Introduction
Pulmonary heart disease (PHD), idiopathic pulmonary
arterial hypertension (IPAH, formerly called primary pulmonary
hypertension) and coronary heart disease (CHD) are cardiovascular
diseases with severe effects upon human health. CHD and PHD have a
high prevalence and severe complications. The median survival time
for patients with IPAH who do not receive effective drug
intervention is 2.8 years, and various therapeutic strategies for
IPAH are derived from treatment for pulmonary arterial hypertension
(1,2). Previous studies have identified in
part the etiology, pathology and genetic characteristics of these
conditions, which has provided guidance for prevention and
treatment (3–6). However, the exact mechanisms of these
diseases remain unclear, and further investigation of the
biological characteristics is required in order to determine their
pathological mechanisms. Previous studies have reported that the
pathological processes of these diseases are not limited to the
coronary or pulmonary vasculature, and that there may be systemic
vasculature and complex genetic involvement during the course of
these diseases (7,8). Treatments with angiotensin converting
enzyme inhibitors (ACEIs), vasodilators, anticoagulants, diuretics,
calcium channel blockers and inotropic agents have been used to
alleviate the common symptoms of PHD, IPAH, and CHD (2,9), and
this information enables us to explore the associations between
them.
Networks can reflect the associations between genes,
pathways and the diseases using network construction and modular
analysis (10). A previous study
based on genome-wide linkage analysis demonstrated that alstrom
syndrome protein 1 is a novel genetic risk marker for early-onset
myocardial infarction (a type of CHD) (11). An additional study using network
analysis identified that patients with CHD with elevated vascular
endothelial growth factor A (VEGFA) levels at baseline have an
increased mortality rate compared with those with lower levels
(12). Considering the complexity
of the processes involved, integrated gene network analysis was
used to investigate the multi-level correlation characteristics
among the three diseases.
Materials and methods
Obtaining the genes and network
construction
The terms 'IPAH', 'PHD' or 'CHD' were inputted into
to the search box of the Online Mendelian Inheritance in Man (OMIM)
database, (http://www.ncbi.nlm.nih.gov/omim), a knowledge
database of human genes and genetic disorders (13). Disease-associated genes were then
submitted to Agilent Literature Search software, version 2.82
(http://www.agilent.com/labs/research/litsearch.html),
and an overview network of gene/protein associations was
obtained.
Network analysis
Cytoscape software version 2.71 (http://www.cytoscape.org/) was used for visualization
of disease-associated networks and analysis of the network
properties. Network parameters including the clustering
coefficient, network diameter, network centralization and network
radius were determined.
Identification of modules
MCODE (version 1.32) is programme that is used for
network module division (http://baderlab.org/Software/MCODE). Subsequent to the
disease network data being obtained, each disease network was
imported and MCODE was used to divide it into several modules using
the following parameters: Connectivity threshold, 2; core threshold
K, 2; node score threshold, 0.2.
Functional enrichment analysis
Hypergeometric distribution tests were performed to
analyze the function of the modules that contained the most genes
in each network (IPAH-associated, PHD-associated and CHD-associated
gene networks) using the Database for Annotation, Visualization and
Integrated Discovery (http://david.abcc.ncifcrf.gov/). The following
parameters were used: Count, 2; EASE, 0.01; and species and
background, Homo sapiens. Using the gene ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes annotation, the biological
processes and pathway corresponding to the modules were identified,
and the P-values were ranked.
Results
PHD, IPAH and CHD-associated genes in the
OMIM data-base
Subsequent to searching the OMIM database (on May
23, 2014), a total of 295 PHD-associated, 132 IPAH-associated and
212 CHD-associated genes were identified (Table I). A total of 29 overlapping genes
were detected among the three diseases, which accounted for 9.33%
(29/295) of the identified PHD-associated genes, 21.97% (29/132) of
IPAH-associated genes, 13.68% (29/212) of CHD-associated genes and
5.8% (29/500) of the total number of genes associated with the
three diseases.
 | Table IAssociated genes for the three
diseases. |
Table I
Associated genes for the three
diseases.
| Disease | Gene |
|---|
| IPAH | FGA, APOA1, LYZ,
ACE, COL1A1, MMP2, FBL, FBN1, GYS1, HBB, TBX5, IL6, ALOX5, FOXC2,
