Introduction
Percutaneous coronary intervention (PCI) is
breakthrough for the treatment of ischemic heart disease. It is
also one of the most effective methods used in coronary disease.
However, the vessel wall damage and inflammatory reaction resulting
from stent placement results in restenosis and affects the
long-term curative effects of PCI (1). Multiple studies have demonstrated
that vascular remodeling is a complex dynamic process that is
closely associated with numerous factors such as inflammation,
proliferation and apoptosis of smooth muscle cells (SMCs),
endothelial dysfunction and thrombosis (2). Endothelial cell damage is followed by
restenosis and constitutes the pathological foundation for vascular
SMC proliferation, migration, apoptosis, in addition to synthesis
and degradation of the extracellular matrix (3). Restenosis subsequent to coronary
artery stent implantation is a process involving intimal
hyperplasia and vascular remodeling following vessel damage. The
vessel smooth muscle is in the tunica media surrounded by the cell
matrix, which can act as a biological and mechanical barrier and
leads to smooth muscle contraction, in order to avoid movement.
When the vessel is damaged, inflammatory mediators are released and
induce certain types of SMCs to migrate and gather in the tunica
intima (4). These cells
proliferate and secrete extracellular matrix, promoting new tunica
intima formation for vessel reconstruction. Vascular SMC migration
is the pathological foundation of common vasculopathies including
atherosclerosis, angioplasty and restenosis following coronary
artery stent implantation (5).
Therefore, investigation into the mechanism of SMC migration is
critical to the effective treatment of vascular diseases and the
prevention of restenosis subsequent to coronary artery stent
implantation.
The different functions that SMCs can exert result
in the presence of diverse SMC phenotypes, ranging from contractile
to synthetic, which exhibit differing morphologies, expression
levels of SMC marker genes, proliferative potential and migratory
properties (6). The heterogeneity
of vascular SMCs has been previously demonstrated (7). Contractile and synthetic SMCs
represent the two ends of a spectrum of SMCs with intermediate
phenotypes (8). Morphologically,
contractile SMCs are elongated and spindle-shaped, whereas
synthetic SMCs are less elongated (8). In addition, synthetic SMCs in general
grow faster and exhibit higher levels of migratory acivity than
contractile SMCs (9).
Gap junctions are clustered channels between
contacting cells through which direct intercellular communication
via diffusion of ions and metabolites can occur (10). These structures exist in almost all
mammalian tissues, where they predominantly mediate ion and
chemical material delivery and promote coupling of different cell
types (11,12). Gap junctions are formed by
cell-specific expression patterns of the vascular connexins Cx37,
Cx40, Cx43 and Cx45 (13). Cx37
and Cx40 are expressed by vascular endothelial cells and the
majority of SMCs express Cx43, with little Cx37 and Cx45
expression, with only certain SMCs expressing Cx40 (14). These observations suggest an
association between these connexins and the specific properties
and/or phenotypes of the SMCs, although this remains to be
experimentally demonstrated.
The present study aimed to assess the relevance of
Cx43 in the intimal hyperplasia of coronary arteries by evaluating
the association between the signal transduction protein Cx43 and
SMC phenotypic transformation in porcine coronary arteries.
Materials and methods
Porcine coronary artery samples
All animal procedures were approved by the Animal
Care Committee of the East Hospital at Tongji University. A total
of 15 pigs Shanghai White pigs (gender, 6 female and 9 male; age
range, 3–4 months; weight, 35–40 kg) were purchased from Shanghai
Animal Administration Center. The stent implantation procedure was
performed in the Zhongshan Hospital (Zhongshan, China). Bare metal
stents [MicroPort NeuroTech (Shanghai) Co., Ltd., Shanghai, China]
were implanted into the right anterior descending coronary artery,
left anterior descending artery or left circumflex artery of
3-month-old pigs (n=5 per group). Pigs with restenosis were
confirmed by coronary angiography (15) 1 month subsequent to stent
placement, and were sacrificed subsequent to anesthesia. Restenosis
coronary artery samples were then collected for histology or SMC
isolation from the tunica media. Control 3-month-old pigs were
sacrificed by an intravenous injection of KCl following anesthesia
with ketamine and diazepam. Subsequently, the anterior descending,
circumflex and right coronary arteries were separated. Tunica media
cells were isolated from the arteries and cultured.
Primary cell culture and subculture
Primary cells were isolated using tissue
explantation and trypsin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) enzymatic digestion as described previously
(8). SMCs were isolated into two
groups (n=6) and maintained in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 20% fetal
calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc.) in a
humidified environment at 37°C with 5% CO2. Subsequent
to 3 days incubation, rhomboid-shaped cells grew from the edges of
explants, in a radial pattern. Following 7 days of culture, tissue
fragments were removed. The remaining SMCs reached near confluence
following 7–10 days incubation, exhibiting two distinct populations
under an inverted microscope (Leica DMI3000 B, Leica Microsystems,
Wetzlar, Germany): Spindle-shaped and rhomboid cells as previously
described (7). The two cell types
were separately seeded and proliferation rates were monitored by
cell counting (Scepter 2.0, Merck Millipore, Ltd., Carrigtwohill,
Ireland).
Platelet-derived growth factor (PDGF)
treatment of SMCs from normal coronary arteries
SMC primary cultures were maintained in DMEM
supplemented with 20% FCS. At 90% confluence, cells were seeded
into individual wells of 6-well tissue culture plates subsequent to
trypsin digestion and incubation for 24 h at 37°. Cultured SMCs
were treated with 10 ng/ml PDGF-BB (Roche Diagnostics GmbH,
Mannheim, Germany) for 24 h, followed by incubation in the presence
or absence of a gap junction blocker (100 μmol/l
18α-glycyrrhetinic acid; Sigma-Aldrich, St. Louis, MO, USA) for 48
h.
Immunohistochemistry
Cultured porcine SMCs were washed twice with
phosphate-buffered saline (PBS; Corning, New York, NY, USA) and
fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min.
