Introduction
Interstitial fibrosis is a common pathway of
progressive renal disease, leading to end-stage renal failure
regardless of the etiology (1).
Previous studies have indicated that the process of tubular
epithelial-mesenchymal transition (EMT) contributes to the
predominance of fibroblasts in idiopathic nephrotic syndrome
(2). The pathological mechanism of
EMT remains to be elucidated (3);
however, recent studies have suggested that homeobox protein
HOX-A13 (HOXA13) is involved in urogenital development and renal
fibrosis (4). Furthermore,
Williams et al (5) reported
that HOXA13 exerts an inhibitory effect on transforming growth
factor-β1 (TGF-β1) signaling, which has been widely demonstrated to
induce EMT (6). Thus, HOXA13 is
hypothesized to be involved in regulating EMT and renal fibrosis,
however, the molecular mechanism underlying these effects remains
to be elucidated.
Resistance to glucocorticoid (GC) treatment is a key
clinical problem in multiple diseases, including idiopathic
nephrotic syndrome (7). The
majority of the effects of GC are mediated by the glucocorticoid
receptor (GR). GR is known to inhibit the activity of a number of
immune-regulating transcription factors (8). Abnormalities in the number and
affinity of GRs have been demonstrated to be associated with
peripheral blood mononuclear cell (PBMC) proliferation and cytokine
secretion, which are important in the development of idiopathic
nephrotic syndrome and renal failure (7,9,10).
However, the effect of GR signaling on EMT has not yet, to the best
of our knowledge, been reported. Thus, the aim of the present study
was to investigate the potential function of HOXA13 in human serum
albumin (HSA)-induced EMT and the GR signaling pathway in HKC human
renal tubular epithelial cells.
Materials and methods
Reagents
Mouse anti-β-actin monoclonal antibody (cat no.
6008-1), rabbit anti-vimentin polyclonal antibody (cat no. 10366),
rabbit anti-cytokeratin (CK) polyclonal antibodies (cat no. 10830)
and rabbit anti-GR polyclonal antibody (cat no. 23978) were
obtained from ProteinTech Group, Inc. (Chicago, IL, USA). Rabbit
anti-HOXA13 polyclonal antibodies (cat no. sc-66922) were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit
anti-KAT3A/cAMP response element binding protein (CBP) polyclonal
antibody (cat no. sc-ab119488) was obtained from Abcam (Cambridge,
UK). The plasmids pGV230-eGFP-HOXA13 and pGV230-eGFP vector were
provided by Shanghai Genechem Co., Ltd. (Shanghai, China).
Dulbecco's modified Eagle's medium (DMEM) and Fetal bovine serum
(FBS) were obtained from Wuhan Procell Power Technology Co., Ltd.
(Wuhan, China).
Cell culture and HSA treatment
HKC cells were obtained from the Nephropathy
Laboratory at the Second Xiangya Hospital, Central South University
(Changsha, China). HKC cells were cultured in DMEM supplemented
with 10% FBS and maintained at 37°C in a humidified chamber of 5%
CO2. When the cells reached the appropriate confluence
(50%), cells were seeded (1×106 cells/ml) in 6-well
plates. When they reached 80% confluence, cells were washed with
sterile phosphate-buffered saline (PBS) and serum-starved for 24 h.
One group of cells was treated with medium containing different HSA
concentrations (0, 1, 5, 10, 20 and 30 mg/ml) in serum-free medium
(SFM) for 48 h, another group cells were cultured in SFM containing
20 mg/ml HSA for 0, 12, 24, 48 and 72 h.
Cell viability studies
Adherent and floating cells were harvested every 12
h up to 72 h after exposure to either SFM alone (control) or HSA
preparations (0–30 mg/ml). Cell viability was assessed using the
trypan blue exclusion assay (Shanghai Biyuntian Biological Co.,
Ltd., Shanghai, China). The number of live and dead cells (that
were stained blue) was counted, and the viability was expressed as
the percentage of live cells within the total number of cells
counted according to a previous report (6). Cells were visualized using an
inverted biological microscope, DSZ-2000X Series; Nikon
Corporation, Tokyo, Japan)
Plasmid transfection
Plasmid transfection was conducted with
Lipofectamine 2000 according to the manufacturer's protocols
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cells were incubated for 48 h 37°C in a humidified chamber of 5%
CO2 and then subjected to the collection of cells,
supernatants, proteins, and total RNA according to previous reports
(4,6). The cells for plasmid transfection
were subsequently exposed to DMEM containing 10% FBS and 20 mg/ml
HSA for a further 48 h. Cells were divided into five groups: i) A
control group; ii) a HSA group in which cells were treated with 20
mg/ml HSA and received non-transfected plasmid; iii) a HOXA13
transfection group in which cells were treated with 20 mg/ml HSA
and received transfected plasmid pGV230-eGFP-HOXA13 (Shanghai Jikai
Gene Technology Co., Ltd. Shanghai, China); iv) a negative control
group in which cells were transfected with plasmid pGV230-eGFP
vector and received 20 mg/ml HSA; and v) a CBP group in which cells
were treated with 20 mg/ml HSA and transfected with plasmid
pGV230-eGFP-HOXA13 and treated with 1 µg CBP. CBP, a GR
inhibitor, was used to investigate the GR signaling pathway.