PRKAR1A, MDK, MSH2, NF1, NOS3, NOS2, SDHD, PF4, PKD2, F2, SMAD9,
BMPR2, SFTPB, SLC6A4, ENG, TGFB1, PAX2, RET, UPK3A, VEGFA, VIP,
WBSCR22, ELN, GTF2I, GTF2IRD1, WT1, ALMS1, GALNT3, FGF23, KL, AGK,
KRT8, KRT18, FIG4, CFTR, IKBKAP, GBA, G6PC, SMARCAL1, RMRP, COL1A2,
PKHD1, ABCC6, XYLT1, XYLT2, DHCR7, LIPA, GLA, DMD, MYF6, PDE4D,
SCN5A, SCNN1A, NOTC H3, ACVRL1, SCNN1B, CAV1, HTR2B, PKD1, PPARG,
DYNLT1, SMAD1, ABCA3, GATA6, INVS, NFIX, KCNK3, BIRC5, TRPC6,
ZFPM2, DDAH1, CRTAP, RETN, GCSH, GLDC, AMT, MTHFR, NCF1, AGPAT2,
MYL3, DEL3Q29, G6PC3, TBX20, CTC1, ACTA2, SARS2, PDSS1, FGFR3,
IFNGR1, TPM1, CDKN1A, SERPINA6, NDN, SNRPN, CRE, BBP, EP300, HRAS,
IKBKG, BMPR1A, SEPN1, HIF1A, ABI2, SATB2, DIH1, MAFB, ELMOD2,
SFTPC, SFTPA1, TERC, TERT, MUC5B, SFTPA2, TNNT2, ENPP1, FLNA, SC,
NN1G, CPS1 and ACTC1 |
| CHD | ALDH2, ACTA2, ACE,
APOA1, APOB, APOE, TGFB3, SCN5A, HBN1, LMNA, MYBPC3, CRP, IL10,
EDN1, MYH11, SH2B3, EPOR, JAK2, ESR1, PPP1R17, ABCA1, EPHX2, APOA2,
ITIH4, LDLR, APOA5, LIPI, HTGS, RP1, IRS1, PTPN11, LPA, MYH9,
MMVP1, CCL2, NOS3, PON1, ENPP1, SERPINE1, CD36, ZMPSTE24, MMP3,
ENG, TNF, UGT1A1, VEGFA, TBX1, SLC25A4, MYH6, MYH7, ACTC1, TNNC1,
MYLK2, CAV3, WBSCR22, ELN, GTF2I, GTF2IRD1, HGD, ALMS1, NT5E, HRAS,
KRAS, GLB1, GBA, CBS, SGCG, ABCC6, XYLT1, XYLT2, CHDS3, GLA, MYMY4,
NSDHL, DEL8Q13, GATA4, MEF2A, CX3CR1, PPARG, PLA2G7, PON2,
TNFRSF11B, LRP6, KALRN, AKAP10, IDUA, MTHFR, ADRB1, CHDS1, SMPD1,
PCSK9, CHDS2, CHDS4, C10orf2, LPL, CHDS8, ITPKC, CHDS9, KIF6,
KIAA1462, STAT3, AGT, AGTR1, CACNA1C, CTNNB1, CETP, MMP9, CYP2C19,
CFH, FGB, NR3C1, GPX1, GPD1, GNAI2, HMGCR, USF1, HYT5, HYT6, HYT1,
ADD1, CYP3A5, ECE1, GNB3, HYT3, HYT4, ATP1B1, NOS2, HYT2, PTGIS,
SELE, RGS5, IGF2, LDHB, ALOX5, LGV1, YTHDF2, TLR4, MIF, MB, NFKB1,
CTSC, ITGB3, SELP, TTR, PAPPA, F2, CLU, THBD, TNNI3, TNNT2, TH,
MGP, CPS1, FOXF1, AGTR2, TMSB4X, DMD, CD40LG, AR, CDKN2A, SCN1B,
FABP4, THBS4, IL18, CYP2C9, SGCD, LTB4R, RAC1, ILK, TNFSF12, NPHS1,
PRKAA1, LIPG, ZFPM2, LD, LRAP1, ANGPTL3, ADAMTS7, ADIPOQ, MTTP,
AOMS1, FADS1, TXNIP, HDLCQ1, BMIQ1, BMIQ2, BMIQ3, BMIQ4, BMIQ5,
BMIQ6, UCP2, HDLCQ2, HDLC3, GNPTAB, ABCC9, MIB1, TELM, MIR1-1,
TPM1, BMIQ13, LIPC, CDKN2B-AS1, BANF1, ADORA1, GJA4, NPY, INS,
MMP12, F5, ALOX5AP, PRKCH, SORT1 and VIMP |
| PHD | AOS, ARHGAP31,
TAPVR1, NKX2-5, NOTCH1, CECR, JAG1, GJA1, TBX5, FOXC2, FBN1, MMVP1,
CCL2, MSH2, NF1, NOS3, SMAD4, ELMOD2, SFTPC, SFTPA1, TERC, TERT,
MUC5B, SFTPA2, SMAD9, BMPR2, ELN, ENG, TBX1, WBSCR22, GTF2I,
GTF2IRD1, HPS5, HPS1, BLOC1S3, HPS6, DTNBP1, HPS3, HPS4, ALMS1,
ATD, CHD7, SE, MA3E, FIG4, NKX2-6, GATA6, GDF1, CFC1, CFTR, TGFB1,
LRP2, TTC37, ZEB2, HYLS1, MGP, B3GAT3, SMN1, FKTN, DIP, PKHD1,
NEK1, DYNC2H1, CPS1, FOXF1, NOD, AL, IV, WNT3, GLA, ZIC3, RBM10,
FLNA, ACVRL1, GATA4, STRA6, DEL18Q, SEPN1, SLC29A3, KAT6B, CRELD1,
SMPD1, GBA, HHT4, ABCA3, CTEPH1, LTBP4, MYH7, FADD, SMAD3, ACTA2,
BANF1, IFT43, CCDC11, LTBP2, NOTCH2, ACE, SALL1, KRAS, MAP2K1,
MAP2K2, BRAF, MMP1, NIPBL, FBLN5, IL10, COL3A1, EDNRB, GY, S1, HBB,
CHST3, OGS2, RYR1, PTPN11, SF3B4, AFD1, PRKAR1A, COL1A1, COL1A2,
SDHD, KCNJ2, PKD2, CREBBP, EP300, ZFPM2, DGCR, DCR, GATA1, MTR,
TNNT2, TSC2, IFNG, TSC1, WHCR, WHSC1, GLI3, KIF7, ACLS, ENPP1, AGK,
PEX1, PEX6, PEX10, PEX12, PEX13, PEX14, PEX26, PEX19, PEX2, PEX5,
PEX3, HRAS, EFEMP2, LOX, PLOD1, ETFA, ETFB, ETFDH, CBS, DNAI1,
MKS1, ARSB, PSMB8, HPGD, B3GALTL, ABCC6, XYLT1, XYLT2, ROR2, DHCR7,
ZMPSTE24, LMNA, ADAMTS10, MID1, COX7B, DMD, MYF6, GPC3, SCN5A,
PSEN2, PKD1, INVS, TIRAP, IDUA, MTHFR, SHOC2, DUP22Q11.2, TGFBR1,
TGFBR2, EFTUD2, BAG3, ACTC1, STAT3, ADORA1, FGA, APOA1, LYZ, TPM1,
TNNI3, MMP9, C3, COL5A1, COL5A2, EPHX1, FASLG, DIH1, SOD1, WNT2,
ALOX5, MIF, MBL2, NOS1, PLG, PDGFRA, PTGER3, F2, PRKCE, RARB,
COL2A1, THBD, TP53, SCGB1A1, V EGFA, VCL, CD96, SLC26A2, FANCA,
FANCC, FANCD2, FANCE, FANCB, FANCF, FANCG, BRCA2, DIS3L2, FAH,
MMACHC, FOXP3, FGD1, LOAS, PDE4D, NRXN1, SHH, MMP12, LATD, SMAD2,
PPARG, HOXA1, PITX2, S1PR1, FGF10, FOXO3, TLR4, HIF1A, RPL5, TRPC6,
STAT5B, DLL4, CXCL16, ADIPOQ, PRDM16, AVSD1, DICER1, LOXL2, WT1,
FLCN, PDA1, AGPAT2, MED13L, TPM2, SOS1, G6PC3, TBX20, DEL6Q24Q25,
TAB2, GJA5, MYH6, FGFR3, EGR1, NDN, SNRPN, ATM, AAAS, IL18, BMPR1A,
FOXA1, SERPINA10, NS2, TLR9, CRTAP, BPIFA1, MYL3, RTEL1, TK2,
SUCLA2, DEL16P13.3 and DARC |
Topological distribution of diseases
networks
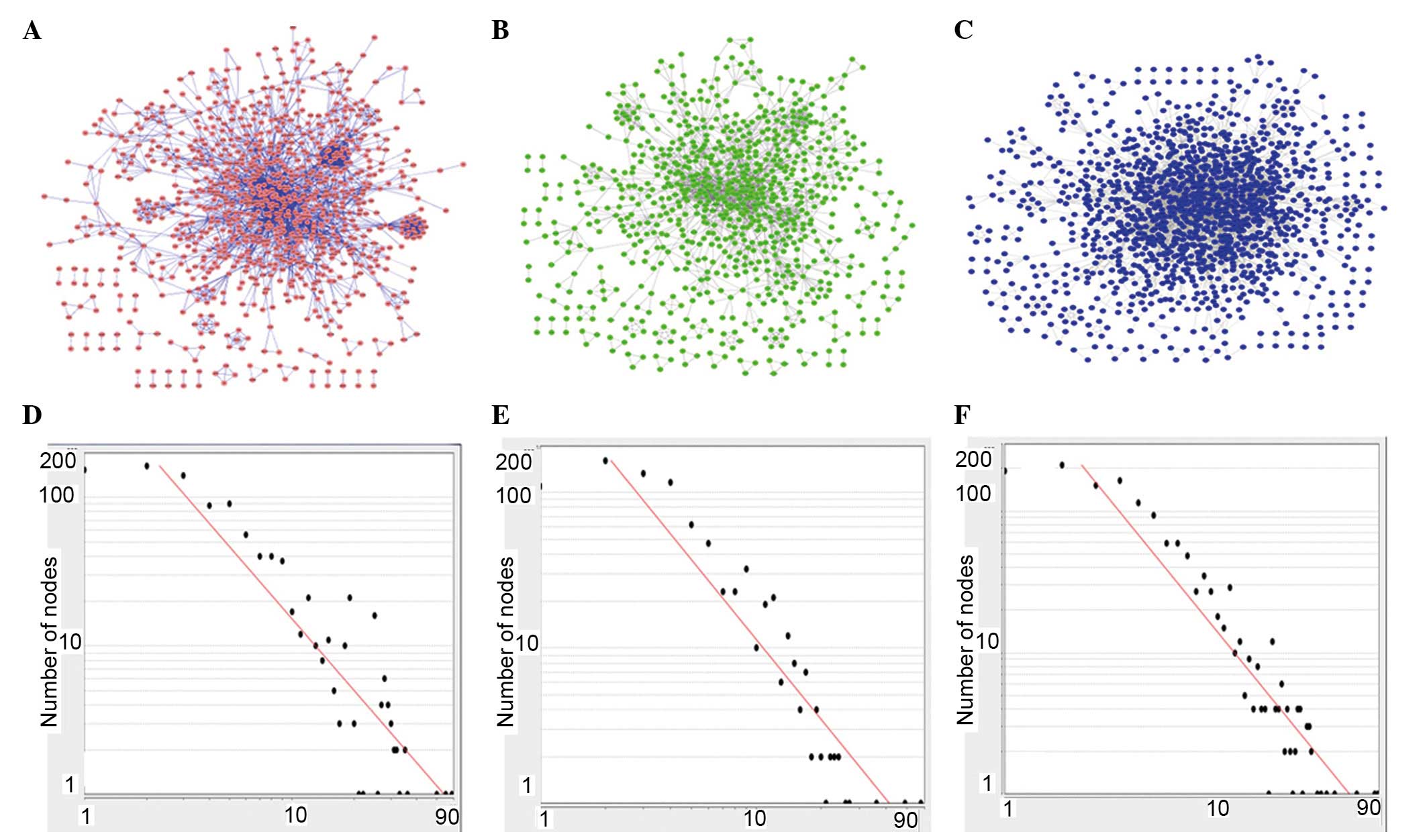
Global maps of the CHD network (Fig. 1A), IPAH network (Fig. 1B) and PHD network (Fig. 1C) exhibit a similar center-outward
diffuse landscape. The multiple topological parameters of the three
disease networks are listed in (Table
II). Based on the 295 PHD-associated genes, PHD-associated
networks contained 1,363 nodes (genes) and 4,592 edges
(interactions), which was more complex than the other networks.
