Introduction
Diabetic retinopathy (DR) is the most common chronic
microvascular complication of diabetes mellitus (DM) and is a
leading cause of visual loss among working-age individuals. DR is
an ocular manifestation of DM, affecting ≤80% of all patients that
have had DM for ≥10 years (1). The
normal functions of the retinal vasculature, neurons and resident
glial cells are affected by DR. Several factors, including
hyperglycaemia, advanced glycation end-products (AGEs) and
cytokines, including vascular endothelial growth factor, have been
implicated in the disease pathogenesis (2). Although the mechanism of DR has not
been fully elucidated, it is understood that the oxidative damage
induced by these factors contributes to the development of DM and
DR (3). In summary, decreasing
oxidative damage may be a therapeutic strategy for DR.
Thioredoxin (Trx) is a 12-kDa protein with a
redox-active dithiol in its active site, -Cys-Gly-Pro-Cys-. It is a
ubiquitous antioxidant enzyme with an essential role in various
cellular functions. Trx was first identified in Escherichia
coli in 1964 by Laurent et al (4). Trx has different forms depending on
its cellular environment and is a major antioxidant important for
the maintenance of redox balance within the cell (5). Furthermore, Trx is important in
anti-apoptotic signalling and regulates the expression of certain
transcription factors. It is also a critical regulator of apoptosis
signal-regulating kinase 1 (ASK1) function.
Pyridoxamine (PM), a vitamin B6 metabolite, has been
demonstrated to be a potent inhibitor of AGE formation in
vitro and in animal models (6). PM was previously demonstrated to
inhibit several oxidative and glycoxidative pathways that can cause
protein damage (7). Additionally,
PM is a prospective drug for the treatment of diabetic nephropathy
(8). However, little has been
reported regarding the effects of PM on the retina.
Based on previous studies, the present study treated
type II diabetic (T2D) mice exposed to light damage with PM to
investigate the protective effects and mechanism of action of PM on
light-induced retinal photoreceptor cell damage in diabetic
mice.
Materials and methods
Animals
All experimental procedures were conducted in
accordance with institutional guidelines for the Care and Use of
Laboratory Animals, and protocols were approved by the
Institutional Animal Care and Use Committees of Dalian Medical
University Laboratory Animal Center (Dalian, China). A total of 42
male inbred BALB/c mice (Dalian Medical University Laboratory
Animal Center) weighing ~35–45 g, aged 6 weeks, were housed in an
animal colony facility for 2 weeks, with 6 mice per cage in each
group. The animals were maintained in a room with a constant
temperature (22±2°C). All animals were born and raised in a 12-h
on/off cyclic light environment at average (mean) illumination of
80 lx. Tap water and food pellets (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) were available ad
libitum. After normal feeding for 4 weeks, mice in the T2D
group (n=6) were injected intraperitoneally with 80 mg/kg
streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA) dissolved
in cold 50 mM citric acid buffer (pH 4.5; Beijing Solarbio Science
& Technology Co., Ltd.) and fed a high fat diet (10% lard, 20%
yolk, 1% cholesterol, 0.5% cholate, 20% sucrose and 48.5% standard
diet) to induce T2D. When the fasting blood glucose (FBG; measured
using a glucometer; Sinocare, Inc., Changsha, China) levels of the
mice reached 11.1 mmol/l, the model was considered to be
successfully established. Experiments were conducted between 10:00
a.m. and 2:00 p.m. PM (Sigma-Aldrich) was dissolved in normal
saline and injected intraperitoneally at concentrations of 25 mg/kg
(PMI group), 50 mg/kg (PMII group) and 100 mg/kg (PMIII group). The
light damage was performed at 5,000 lux for 72 h. Animals were
sacrificed by CO2 asphyxiation after treatment.
HOMA-IR of serum insulin
Fasting serum insulin (FIN) levels were detected
using an enzyme-linked immunosorbant assay kit (cat. no. BP-E20353;
Shanghai Lengton Bioscience Co., Ltd., Shanghai, China). The
insulin resistance index was determined based on the following
equation: HOMA-IR = (FBG × FIN) / 22.5.