Subsequent to incubation with primary (anti-Cx40, anti-Cx43,
anti-S100A4 and anti-α-smooth muscle actin (SMA); Abcam, Cambridge,
MA, USA) and polymer helper and poly-peroxidase anti-mouse/rabbit
immunoglobulin G secondary antibodies (PV-9000 kit; GBI Labs,
Mukilteo, WA USA) were added for 1 h, and diaminobenzidine (Roche
Diagnostics GmbH) was used for signal detection. The slides were
washed under running water and tissue samples were counterstained
with hematoxylin. Sections were observed and imaged using an
Olympus CX31 microscope (Olympus Corporation, Tokyo, Japan).
Western blotting
Western blotting of proteins extracted from coronary
artery SMCs was performed as described previously (16,17).
Briefly, proteins were separated by sodium dodecyl
sulfate-polyacrylimide gel electrophoresis (10%; Beyotime Institute
of Biotechnology Co., Shanghai, China) and electroblotted onto
nitrocellulose membranes (Merck Millipore, Ltd.). Subsequent to
blocking in TBST containing 5% w/v fat-free milk, membranes were
incubated 1 h each with the primary antibodies rabbit anti-pig
polyclonal anti-Cx43 (GJA1; cat. no., ab11370; dilution, 1:1,000;
Abcam), rabbit anti-pig polyclonal anti-Cx40 (GJA5; cat. no.,
ab38580; dilution, 1:1,000; Abcam), rabbit anti-pig polyclonal
anti-α-SMA (cat. no., ab5694; dilution, 1:1,000; Abcam), rabbit
anti-pig polyclonal anti-S100A4 (cat. no., ab27957; dilution,
1:1,000; Abcam) and mouse anti-pig monoclonal anti-β-actin (cat.
no. ab10024; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing,
China), and the HRP-conjugated goat anti-rabbit or anti-mouse
secondary antibodies (cat. nos., sc-2030 or sc-2302; dilution,
1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) secondary
antibody at 37°. The Enhanced Chemiluminescence reagent kit (Pierce
Biotechnology, Inc., Rockford, IL, USA) was used for detection and
the membranes were exposed to film for autoradiography (Allura xper
F D 20; Philips Medical Systems Nederland B.V., Eindhoven, The
Netherlands).
Immunofluorescence and hematoxylin-eosin
staining
Serial cryosections (section thickness, 6
μm;) were obtained using Leica CM3050 S (Leica Microsystems)
from coronary artery great vessels with stent implantation or
normal controls. Sections were stained with hematoxylin and eosin
(Beyotime Institute of Biotechnology Co.,) and examined by light
microscopy.
For immunofluorescent labeling, coverslips were
incubated overnight with the rabbit anti-pig polyclonal anti-Cx43,
rabbit anti-pig polyclonal anti-Cx40, rabbit anti-pig polyclonal
anti-α-SMA, rabbit anti-pig polyclonal anti-S100A4 and mouse
anti-pig monoclonal anti-β-actin (cat. no., ab10024; Beijing
Biosynthesis Biotechnology Co., Ltd.) primary antibodies at a
dilution of 1:1,000, followed by fluorescein isothiocyanate
(FITC)-conjugated polyclonal goat anti-rabbit secondary antibody
(cat. no. bs-0295G; Bioss Antibodies, Woburn, MA, USA) and rabbit
anti-mouse secondary antibody (cat. no. bs-0368R; Bioss Antibodies)
for 4 h at 37°. All steps were performed at room temperature and
the cells were washed with PBS in between the steps. Cells were
examined under a fluorescence microscope (FV300; Olympus
Corporation) equipped with the appropriate filters.
Flow cytometry analysis
Subsequent to three washes in PBS, 1×106
SMCs were incubated with the primary antibodies for 30 min at room
temperature. Following another washing step cells were incubated
with the FITC-labeled secondary antibody for 30 min at room
temperature. Then, cells were washed three times with PBS and
resuspended. Cell fluorescence was analyzed on a FACSCalibur flow
cytometer with CellQuest software version 5.1 (BD Biosciences,
Franklin Lakes, CA, USA). Gates for forward and side scatter
measurements were set and a total of 10,000 events were acquired
per sample.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 50–100 mg of tissue
using the TRI Reagent (Molecular Research Center, Inc., Cincinnati,
OH, USA); RNA concentrations were determined by spectrophotometry
at 260 nm. RT was conducted using the M-MLV1 reverse transcription
kit (Promega Corporation, Madison, WI, USA). First strand cDNA was
synthesized from 1 μg RNA using oligo (dT) 12–18 primers.
Then, RT-qPCR was performed in triplicate using FastStart Universal
SYBR Green Master (Rox) from Roche Diagnostics GmbH for 15 min at
95°C for initial denaturation, followed by 40 cycles of 95°C for 30
sec and 60 C for 30 sec on an ABI PRISM 7900HT Fast Real Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. The primer pairs used
are presented in Table I. The
quantification of target gene relative expression was conducted
using the ΔCq method (18), with
glyceraldehyde 3-phosphate dehydrogenase as the internal control.
All reactions were performed in triplicate.
 | Table IPrimers pairs used in reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers pairs used in reverse
transcription-quantitative polymerase chain reaction.
