retrieved from the Protein Data Bank (PDB ID: 4PQE) was conducted,
using the molecular docking simulation protocol. Subsequently, a
protein-ligand interaction analysis was performed with 20 ns
molecular dynamics (MD) simulation.
Materials and methods
AChE inhibitory assay
The following plant samples with medicinal
properties were collected: Allium sativum, Areca catechu, Camellia
sinensis, Curcuma longa, Lobelia chinensis, Nelumbo nucifera,
Portulaca oleracea, Uncaria rhynchophylla and Zingiber officinale.
The majority of the collected samples are commonly available in
vegetable and herbal medicine markets. Approximately 120 g of each
of the plant samples was crushed to a powder using a pestle and
mortar. Each of the samples was then treated with 95% ethanol and
was refluxed for 2 h to collect the alcohol extract. The extract
was further evaporated, air-dried and suspended in deionized water.
The pH of the suspension was adjusted to 2.0. The aqueous solution
from the alkaloidal extracts, which is acidic in nature, was
filtered following an overnight incubation, the pH was adjusted to
10.0 and the extract was obtained using chloroform. The layers of
chloroform were evaporated and air dried. The final alkaloidal
extracts obtained were used to conduct the AChE inhibitory assay,
which was purchased from Sigma-Aldrich (St. Louis, MO, USA). The
AChE inhibitory assay was performed according to the protocol
developed by Ellman et al (23)
with slight modifications. Briefly, a mixture containing 100 µl
0.1M NaH2PO4 (pH 8.0), 25 µl plant extract
solution (1 mg/ml) and 20 µl AChE enzyme (0.20 U/ml) was incubated
for 30 min at 4°C. Subsequently, ~15 µl 0.01 M
5,5′-dithiobis(2-nitrobenzoic acid) mixed with 10 µl 0.05 M
acetylthiocholine iodide was incubated with the mixture at 37°C for
30 min. The optical density of the final solution was measured at
405 nm (ELC800 Absorbance Microplate Reader; BioTek Instruments,
Inc., Winooski, VT, USA), and enzyme activity was calculated by
comparing the reaction rate of the samples relative to a blank
solution. The percentage of inhibitory activity was calculated by
subtracting the enzyme activity percentage from 100%.
Molecular docking
The molecular docking simulation was performed using
the Molegro Virtual Docker 5.0 (CLC Bio, Aarhus, Denmark). Briefly,
the 2D structures of the compounds present in the plant samples
were retrieved from the National Center for Biotechnology
Information (NCBI) PubChem database (https://pubchem.ncbi.nlm.nih.gov). Their geometries
were optimized using the MM2 force field and were converted to a 3D
format (sybyl mol2 file format). AChE retrieved from the PDB (ID:
4PQE) was then loaded into the Molegro Virtual Docker. The bind
site was set having a sphere of 15 Å radius and the following
coordinates: X, −27.90; Y, 22.91; Z, −10.18.
For the molecular docking simulation, the bonds of
all the compounds were set flexible, and for the protein the
residues in the binding site were set flexible with a tolerance of
1.0 and strength of 0.80. The side chain and torsional degrees of
freedom for the flexible residues and ligands were subjected to
2,000 steps of energy minimization. Flexible molecular docking
simulations were performed setting the MolDock Grid scoring
function (24) with a grid
resolution of 0.30 Å. The search algorithm was set for MolDock SE
with 20 runs for each compound, with a maximum of 1,500 iterations
and population size of 50. The in-depth molecular interaction was
inspected using a ligand energy inspector. The ligand energy
inspector evaluates the energy interactions for a given docked
ligand with the interacting amino acids. In the present study, the
in-depth molecular interaction was inspected using a ligand energy
inspector. The ligand energy inspector evaluates the energy
interactions for a given docked ligand with the interacting amino
acids.
MD simulation
MD simulations were performed using the GROMACS 5.0
(Royal Institute of Technology, Stockholm, Sweden; and Uppsala
University, Uppsala, Sweden) installed in Ubuntu Linux 14.0 LTS
Intel i5 processor with the standard GROMOS96 43a1 force field
(25). MD simulation was performed
for the docked protein-ligand complexes and AChE (PDB ID: 4PQE).
For the protein and the protein-ligand docked complexes, initially,
the system was immersed in a cubic water box and the energy of the
complexes was minimized using the steepest descent approach.
Following energy minimization, the systems were equilibrated for
100 ps with NVT (number of particles, volume and temperature;
canonical) and NPT (number of particles, temperature and pressure;
isothermal-isobaric) ensemble equilibration protocol for ~5,000
steps. Finally, the equilibrated systems were run for 20 ns of MD
simulation production under a constant number of particles at 310 K
and 1 bar pressure. The trajectory was analyzed and a graph was
plotted for the root mean square deviation (RMSD) backbone of AChE
and the AChE-ligand complexes.