Morphological analysis by quantitative
histology
The enucleated eyes of each mouse were immersed in
4% paraformaldehyde, containing 20% isopropanol, 2% trichloroacetic
acid and 2% zinc chloride, for 24 h and then in 70% ethanol for
24–60 h. Following alcohol dehydration, the eyes were embedded in
paraffin and 5 µm-thick sagittal sections containing the
entire retina, including the optic disc, were sliced. The retinal
sections were stained with hematoxylin-eosin (Beijing Solarbio
Science & Technology Co., Ltd.). In each of the superior and
inferior hemispheres, the outer nuclear layer (ONL) thickness was
measured at nine defined points. Each point was centered on
adjacent 220-µm lengths of the retina. The first point of
measurement was ~220 µm from the optic nerve head, and
subsequent measurement points were located more peripherally. The
mean ONL thickness was determined based on 18 measurement points in
each section.
Western blotting
Western blot analysis was performed as previously
described (9). Briefly, retinas
were lysed in radioimunoprecipitation assay buffer, and protein was
quantified using a bicinchoninic acid kit (both purchased from
Beyotime Institute of Biotechnology, Haimen, China). Then, protein
was loaded (30 µg protein/lane), separated by 10% SDS-PAGE
and transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
non-fat milk for 1 h at room temperature and were then incubated
overnight at 4°C with the following primary antibodies: Monoclonal
rabbit phospho-extracellular signal-regulated kinase 1/2 (p-Erk1/2;
1:1,000 dilution; cat. no. 4376; Cell Signaling Technology, Inc.,
Danvers, MA, USA); polyclonal mouse nuclear factor erythroid
2-related factor 2 (Nrf2; 1:1,000 dilution; cat. no. sc-722; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA); and monoclonal β-actin
(1:1,000 dilution; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc.). The membranes were washed three times with 1X Tris-buffered
saline-0.1% Tween 20 (TBS-T; Beijing Solarbio Science &
Technology Co., Ltd.) for 10 min. Subsequently, the membranes were
incubated with goat anti-rabbit or goat anti-mouse horseradish
peroxidase-conjugated IgG for 1 h at room temperature and washed 3
times with 1X TBS-T for 15 min. The membranes were then developed
using an enhanced chemiluminescence system and exposed to X-ray
film (both purchased from Beijing Solarbio Science & Technology
Co., Ltd.). The intensities of the bands were measured using
LabWorks 4.5 software (Perkin Elmer, Waltham, MA, USA). All primary
and secondary antibodies were diluted in TBS-T with 2.5% non-fat
dry milk.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was obtained from each retina sample using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). RT was performed with the PrimeScript RT Reagent kit (Perfect
Real Time; Takara Bio, Inc., Otsu, Japan). RT-qPCR was performed to
measure Trx mRNA expression using SYBR Premix DimerEraser (Takara
Bio, Inc.), with reverse-transcribed cDNA as the template. All PCR
reactions were conducted in a final volume of 20 µl. The
amplification was performed using an ABI Prism 7000 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) under the following conditions: 95°C for 30 sec followed by
40 cycles of 95°C for 3 sec, 72°C for 30 sec and 55°C for 30 sec.
GAPDH was used as an internal reference gene. The primers used were
as follows: Forward, 5′-GGA ATG GTG AAG CAG ATC GAG-3′ and reverse,
5′-ACG CTT AGA CTA ATT CAT TAAT-3′ for Trx; forward, 5′-TGT GAT GGG
TGT GAA CCA CGA GAA-3′ and reverse, 5′-GAG CCC TTC CAC AAT GCC AAA
GTT-3′ for GAPDH. The 2−ΔΔCq method was used to quantify
the results (10).