| Target | Primer sequences | Length (bp) | Tm (°C) |
|---|
| CX43 |
5′-CTGAGCCCCTCCAAAGACTG-3′ | 101 | 60.04 |
|
5′-TTGTATCCGGGAGGGGACAT-3′ | | 60.03 |
| CX40 |
5′-GGACAAGCTCTTCGGCTTCT-3′ | 126 | 60.04 |
|
5′-TCGCTGGTACAGGTCGAGTA-3′ | | 60.04 |
| GAPDH |
5′-GGAGAACGGGAAGCTTGTCA-3′ | 138 | 59.97 |
|
5′-GCCTTCTCCATGGTCGTGAA-3′ | | 60.04 |
Statistical analysis
Prism version 5.0 (GraphPad Software, Inc., San
Diego, CA, USA) was used for analysis. The data are presented as
the mean ± standard deviation and the one-way analysis of variance
was used for comparisons among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Primary culture of porcine artery
cells
Subsequent to trypsin digestion, two distinct
phenotypes of normal coronary artery SMCs were isolated: Rhomboid
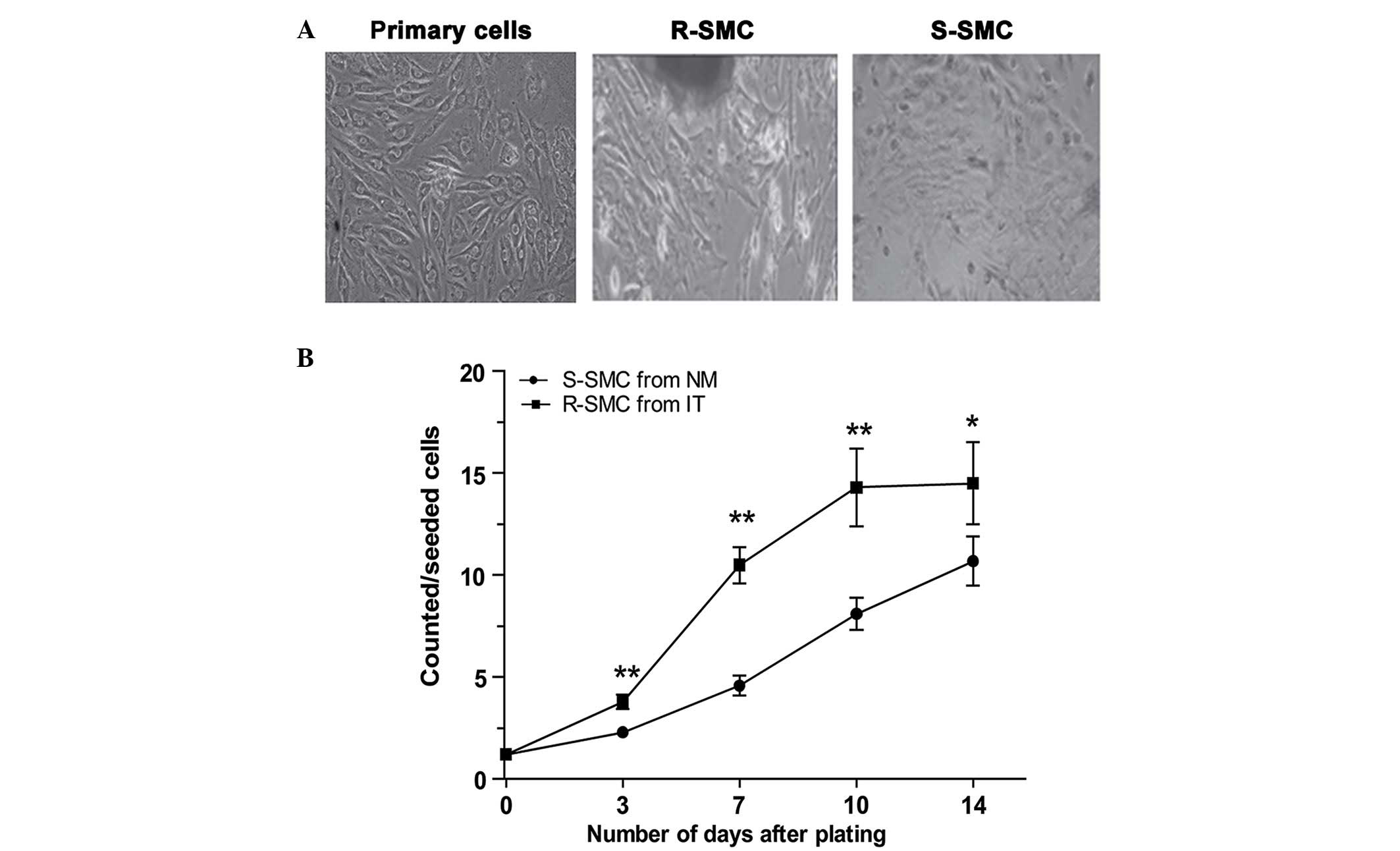
and spindle-shaped. As presented in Fig. 1, the in vitro proliferation
rate was different for the two cell types. Spindle-shaped SMCs
(S-SMCs), the major constituents of regular coronary artery tissue,
grew in a ''hills-and valleys'' pattern, with a significantly lower
proliferation rate. In contrast, rhomboid SMCs (R-SMCs) grew in a
monolayer or multilayer pattern, with a faster proliferation
rate.