Western blot analysis
Western blot analysis was performed as described in
previous studies (11,12). Briefly, 1×106 cells from
each group were collected and total protein was extracted, as
described previously (11,12). For each sample, 20 µl total
protein was subjected to 15% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (Kaiji Biotechnology., Shanghai, China) and
then transferred to a nitrocellulose membrane (Kaiji
Biotechnology.). Membranes were blocked in 5% fat-free dry milk
(Biyuntian, Shanghai, China) and then incubated overnight at 4°C
with anti-CK (at 1:200 dilution), anti-vimentin (at 1:1,000
dilution), anti-HOXA13 (at 1:200 dilution), anti-GR (at 1:1,000
dilution), anti-CBP (at 1:500 dilution) or anti-β-actin (at 1:4,000
dilution). Secondary antibody (goat anti-rabbit immunoglobulin G
labeled with horseradish peroxidase; at 1:4,000 dilution) was added
to membranes following washing with TBS-T (Shanghai Biyuntian
Biological Co., Ltd.) and incubated for 1 h at room temperature as
previously described (11,12). Following washing, Luminata Forte
Western HRP substrate (EMD Millipore; Billerica, MA, USA) was added
and allowed to develop for 2 min in a darkroom, and X-ray film (Gel
Doc Efficient Zeitgeist, Bio-Rad, USA) was then exposed to the
membranes. The β-actin signal was set as the internal reference.
Relative intensities of protein bands were quantified using an
image analysis system (Image Lab Software; version 4.0; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