However, the IPAH-associated network had the smallest number of
genes (132 IPAH-associated genes) and the biggest network
centralization value (0.065) and clustering coefficient (0.658).
The increase in the node degree (the number of node-edges in the
network) in the CHD (Fig. 1A and
D), IPAH (Fig. 1B and E) and
PHD (Fig. 1C and F) networks
followed a power-law distribution.
 | Table IITopological parameters of the three
disease networks. |
Table II
Topological parameters of the three
disease networks.
| Parameter | CHD | IPAH | PHD |
|---|
| Clustering
coefficient | 0.611 | 0.658 | 0.614 |
| Network
diameter | 13 | 13 | 12 |
| Network radius | 1 | 1 | 1 |
| Network
centralization | 0.045 | 0.065 | 0.058 |
| Number of
nodes | 974 | 812 | 1363 |
| Number of
edges | 3040 | 2067 | 4592 |
| Gene number | 212 | 132 | 295 |
| Overlapping
gene/gene | 0.13 (13.68%) | 0.21 (21.97%) | 0.09 (9.83%) |
| Modules | 74 | 91 | 110 |
| Average size | 7.081 | 5.374 | 6.964 |
| Maximum size | 78 | 53 | 49 |
| Minimum size | 3 | 3 | 3 |
| Modularity | 0.445 | 0.47 | 0.415 |
| Overlapping
module/module | 0.04 (4.05%) | 0.03 (3.30%) | 0.02 (2.73%) |
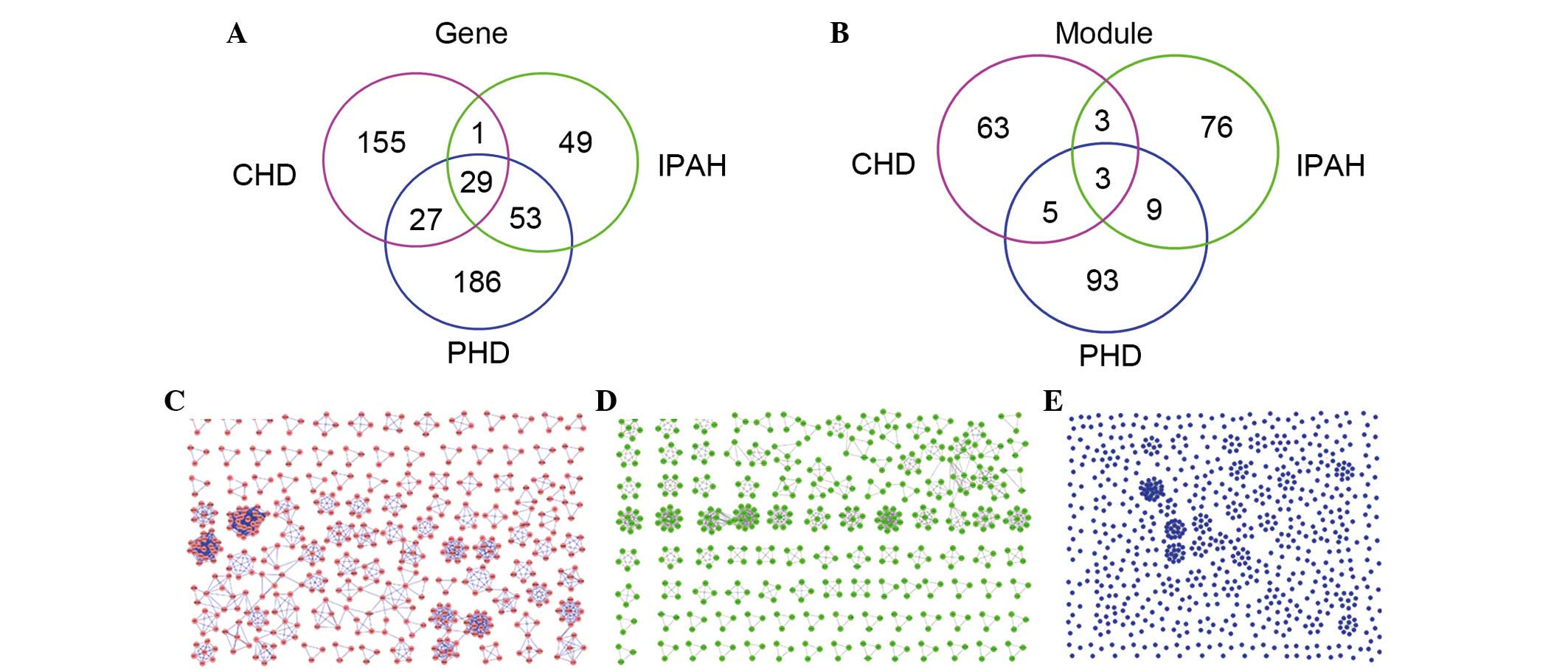
Overlapping distributions of genes in the
disease networks
The 29 overlapping genes accounted for 13.68% of
CHD-associated genes, 9.83% of PHD-associated genes, and 21.97% of
IPAH-associated genes, 12 of which were in the 7 CHD network
modules, 8 were in the 7 PHD network modules and 15 were in the 14
IPAH network modules. In addition, arachidonate 5-lipoxygenase
(ALOX5) was identified in all three disease network modules.
Different overlapping distribution of
modules among disease networks
The 3 overlapping modules accounted for 10.81% of
the CHD modules, 2.71% of the PHD modules and 3.29% of the IPAH
modules. Compared with other modules in the three diseases,
MC9I9P13 and MC19I13P25 ranked in a higher
position.