The expression of gap junction proteins
Cx40 and Cx43 is associated with the SMC type
The expression of two types of gap junction
proteins, Cx40 and Cx43, was assessed in the isolated SMCs. In
primary SMC cultures, where S-SMCs represent the majority of cells,
high expression levels of Cx40 protein were detected by
immunochemical staining, and this was confirmed by the amount of
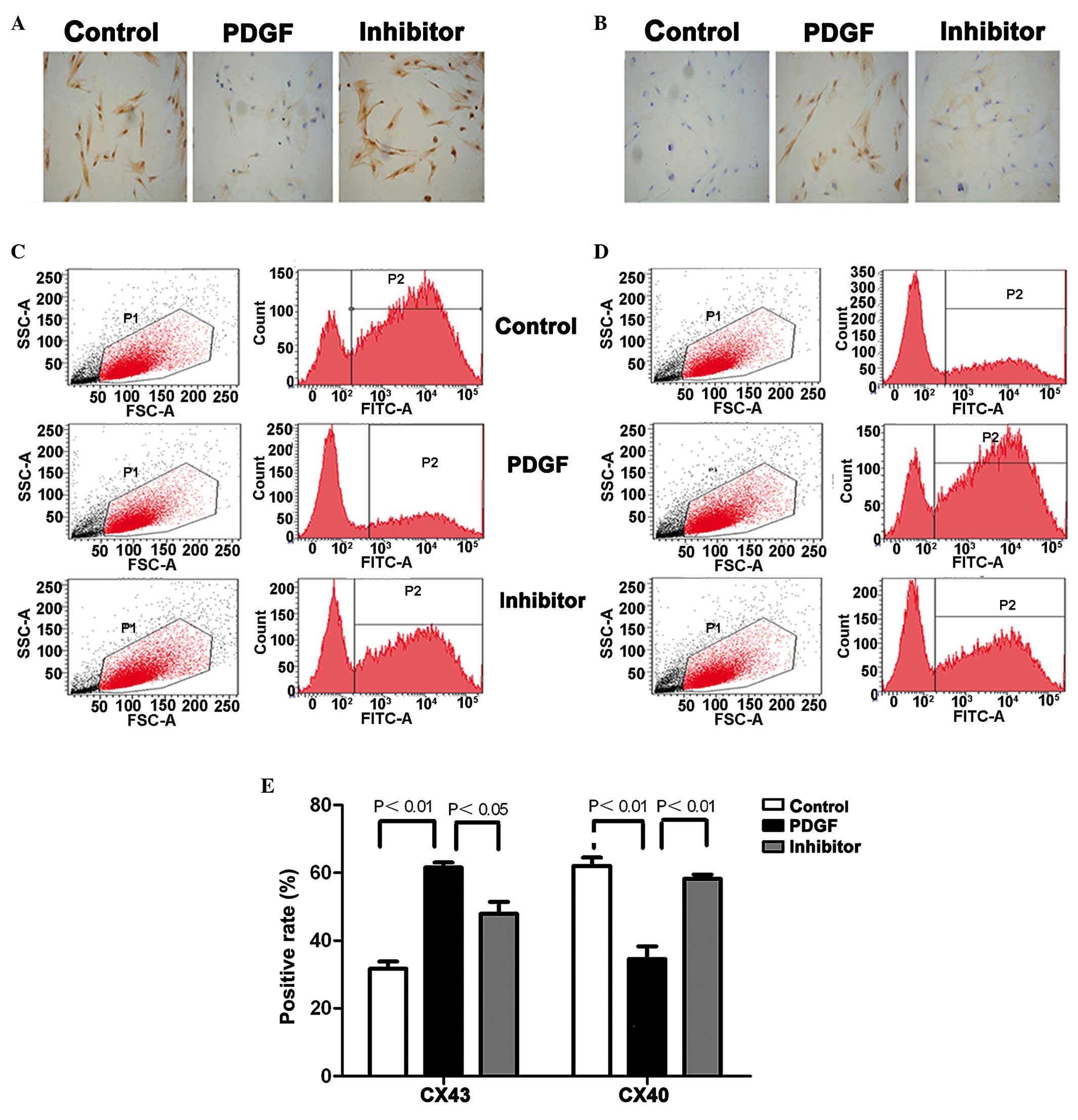
Cx40-positive cells obtained by flow cytometry (Fig. 2). Incubation with PDGF-BB induced
S-SMC differentiation towards R-SMC and cells switched from a
rhomboid shape to an oval shape. In agreement, a reduction in the
Cx40 protein levels and increased Cx43 protein levels were
observed. Notably, treatment with the Cx43 inhibitor
18α-glycyrrhetinic acid reversed the PDGF-BB effects: R-SMC
switched to S-SMC; reduced Cx43 protein levels and increased Cx40
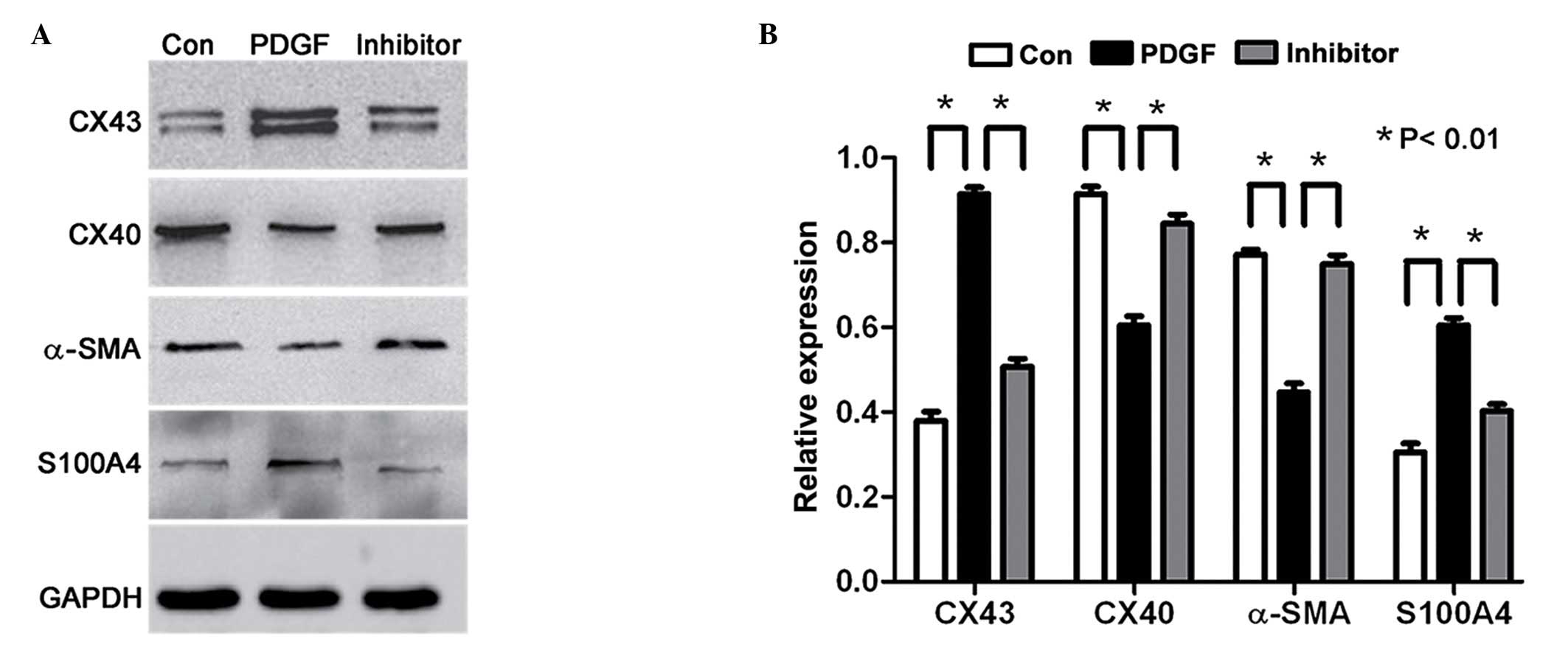
protein levels were observed in the SMCs. Western blot analysis
(Fig. 3) confirmed the reduced
Cx40 and increased Cx43 protein expression subsequent to PDGF-BB
treatment. Notably, PDGF-BB also reduced α-SMA while increasing
S100A4 protein expression in SMCs. All the PDGF-BB effects were
reversed subsequent to treatment with the Cx43 inhibitor.
