Introduction
Cervical cancer is a key threat to women's health,
with its incidence second to breast cancer in women worldwide
(1). According to a report from
the World Health Organization there are approximately 500,000 new
cases of cervical cancer worldwide each year, of which 80% occur in
developing countries (2,3).
MicroRNAs (miRNAs) are endogenous, single-stranded
RNA molecules, with an approximate length of 22 nucleotides. miRNAs
are widely present in eukaryotic cells and function to regulate
gene expression, in addition to serving roles in a variety of
important physiological and pathological processes (4). Previous studies have indicated that
miRNAs are associated with the occurrence and development of
various tumors, however there have been few reports about the role
of miRNAs in cervical cancer (2,5).
The occurrence of cervical cancer is a complex
process with multiple stages and steps, and is associated with the
expression of various oncogenes and tumor suppressor genes, in
addition to the regulation of apoptosis (6). Bcl-2 protein is predominantly
localized in the mitochondria and rough endoplasmic reticulum,
where it functions as an anti-apoptotic protein by inhibiting the
oligomerization of Bax and Bak, thereby prolonging the life cycle
of cells (7). Overexpression of
Bcl-2 inhibits apoptosis, thereby inducing immortality in damaged
cells and promoting the development of tumors, acting in
combination with genes regulating proliferation and inhibiting
growth (8).
Divaris et al (9) first used 5-aminolevulinic acid
photodynamic therapy (ALA-PDT) for the treatment of skin diseases
in 1990. At present, topical ALA-PDT is predominantly used for the
treatment of benign and malignant skin tumors, including solar
keratoses, seborrheic keratoses, basal cell epithelioma, Bowen's
disease and squamous cell carcinoma (10). In addition, topical ALA-PDT has
been demonstrated to exhibit a unique effect on certain infections
of the skin, such as acne vulgaris and warts caused by human
papillomavirus (HPV) infection (11). Thus, due to the high selectivity
and broad application prospects of ALA-PDT, it has received
increased attention from clinicians (12,13).
In the current study, the potential anticancer effect of ALA-PDT on
HeLa human cervical cancer cells was investigated, by assessing its
effects on the proliferation, cytotoxicity and apoptosis of HeLa
cells. In addition, the mechanisms underlying the effect of ALA-PDT
on HeLa cells were investigated.
Materials and methods
Reagents
Gibco Dulbecco's modified Eagle's medium (DMEM) and
fetal bovine serum (FBS) were obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma-Aldrich (St. Louis, MO, USA). An Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection kit was purchased from Bestbio, Co. (Shanghai, China).
TRIzol® reagent and TaqMan miRNA Real-Time Quantitative
PCR assays were purchased from Thermo Fisher Scientific, Inc
(Invitrogen). A bicinchoninic acid (BCA) Protein Assay kit was
purchased from Beyotime Institute of Biotechnology (Nanjing,
China).
Cell culture
HeLa human cervical carcinomas cells were obtained
from the experimental center of Qilu Hospital (Jinan, China). HeLa
cells were cultured in DMEM containing 10% FBS, 100 U/ml penicillin
and 100 U/ml streptomycin (both Beyotime Institute of
Biotechnology) at 37°C and 5% CO2. Culture media was
replaced every 2–3 days.
Cell treatments and cell proliferation
assay
HeLa cells (1.5×104 cells/well) were
seeded in a 96-well plate and cultured for 24 h. Subsequently, HeLa
cells were treated with various concentrations of ALA (0, 0.1,
0.25, 0.5, 1.0, 2.0 and 4.0 µM; Sigma-Aldrich) for 24 h as
previously described (14).
Subsequently, PDT was conducted using a Aila laser generator
apparatus (XD-635AB; Shanghai Fudan-Zhangjiang Bio-Pharmaceutical,
Co., Ltd., Shanghai, China), with a dose of 5 J/cm2 HeLa
cells. A total of 20 µl MTT was added to each well and
incubated for 4 h. Subsequently, 150 µl dimethyl sulfoxide
(Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) was added
to each well followed by gentle agitation for 20 min. The
absorbance of each well was measured at a wavelength of 570 nm
using the LabSystems Multiskan MS Microplate Reader (Thermo Fisher
Scientific, Inc.).
Lactate dehydrogenase (LDH) assay for
cytotoxicity
HeLa cells (1.5×104 cells/well) were
seeded in a 96-well plate and cultured for 24 h. Subsequently HeLa
cells were treated with different concentrations of ALA (0, 0.25,
0.5 and 1.0 µM) for 24 h, after which PDT was conducted
using a dose of 5 J/cm2 of HeLa cells. A total of 100
µl LDH solution (Shanghai Enzyme-linked Biotechnology, Co.,
Ltd., Shanghai, China) was added to each well and incubated for 30
min. The absorbance was read at a wavelength of 490 nm using the
LabSystems Multiskan MS Microplate Reader.
Annexin V-FITC/PI apoptosis assay
HeLa cells (1×106 cells/well) were seeded
in a 6-well plate and cultured for 24 h. Subsequently, HeLa cells
were treated with different concentrations of ALA (0, 0.25, 0.5 and
1.0 µM) for 24 h, after which PDT was conducted using a dose
of 5 J/cm2 of HeLa cells. A total of 10 µl
Annexin-V FITC was added to each well and incubated for 10 min in
the dark. Then, 10 µl PI was added to each well and
incubated for 30 min in the dark. Flow cytometry was conducted
using a FACSCalibur flow cytometer and CellQuest™ Pro software,
version 5.1 (BD Biosciences, San Jose, CA, USA) to analyze
apoptosis.
Reverse transcription-quantitative PCR
analysis of miR-143 expression
HeLa cells (1×106 cells/well) were seeded
in a 6-well plate and cultured for 24 h at 37°C and 5%
CO2. Subsequently, HeLa cells were treated with various
concentrations of ALA (0, 0.25, 0.5 and 1.0 µM) for 24 h,
after which PDT was conducted using a dose of 5 J/cm2
HeLa cells. Total RNA was extracted from HeLa cells using
TRIzol® reagent, and 1 ng RNA was converted to cDNA
using the SuperScript III First-Strand Synthesis System for RT-PCR
(Thermo Fisher Scientific, Inc.). The miRNAs were measured using
TaqMan miRNA Real-Time PCR assays and the Rotor-Gene 3000 thermal
cycler (Qiagen China Co., Ltd., Shanghai, China). The PCR cycling
conditions were as follows: 95°C for 1 min, followed by 40 cycles
at 95°C for 30 sec, 60°C for 30 sec, 95°C for 45 min, 55°C for 1
min and 55°C for 10 sec. The primers used were as follows miR-143,
forward 5′-UGAGAUGAAGCACUGUAGCUC-3′ and reverse
5′-TGAGATGAAGCACTGTAGCTCT-3′; U6 small nuclear (sn)RNA, forward
5′-CGCTTCGGCAGCACATATACTA-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCA-3′ (Sangon Biotech Co., Ltd., Shanghai,
China). The relative expression levels of miR-143 were calculated
using the 2−ΔΔCq method, following normalization to U6
snRNA.