CK, vimentin, and HOXA13 mRNA expression levels were
detected by qPCR, according to a previous report (11). Approximately 1×106
cells/ml from each group were collected from six-well plates for
qPCR detection. The gene sequences were used to design primers and
these were synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.), as follows: Sense, 5′-ATCCACGAGCACAGTCCACAT-3′ and
antisense, 5′-AGGAGACGGTGACCAGGGTT-3′ for CK; sense,
5′-TGACCGCTTCGCCAACTACAT-3′ and antisense,
5′-TCCCGCATCTCCTCCTCGTA-3′ for vimentin; sense,
5′-CACTCTGCCCGACGTGGTCT-3 and antisense, 5′-CCGCCTCCGTTTGTCCTTA-3′
for HOXA13; and sense, 5′-CGACAGGATGCAGAACGAGA-3′ and antisense,
5′-AGTGAGGACCCTGGATGTGA-3′ for β-actin.
Total RNA isolation and reverse transcription were
conducted according to previous reports (13,14)
and using SYBR Green PCR Mix (Takara Bio, Inc., Otsu, Japan).
Double-distilled water used instead of a template served as a
negative control. The number of β-actin transcripts was used as a
reference of endogenous RNA, and the quantification of test genes
for each sample was standardized relative to the number of β-actin
transcripts. The 2−ΔΔCq cycle threshold formula was used
to calculate the relative abundance of transcripts (13,14).
Statistical analysis
The SPSS software package (version 17.0; SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Values are
expressed as the mean ± standard deviation. Differences between