Stent implantation induced restenosis and
SMC phenotype alterations in coronary artery tissues were
associated with gap junction protein expression patterns
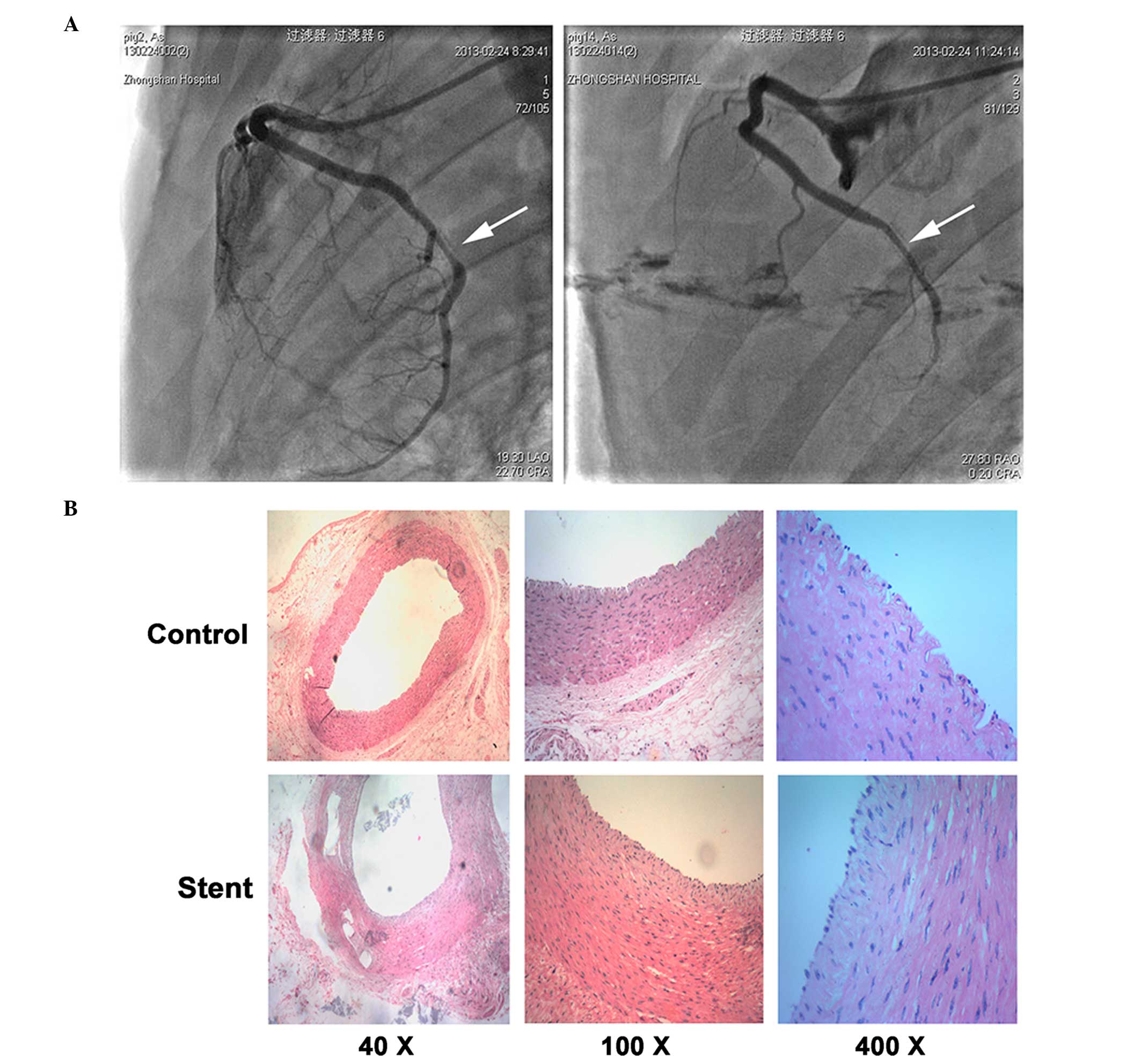
Using angiography technology, it was confirmed that
stent implantation resulted in restenosis in the coronary artery
(Fig. 4A). A comparison between
restenosis and normal coronary artery tissues revealed a different
composition of SMCs. In normal artery tissues, S-SMCs were the most
abundant cells, whereas higher numbers of R-SMC were observed in
the restenosis tissues (Fig. 4B).