Using the MCODE, version 1.32 for each disease
network, there are 29 overlapping genes (Fig. 2A) and 3 overlapping modules in the
three disease networks (Fig. 2B).
Furthermore, 110 modules were identified from the CHD network
(Fig. 2C), 91 modules from the
IPAH network (Fig. 2D) and 74
modules from the PHD network (Fig.
2E). Notably, the three overlapping modules were identified
among the three disease networks (Fig.
2B). In addition, 3, 5 and 9 additional modules, respectively,
were identified between pairs of the diseases, for example
MP1C1 between PHD and CHD, MI3P3 between IPAH
and PHD and MC48I66 between CHD and IPAH networks.
Excluding the overlapping modules, the PHD network had the most
unique modules (n=93) compared with the other networks.
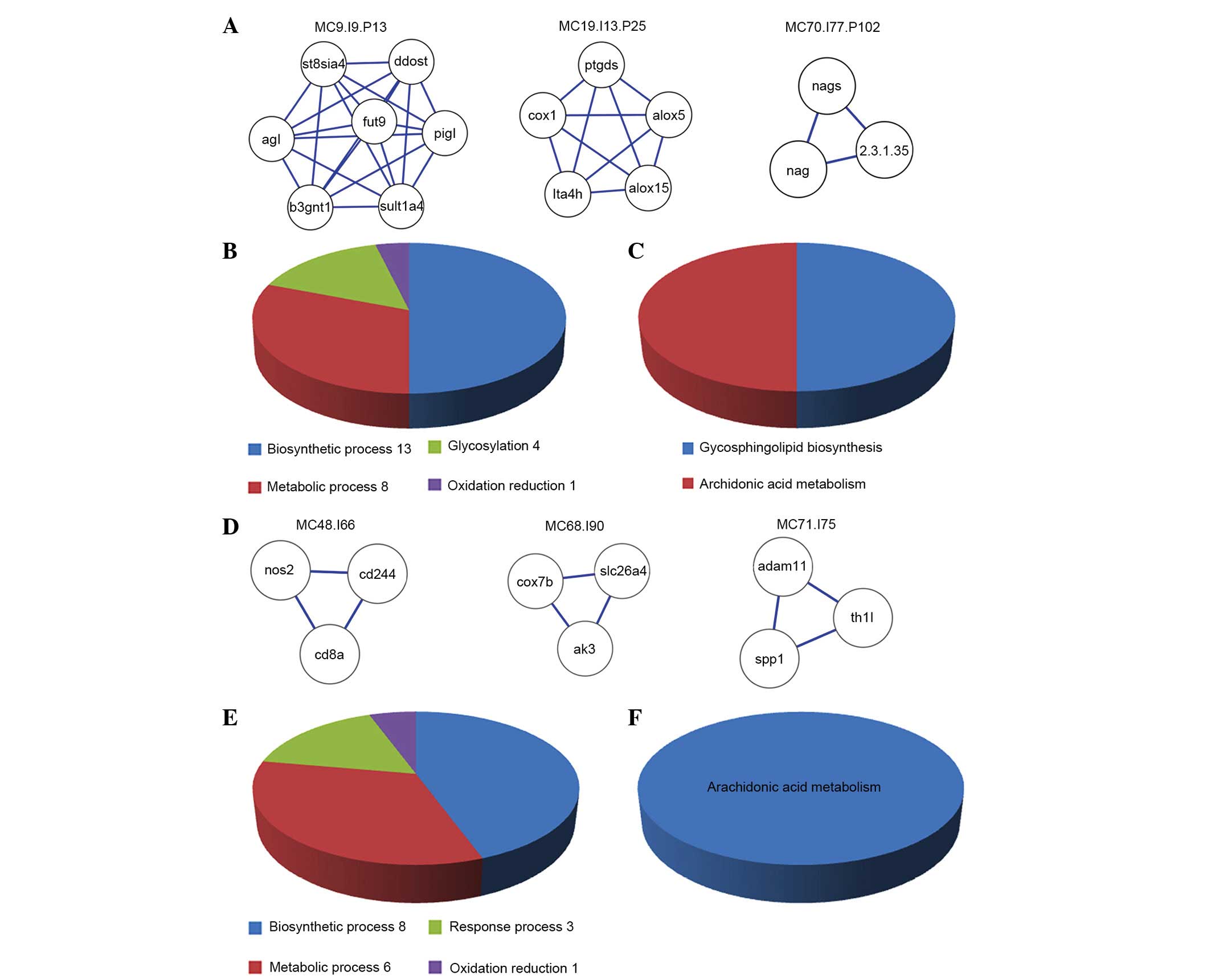
Functional enrichment analysis of three
overlapping modules
Based on the GO functional enrichment analysis of
the 3 overlapping functional modules in IPAH, CHD and PHD (Fig. 3A), the 3 overlapping modules were
observed to contain 26 overlapping functional annotations (Fig. 3B), including 13 for biosynthetic
processes (such as the cellular carbohydrate biosynthetic process
and other biosynthetic processes), 8 for metabolic processes (the
glycoprotein metabolic process and other metabolic processes), 4
for glycosylation processes (glycosylation, biopolymer
glycosylation, protein amino acid glycosylation and protein amino
acid N-linked glycosylation) and 1 for oxidation reduction.
Biosynthetic processes and metabolic processes accounted for 50.00%
(13/26) and 30.77% (8/26) of all biological processes,
respectively. The three overlapping modules also have two pathways
(Fig. 3C), including
glycosphingolipid biosynthesis and the arachidonic acid
metabolism.
Modules between IPAH and CHD
IPAH and CHD shared 3 other overlapping modules in
addition to the three overlapping modules for IPAH, CHD and PHD
(Fig. 3D). The 3 overlapping
modules all consisted of 3 nodes and 3 edges, and had 18 functional
annotations (Fig. 3E), including
the eicosanoid biosynthetic process, the eicosanoid metabolic
process, the fatty acid biosynthetic process and the cellular
alkene metabolic process. The arachidonic acid metabolism was the
only pathway identified (Fig.
3F).
Modules between CHD and PHD
In addition to the three overlapping modules, CHD
and PHD shared 5 additional common modules (Fig. 4A), which consisted of a minimum of
3 nodes and 3 edges. The largest one, MP1C1, comprises
26 nodes and 325 edges. The 5 overlapping modules have 176
functional annotations (Fig. 4B)
and 5 pathways (Fig. 4C).
Regulatory processes, cellular processes and development processes
have the most functional annotations. The most significant module,
MP1C1, is the highest ranked module for PHD and CHD
(ranked using the MCODE score) and includes 114 functional
annotations, 37 for cellular processes (cell motility, cell
migration and other cell processes), and 7 for response processes
(for example the response to estrogen stimulus and positive
regulation of response to stimuli).