groups were evaluated by one-way analysis of variance followed by
Duncan's test by correction for multiple comparison. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell viability
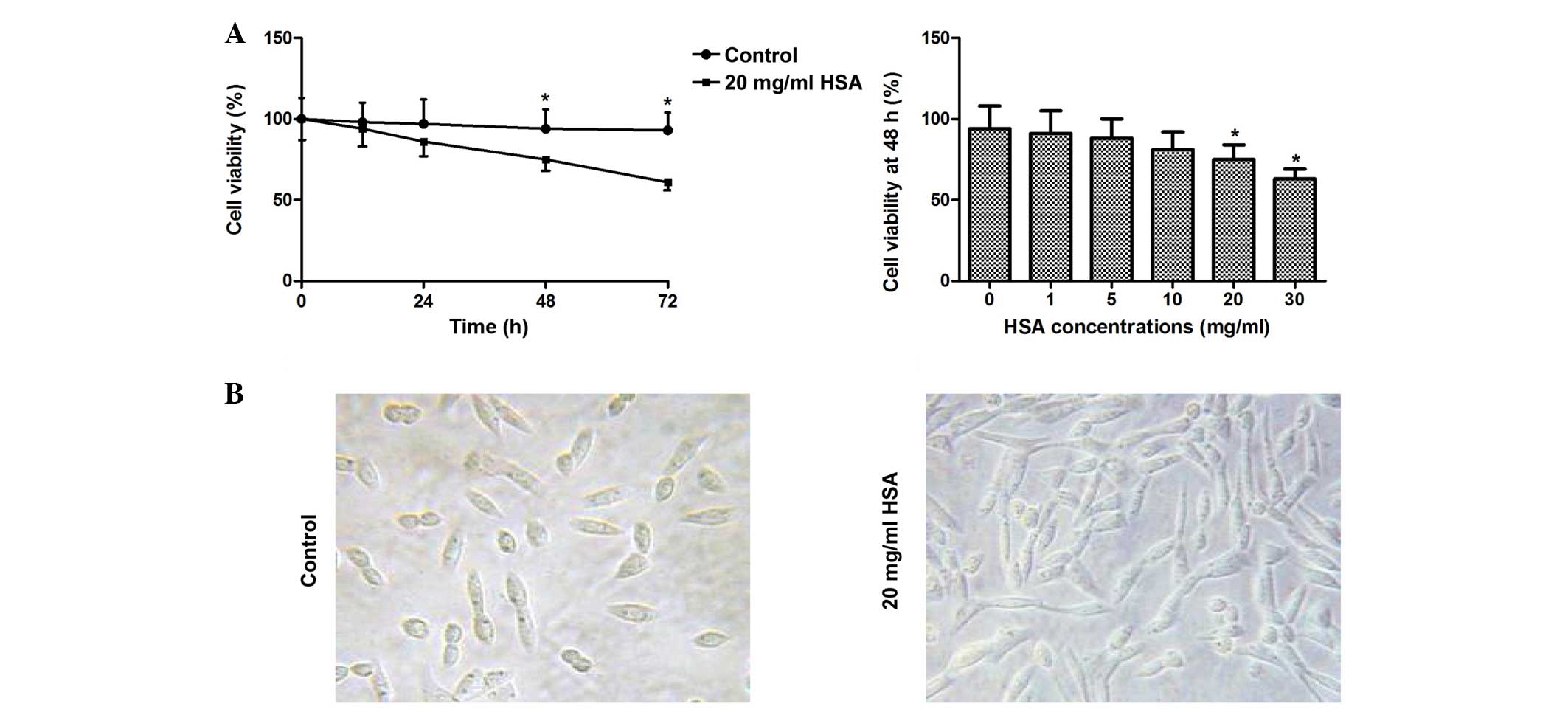
The results demonstrated that incubation with HSA
(0–30 mg/ml) treatment for 48 h reduced cell viability in a
dose-dependent manner, and 20 and 30 mg/ml significantly decreased
cell viability (P<0.05). Furthermore, incubation with 20 mg/ml
HSA significantly reduced cell viability at 48 and 72 h (P<0.05;
Fig. 1). The results of cell
morphology analysis demonstrated that cells treated with 20 mg/ml
HSA for 48 h were spindle shaped and exhibited a fibroblast-like
morphology compared with the control group (Fig. 1).
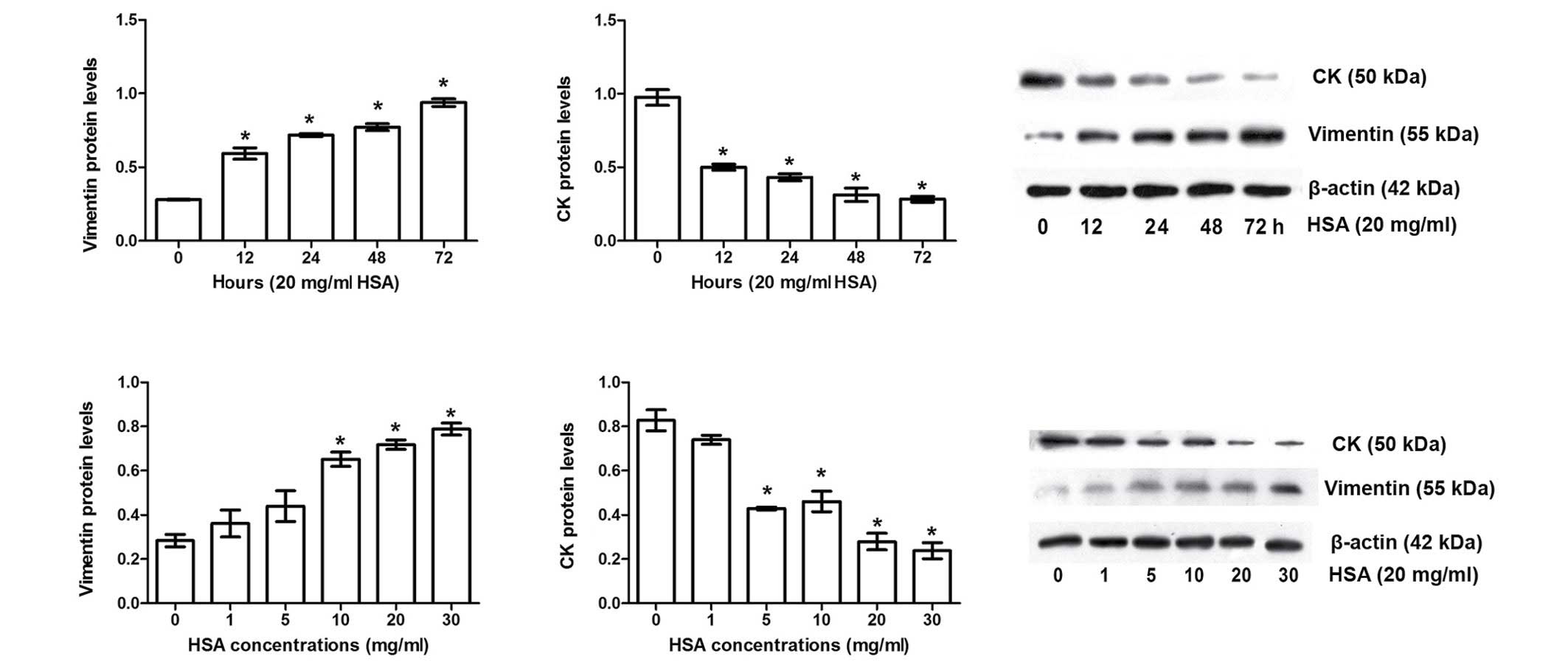
Albumin induces EMT in HKC cells
Vimentin (a mesenchymal cell marker) and CK (an
epithelial cell marker) have been widely used to evaluate EMT
(15). Western blotting data in
the present study indicated that HSA significantly increased
vimentin expression levels and lowered CK expression levels in a
time- and dose-dependent manner (P<0.05; Fig. 2), indicating that albumin treatment
induces EMT in HKC cells.
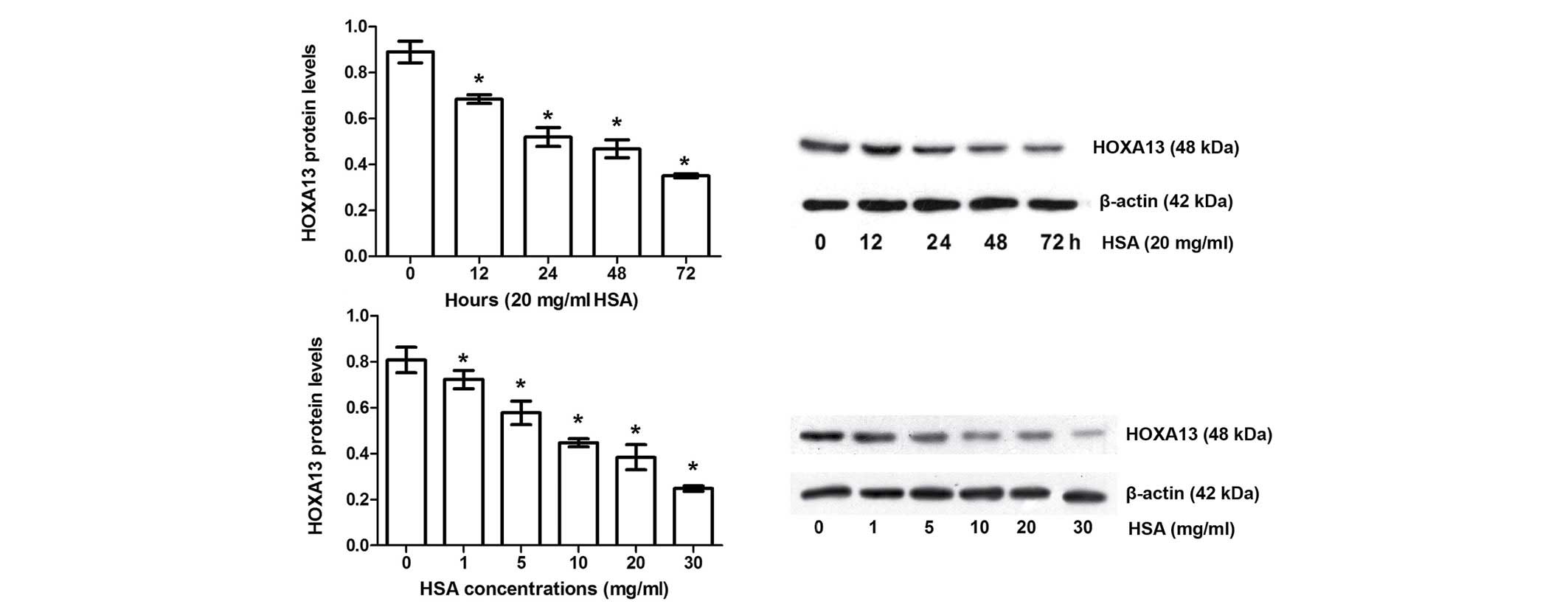
Expression of HOXA13 in albumin-induced
EMT in HKC cells
Effects of albumin-induced EMT on HOXA13 expression
are shown in Fig. 3 and Table I. HSA significantly inhibited
HOXA13 in a time- and dose-dependent manner compared with the
control group (P<0.05).