Results
The AChE inhibitory activities of the plant extracts
are presented in Table I. Total
alkaloidal extracts from Nelumbo nucifera, Uncaria
rhynchophylla and Portulaca oleracea exhibited 76.5,
78.4 and 74.2% inhibition against AChE, respectively (Table I). The compounds present in the
herbal plants were retrieved from the NCBI PubChem database, and
are presented in Table II. The
molecular docking scores of the bioactive compounds present in the
herbal plants against AChE are shown in Table III. The scores and results were
based on the Rerank and MolDock scores, and interaction energy
(24). Rerank Score, MolDock Score
and Interaction Energy are measure based on the energy parameters E
inter (steric, van der Waals, hydrogen bonding and electrostatic)
between the ligand and the protein, and E intra (torsion, SP2,
hydrogen bonding, van der Waals and electrostatic) (24). These energy terms are generated by
the MolDock SE algorithm and based on the most stable E inter and E
intra; the compounds were ranked accordingly. The results of the
ligand-protein interaction analyses of the top docked compounds
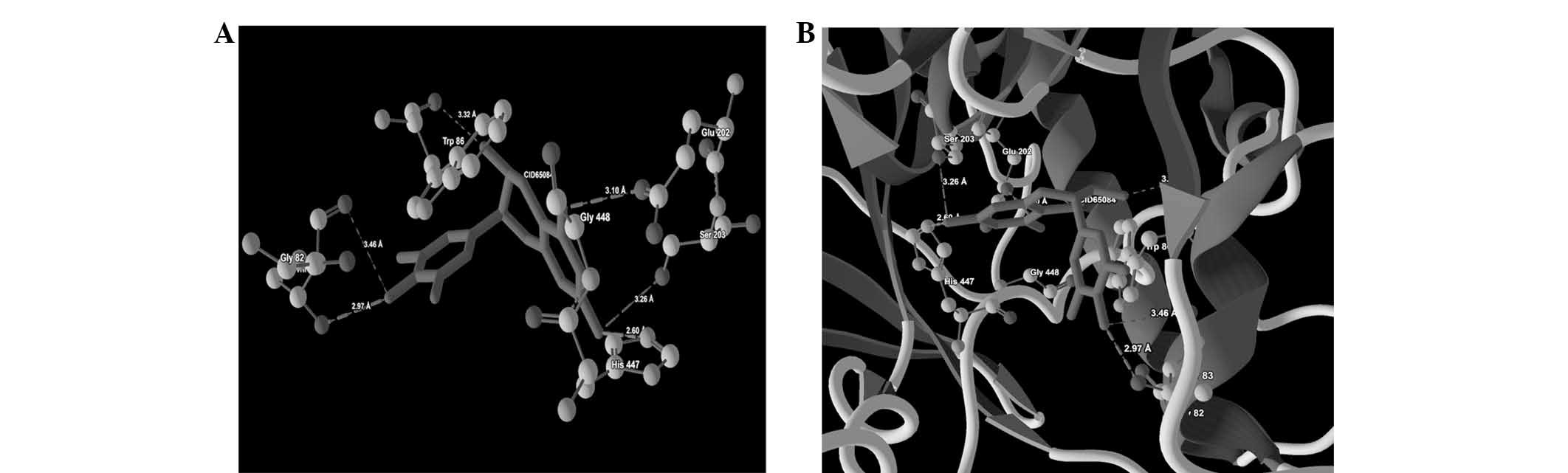
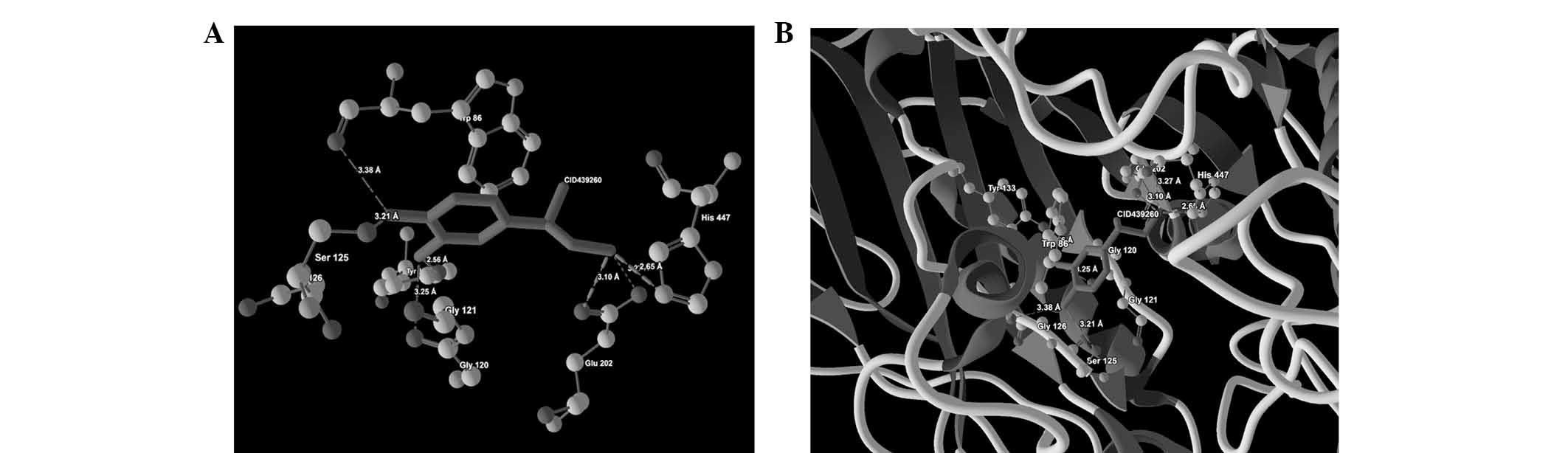