Western blotting of Bcl-2 and Bax protein
expression
HeLa cells (1×106 cells/well) were seeded
into a 6-well plate and cultured for 24 h at 37°C in 5%
CO2. Subsequently, HeLa cells were treated with various
concentrations of ALA (0, 0.25, 0.5 and 1.0 µM) for 24 h,
after which PDT was conducted using a dose of 5 J/cm2 of
HeLa cells. HeLa cells were then lysed on ice using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) and cultured for 30 min at 4°C. The protein
concentration was determined using a BCA Protein Assay kit.
Equivalent volumes of protein were separated by 10% SDS-PAGE
(Beyotime Institute of Biotechnology) and transferred onto
polyvinylidine difloride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked for 1 h in 5% nonfat dried milk in
Tris-buffered saline-Tween-20 (TBST; Jiancheng Bioengineering
institute, Nanjing, China). The membranes were then incubated with
mouse anti-Bcl-2 (1:1,000; cat. no. sc-7382; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-Bax (1:1,000; cat. no.
sc-23959; Santa Cruz Biotechnology, Inc.) and anti-β-actin (1:500;
cat. no. AF0003; Beyotime Institute of Biotechnology) monoclonal
antibodies overnight at 4°C. Subsequently, the membranes were
incubated with alkaline phosphatase-conjugated goat anti-mouse IgG
secondary antibodies (1:5,000; cat. no. A0258; Beyotime Institute
of Biotechnology), and observed using an Enhanced Chemiluminscence
Advanced Western Blot Detection kit (cat. no. P0202; Beyotime
Institute of Biotechnology). The proteins were analyzed using
ImageJ 1.37 software (National Institutes of Health, Bethesda, MA,
USA).
Transfection of miR-143 and
anti-miR-143
miR-143, anti-miR-143 and negative plasmids were
obtained from Sangon Biotech Co., Ltd. HeLa cells (1×106
cells/well) were seeded into a 6-well plate and cultured for 24 h
at 37°C in 5% CO2. Subsequently, the plasmids (100
nmol/l) were transfected using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) into HeLa cells.
Statistical analysis
Statistical analysis was conducted using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). All
experiments were performed three times and data were presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
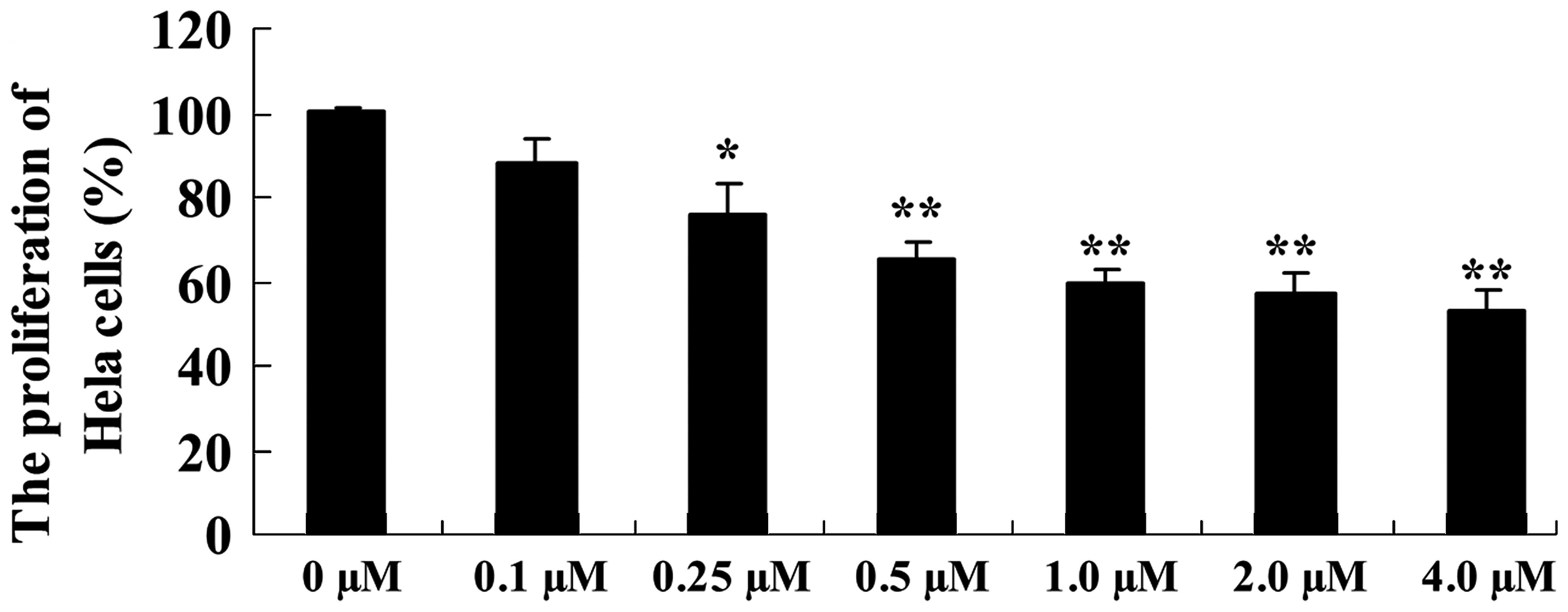
ALA-PDT inhibits the proliferation of
HeLa cells
As presented in Fig.
1, the proliferation of HeLa cells was inhibited following
treatment with ALA-PDT in a dose-dependent manner. Following
treatment with ALA-PDT for 24 h, the proliferation of HeLa cells
was significantly reduced, compared with the 0 µM ALA-PDT
treatment group (0.25 µM, P<0.05; 0.5–4.0 µM,
P<0.01; Fig. 1).