Immunohistochemical analysis
Paraffin-embedded retinal sections (5 µm)
were deparaffinised, rehydrated and subjected to antigen retrieval
by boiling the sections in 10 mmol/l citrate buffer (pH 6.0;
Beyotime Institute of Biotechnology) for 20 min and washing twice
with water and twice with phosphate buffered saline (PBS). Sections
were then blocked with bovine serum albumin (Beyotime Institute of
Biotechnology) for 20 min and incubated with primary anti-ASK1
rabbit monoclonal antibody (dilution 1:50; cat. no. ab45178; Abcam,
Cambridge, UK) at 4°C overnight. For the analysis of ASK1
expression, after rinsing twice with PBS for 5 min, the sections
were incubated with peroxidase-conjugated goat anti-rabbit
immunoglobulin (dilution, 1:200; cat. no. ZB-2031; ZSGB-BIO,
Beijing, China) at room temperature for 1 h. The sections were
finally rinsed twice for 5 min in PBS and stained with
3,3′-diaminobenzidine (ZSGB-Bio). Image Pro Plus version 5.0
(Olympus Corporation, Tokyo, Japan) was used to analyze the
integrated optical density index for each group.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The statistical analyses were performed using one-way
analysis of variance for continuous variables. SPSS software
version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for all of the
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
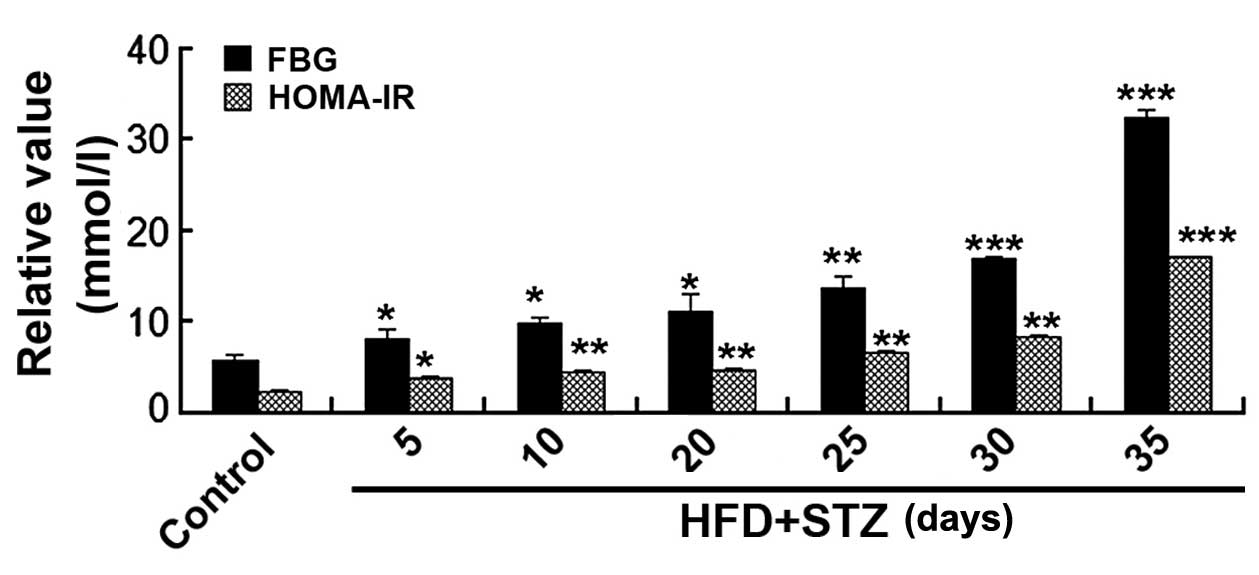
FBG, homeostatic model assessment-insulin
resistance (HOMA-IR) and establishment of the T2D mouse model
FBG levels and HOMA-IR were significantly increased
5 days after STZ injection (P<0.05). The T2D animal model was
successfully constructed at day 20 after STZ injection, when FBG
levels reached 11.1 mmol/l (Fig.
1).