against AChE (PDB ID: 4PQE) are presented in Table IV. Images depicting the molecular
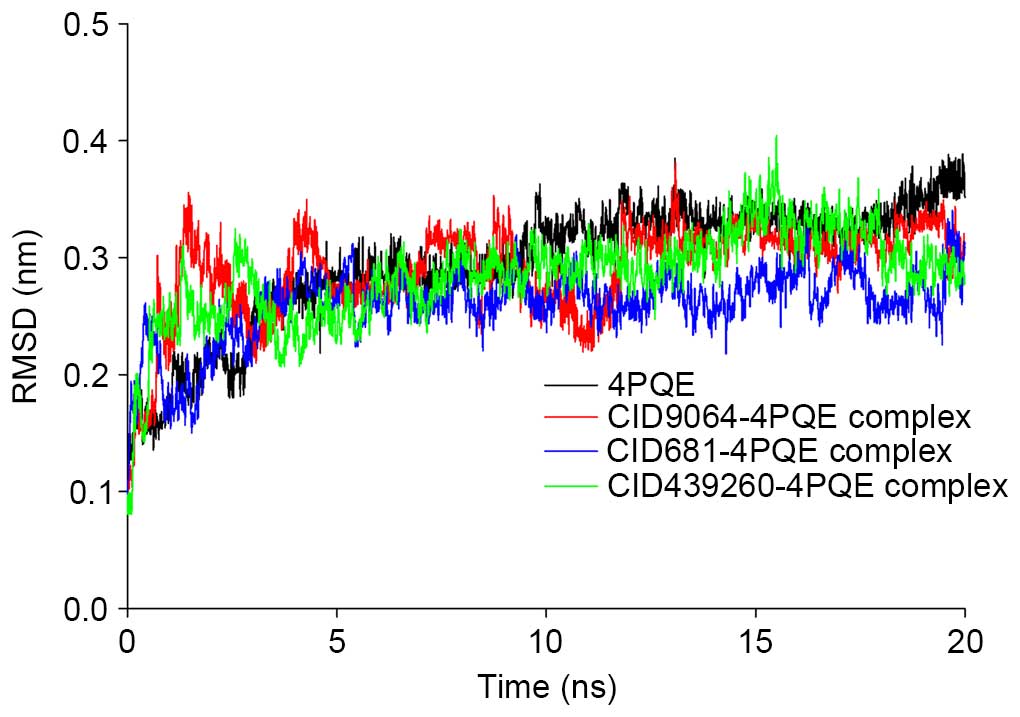
interactions for the top docking hits are presented in Figs. 1Figure 2Figure 3Figure 4Figure 5–6, and the MD simulation investigating
stability of the RMSD backbones of PDB ID: 4PQE and protein-ligand
complexes is demonstrated in Fig.
7.
 | Table IInhibitory activity of the total
alkaloidal extracts (100 µg/ml final concentration) against
AChE. |
Table I
Inhibitory activity of the total
alkaloidal extracts (100 µg/ml final concentration) against
AChE.
| SN | Scientific name | Parts used | Yield | AChE inhibition
(%) |
|---|
| 1 | Allium
sativum | Bulb | 0.44 | 33.8 |
| 2 | Areca
catechu | Fruit | 0.66 | 19.3 |
| 3 | Camellia
sinensis | Leaves | 0.51 | 40.2 |
| 4 | Curcuma
longa | Root | 0.27 | 35.8 |
| 5 | Lobelia
chinensis | Whole plant | 0.21 | 28.2 |
| 6 | Nelumbo
nucifera | Leaves | 0.34 | 76.5 |
| 7 | Portulaca
oleracea | Stem | 0.24 | 74.2 |
| 8 | Uncaria
rhynchophylla | Stem | 0.41 | 78.4 |
| 9 | Zingiber
officinale | Root | 0.43 | 60.5 |
 | Table IICompounds associated with the herbal
plants and their NCBI Pubchem ID. |
Table II
Compounds associated with the herbal
plants and their NCBI Pubchem ID.