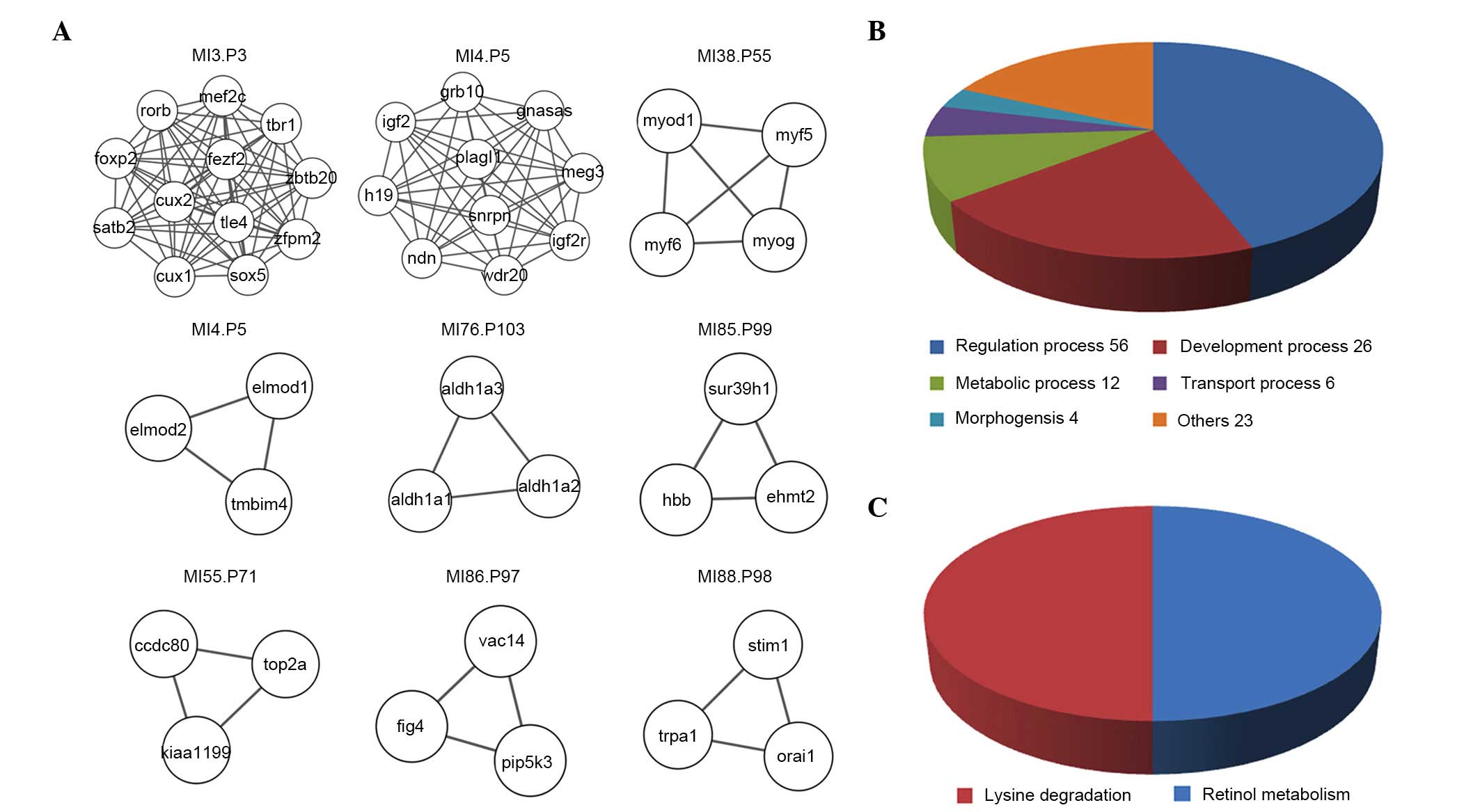
Modules between IPAH and PHD
IPAH and PHD shared 9 other common modules in
addition to the three overlapping modules (Fig. 5A). These were the modules that
consisted of a minimum of 3 nodes and 3 edges. The most significant
module, MI3P3, comprises 12 nodes and 66 edges. The 9
overlapping modules have 127 functional annotations (Fig. 5B) and 2 pathways (Fig. 5C). Regulation processes,
development processes and metabolic processes have the most
functional annotations. The most significant module,
MI3P3, ranks third in the PHD and IPAH modules (ranked
by MCODE score), including 36 functional annotations, including 10
for transcription (for example regulation of transcription and
other transcriptional processes), and 9 for metabolic processes
(for example the nitrogen compound metabolic process and other
metabolic processes).
Modules between IPAH and CHD
IPAH and CHD shared 3 other overlapping modules in
addition to the three overlapping modules for IPAH, CHD and PHD
(Fig. 3D). The 3 overlapping
modules all consisted of 3 nodes and 3 edges, and had 18 functional
annotations (Fig. 3E), including
the eicosanoid biosynthetic process, the eicosanoid metabolic
process, the fatty acid biosynthetic process and the cellular
alkene metabolic process. The arachidonic acid metabolism was the
only pathway identified (Fig.
3F).
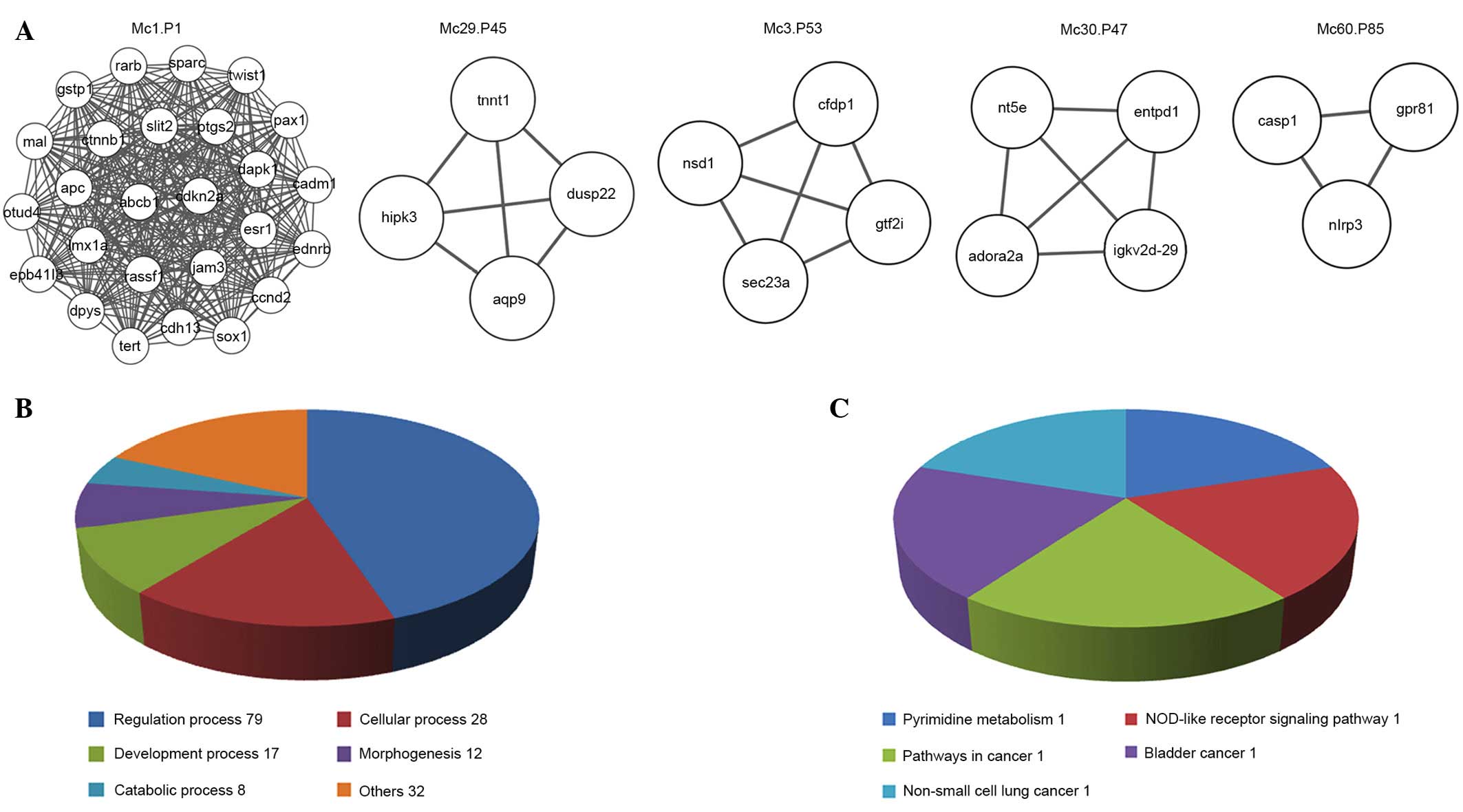
Unique disease modules contribute their
own functions and pathways
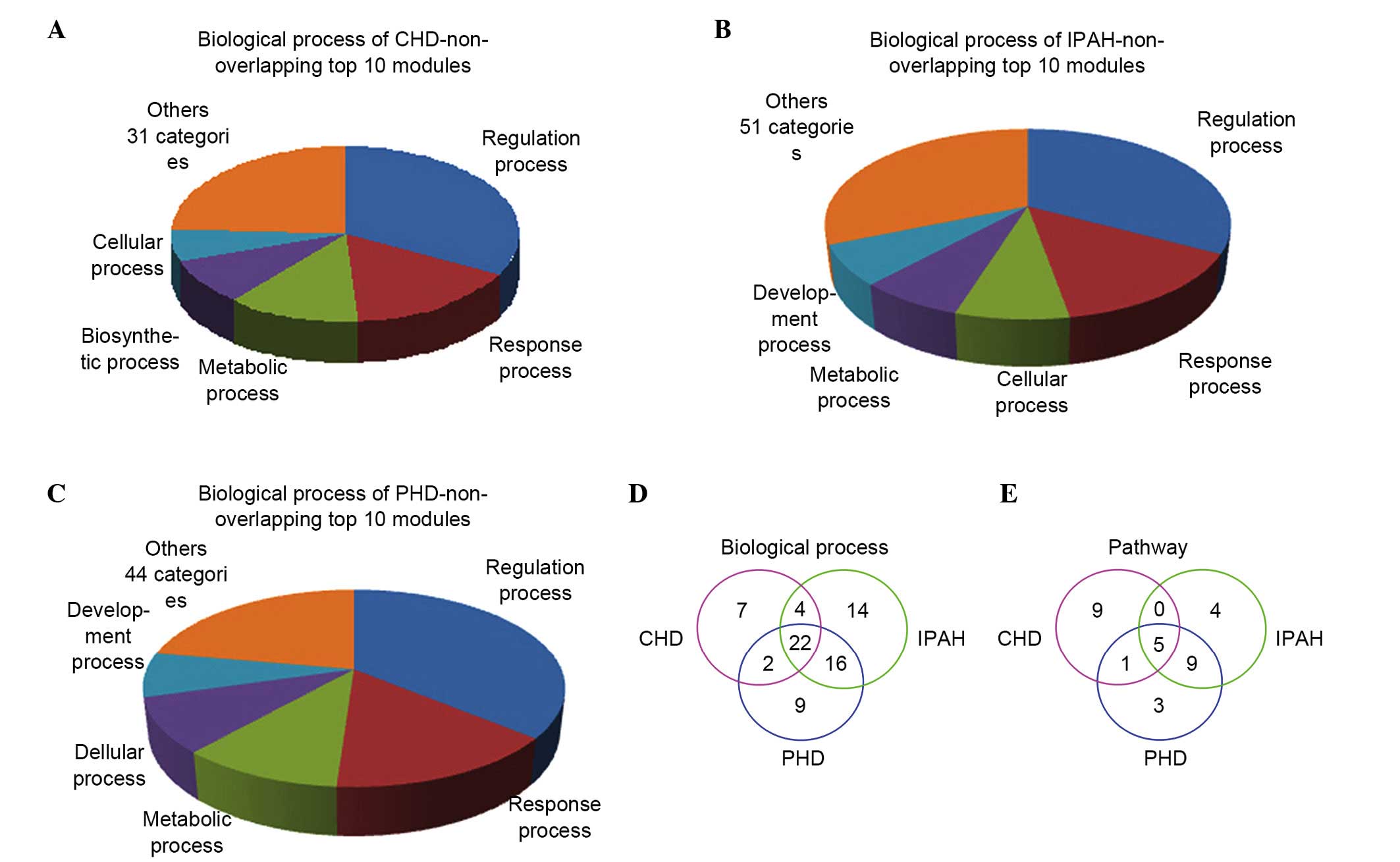
The top 10 non-overlapping modules of the
CHD-associated network (Fig. 6A),
the IPAH-associated network (Fig.
6B) and the PHD-associated network (Fig. 6C) shared 22 common biological
processes (Fig. 6D), including
regulation, response and cellular processes. They shared 5 types of
pathways (Fig. 6E), including the
metabolic pathway, a cancer-associated pathway and an
apoptosis-associated pathway.
Discussion
The current study identified numerous biological
characteristics among IPAH, CHD and PHD. There were 29 overlapping
genes that demonstrated genetic similarity between the three
diseases. Genes including nitric oxide synthase 3 (NOS3),
angiotensin converting enzyme (ACE), ALOX5, VEGFA, apolipoprotein
A-I and endoglin have been identified as biomarkers or therapeutic
targets for IPAH, CHD and PHD based on direct evidence from the
Comparative Toxicogenomics Database (http://ctdbase.org/), which provides manually curated
information regarding the association between genes and disease
(14). Previous studies have
investigated these biological processes, improving the
understanding of their roles in the diseases investigated in the
current study. For example, studies have demonstrated that NOS3