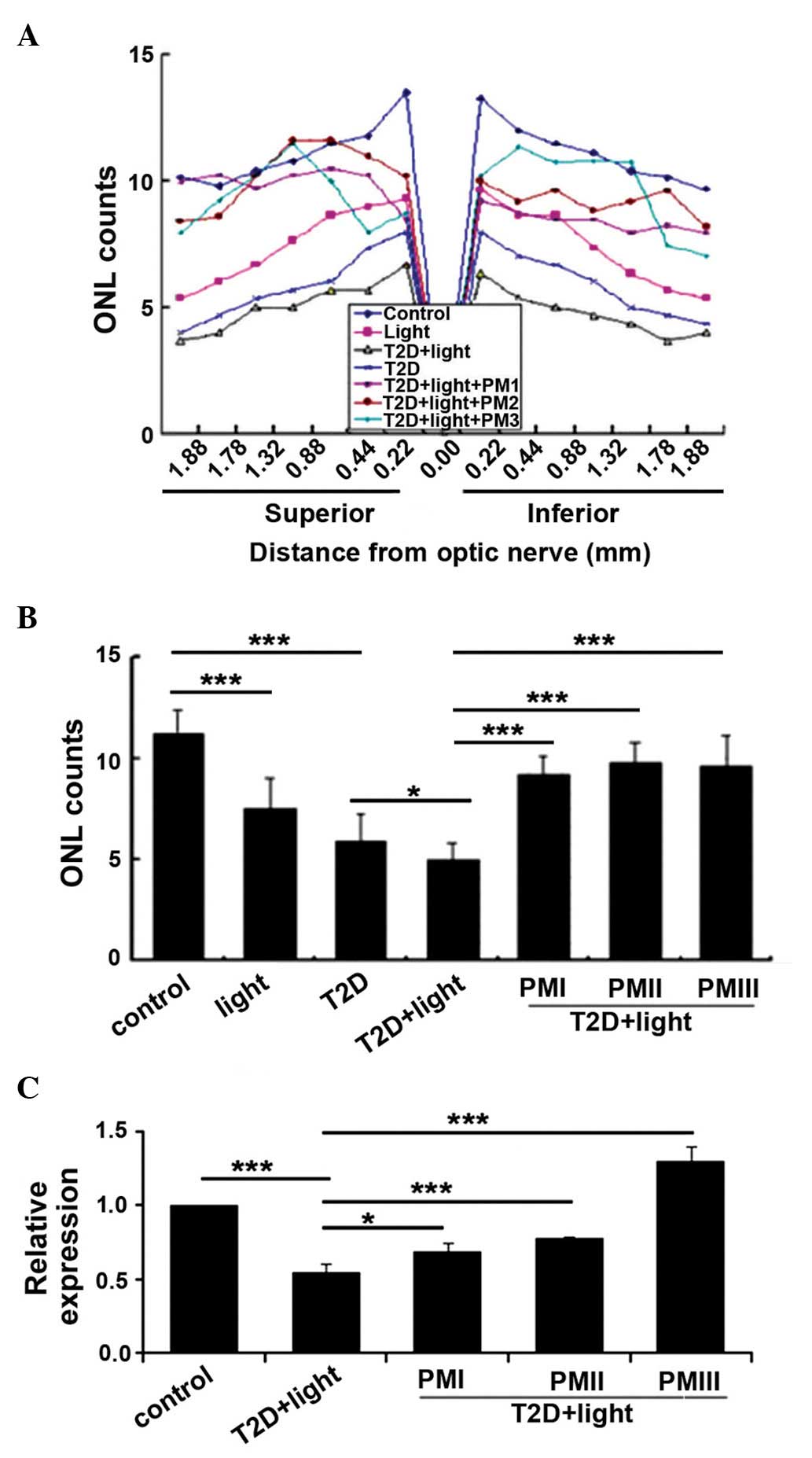
PM protects the retinal photoreceptor
cells of mice with T2D
To investigate the protective effects of PM on the
retinal photoreceptor cells of T2D mice, T2D mice were treated with
different concentrations of PM (25, 50 or 100 mg/kg). Following
treatment, morphological observation was conducted in all groups.