| SN | Plant | Chemical
composition | Pubchem ID |
|---|
| 1 | Nelumbo
nucifera | Miquelianin | CID 5274585 |
| | Coclaurine | CID 160487 |
| | Higenamine | CID 114840 |
| | Nuciferine | CID 3108374 |
| 2 | Portulaca
oleracea | Norepinephrine | CID 439260 |
| | Dopamine | CID 681 |
| | L-DOPA | CID 6047 |
| 3 | Uncaria
rhynchophylla | Catechin | CID 9064 |
| |
Rhynchophylline | CID 3033948 |
| 4 | Areca
catechu | Arecaidine | CID 10355 |
| | Arecoline | CID 2230 |
| 5 | Lobelia
chinensis | Lobeline | CID 101616 |
| | Lobelanine | CID 442647 |
| | Lobelanidine | CID 96946 |
| 6 | Curcuma
longa | Curcumin | CID 969516 |
| 7 | Zingiber
officinale | Zingerone | CID 31211 |
| | Shogaol | CID 5281794 |
| | Gingerol | CID 442793 |
| 8 | Allium
sativum | Allicin | CID 65036 |
| | Ajoene | CID 5386591 |
| 9 | Camellia
sinensis | Gallocatechol | CID 65084 |
 | Table IIIMolecular docking scores of the
compounds. |
Table III
Molecular docking scores of the
compounds.
| Ligand | Source | Rerank score | MolDock score | Interaction | HBond |
|---|
| CID 9064 | Uncaria
rhynchophylla | −67.43 | −91.57 | −114.847 | −11.47 |
| CID 681 | Portulaca
oleracea | −64.76 | −84.07 | −86.940 | −12.38 |
| CID 439260 | Portulaca
oleracea | −60.40 | −81.88 | −83.460 | −12.14 |
| CID 31211 | Zingiber
officinale | −59.39 | −46.98 | −48.230 | −3.87 |