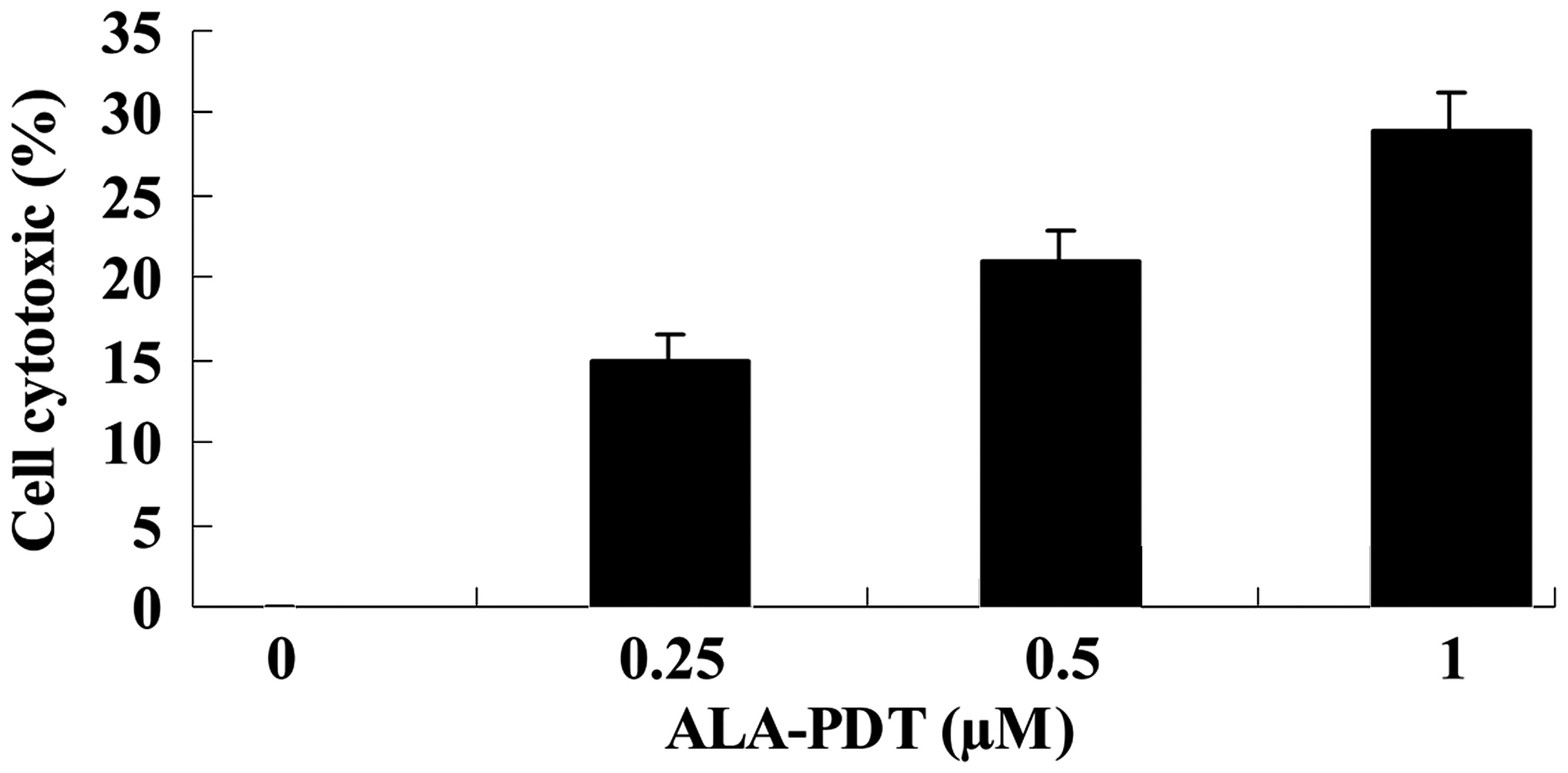
ALA-PDT induces cytotoxicity in HeLa
cells
To investigate the effect of ALA-PDT on HeLa cells,
the level of cytotoxicity was measured using an LDH assay.
Treatment with ALA-PDT increased the level of cytotoxicity observed
in HeLa cells, compared with the 0 µM ALA-PDT treatment
group (0.25 µM, P<0.05; 0.5 and 1.0 µM, P<0.01;
Fig. 2).
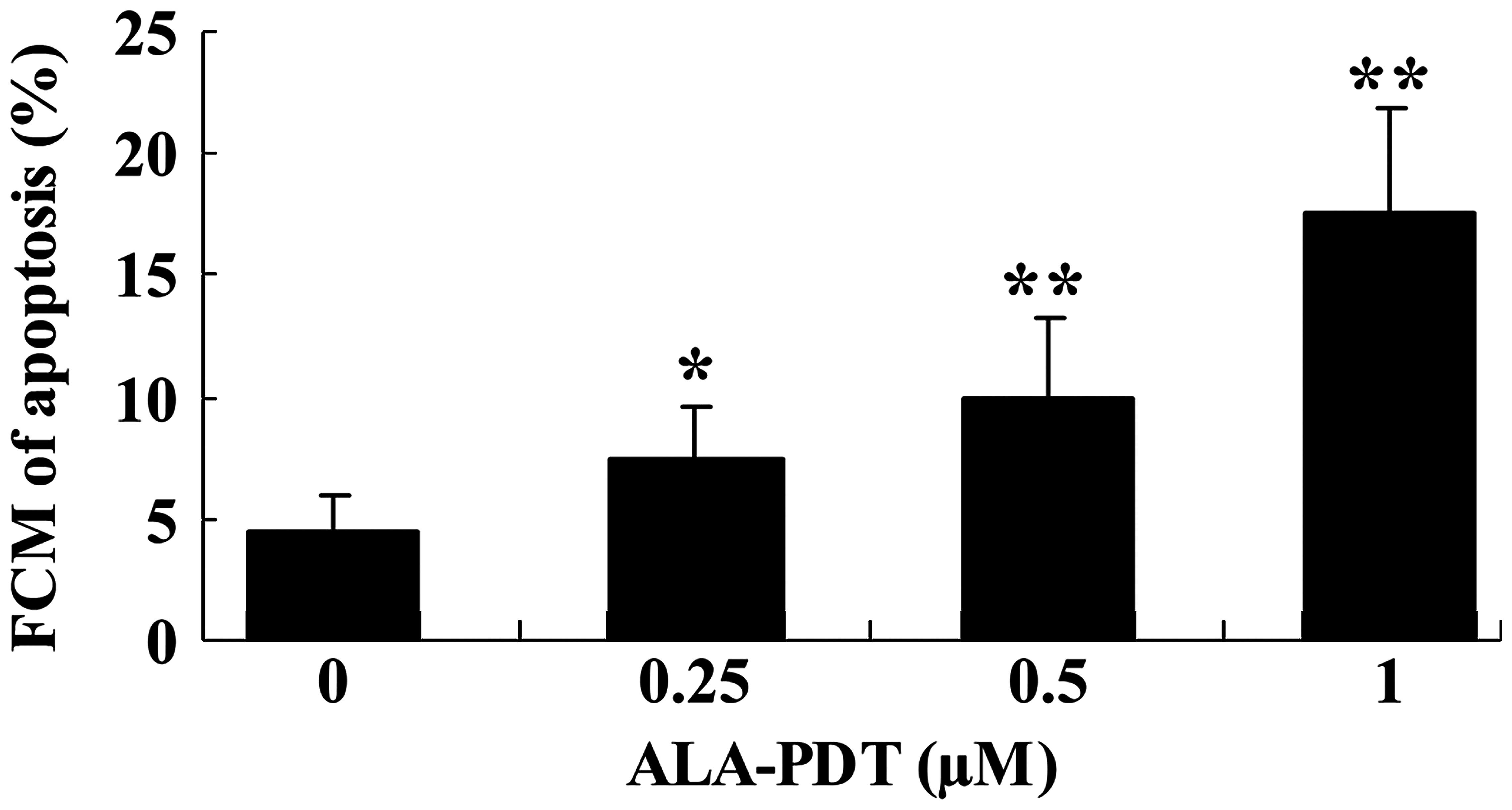
ALA-PDT induces apoptosis in HeLa
cells
To investigate whether apoptosis is promoted
following treatment with ALA-PDT, the level of apoptotic HeLa cells
was assessed using an annexin V-FITC/PI apoptosis assay. Treatment