The mean total number of photoreceptor cells, measured at
equidistant loci of the superior and inferior retina, was decreased
in T2D mice compared with the control mice (P<0.001), whereas
the numbers in the PM-treated groups were increased compared with
the T2D + light group (P<0.001; Fig. 2A and B). To explore this change in
association with gene expression levels, RT-qPCR was performed to
measure Trx mRNA expression. Trx mRNA expression levels were
significantly decreased in the T2D + light group compared with
control mice (P<0.001), whereas, compared with T2D+ light mice,
Trx expression was significantly increased following PM treatment
(25 mg/kg, P<0.05; 50 or 100 mg/kg, P<0.001; Fig. 2C).
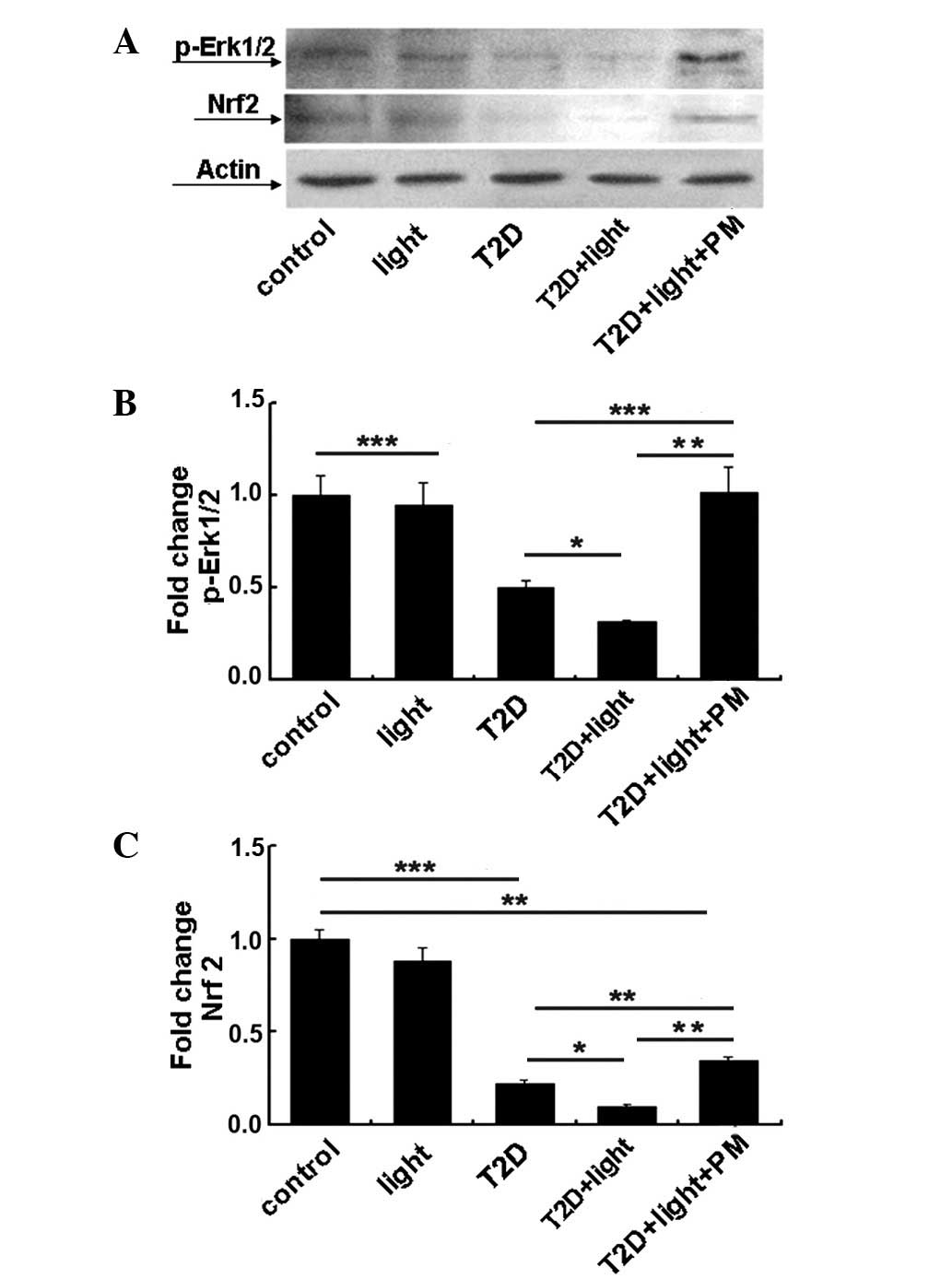
PM effects the expression of signalling
pathway proteins
To explore the protective effects and mechanism of
PM, western blotting and immunohistochemistry were performed to
detect the expression of p-Erk1/2 and Nrf2, and ASK1, respectively.