serves a role in cardiovascular pathology and individual responses
to antihypertensive drugs (15,16),
and that levels of NOS3 vary with increasing CHD and IPAH
occurrence (17,18). Additional studies have suggested
that ACE may contribute to the development of IPAH and CHD
(19,20). Drugs targeted at ACE, such as
ACEIs, are used to treat IPAH, PHD and CHD (21–23).
The common modules for IPAH, PHD and CHD shared two
pathways: Glycosphingolipid biosynthesis and the arachidonic acid
metabolism. Previous studies have indicated that inhibition of
glycosphingolipid synthesis induces a marked reduction of plasma
cholesterol and inhibits atherosclerosis development in mice, which
may alleviate CHD (24–26). The association between
glycosphingolipid biosynthesis and IPAH and PHD remains unclear,
however targeting glycosphingolipid biosynthesis may be a novel
treatment modality for cardiovascular disease. The pathological
processes for IPAH, PHD and CHD involve the arachidonic acid
metabolism. A previous study indicated that a lower ratio of serum
eicosapentaenoic acid to arachidonic acid is associated with a
greater risk of cardiovascular disease, particularly CHD (27). Thromboxane A2 (TXA2) is the major
arachidonic acid metabolite. It serves a key role in normal
physiology and control of vascular tone (28) and is associated with the
development of IPAH (29). TXA2 is
also closely associated with the occurrence of pulmonary embolism,
which leads to PHD (30,31). Biosynthetic processes are the most
functional annotations of the three diseases, and they include the
leukotriene (LT) biosynthetic process and the lipid biosynthetic
process. LTs are lipid mediators that are derived from the
5-lipoxygenase pathway of arachidonic acid metabolism, and which
modulate inflammation in pulmonary arterial hypertension (32). LTs serve a pivotal role in the
pathogenesis of atherosclerosis, have a close association with CHD
(33), and additionally increase
oxygen free radicals involved in the development and maintenance of
heart failure, which is a typical symptom of PHD (34). Due to the fact that LTs are
important in IPAH, CHD and PHD, the use of agents that influence
the LT pathway has potential for the use in treatment for IPAH and
PHD (32,34). LTs have additionally been
identified to be effective in predicting CHD (33). Functional observations including
oxidation reduction, the unsaturated fatty acid biosynthetic
process, the glycoprotein biosynthetic process and the eicosanoid
metabolic process were additionally identified to be associated
with IPAH, CHD and PHD in the current study; however, these
observations require further investigation and discussion.