| CID 65084 | Camellia
sinensis | −53.40 | −71.24 | −78.860 | −9.88 |
| CID 6047 | Portulaca
oleracea | −50.14 | −36.84 | −32.170 | −9.71 |
| CID 65036 | Allium
sativum | −49.10 | −29.47 | −26.750 | −2.02 |
| CID 442793 | Zingiber
officinale | −48.79 | −72.86 | −73.050 | −2.16 |
| CID 114840 | Nelumbo
nucifera | −45.15 | −47.37 | −55.850 | −8.94 |
| CID 160487 | Nelumbo
nucifera | −43.02 | −51.27 | −59.170 | −5.89 |
| CID 5386591 | Allium
sativum | −40.22 | −10.84 | −6.720 | −1.65 |
| CID 5281794 | Zingiber
officinale | −39.29 | −73.70 | −67.630 | −7.49 |
 | Table IVMolecular interaction analysis of the
top hits. |
Table IV
Molecular interaction analysis of the
top hits.
| SN | Ligand | Protein-ligand | Interaction energy
(kJ/mol) | Interaction
distance (Å) |
|---|
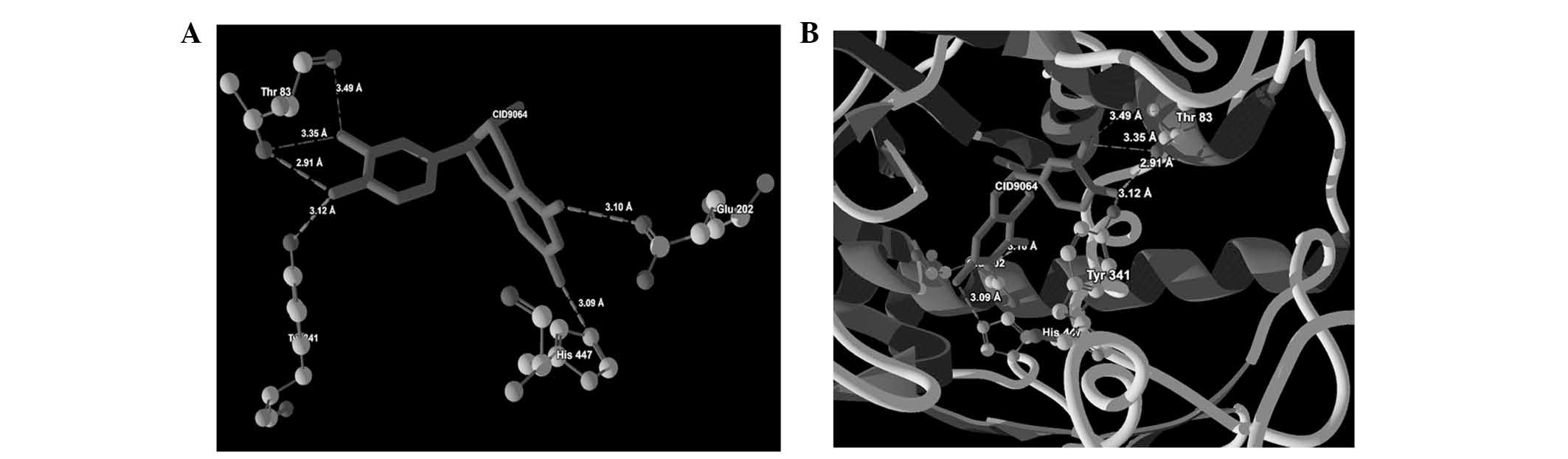
| 1 | CID9064 |
Thr83(OG1)-O(5) | −2.50 | 2.91 |
| |
Thr83(OG1)-O(4) | −1.26 | 3.35 |
| | Thr83(O)-O(4) | −0.55 | 3.49 |
| |
Thr341(OH)-O(5) | −2.40 | 3.12 |
| |
His447(NE2)-O(3) | −2.50 | 3.09 |
| |
Glu202(OE1)-O(2) | −2.50 | 3.10 |
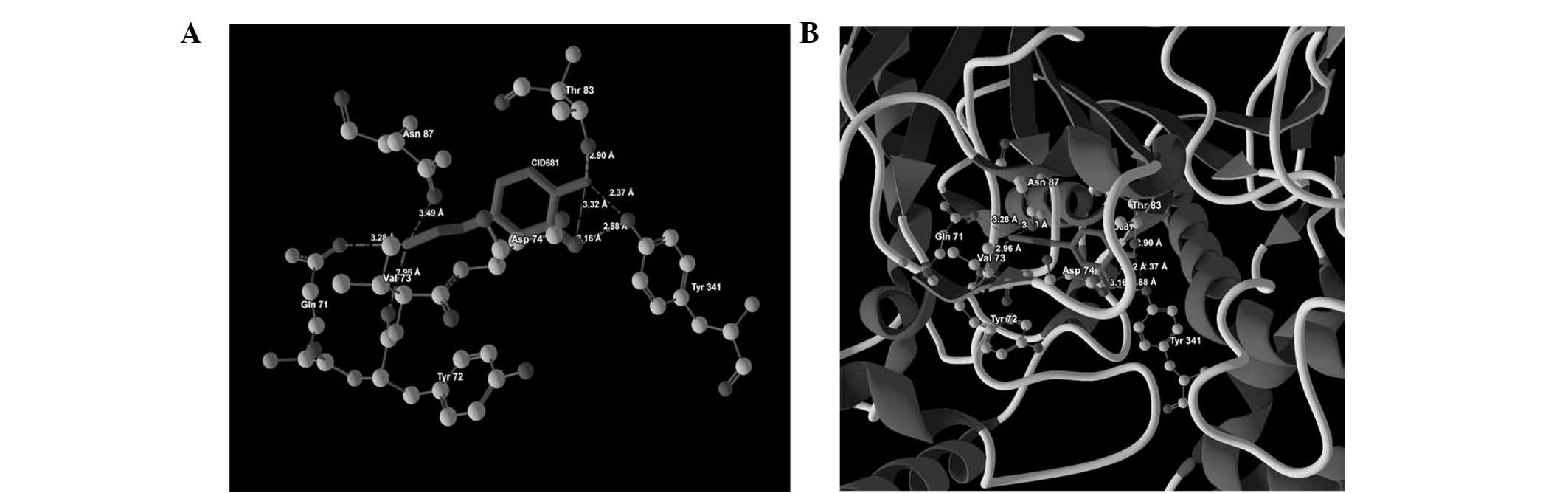
| 2 | CID681 |
Asn87(OD1)-N(2) | −0.57 | 3.49 |
| |
Gln71(OE1)-N(2) | −1.61 | 3.28 |
| | Tyr72(O)-N(2) | −2.50 | 2.96 |
| |
Tyr341(OH)-O(0) | −2.50 | 2.88 |
| |
Asp74(OD2)-O(0) | −2.20 | 3.16 |
| |
Asp74(OD2)-O(1) | −1.40 | 3.32 |