with ALA-PDT for 24 h induced apoptosis in HeLa cells, compared
with the 0 µM ALA-PDT treatment group (0.25 µM
P<0.05; 0.5 and 1.0 µM, P<0.01; Fig. 3).
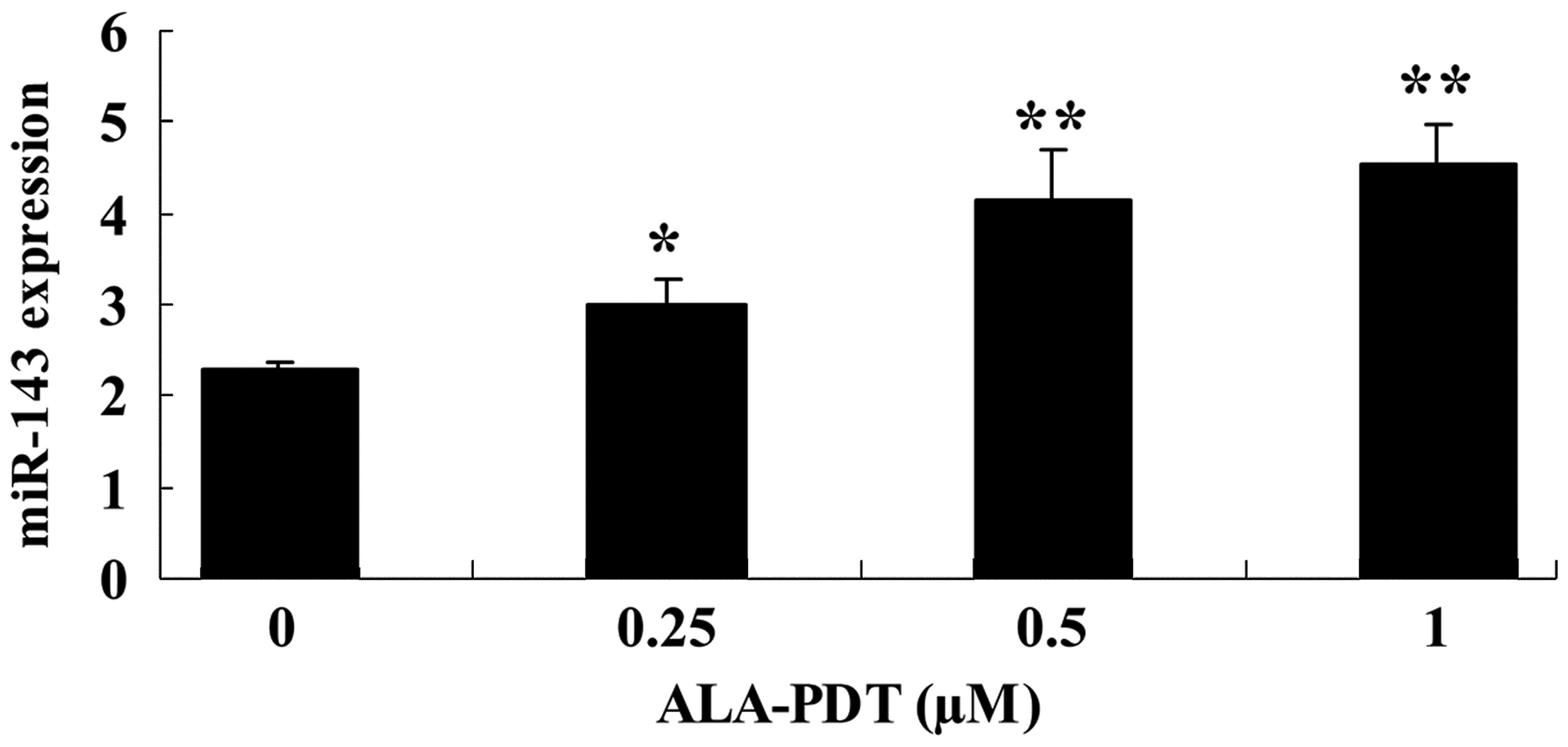
ALA-PDT increases the expression of
miR-143 in HeLa cells
The aim of the investigation was to determine
whether treatment with ALA-PDT alters the expression of miR-143 in
HeLa cells. Following treatment with ALA-PDT for 24 h, the
expression levels of miR-143 were increased in HeLa cells, compared
with the 0 µM ALA-PDT treatment group (0.25 µM,
P<0.05; 0.5 and 1.0 µM, P<0.01; Fig. 4).