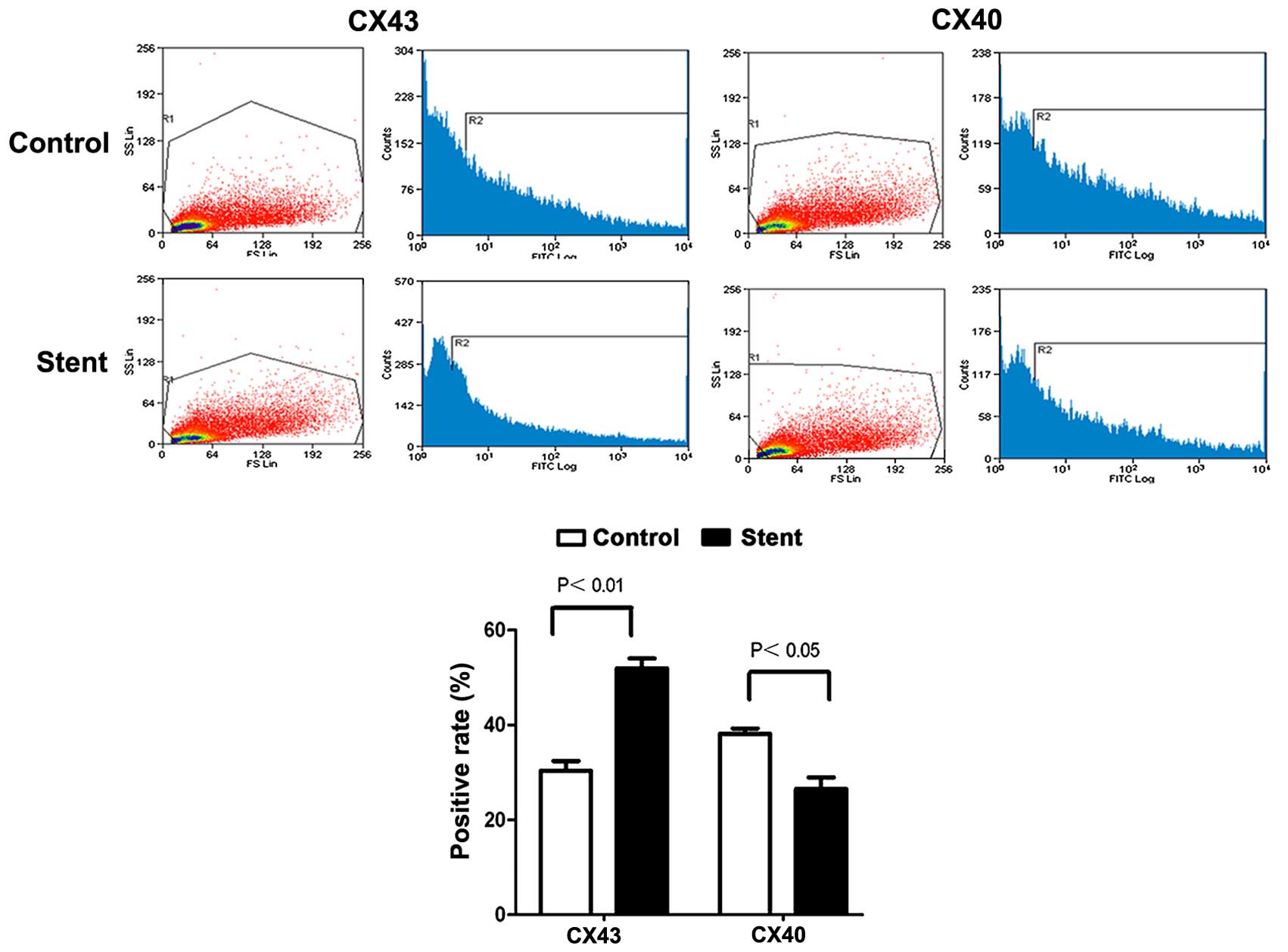
The expression of Cx40 and Cx43 in SMCs isolated from artery
tissues was detected by flow cytometry. As presented in Fig. 5, Cx43 expression was significantly
increased following stent implantation, while the levels of Cx40
expression were significantly reduced. The alterations in Cx40 and
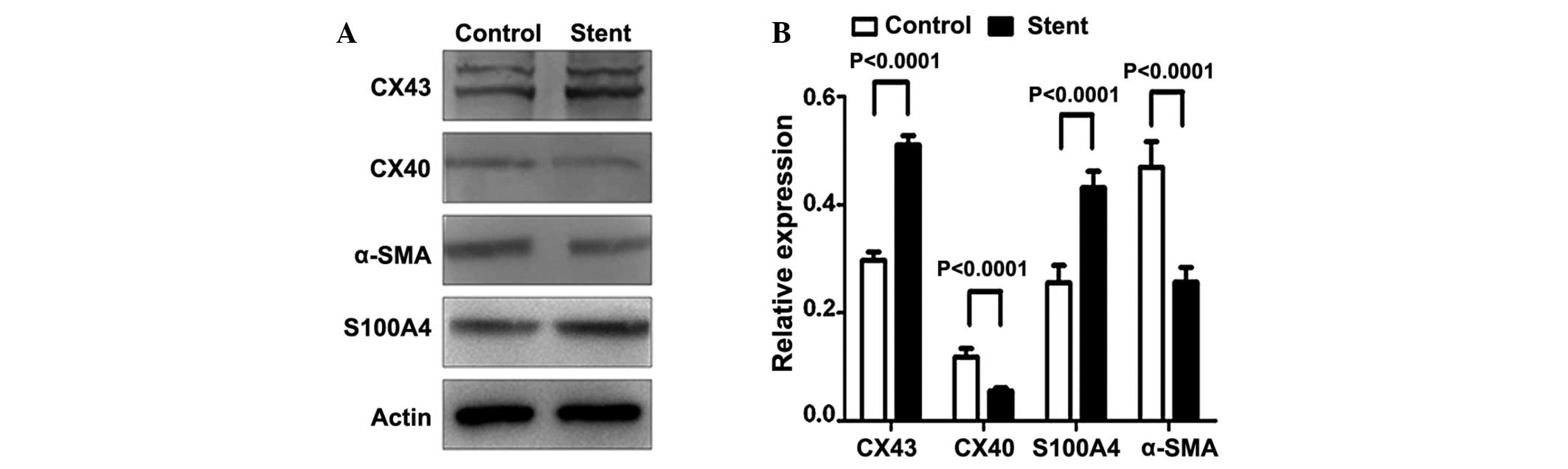
Cx43 expression subsequent to stent implantation at the protein and
mRNA levels were also confirmed by western blotting and RT-qPCR
(Fig. 6). In addition, the
expression levels of α-SMA and S1004A in the SMCs were assessed.