| |
Tyr341(OH)-O(1) | −0.55 | 2.37 |
| |
Thr83(OG1)-O(1) | −2.50 | 2.90 |
| 3 | CID439260 |
Ser125(OG)-O(2) | −2.00 | 3.21 |
| | Trp86(O)-O(2) | −1.10 | 3.38 |
| | Gly120(O)-O(1) | −1.75 | 3.25 |
| |
Tyr133(OH)-O(1) | −2.20 | 2.56 |
| |
His447(NE2)-N(3) | −2.50 | 2.65 |
| |
Glu202(OE2)-N(3) | −0.14 | 3.27 |
| |
Glu202(OE1)-N(3) | −2.50 | 3.10 |
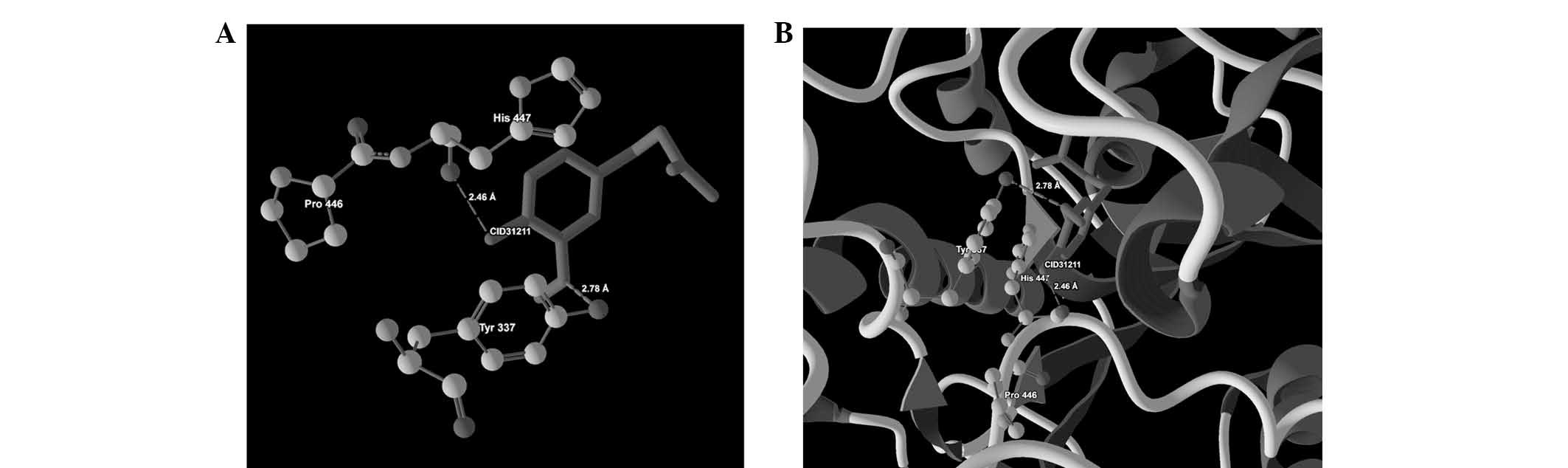
| 4 | CID31211 |
Tyr337(OH)-O(0) | −2.50 | 2.78 |
| | His447(O)-O(1) | −1.37 | 2.46 |
| 5 | CID65084 |
Thr83(OG1)-O(6) | −2.50 | 2.97 |
| | Thr83(O-O(6) | −0.36 | 3.46 |
| | Trp86(O)-O(1) | −0.24 | 3.32 |
| |
Glu202(OE1)-O(2) | −2.50 | 3.10 |
| |
Ser303(OG1)-O(3) | −1.68 | 3.26 |
| |
His447(NE2)-O(3) | −2.50 | 2.60 |
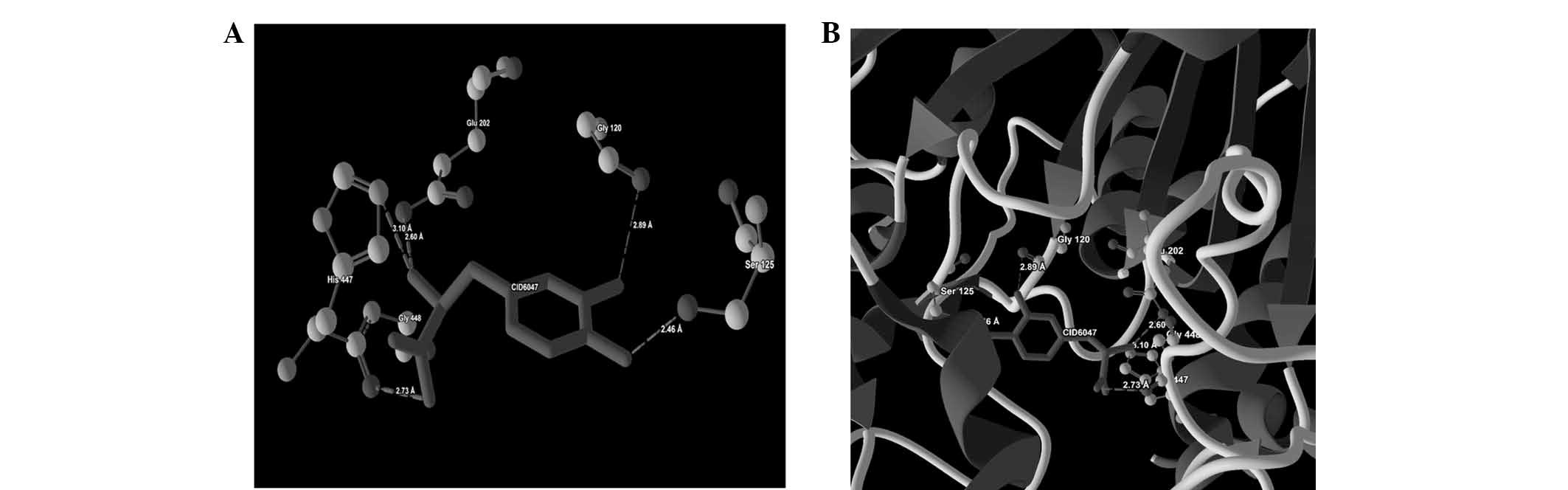
| 6 | CID6047 |
His447(NE2)-N(4) | −2.50 | 3.10 |
| |
Glu202(OE2)-N(4) | −2.50 | 2.60 |
| | His447(O)-O(1) | −2.50 | 2.73 |
| |
Ser125(OG)-O(2) | −1.31 | 2.46 |
| | Gly120(O)-O(0) | −0.90 | 2.89 |
Discussion
In the present study, AChE inhibitory assay of the
plant extracts demonstrated that the total alkaloidal extracts from
Uncaria rhynchophylla (78.4%), Nelumbo nucifera
(76.5%) and Portulaca oleracea (74.2%) exhibited AChE
inhibition (Table I). In addition,
the alkaloidal extract from Areca catechu demonstrated the
poorest AChE inhibition (19.3%; Table
I).