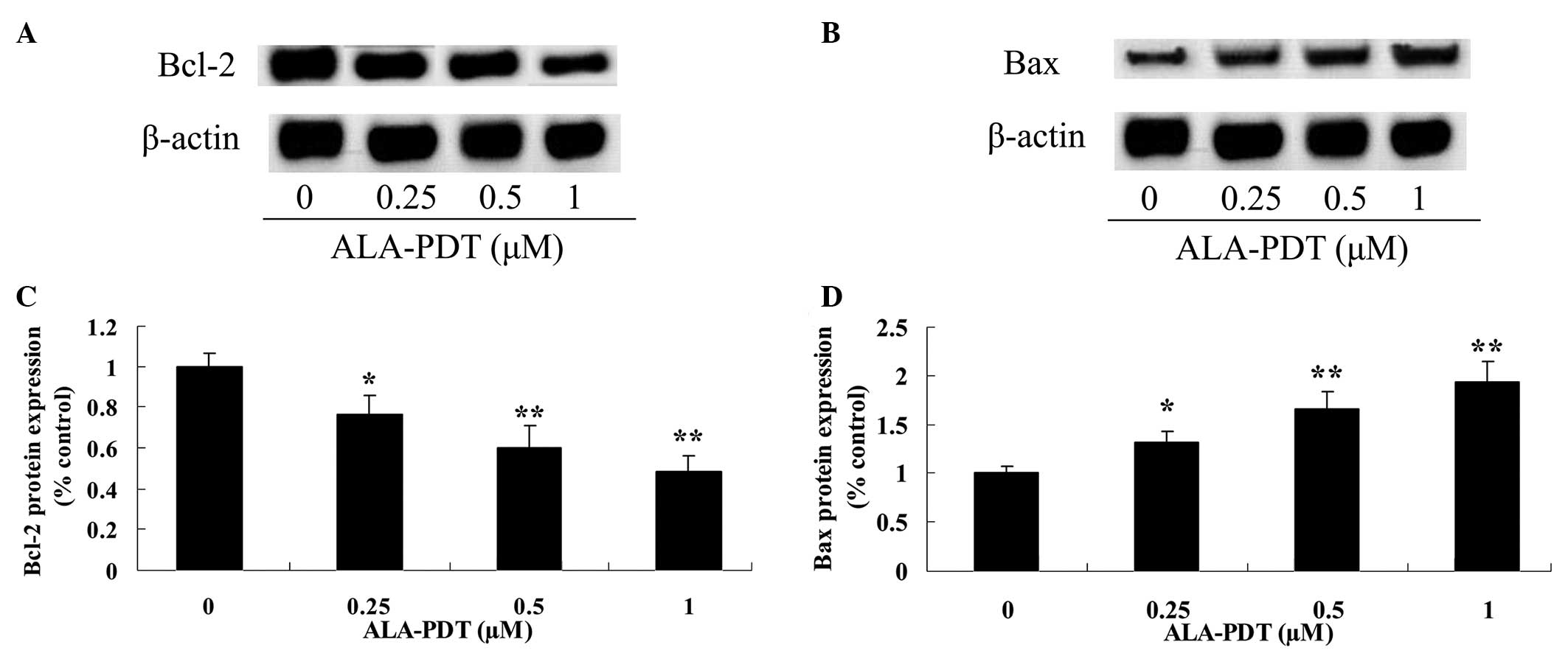
ALA-PDT inhibits the Bcl-2/Bax signaling
pathway in HeLa cells
To investigate the effect of ALA-PDT treatment on
the Bcl-2/Bax signaling pathway in HeLa cells, the expression
levels of Bcl-2 and Bax were analyzed by western blotting (Fig. 5A and B). The results indicated that
Bcl-2 and Bax protein expression levels were reduced and increased,
respectively, following treatment with ALA-PDT for 24 h (0.25
µM, P<0.05; 0.5 and 1.0 µM, P<0.01; Fig. 5C and 5D).
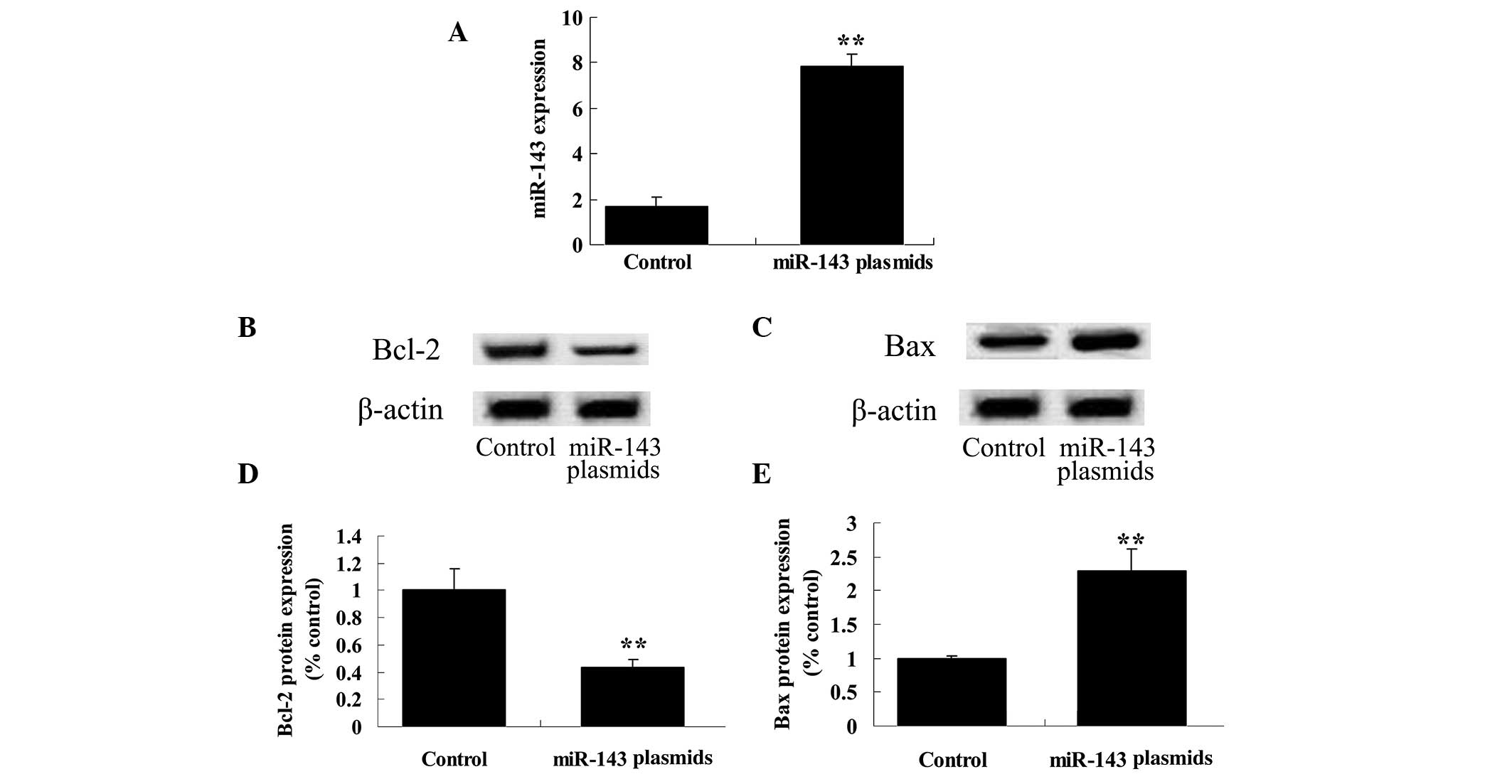
Upregulation of miR-143 expression
downregulates the Bcl-2/Bax signaling pathway in HeLa cells
To further investigate the potential association
between miR-143 expression levels and the effect of ALA-PDT on HeLa
cells, the effect of ALA-PDT on HeLa cells following miR-143
plasmid transfection was analyzed. The results demonstrated that
the miR-143 plasmid markedly increased the expression levels of
miR-143 in HeLa cells (P<0.01; Fig.