Reduced α-SMA expression and increased S1004A levels were observed
subsequent to stent implantation.
Discussion
The isolation of two distinct SMC populations from
normal coronary arteries have been described in the current study:
S-SMCs, which displayed relatively lower proliferation rates and
R-SMCs, which grew faster. These observations are in agreement with
previous studies (19). R-SMCs are
present in higher proportions in SMCs from stent-induced intimal
thickening compared with normal tissues, suggesting that R-SMCs
possess higher proliferative and migratory activities compared with
S-SMCs, and are involved in arterial repair and restenosis. The
data of the current study corroborate with previous studies that
demonstrated that fast growing R-SMCs, which display clear Cx43
expression and a high migratory rate (8), are present in higher proportions from
stent-induced intimal thickening, suggesting that they participate
in the restenotic process (19).
Cytokines, tissue factors and inflammatory factors
are released subsequent to vessel damage. These factors activate
SMCs in media; induce SMC phenotypic modulation, including
contraction and synthesis and the secretion of extracellular
matrix, which promotes SMC proliferation and migration to the
intima (20–23). PDGF is an inflammatory factor and
comprises several members, including PDGF-AA, PDGF-BB, PDGF-AB,
PDGF-CC and PDGF-DD. PDGF is involved in cell signal transduction
and transcriptional activation through the binding of PDGF membrane
receptors, and it can additionally promote cell mitosis and induce
SMC phenotypic modulation (24).
With platelet deposition, SMC phenotypic modulation and new intima
formation are enhanced by platelet-activating factors (25). Migration and proliferation of
vessel SMCs are important cellular processes in the initiation
stage of vascular remodeling, and are the key causes of restenosis
subsequent to stent implantation (5).
Phenotypic modulation, proliferation and migration
of SMCs serve a critical role in restenosis, and SMCs have been
predominantly evaluated in studies describing the association
between connexin and restenosis (26). The current study identified an
association between phenotypic modulation of SMCs from porcine
coronary arteries and the expression of connexins. Two distinct SMC
populations from normal coronary arteries were isolated, S-SMCs and
R-SMCs (7). In the current study,
Cx43 expression was observed to be greater in R-SMCs compared with
S-SMCs in vitro, indicating that Cx43 expression is
phenotype-dependent in SMCs. Notably, PDGF-BB induced the
transition of S-SMCs to R-SMCs, with resulting increased Cx43
expression. However, when Cx43 expression is blocked by antisense
RNA, the phenotypic alterations induced by PDGF-BB are reported to
be reverted with the expression of α-SMA. In accordance with
previous reports (27,28), the number of R-SMCs was reported to
be increased and Cx43 expression was upregulated in the intima of
restenosis subsequent to coronary artery stent implantation. These
studies also revealed that inhibition of Cx43 expression blocks
macrophage infiltration in addition to SMC proliferation and
migration, which is in agreement with previous studies (29,30–32)
describing the phenotypic modulation of porcine coronary
arteries.
The formation of new intima was further inhibited
subsequent to stent implantation. Taken together, the above results
suggest that Cx43 levels are clearly associated with the SMC
phenotypic and migratory patterns, suggesting that Cx43 may be a
potential novel target for restenosis prevention.
Overall, Cx43 is closely associated with restenosis
subsequent to PCI. Although the mechanism underlying these
observations remains unclear, these observations provide a basis
for the use of Cx43 as a molecular target to prevent restenosis
subsequent to stent implantation. Downregulation of Cx43 expression
and activity may influence the pathological process of vascular
remodeling by inhibiting SMC migration at an early stage, thus
improving vascular remodeling and preventing restenosis.
Acknowledgments
The present study was supported by the Scientific
Committee of Pudong New District (grant no. PKJ20112-Y21).
References
|
1
|
Molica F, Burger F, Thomas A, Staub C,
Tailleux A, Staels B, Pelli G, Zimmer A, Cravatt B, Matter CM, et
al: Endogenous cannabinoid receptor CB1 activation promotes
vascular smooth-muscle cell proliferation and neointima formation.
J Lipid Res. 54:1360–1368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shu ZW, Yu M, Chen XJ and Tan XR: Ghrelin
could be a candidate for the prevention of in-stent restenosis.
Cardiovasc Drugs Ther. 27:309–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Werner N, Priller J, Laufs U, Endres M,
Böhm M, Dirnagl U and Nickenig G: Bone marrow-derived progenitor
cells modulate vascular reendothelialization and neointimal
formation: Effect of 3-hydroxy-3-methylglutaryl coenzyme a
reductase inhibition. Arterioscler Thromb Vasc Biol. 22:1567–1572.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng XW, Kuzuya M, Nakamura K, Di Q, Liu
Z, Sasaki T, Kanda S, Jin H, Shi GP, Murohara T, et al:
Localization of cysteine protease, cathepsin S, to the surface of
vascular smooth muscle cells by association with integrin
alpha-nubeta3. Am J Pathol. 168:685–694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai X: Regulation of smooth muscle cells
in development and vascular disease: Current therapeutic
strategies. Expert Rev Cardiovasc Ther. 4:789–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rensen SS, Doevendans PA and van Eys GJ:
Regulation and characteristics of vascular smooth muscle cell
phenotypic diversity. Neth Heart J. 15:100–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hao H, Ropraz P, Verin V, Camenzind E,
Geinoz A, Pepper MS, Gabbiani G and Bochaton-Piallat ML:
Heterogeneity of smooth muscle cell populations cultured from pig
coronary artery. Arterioscler Thromb Vasc Biol. 22:1093–1099. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao H, Gabbiani G and Bochaton-Piallat ML:
Arterial smooth muscle cell heterogeneity: Implications for
atherosclerosis and restenosis development. Arterioscler Thromb
Vasc Biol. 23:1510–1520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chamley-Campbell J, Campbell GR and Ross
R: The smooth muscle cell in culture. Physiol Rev. 59:1–61.