Molecular docking analysis of the bioactive
compounds present in the herbal plants confirmed that certain
compounds in Portulaca oleracea, Zingiber officinale
and Camellia sinensis possessed strong molecular
interactions at the potential ligand binding site of AChE. The
major interactions, including bonded and non-bonded interactions,
were formed between the docked compounds and the binding cavity of
the enzyme. The strength of the ligand-protein interaction was
measured by the Rerank score. The Rerank score is a linear
combination of E-inter (steric, Van der Waals, hydrogen bonding,
electrostatic) between the ligand and the protein, and E-intra
(torsion, sp2-sp2, hydrogen bonding, Van der Waals, electrostatic)
of the ligand weighted by pre-defined coefficients (24). The Rerank score of the docked
compounds along with their MolDock score and interaction energy are
shown in Table III. In the
present molecular docking simulation analysis, CID9064 (Uncaria
rhynchophylla) CID681 (Portulaca oleracea), and
CID439260 (Portulaca oleracea) and CID6047 (Portulaca
oleracea) docked at the binding cavity having a Rerank score of
−67.43, −64.76 and −60.40 kJmol−1, respectively.
Uncaria rhynchophylla and Portulaca oleracea
exhibited 78.4 and 74.2% AChE inhibition, respectively. CID 31211
(Zingiber officinale) demonstrated a Rerank score of −59.39
and exhibited 60.5% AChE inhibition. Finally, CID65084 (Camellia
sinensis) had a Rerank score of −53.40 and exhibited 40.2% AChE
inhibition.
To understand the in-depth molecular interaction of
these compounds with AChE, and its binding mechanism, a
ligand-protein interaction analysis for the top 6 docking hits was
conducted using a ligand energy inspector. The ligand-protein
interaction, including the residues present, their interaction
distances and energy, and the interacting atoms of the protein and
ligand are presented in Table IV.
The molecular docking simulation indicated that the top docking
poses were demonstrated to be docked into the binding cavity
displaying bonded and non-bonded interactions. Images depicting
ligand-protein binding are shown in Fig. 1 (CID9064), Fig. 2 (CID681), Fig. 3 (CID6047) and Fig. 4 (CID31211), Fig. 5 (CID65084) and Fig. 6 (CID439260). The backbone RMSD
values of the protein and protein-ligand complexes during 20 ns of
MD simulation are presented in Fig.
7, and indicated that the RMSD values for AChE-ligand complexes
were more stable suggesting a conformational flexibility and
stability in dynamic behavior. Numerous herbal compounds have been
demonstrated to have anti-cancer, anti-microbial and
anti-inflammatory potential. However, this is not in case of AChE
inhibition. In fact, there are few reports on the investigation of
herbal compounds as inhibitors of AChE inhibition (26).
In conclusion, Uncaria rhynchophylla, Nelumbo
nucifera and Portulaca oleracea possessed strong AChE
inhibition. In addition, the molecular docking simulation analyses
demonstrated that the active compounds present in Uncaria
rhynchophylla (CID9061) and Portulaca oleracea (CID681
and CID439260) had strong molecular interactions, as evidenced by
the molecular docking scores and ligand-protein interaction energy
analyses. Furthermore, the MD simulation confirmed the stability of
the protein-ligand docked complexes. The results of the present
study suggested that CID9064 (catechin), CID681 (dopamine) and
CID439260 (norepinephrine) may be key bioactive ingredients that
may be prescribed to patients with AD.