It was observed that p-Erk1/2 and Nrf2 protein expression levels
were significantly increased following PM treatment compared with
the levels in untreated T2D + light mice (P<0.01; Fig. 3). By contrast, ASK1 expression
levels were significantly decreased following PM treatment compared
with untreated T2D + light mice (P<0.001; Fig. 4). The results of the present study
indicate that PM can protect retinal photoreceptor cells in T2D
mice and that the mechanism of PM is associated with the
upregulation of p-Erk1/2 and Nrf2 expression, and the
downregulation of ASK1 expression.
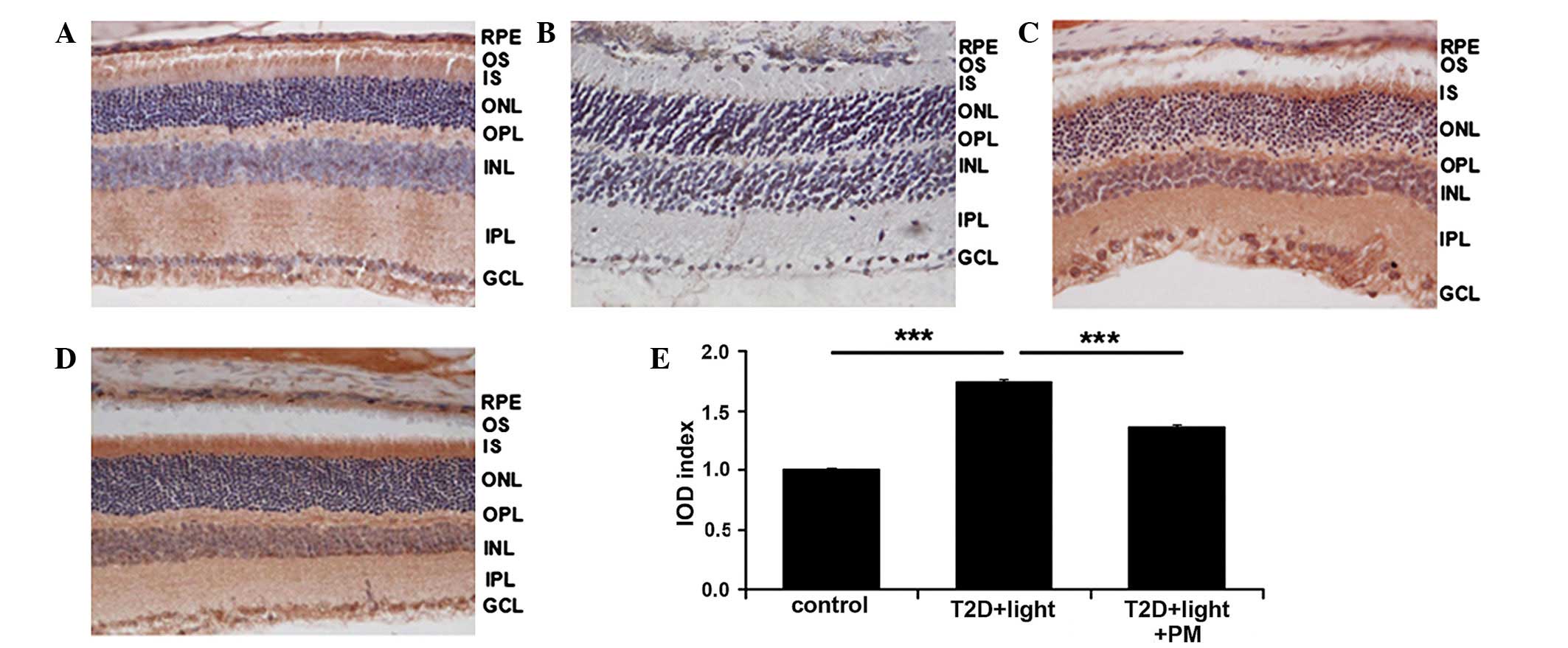 | Figure 4PM (50 mg/kg) increases the expression
of antioxidant-associated proteins in the retinas of T2D mice.