6A). In addition, the miR-143 plasmid downregulated the
Bcl-2/Bax signaling pathway in HeLa cells (P<0.01; Fig. 6B–E).
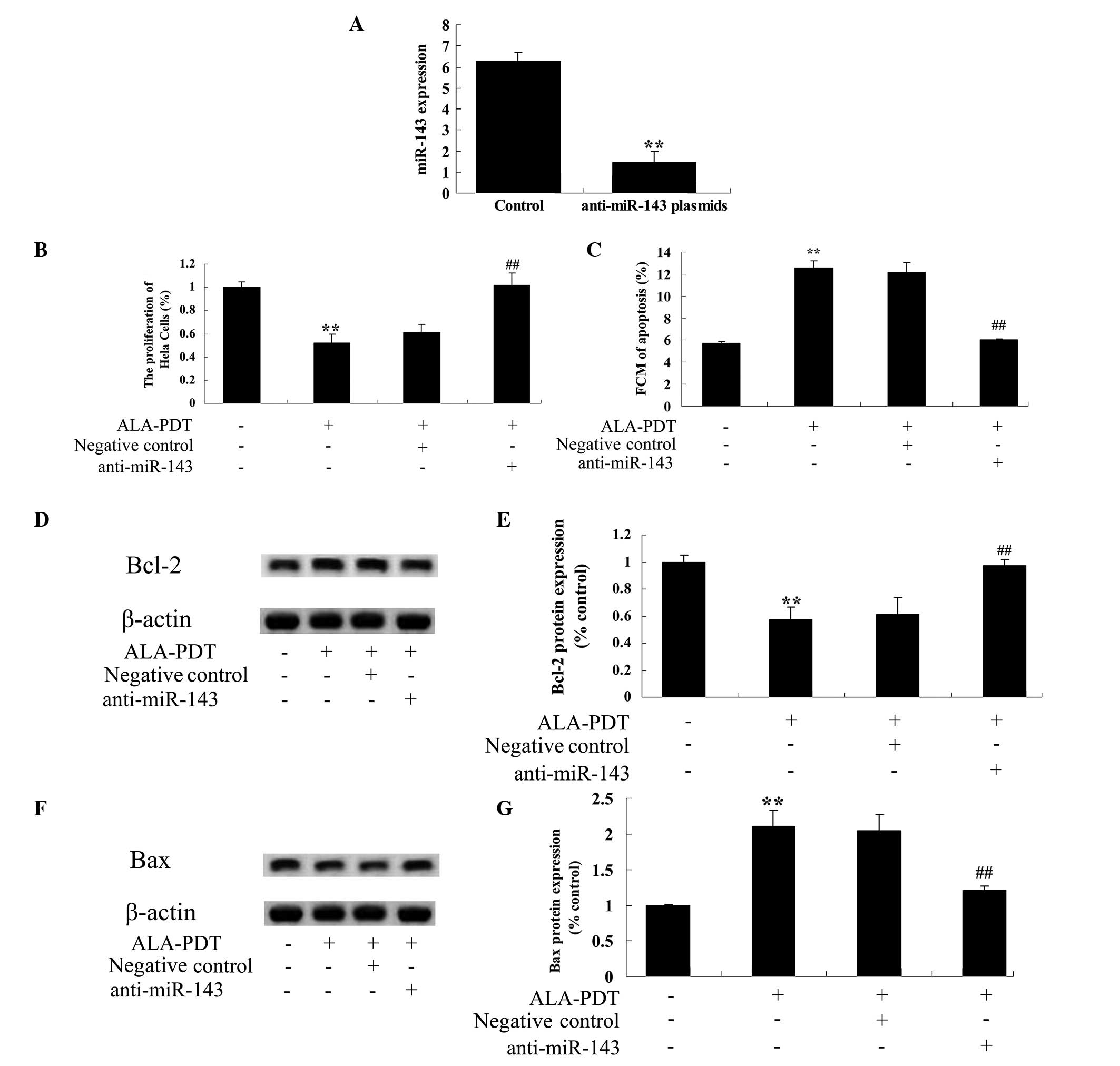
Downregulation of miR-143 expression
reverses the effect of ALA-PDT on HeLa cells and upregulates the
Bcl-2/Bax signaling pathway in HeLa cells
To investigate the mechanism underlying the effect
of ALA-PDT treatment on apoptosis in HeLa cells, anti-miR-143
plasmids were transfected into HeLa cells. The results demonstrated
that the anti-miR-143 plasmid significantly reduced the miR-143
expression levels in HeLa cells (P<0.01; Fig. 7A). In addition, the anti-miR-143
plasmid significantly inhibited the effect of ALA-PDT on
proliferation (P<0.01, Fig. 7B)
and apoptosis (P<0.01; Fig. 7C)
in HeLa cells. Furthermore, the anti-miR-143 plasmid significantly
blocked the effect of ALA-PDT on Bcl-2 and Bax levels in HeLa cells
(P<0.01; Fig. 7D–G).