1979.PubMed/NCBI
|
|
10
|
Willecke K, Eiberger J, Degen J, Eckardt
D, Romualdi A, Güldenagel M, Deutsch U and Söhl G: Structural and
functional diversity of connexin genes in the mouse and human
genome. Biol Chem. 383:725–737. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vinken M, Vanhaecke T, Papeleu P, Snykers
S, Henkens T and Rogiers V: Connexins and their channels in cell
growth and cell death. Cell Signal. 18:592–600. 2006. View Article : Google Scholar
|
|
12
|
Venance L, Glowinski J and Giaume C:
Electrical and chemical transmission between striatal GABAergic
output neurones in rat brain slices. J Physiol. 559:215–230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurtz L, Madsen K, Kurt B, Jensen BL,
Walter S, Banas B, Wagner C and Kurtz A: High-level connexin
expression in the human juxtaglomerular apparatus. Nephron Physiol.
116:p1–p8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shav D, Gotlieb R, Zaretsky U, Elad D and
Einav S: Wall shear stress effects on endothelial-endothelial and
endothelial-smooth muscle cell interactions in tissue engineered
models of the vascular wall. PLoS One. 9:e883042014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen L, Wu Y, Zhang F, Wu L, Dong C, Gao
Y, Sun A, Zou Y, Qian J, Sun J, et al: Assessment of an
asymmetrical coating stent with sirolimus released from ablumial
matrix in porcine model. Clin Res Cardiol. 101:917–927. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chadjichristos CE, Matter CM, Roth I,
Sutter E, Pelli G, Lüscher TF, Chanson M and Kwak BR: Reduced
connexin43 expression limits neointima formation after balloon
distension injury in hypercholesterolemic mice. Circulation.
113:2835–2843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brisset AC, Hao H, Camenzind E, Bacchetta
M, Geinoz A, Sanchez JC, Chaponnier C, Gabbiani G and
Bochaton-Piallat ML: Intimal smooth muscle cells of porcine and
human coronary artery express S100A4, a marker of the rhomboid
phenotype in vitro. Circ Res. 100:1055–1062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Chadjichristos CE, Morel S, Derouette JP,
Sutter E, Roth I, Brisset AC, Bochaton-Piallat ML and Kwak BR:
Targeting connexin43 prevents platelet-derived growth
factor-BB-induced phenotypic change in porcine coronary artery
smooth muscle cells. Circ Res. 102:653–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bujo H and Saito Y: Modulation of smooth
muscle cell migration by members of the low-density lipoprotein
receptor family. Arterioscler Thromb Vasc Biol. 26:1246–52. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thyberg J and Hultgårdh-Nilsson A:
Fibronectin and the basement membrane components laminin and
collagen type IV influence the phenotypic properties of subcultured
rat aortic smooth muscle cells differently. Cell Tissue Res.
276:263–271. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Van Putten V, Zarinetchi F, Nicks
ME, Thaler S, Heasley LE and Nemenoff RA: Suppression of
smooth-muscle alpha-actin expression by platelet-derived growth
factor in vascular smooth-muscle cells involves Ras and cytosolic
phospholipase A2. Biochem J. 327:709–716. 1997. View Article : Google Scholar
|
|
23
|
Su B, Mitra S, Gregg H, Flavahan S,
Chotani MA, Clark KR, Goldschmidt-Clermont PJ and Flavahan NA:
Redox regulation of vascular smooth muscle cell differentiation.
Circ Res. 89:39–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andrae J, Gallini R and Betsholtz C: Role
of platelet-derived growth factors in physiology and medicine.
Genes Dev. 22:1276–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deaton RA, Gan Q and Owens GK:
Sp1-dependent activation of KLF4 is required for PDGF-BB-induced
phenotypic modulation of smooth muscle. Am J Physiol Heart Circ
Physiol. 296:H1027–H1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morel S: Multiple roles of connexins in
atherosclerosis- and restenosis-induced vascular remodeling. J Vasc
Res. 51:149–161. 2014. View Article : Google Scholar
|
|
27
|
de Wit C, Wolfle SE and Höpfl B:
Connexin-dependent communication within the vascular wall:
Contribution to the control of arteriolar diameter. Adv Cardiol.
42:268–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeh HI, Lupu F, Dupont E and Severs NJ:
Upregulation of connexin43 gap junctions between smooth muscle
cells after balloon catheter injury in the rat carotid artery.
Arterioscler Thromb Vasc Biol. 17:3174–3184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li P, Liu Y, Yi B, Wang G, You X, Zhao X,
Summer R, Qin Y and Sun J: MicroRNA-638 is highly expressed in
human vascular smooth muscle cells and inhibits PDGF-BB-induced
cell proliferation and migration through targeting orphan nuclear
receptor NOR1. Cardiovasc Res. 99:185–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nomiyama T, Nakamachi T, Gizard F, Heywood
EB, Jones KL, Ohkura N, Kawamori R, Conneely OM and Bruemmer D: The
NR4A orphan nuclear receptor NOR1 is induced by platelet-derived
growth factor and mediates vascular smooth muscle cell
proliferation. J Biol Chem. 281:33467–33476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scott RA, Paderi JE, Sturek M and Panitch
A: Decorin mimic inhibits vascular smooth muscle proliferation and
migration. PLoS One. 8:e824562013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hua Y, Dolence J, Ramanan S, Ren J and
Nair S: Bisdemethoxycurcumin inhibits PDGF-induced vascular smooth
muscle cell motility and proliferation. Mol Nutr Food Res.
57:1611–1618. 2013. View Article : Google Scholar : PubMed/NCBI
|