Immunohistochemical staining of retinal sections for apoptosis
signal-regulating kinase 1 (ASK1) from (A) the control, (B) PBS
treatment, (C) T2D + light treatment and (D) T2D + light + PM
treatment groups. Magnification, ×400; 3,3′-diaminobenzidine
staining. (E) IOD was measured and demonstrated that PM treatment
significantly decreases the protein expression levels of ASK1. Data
are presented as the mean ± standard error of the mean, repeats =
3. ***P<0.001. RPE, retinal pigment epithelium; OS,
outer segment; IS, inner segment; ONL, outer nuclear layer; OPL,
outer plexiform layer; INL, inner nuclear layer; IPL, inner
plexiform layer; GCL, ganglion cell layer; IOD, integrated optical
density; T2D, type 2 diabetes; PM, pyridoxamine. |
Discussion
Since 1966, when Noell established the rat retinal
light damage animal model, researchers have used different types of
animals and light conditions to cause retinal damage (11). The light damage model is a well
established animal model for the analysis of retinal degeneration
diseases (11). As the development
of DR is a slow process, the present study employed a light damage
model to accelerate the process during experimentation.
The present study observed that a decrease in the
number of retinal photoreceptor cells following STZ injection
continued over time. The data demonstrated that DR is a
neurodegenerative disease. Additionally, DR is understood to be a
multifactorial disease that involves a variety of signalling
pathways and active substances. Thus, it is important to identify
novel preventive and therapeutic methods for controlling DR.
A number of studies have reported the existence of
pyridoxine (P) deficiency in T1D and T2D patients, and in
experimental diabetes models (12,13).
P is rapidly taken up by red blood cells, and converted to PM and
pyridoxal phosphate (PP). P, PP and PM form the vitamin B6 compound
group and are interconvertible within the cell. The biochemical
mechanism by which PM exerts its beneficial effects against
cellular damage in diabetes is unclear. Thus, the present study
used PM to treat T2D mice to investigate the mechanisms of
action.
The mitogen-activated protein kinase (MAPK)
signalling pathway is comprised of three subfamilies: c-Jun
N-terminal kinase, Erk1/2 and p38MAPK. These pathways are known to
be activated by oxidative stress. Deviation from the strict
regulation of MAPK signalling via oxidative stress can cause the
development of human diseases, including various neurodegenerative
diseases, DM and cancer (9).
Furthermore, the activity of ASK1, a member of MAPK kinase kinase
family, is regulated by Trx.
Erk1/2 are a major MAPK subfamily and their
signalling is recognized as an important pathway in the
transduction of extracellular signals to induce cellular responses.
Additionally, Erk1/2 are involved in various physiological effects
and pathological processes (14,15).
Numerous cellular activities, including proliferation,
differentiation and development, are associated with the Erk1/2
signalling pathway (16). However,
inappropriate and continuous pathway activation contributes to
oncogenesis, diabetic complications and angiogenesis (17). In previous studies, sulforaphane
(SF), an inducer of phase II detoxification enzymes and an
inhibitor of phase I enzymes, has been demonstrated to inhibit
retinal degeneration (9).