Discussion
Cervical cancer is the most prevalent type of
malignant tumor of the female genital tract in Chinese women, with
140,000 new cases occurring annually in China, approximately 28.8%
of the newly diagnosed cases of cervical cancer worldwide (15). In recent years, the increase in
cervical cancer screening has led to significant reductions in its
incidence and mortality. However, the increase in the of HPV
infection rate in addition to alterations in lifestyle have
resulted in an increase in the incidence, and a reduction in the
age of onset of cervical cancer (16). The current study demonstrated that
ALA-PDT inhibited proliferation and increased cytotoxicity and
apoptosis in HeLa cells. Previous studies have demonstrated the
cytotoxic effects of ALA-PDT against skin cancer cells (17,18).
ALA-PDT has previously been demonstrated to be cytotoxic against
HeLa cervical cancer cells, suppressing tumor growth and inducing
apoptosis (19). Therefore,
ALA-PDT may represent a potential anticancer therapy for cervical
cancer.
Numerous previous studies have indicated the
potential role of miRNAs in cancer; studies in C. elegans
and Drosophila demonstrated that miRNAs regulate cell
proliferation and apoptosis, suggesting that miRNAs are associated
with proliferation-associated diseases such as cancer (20,21).
In addition, numerous miRNA genes are located in genomic regions
which are frequently mutated, with variation in these regions
commonly accompanied by the occurrence of cancer (22,23).
Furthermore, compared with normal tissue, there is differential
expression of miRNAs in malignant tumors and tumor cell lines
(24,25). The current study demonstrated that
ALA-PDT increased the expression levels of miR-143 in HeLa cells.
Liu et al (26)
demonstrated in vitro that overexpression of miR-143
significantly inhibited proliferation, promoted apoptosis and
reduced the levels of Bcl-2 in HeLa cervical cancer cells. However,
how ALA-PDT influences miR-143 expression remains to be fully
elucidated.
Apoptosis, known as programmed cell death, is the
automatic and orderly self-destruction of the cell, and is
controlled by genes, involving a series of gene activation,
expression and regulation. The Bcl-2 gene is a cancer-associated
gene with interest in the study of apoptosis, in which Bcl-2/Bax is
of particular importance (27).
The predominant biological function of Bax is to promote apoptosis,
thereby inhibiting tumorigenesis. Bcl-2 and Bax in vivo
serve a role as a dimer protein (28). Homodimers of Bcl-2 inhibit
apoptosis whilst homodimers of Bax promote apoptosis. When a
Bcl-2/Bax heterodimer is formed, Bax is able to inhibit the
anti-apoptotic function of Bcl-2 resulting in the promotion of
apoptosis (29). In the current
study, the results demonstrated that ALA-PDT reduces Bcl-2 and
increases Bax protein expression in HeLa cells. He et al
(30) demonstrated that ALA-PDT
reduced proliferation and induced apoptosis of the Mel80 cervical
cancer cell line via the suppression of Bcl-2 and the activation of
Bax. Wei et al (14)
additionally suggested that administration of ALA-PDT in
combination with low-dose cisplatin was an effective and feasible
therapy for cervical cancer though alterations in the expression of
p21, Bcl-2 and Bax.
To further investigate the potential association of
the effect of ALA-PDT on HeLa cells, miR-143 expression and the
Bcl-2/Bax signaling pathway, the present study analyzed the
anticancer effect of ALA-PDT on HeLa cells following transfection
of miR-143 and anti-miR-143 plasmids. The data collected
demonstrated that overexpression of miR-143 inhibited the Bcl-2/Bax
signaling pathway. Furthermore, downregulation of miR-143
expression reduced the anticancer effect of ALA-PDT on HeLa cells
through the upregulation of the Bcl-2/Bax signaling pathway. These
data are supported by previous studies. Liu et al (26) demonstrated that overexpression of
miR-143 significantly inhibited cell proliferation and promoted
apoptosis, through the reduction in Bcl-2 levels, with
downregulated expression of miR-143 increasing Bcl-2 levels in
cervical cancer.
In conclusion, the results of the present study
suggested that the anticancer effect of ALA-PDT may be used as a
novel therapy for the treatment of human cervical cancer, by
increasing the expression of miR-143 and downregulating the
Bcl-2/Bax signaling pathway. Further studies are required to fully
elucidate the precise anticancer effect of ALA-PDT in animal models
and as a clinical treatment.
References
|
1
|
Zeng T and Li G: MicroRNA-10a enhances the
metastatic potential of cervical cancer cells by targeting
phosphatase and tensin homologue. Mol Med Rep. 10:1377–1382.
2014.PubMed/NCBI
|
|
2
|
Shi YA, Zhao Q, Zhang LH, Du W, Wang XY,
He X, Wu S and Li YL: Knockdown of hTERT by siRNA inhibits cervical
cancer cell growth in vitro and in vivo. Int J Oncol. 45:1216–1224.
2014.PubMed/NCBI
|
|
3
|
World Health Organization (WHO): WHO
guidelines for screening and treatment of precancerous lesions for
cervical cancer prevention world health organization. WHO; Geneva,
Switzerland: 2013
|
|
4
|
Wang N, Xu Z, Wang K, Zhu M and Li Y:
Construction and analysis of regulatory genetic networks in
cervical cancer based on involved microRNAs, target genes,
transcription factors and host genes. Oncol Lett. 7:1279–1283.
2014.PubMed/NCBI
|
|
5
|
González-Quintana V, Palma-Berré L,
Campos-Parra AD, López-Urrutia E, Peralta-Zaragoza O, Vazquez-Romo
R and Pérez-Plasencia C: MicroRNAs are involved in cervical cancer
development, progression, clinical outcome and improvement
treatment response (Review). Oncol Rep. Oct 30–2015.Epub ahead of
print. View Article : Google Scholar
|
|
6
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014.PubMed/NCBI
|
|
7
|
Tarantino G, Scopacasa F, Colao A, Capone
D, Tarantino M, Grimaldi E and Savastano S: Serum Bcl-2
concentrations in overweight-obese subjects with nonalcoholic fatty
liver disease. World J Gastroenterol. 17:5280–5288. 2011.