Acknowledgments
The authors would like to acknowledge the 5th
People's Hospital of Wuhan (Wuhan, Hubei, P.R. China) for necessary
support.
References
|
1
|
Burns A and Iliffe S: Alzheimer's disease.
BMJ. 338:b1582009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Querfurth HW and LaFerla FM: Alzheimer's
disease. New Eng J Med. 362:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Todd S, Barr S, Roberts M and Passmore AP:
Survival in dementia and predictors of mortality: A review. Int J
Geriatr Psychiatry. 28:1109–1124. 2013.PubMed/NCBI
|
|
4
|
Ballard C, Gauthier S, Corbett A, Brayne
C, Aarsland D and Jones E: Alzheimer's disease. Lancet.
377:1019–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson CA, Spilsbury K, Hall J, Birks Y,
Barnes C and Adamson J: Systematic review of information and
support interventions for caregivers of people with dementia. BMC
Geriatr. 7:182007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forbes D, Thiessen EJ, Blake CM, Forbes SC
and Forbes S: Exercise programs for people with dementia. Cochrane
Database Syst Rev. 12:CD0064892013.PubMed/NCBI
|
|
7
|
Mendez MF: Early-onset Alzheimer's
disease: Nonamnestic subtypes and type 2 AD. Arch Med Res.
43:677–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berchtold NC and Cotman CW: Evolution in
the conceptualization of dementia and Alzheimer's disease:
Greco-Roman period to the 1960s. Neurobiol Aging. 19:173–189. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonin-Guillaume S, Zekry D, Giacobini E,
Gold G and Michel JP: The economical impact of dementia. Presse
Med. 34:35–41. 2005.In French. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meek PD, McKeithan K and Schumock GT:
Economic considerations in Alzheimer's disease. Pharmacotherapy.
18:68–73; discussion 79–82. 1998.PubMed/NCBI
|
|
12
|
Francis PT, Palmer AM, Snape M and Wilcock
GK: The cholinergic hypothesis of Alzheimer's disease: A review of
progress. J Neuro Neurosurg Psychiatry. 66:137–147. 1999.
View Article : Google Scholar
|
|
13
|
Houghton PJ, Ren YH and Howes MJ:
Acetylcholinesterase inhibitors from plants and fungi. Nat Prod
Rep. 23:181–199. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hardy J and Allsop D: Amyloid deposition
as the central event in the aetiology of Alzheimer's disease.
Trends Pharmacol Sci. 12:383–388. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mudher A and Lovestone S: Alzheimer's
disease-do tauists and baptists finally shake hands? Trends
Neurosci. 25:22–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quinn DM: Acetylcholinesterase: Enzyme
structure, reaction dynamics, and virtual transition states. Chem
Rev. 87:955–979. 1987. View Article : Google Scholar
|
|
17
|
Taylor P and Radić Z: The cholinesterases:
From genes to proteins. Ann Rev Pharmacol Toxicol. 34:281–320.
1994. View Article : Google Scholar
|
|
18
|
Sussman JL, Harel M, Frolow F, Oefner C,
Goldman A, Toker L and Silman I: Atomic structure of
acetylcholinesterase from Torpedo californica: A prototypic
acetylcholine-binding protein. Science. 253:872–879. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radić Z, Gibney G, Kawamoto S,
MacPhee-Quigley K, Bongiorno C and Taylor P: Expression of
recombinant acetylcholinesterase in a baculovirus system: Kinetic
properties of glutamate 199 mutants. Biochemistry. 31:9760–9767.
1992. View Article : Google Scholar
|
|
20
|
Whittaker VP: The contribution of drugs
and toxins to understanding of cholinergic function. Trends
Pharmacol Sci. 11:8–13. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Purves D, Augustine GJ, Fitzpatrick D,
Hall WC, LaMantia AS, McNamara JO and White LE: Neuroscience. 4th
edition. Sinauer Associates; Sunderland, MA: pp. 121–122. 2008
|
|
22
|
Pohanka M: Alpha7 nicotinic acetylcholine
receptor is a target in pharmacology and toxicology. Inter J Mol
Sci. 13:2219–2238. 2012. View Article : Google Scholar
|
|
23
|
Ellman GL, Courtney KD, Andres V Jr and
Feather-Stone RM: A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomsen R and Christensen MH: MolDock: A
new technique for high-accuracy molecular docking. J Med Chem.
49:3315–3321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Der Spoel D, Lindahl E, Hess B,
Groenhof G, Mark AE and Berendsen HJ: GROMACS: Fast, flexible, and
free. J Comput Chem. 26:1701–1718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin HQ, Ho MT, Lau LS, Wong KK, Shaw PC
and Wan DC: Anti-acetylcholinesterase activities of traditional
Chinese medicine for treating Alzheimer's disease. Chem Biol
Interact. 175:352–354. 2008. View Article : Google Scholar : PubMed/NCBI
|