Furthermore, increased expression of Nrf2, a transcription factor
that binds to the antioxidant responsive element, also protects
retinal cells from oxidative stress by inducing phase II enzymes
(5). Trx induction is mediated by
Erk signalling. The Erk signalling pathway has previously been
demonstrated to be involved in the SF-mediated upregulation of
Trx/Trx reductase 1/Nrf2 expression in vivo (9). Thus, the present study investigated
the expression of proteins associated with this pathway. The data
of the current study demonstrated that PM significantly increases
the expression of Trx, Nrf2 and p-Erk1/2 compared with untreated
T2D mice. These data indicate that PM exerts effects similar to
those of antioxidant enzymes by upregulating the expression of a
phase II enzyme (Trx) and Nrf2.
In conclusion, the results of the present study
demonstrated that PM protects retinal photoreceptor cells in T2D
mice exposed to light damage and that its protective mechanism of
action may be associated with the upregulation of Trx, p-Erk1/2 and
Nrf2, and downregulation of ASK1.
Acknowledgments
The present work was supported by grants from the
National Nature Science Foundation of China (nos. 30850001,
31371218 and 31300812).
References
|
1
|
Stitt AW, Lois N, Medina RJ, Adamson P and
Curtis TM: Advances in our understanding of diabetic retinopathy.
Clin Sci (Lond). 125:1–17. 2013. View Article : Google Scholar
|
|
2
|
Tarr JM, Kaul K, Chopra M, Kohner EM and
Chibber R: Pathophysiology of diabetic retinopathy. ISRN
Ophthalmol. 2013:3435602013.
|
|
3
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laurent TC, Moore EC and Reichard P:
Enzymatic synthesis of deoxyribonucleotides. Iv. Isolation and
characterization of thioredoxin, the hydrogen donor from
Escherichia coli B. J Biol Chem. 239:3436–3444. 1964.PubMed/NCBI
|
|
5
|
Johnson J, Maher P and Hanneken A: The
flavonoid, eriodictyol, induces long-term protection in ARPE-19
cells through its effects on Nrf2 activation and phase 2 gene
expression. Invest Ophthalmol Vis Sci. 50:2398–2406. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turgut F and Bolton WK: Potential new
therapeutic agents for diabetic kidney disease. Am J Kidney Dis.
55:928–940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voziyan PA, Metz TO, Baynes JW and Hudson
BG: A post-Amadori inhibitor pyridoxamine also inhibits chemical
modification of proteins by scavenging carbonyl intermediates of
carbohydrate and lipid degradation. J Biol Chem. 277:3397–3403.
2002. View Article : Google Scholar
|
|
8
|
Williams ME, Bolton WK, Khalifah RG,
Degenhardt TP, Schotzinger RJ and McGill JB: Effects of
pyridoxamine in combined phase 2 studies of patients with type 1
and type 2 diabetes and overt nephropathy. Am J Nephrol.
27:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruta LM, Magliano DJ, Lemesurier R, Taylor
HR, Zimmet PZ and Shaw JE: Prevalence of diabetic retinopathy in
Type 2 diabetes in developing and developed countries. Diabetic
Med. 30:387–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
11
|
Organisciak DT, Darrow RM, Barsalou L,
Darrow RA, Kutty RK, Kutty G and Wiggert B: Light history and
age-related changes in retinal light damage. Invest Ophthalmol Vis
Sci. 39:1107–1116. 1998.PubMed/NCBI
|
|
12
|
Jain SK and Lim G: Pyridoxine and
pyridoxamine inhibits superoxide radicals and prevents lipid
peroxidation, protein glycosylation, and (Na+ +
K+)-ATPase activity reduction in high glucose-treated
human erythrocytes. Free Radic Biol Med. 30:232–237. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marcucci R, Zanazzi M, Bertoni E, Rosati
A, Fedi S, Lenti M, Prisco D, Castellani S, Abbate R and Salvadori
M: Vitamin supplementation reduces the progression of
atherosclerosis in hyperhomocysteinemic renal-transplant
recipients. Transplantation. 75:1551–1555. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
15
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan Y, Ichikawa T, Li J, Si Q, Yang H,
Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L and Cui T: Diabetic
downregulation of Nrf2 activity via ERK contributes to oxidative
stress-induced insulin resistance in cardiac cells in vitro and in
vivo. Diabetes. 60:625–633. 2011. View Article : Google Scholar : PubMed/NCBI
|