View Article : Google Scholar
|
|
8
|
Li Z, Qu L, Zhong H, Xu K, Qiu X and Wang
E: Low expression of Mig-6 is associated with poor survival outcome
in NSCLC and inhibits cell apoptosis via ERK-mediated upregulation
of Bcl-2. Oncol Rep. 31:1707–1714. 2014.PubMed/NCBI
|
|
9
|
Divaris DX, Kennedy JC and Pottier RH:
Phototoxic damage to sebaceous glands and hair follicles of mice
after systemic administration of 5-aminolevulinic acid correlates
with localized protoporphyrin IX fluorescence. Am J Pathol.
136:891–897. 1990.PubMed/NCBI
|
|
10
|
Ji J, Fan Z, Zhou F and Wang X, Shi L,
Zhang H, Wang P, Yang D, Zhang L, Chen WR and Wang X: Improvement
of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for
skin squamous cell carcinoma. Oncotarget. 6:17135–17146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HW, Zhang LL, Miao F, Lv T, Wang XL
and Huang Z: Treatment of HPV infection-associated cervical
condylomata acuminata with 5-aminolevulinic acid-mediated
photodynamic therapy. Photochem Photobiol. 88:565–569. 2012.
View Article : Google Scholar
|
|
12
|
Kennedy JC, Pottier RH and Pross DC:
Photodynamic therapy with endogenous protoporphyrin IX: Basic
principles and present clinical experience. J Photochem Photobiol
B. 6:143–148. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin HT, Kim JH, Shim J, Lee JH, Lee DY,
Lee JH and Yang JM: Photodynamic therapy using a new formulation of
5-aminolev-ulinic acid for wrinkles in Asian skin: A randomized
controlled split face study. J Dermatolog Treat. 26:246–51. 2015.
View Article : Google Scholar
|
|
14
|
Wei XQ, Ma HQ, Liu AH and Zhang YZ:
Synergistic anticancer activity of 5-aminolevulinic acid
photodynamic therapy in combination with low-dose cisplatin on HeLa
cells. Asian Pac J Cancer Prev. 14:3023–3028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang P, Shen K, Wang X, Song H, Yue Y and
Liu T: TPX2 regulates tumor growth in human cervical carcinoma
cells. Mol Med Rep. 9:2347–2351. 2014.PubMed/NCBI
|
|
16
|
Yu Y, Zhang Y and Zhang S: MicroRNA-92
regulates cervical tumorigenesis and its expression is upregulated
by human papillo-mavirus-16 E6 in cervical cancer cells. Oncol
Lett. 6:468–474. 2013.PubMed/NCBI
|
|
17
|
Hadizadeh M and Fateh M: Synergistic
Cytotoxic Effect of Gold Nanoparticles and 5-Aminolevulinic
Acid-Mediated Photo-dynamic Therapy against Skin Cancer Cells. Iran
J Med Sci. 39:452–458. 2014.PubMed/NCBI
|
|
18
|
Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi
L, Zhou F, Chen WR, Wang H and Wang X: Stimulation of dendritic
cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget. Nov
26–2015.Epub ahead of print.
|
|
19
|
Gui T, Wang Y, Mao Y, Liu J, Sun S, Cao D,
Yang J and Shen K: Comparisons of 5-aminolevulinic acid
photodynamic therapy and after-loading radiotherapy in vivo in
cervical cancer. Clin Transl Oncol. 15:434–442. 2013. View Article : Google Scholar
|
|
20
|
Koczorowska MM, Kwasniewska A and
Gozdzicka-Jozefiak A: IGF1 mRNA isoform expression in the cervix of
HPV-positive women with pre-cancerous and cancer lesions. Exp Ther
Med. 2:149–156. 2011.PubMed/NCBI
|
|
21
|
Masliah-Planchon J, Garinet S and Pasmant
E: RAS-MAPK pathway epigenetic activation in cancer: miRNAs in
action. Oncotarget. Dec 5–2015.Epub ahead of print. PubMed/NCBI
|
|
22
|
Ju H, Yang Y, Sheng A and Jiang X: Role of
microRNAs in skeletal muscle development and rhabdomyosarcoma
(review). Mol Med Rep. 11:4019–4024. 2015.PubMed/NCBI
|
|
23
|
Jamali Z, Asl Aminabadi N, Attaran R,
Pournagiazar F, Ghertasi Oskouei S and Ahmadpour F: MicroRNAs as
prognostic molecular signatures in human head and neck squamous
cell carcinoma: A systematic review and meta-analysis. Oral Oncol.
51:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nair VS, Maeda LS and Ioannidis JP:
Clinical outcome prediction by microRNAs in human cancer: A
systematic review. J Natl Cancer Inst. 104:528–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang X and Jia Z: Construction of
HCC-targeting artificial miRNAs using natural miRNA precursors. Exp
Ther Med. 6:209–215. 2013.PubMed/NCBI
|
|
26
|
Liu L, Yu X, Guo X, Tian Z, Su M, Long Y,
Huang C, Zhou F, Liu M, Wu X and Wang X: miR-143 is downregulated
in cervical cancer and promotes apoptosis and inhibits tumor
formation by targeting Bcl-2. Mol Med Rep. 5:753–760. 2012.
|
|
27
|
Qiao WL, Wang GM, Shi Y, Wu JX, Qi YJ,
Zhang JF, Sun H and Yan CD: Differential expression of Bcl-2 and
Bax during gastric ischemia-reperfusion of rats. World J
Gastroenterol. 17:1718–1724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu LH, Zhou YJ, Ding L, Zhang SZ, Sun J
and Cao JG: Induction of apoptosis by VB1 in breast cancer cells:
The role of reactive oxygen species and Bcl-2 family proteins. Int
J Mol Med. 33:423–430. 2014.
|
|
29
|
Deng B, Zhang XF, Zhu XC, Huang H, Jia HL,
Ye QH, Dong QZ and Qin LX: Correlation and prognostic value of
osteopontin and Bcl-2 in hepatocellular carcinoma patients after
curative resection. Oncol Rep. 30:2795–2803. 2013.PubMed/NCBI
|
|
30
|
He GF, Bian ML, Zhao YW, Xiang Q, Li HY
and Xiao C: A study on the mechanism of 5-aminolevulinic acid
photodynamic therapy in vitro and in vivo in cervical cancer. Oncol
Rep. 21:861–868. 2009.PubMed/NCBI
|