 | Table IEffects of HOXA13 transfection on gene
expression levels in HSA-induced epithelial-mesenchymal
transition. |
Table I
Effects of HOXA13 transfection on gene
expression levels in HSA-induced epithelial-mesenchymal
transition.
| Gene | Control | HSA group | HOXA13
transfection | Negative control |
|---|
| HOXA13 | 1.00±0.05 | 0.27±0.01a | 0.95±0.06b | 0.33±0.01 |
| Cytokeratin | 1.00±0.05 | 0.34±0.01a | 0.77±0.03b | 0.29±0.01 |
| Vimentin | 1.00±0.06 | 2.65±0.12a | 1.07±0.06b | 2.90±0.13 |
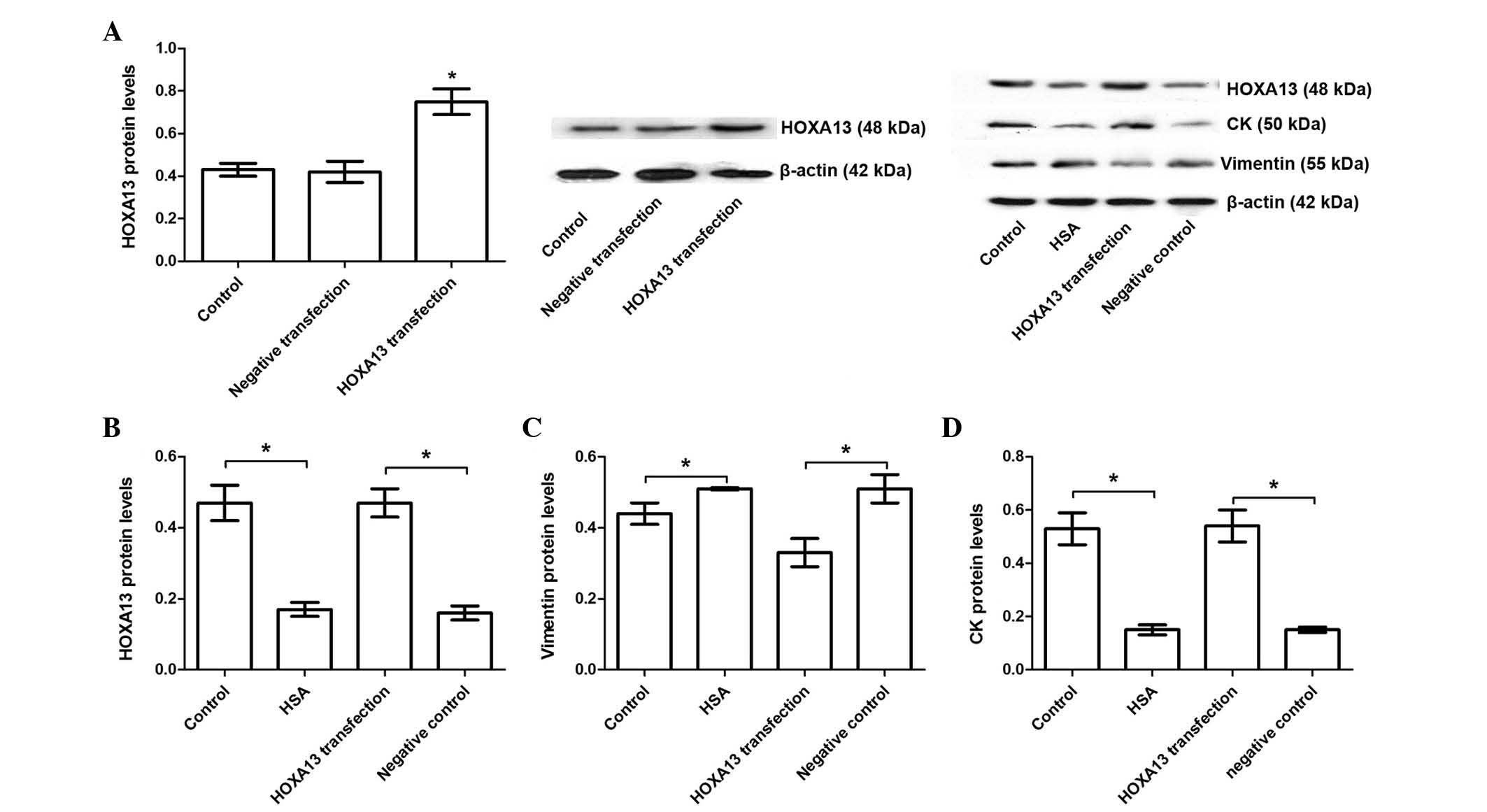
Albumin-induced EMT is restored by
overexpression of HOXA13 in HKC cells
To investigate the protective role of HOXA13 in
HSA-induced EMT, HKC cells were transfected with pGV230-eGFP-HOXA13
and pGV230-eGFP, and the results demonstrated that HOXA13
transfection significantly increased HOXA13 protein abundance
compared with the cells transfected with pGV230-eGFP vector
(P<0.05; Fig. 4A). HKC cells
were transfected with pGV230-eGFP-HOXA13 and then treated with 20
mg/ml HSA, and the results demonstrated that HOXA13 transfection
significantly upregulated HOXA13 expression compared with
HSA-treated negative control group (P<0.05; Fig. 4B). Furthermore, the effects of
HOXA13 on albumin-induced upregulation of EMT were measured and the
results demonstrated that upregulation of HOXA13 reversed
albumin-induced EMT as suggested by downregulation of vimentin and
upregulation of CK (P<0.05; Figs.