A greater number of overlapping functional modules
and functional annotations were identified between pairs of the
diseases. The most significant CHD and PHD module was
Mp1c1, and the most important functional annotations of
this module are cellular processes and response processes.
Regulation of the response to external stimuli and negative
regulation of the defense response may be important in regulating
the positive and negative feedback loops that maintain cellular
status (35). Cell migration, cell
motility, regulation of locomotion and positive regulation of
locomotion are considered characteristic aspects in CHD (36). Certain treatments influence cell
function. Treatments including atorvastatin, which increases the
migration of endothelial progenitor cells, can reduce pulmonary
arterial pressure in patients with PHD (37).
The most significant IPAH and PHD module is
MI3P3, and transcription is the major pathway.
Regulation of transcription, negative regulation of the RNA
metabolic process and positive regulation of transcription from the
RNA polymerase II promoter have been identified to participate in
regulation of the DNA transcription into messenger RNA by
transcription factors. Therapeutic strategies aimed at
transcription factors may be a novel treatment technique for IPAH
(38).
There are three common IPAH and CHD modules. There
are 18 biological processes, some of which are pathways involved in
the development of IPAH and CHD, such as the inflammatory response
and the response to wounding (39–41).
These two diseases are not connected as closely as those described
above, potentially due to the fact that the course of IPAH is
shorter than that of CHD, and numerous patients are diagnosed with
IPAH at a younger age (42).
Patients with CHD are predominantly over the age of 40 years
(43), and thus there is a lack of
overlap for CHD and IPAH.
Medication aimed at common target genes and pathways
has been used to treat IPAH and CHD. HMG-coenzyme-A reductase
inhibitors (statins) (44,45) and ACEIs (46,47)
are used to treat patients with CHD. It has been previously
identified that statins reduce pulmonary hypertension in patients
with pulmonary heart disease by improving endothelial progenitor
cell function (37), and ACEIs are
also used to relieve symptoms of IPAH (48,49).
The nonselective endothelin (ET) receptor antagonist bosentan,
which is recommended as a first-line agent for the treatment of
IPAH, improves the hemodynamics and exercise capacity in patients
with PHD (50). It has been
observed that ET-1 expression is enhanced in human atherosclerotic
lesions (51), and ET-1 plasma
levels are increased in patients with acute myocardial infarction
(a type of CHD) (52). Long-term
administration of ET receptor antagonists improves coronary
endothelial function in patients with early atherosclerosis
(53), and it provides a new
approach for prevention and therapy of CHD.
The top 10 non-overlapping IPAH, CHD and PHD modules
shared 22 types of similar biological processes, including
regulation processes, response processes and cellular processes,
which were discussed above and their correlation was presented with
the three diseases (35–37). The apoptosis pathway participates
in the development of CHD, IPAH and PHD (54–56).
Although these top 10 modules are unique, there are also several
common types of biological processes and some common pathways,
including apoptosis, cell cycle and metabolism. The unique part of
the biological processes was also identified to be associated with
the disease. Regeneration-associated functions are uncommon in CHD,
and intracoronary autologous bone marrow cell transplantation has
been reported to result in regeneration of function of human
infarcted hearts in patients with CHD (57). The current study demonstrated that
there are correlations between IPAH, CHD and PHD, which confirms
the necessity and feasibility of the study.
Due to the fact that numerous overlapping genes,
biological processes and pathways were identified in the present
study, further insight into the pathological mechanisms of IPAH,
CHD and PHD was gained, and novel focuses for future research and
potential drug targets were identified. The results of the current
study suggest that drug indications may be broadened because of
these potential targets.
With the variation present between the large amount
of online data available, the results of network analyses may
slightly differ. In addition, certain results cannot be fully
understood due to the limitations present in the current
literature. The current study was based on gene network analysis
constructing a system network to provide comprehensive insight into
IPAH, PHD and CHD, instead of focusing on a single gene or pathway
as previous studies have done.
Overlapping genes and modules in IPAH, PHD and CHD
were investigated in the present study. A disease network was
constructed to determine the common characteristics between IPAH,
PHD and CHD. The three common modules, MC9I9P13,
MC19I13P25 and MC70I77P102, can be studied
together when observing intervention results, and multi-gene
alterations in IPAH, PHD and CHD can be studied. In addition, the
observations of the current study suggest that drug indications may
be broadened due to common targets. For example, medicine aimed at
ET to treat IPAH may additionally be used for PHD and CHD.
References
|
1
|
Souza R, Jardim C and Humbert M:
Idiopathic pulmonary arterial hypertension. Semin Respir Crit Care
Med. 34:560–567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo YH, Su LX, Guo N and Liu CT: Novel
therapy for idiopathic pulmonary arterial hypertension: Can
hepatocyte growth factor be beneficial? J Geriatr Cardiol.
9:211–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janczura M, Bochenek G, Nowobilski R,
Dropinski J, Kotula-Horowitz K, Laskowicz B, Stanisz A, Lelakowski
J and Domagala T: Correction: The relationship of metabolic
syndrome with stress, coronary heart disease and pulmonary function
- An occupational cohort-based study. PLoS One. 10:e01394082015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Runo JR and Loyd JE: Primary pulmonary
hypertension. Lancet. 361:1533–1544. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel RS and Ye S: Genetic determinants of
coronary heart disease: new discoveries and insights from
genome-wide association studies. Heart. 97:1463–1473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewczuk J: Pulmonary heart disease –
cardiologist's point of view. Pneumonol Alergol Pol. 80:541–545.
2012.In Polish.
|
|
7
|
Mikhail G, Chester AH, Gibbs JS, Borland
JA, Banner NR and Yacoub MH: Role of vasoactive mediators in
primary and secondary pulmonary hypertension. Am J Cardiol.
82:254–255. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han MK, McLaughlin VV, Criner GJ and
Martinez FJ: Pulmonary diseases and the heart. Circulation.
116:2992–3005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fihn SD, Gardin JM, Abrams J, Berra K,
Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC,
Hinderliter AL, et al: 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS
guideline for the diagnosis and management of patients with stable
ischemic heart disease: A report of the American College of
Cardiology Foundation/American Heart Association task force on
practice guidelines, and the American College of Physicians
American Association for Thoracic Surgery, Preventive
Cardiovascular Nurses Association, Society for Cardiovascular
Angiography and Interventions, and Society of Thoracic Surgeons.
Circulation. 126:e354–e471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Csermely P, Korcsmáros T, Kiss HJ, London
G and Nussinov R: Structure and dynamics of molecular networks: A
novel paradigm of drug discovery: A comprehensive review. Pharmacol
Ther. 138:333–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ichihara S, Yamamoto K, Asano H, Nakatochi
M, Sukegawa M, Ichihara G, Izawa H, Hirashiki A, Takatsu F, Umeda
H, et al: Identification of a glutamic acid repeat polymerphism of
ALMS1 as a novel genetic risk marker for early-onset myocardial
infarction by genome-wide linkage analysis. Circ Cardiovasc Genet.
6:569–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eaton CB, Gramling R, Parker DR, Roberts
MB, Lu B and Ridker PM: Prospective association of vascular
endothelial growth factor-A (VEGF-A) with coronary heart disease
mortality in southeastern New England. Atherosclerosis.