4C and D). Furthermore, the results were validated by qPCR
(Table I), indicating that HOXA13
has a protective role against HSA-induced EMT in HKC cells through
mediating vimentin and CK levels.
Effects of HOXA13 on GR in
albumin-induced EMT
The GR signaling pathway was also investigated in
albumin-induced EMT using CBP, a GR inhibitor. GR expression levels
in albumin-induced EMT were measured and the results demonstrated
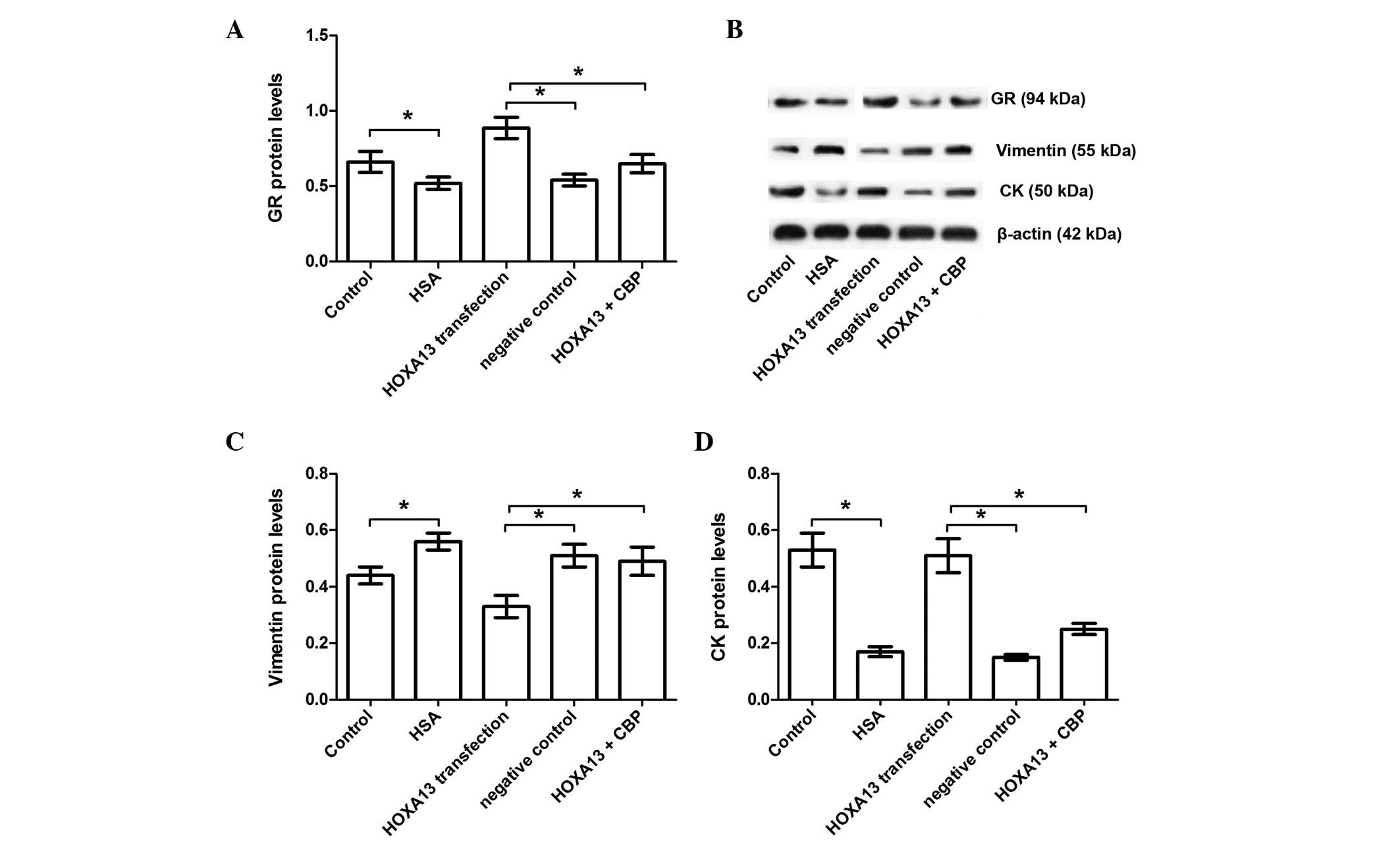
that HSA treatment significantly inhibited GR (P<0.05; Fig. 5). HOXA13 transfection reversed the
GR inhibition that resulted from HSA treatment; however, this
effect was inhibited by CBP treatment (P<0.05; Fig. 5). Furthermore, the present data
also demonstrated that CBP inhibited the protective function of
HOXA13 transfection as suggested by its ability to reduce vimentin
and enhance CK expression (P<0.05; Fig. 5). Thus, it was hypothesized that
the protective function of HOXA13 transfection involves the GR
signaling pathway.
Discussion
The present study demonstrated that albumin-induced
EMT in HKC cells was characterized by downregulation of CK and
upregulation of vimentin in a time- and dose-dependent manner. In
addition, HOXA13, as a nuclear transcription factor, decreased CK
and increased vimentin production, which contributed to the
beneficial role in albumin-induced EMT of HKC cells. Furthermore,
the present study suggests that the GR signaling pathway is
involved in the protective function of HOXA13 in albumin-induced
EMT.
Emerging evidence demonstrates that EMT is key in
renal tubulointerstitial fibrosis (1,3). In
the present study, a prolonged albumin overload model was used to
mimic the effects of chronic proteinuria to induce EMT, the results
demonstrated that there was a significant decrease in the viability
of HKC cells exposed to HSA, and HSA treatment reduced the
expression levels of the epithelial marker CK and increased the
expression levels of the mesenchymal marker vimentin, suggesting
that HSA induced EMT in HKC cells, which is consistent with the
results of previous studies (16,17).
Members of the HOX family contain a highly conserved
DNA sequence and encode the nuclear transcription regulatory
proteins, which are important in the regulation of the
differentiation and proliferation of adult tissue (18). The HOXA13 gene is important in
mammalian embryonic development and is associated with limb
formation and reproductive development (19). A previous study indicated that
HOXA13 exerts a protective effect against renal fibrosis (4). Williams et al reported that
HOXA13 inhibits TGF-β1-mediated transcriptional activity, which has
been demonstrated to induce EMT (5). In the current study, HSA was observed
to induce EMT and significantly inhibit HOXA13 expression, and the
reduced HOXA13 expression may serve as a marker for EMT. To further
elucidate the role of HOXA13 in EMT, the expression of HOXA13 was
upregulated and the results demonstrated that liposomal
transfection with HOXA13 significantly reversed HSA-induced CK
reduction and vimentin overexpression, suggesting a protective role
of HOXA13 against EMT in HKC cells as a result of CK reduction and
vitmentin upregulation involved in EMT.
GR is known to inhibit the activity of a growing
number of immune-regulating transcription factors (8). Abnormalities of number and affinity
of GR have been demonstrated to be associated with PBMC
proliferation and cytokine secretion, which are important in the
development of idiopathic nephrotic syndrome and renal failure
(7,9). The current study demonstrated that
HSA-induced EMT significantly inhibited GR signaling, while
upregulation of HOXA13 activated the GR signaling pathway. It has
been demonstrated that CBP is a negative regulator of GR activity
(20). Thus, CBP was used to
clarify the underlying mechanism of GR in the protective role of
HOXA13 in HSA-induced EMT and the results demonstrated that CBP
treatment inactivated GR signaling and blocked the beneficial
effect of HOXA13 in HSA-induced EMT (demonstrated by CK and
vimentin expression levels).
In conclusion, albumin overload induces EMT in HKC
cells and downregulates HOXA13 expression. Transfection experiments
demonstrate that HOXA13 exerts a protective effect in HSA-induced
EMT, which may involve the GR signaling pathway. The results of the
present study suggested that HOXA13 may be a novel target for the
therapy of renal interstitial fibrosis.
References
|
1
|
Zununi Vahed S, Samadi N and Ardalan M:
Diagnosis of interstitial fibrosis and tubular atrophy in kidney
allograft: Implementation of microRNAs. Iran J Kidney Dis. 8:4–12.
2014.PubMed/NCBI
|
|
2
|
Grande MT and López-Novoa JM: Fibroblast
activation and myofibroblast generation in obstructive nephropathy.
Nat Rev Nephrol. 5:319–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Sun Y, Liu F, Sun L, Li J, Duan S,
Liu H, Peng Y, Xiao L, Liu Y, et al: Norcantharidin inhibits renal
interstitial fibrosis by blocking the tubular
epithelial-mesenchymal transition. PLoS One. 8:e663562013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamasaki Y, Doi K, Okamoto K, Ijichi H,
Seki G, Maeda-Mamiya R, Fujita T and Noiri E:
3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor
simvastatin ameliorates renal fibrosis through HOXA13-USAG-1
pathway. Lab Invest. 92:1161–1170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams TM, Williams ME, Heaton JH,
Gelehrter TD and Innis JW: Group 13 HOX proteins interact with the
MH2 domain of R-Smads and modulate Smad transcriptional activation
functions independent of HOX DNA-binding capability. Nucleic Acids
Res. 33:4475–4484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tirino V, Camerlingo R, Bifulco K, Irollo
E, Montella R, Paino F, Sessa G, Carriero MV, Normanno N, Rocco G
and Pirozzi G: TGF-β1 exposure induces epithelial to mesenchymal
transition both in CSCs and non-CSCs of the A549 cell line, leading
to an increase of migration ability in the CD133+ A549
cell fraction. Cell Death Dis. 4:e6202013. View Article : Google Scholar
|
|
7
|
Chen P, Jiang T, Ouyang J and Cui Y:
Glucocorticoid receptor auto-upregulation and its relation with
glucocorticoid sensitivity in idiopathic nephrotic syndrome. Int
Urol Nephrol. 43:167–174. 2011. View Article : Google Scholar
|
|
8
|
Ratman D, Vanden Berghe W, Dejager L,
Libert C, Tavernier J, Beck IM and De Bosscher K: How
glucocorticoid receptors modulate the activity of other
transcription factors: A scope beyond tethering. Mol Cell
Endocrinol. 380:41–54. 2013. View Article : Google Scholar
|
|
9
|
Carlotti AP, Franco PB, Elias LL,
Facincani I, Costa EL, Foss N, Moreira AC and de Castro M:
Glucocorticoid receptors, in vitro steroid sensitivity and cytokine
secretion in idiopathic nephrotic syndrome. Kidney Int. 65:403–408.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, He QN, Zhu M, Zhou G, Ding JJ,
Zhou P, Wu XC and Yi ZW: Effect of glucocorticoid receptor beta on
glucocorticoid action in glomerular mesangial cells. Zhong Nan Da
Xue Xue Bao Yi Xue Ban. 32:941–948. 2007.
|
|
11
|
Yin J, Ren W, Liu G, Duan J, Yang G, Wu L,
Li T and Yin Y: Birth oxidative stress and the development of an
antioxidant system in newborn piglets. Free Radic Res.
47:1027–1035. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin J, Duan J, Cui Z, Ren W, Li T and Yin
Y: Hydrogen peroxide-induced oxidative stress activates NF-κB and
Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv.
5:15479–15486. 2015. View Article : Google Scholar
|
|
13
|
Yin J, Ren W, Duan J, Wu L, Chen S, Li T,
Yin Y and Wu G: Dietary arginine supplementation enhances
intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth
depression in mycotoxin-challenged pigs. Amino Acids. 46:883–892.
2014. View Article : Google Scholar
|
|
14
|
Yin J, Wu MM, Xiao H, Ren WK, Duan JL,
Yang G, Li TJ and Yin YL: Development of an antioxidant system
after early weaning in piglets. J Anim Sci. 92:612–619. 2014.
View Article : Google Scholar
|
|
15
|
Kallergi G, Papadaki MA, Politaki E,
Mavroudis D, Georgoulias V and Agelaki S: Epithelial to mesenchymal
transition markers expressed in circulating tumour cells of early
and metastatic breast cancer patients. Breast Cancer Res.
13:R592011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Igleslas J, Abernethy VE, Wang Z,
Lieberthal W, Koh JS and Levine JS: Albumin is a major serum
survival factor for renal tubular cells and macrophages through
scavenging of ROS. Am J Physiol. 277:F711–F722. 1999.
|
|
17
|
Ibrini J, Fadel S, Chana RS, Brunskill N,
Wagner B, Johnson TS and El Nahas AM: Albumin-induced epithelial
mesenchymal transformation. Nephron Exp Nephrol. 120:e91–e102.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dressler GR: An update on the vertebrate
homeobox. Trends Genet. 5:129–131. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Utsch B, Becker K, Brock D, Lentze MJ,
Bidlingmaier F and Ludwig M: A novel stable polyalanine [poly(A)]
expansion in the HOXA13 gene associated with hand-foot-genital
syndrome: Proper function of poly(A)-harbouring transcription
factors depends on a critical repeat length? Hum Genet.
110:488–494. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kino T, Nordeen SK and Chrousos GP:
Conditional modulation of glucocorticoid receptor activities by
CREB-binding protein (CBP) and p300. J Steroid Biochem Mol Biol.
70:15–25. 1999. View Article : Google Scholar : PubMed/NCBI
|