200:221–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamosh A, Scott AF, Amberger JS, Bocchini
CA, Valle D and McKusick VA: Online mendelian inheritance in Man
(OMIM), a knowledgebase of human genes and genetic disorders.
Nucleic Acids Res. 30:52–55. 2002. View Article : Google Scholar :
|
|
14
|
Mattingly CJ, Colby GT, Forrest JN and
Boyer JL: The Comparative Toxicogenomics Database (CTD). Environ
Health Perspect. 111:793–795. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der Wal AC: Coronary artery pathology.
Heart. 93:1484–1489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooke GE, Doshi A and Binkley PF:
Endothelial nitric oxide synthase gene: Prospects for treatment of
heart disease. Pharmacogenomics. 8:1723–1734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Colombo MG, Paradossi U, Andreassi MG,
Botto N, Manfredi S, Masetti S, Biagini A and Clerico A:
Endothelial nitric oxide synthase gene polymorphisms and risk of
coronary artery disease. Clin Chem. 49:389–395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aytekin M, Aulak KS, Haserodt S,
Chakravarti R, Cody J, Minai OA and Dweik RA: Abnormal platelet
aggregation in idiopathic pulmonary arterial hypertension: Role of
nitric oxide. Am J Physiol Lung Cell Mol Physiol. 302:L512–L520.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orte C, Polak JM, Haworth SG, Yacoub MH
and Morrell NW: Expression of pulmonary vascular
angiotensin-converting enzyme in primary and secondary plexiform
pulmonary hypertension. J Pathol. 192:379–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mattu RK, Needham EW, Galton DJ, Frangos
E, Clark AJ and Caulfield M: A DNA Variant at the
angiotensin-converting enzyme gene locus associates with coronary
artery disease in the caerphilly heart study. Circulation.
91:270–274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanno S, Wu YJ, Lee PC, Billiar TR and Ho
C: Angiotensin-converting enzyme inhibitor preserves p21 and
endothelial nitric oxide synthase expression in
monocrotaline-induced pulmonary arterial hypertension in rats.
Circulation. 104:945–950. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCullough PA: Chronic kidney disease:
Tipping the scale to the benefit of angiotensin-converting enzyme
inhibitors in patients with coronary artery disease. Circulation.
114:6–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zisman LS, Asano K, Dutcher DL, Ferdensi
A, Robertson AD, Jenkin M, Bush EW, Bohlmeyer T, Perryman MB and
Bristow MR: Differential regulation of cardiac angiotensin
converting enzyme binding sites and AT1 receptor density in the
failing human heart. Circulation. 98:1735–1741. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bietrix F, Lombardo E, van Roomen CP,
Ottenhoff R, Vos M, Rensen PC, Verhoeven AJ, Aerts JM and Groen AK:
Inhibition of glycosphingolipid synthesis induces a profound
reduction of plasma cholesterol and inhibits atherosclerosis
development in APOE*3 Leiden and low-density lipoprotein
receptor−/−mice. Arterioscler Thromb Vasc Biol. 30:931–937. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peters SA, Singhateh Y, Mackay D, Huxley
RR and Woodward M: Total cholesterol as a risk factor for coronary
heart disease and stroke in women compared with men: A systematic
review and meta-analysis. Atherosclerosis. 248:123–131. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chapman MJ: Therapeutic elevation of
HDL-cholesterol to prevent atherosclerosis and coronary heart
disease. Pharmacol Ther. 111:893–908. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ninomiya T, Nagata M, Hata J, Hirakawa Y,
Ozawa M, Yoshida D, Ohara T, Kishimoto H, Mukai N, Fukuhara M, et
al: Association between ratio of serum eicosapentaenoic acid to
arachidonic acid and risk of cardiovascular disease: The Hisayama
Study. Atherosclerosis. 231:261–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ball SK, Field MC and Tippins JR:
Regulation of thromboxane receptor signaling at multiple levels by
oxidative stress-induced stabilization, relocation and enhanced
responsiveness. PLoS One. 5:e127982010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirenallur-S DK, Detweiler ND, Haworth ST,
Leming JT, Gordon JB and Rusch NJ: Furegrelate, a thromboxane
synthase inhibitor, blunts the development of pulmonary arterial
hypertension in neonatal piglets. Pulm Circ. 2:193–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sobieszczyk P, Fishbein MC and Goldhaber
SZ: Acute pulmonary embolism: Don't ignore the platelet.
Circulation. 106:1748–1749. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goldhaber SZ and Elliott CG: Acute
pulmonary embolism: Part I epidemiology, pathophysiology, and
diagnosis. Circulation. 108:2726–2729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian W, Jiang X, Sung YK, Qian J, Yuan K
and Nicolls MR: Leukotrienes in pulmonary arterial hypertension.
Immunol Res. 58:387–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nair J, Shanker J, Jambunathan S, Arvind P
and Kakkar VV: Expression analysis of leukotriene-inflammatory gene
interaction network in patients with coronary artery disease. J
Atheroscler Thromb. 21:329–345. 2014. View Article : Google Scholar
|
|
34
|
Prasad K and Kalra J: Oxygen free radicals
and heart failure. Angiology. 39:417–420. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim D, Kwon YK and Cho KH: Coupled
positive and negative feedback circuits form an essential building
block of cellular signaling pathways. Bioessays. 29:85–90. 2007.
View Article : Google Scholar
|
|
36
|
Karastergiou K, Evans I, Ogston N, Miheisi
N, Nair D, Kaski JC, Jahangiri M and Mohamed-Ali V: Epicardial
adipokines in obesity and coronary artery disease induce
atherogenic changes in monocytes and endothelial cells.
Arterioscler Thromb Vasc Biol. 30:1340–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu HF, Qi XW, Ma LL, Yao DK and Wang L:
Atorvastatin improves endothelial progenitor cell function and
reduces pulmonary hypertension in patients with chronic pulmonary
heart disease. Exp Clin Cardiol. 18:e40–e43. 2013.PubMed/NCBI
|
|
38
|
Crosswhite P and Sun Z: Molecular
mechanisms of pulmonary arterial remodeling. Mol Med. 20:191–201.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hamidi SA, Prabhakar S and Said SI:
Enhancement of pulmonary vascular remodelling and inflammatory
genes with VIP gene deletion. Eur Respir J. 31:135–139. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heusch G, Schulz R and Erbel R:
Inflammatory markers in coronary heart disease: Coronary vascular
versus myocardial origin? Circulation. 108:e42003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Nigris F, Youssef T, Ciafré S, Franconi
F, Anania V, Condorelli G, Palinski W and Napoli C: Evidence for
oxidative activation of c-Myc-Dependent nuclear signaling in human
coronary smooth muscle cells and in early lesions of watanabe
heritable hyperlipidemic rabbits: Protective effects of vitamin E.
Circulation. 102:2111–2117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fraisse A, Jais X, Schleich JM, di Filippo
S, Maragnès P, Beghetti M, Gressin V, Voisin M, Dauphin C, Clerson
P, et al: Characteristics and prospective 2-year follow-up of
children with pulmonary arterial hypertension in France. Arch
Cardiovasc Dis. 103:66–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Page IH, Berrettoni JN, Butkus A and Sones
FM Jr: Prediction of coronary heart disease based on clinical
suspicion, age, total cholesterol, and triglyceride. Circulation.
42:625–645. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vaughan CJ, Gotto AM Jr and Basson CT: The
evolving role of statins in the management of atherosclerosis. J Am
Coll Cardiol. 35:1–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maron DJ, Fazio S and Linton MF: Current
perspectives on statins. Circulation. 101:207–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yusuf S, Sleight P, Pogue J, Bosch J,
Davies R and Dagenais G: Effects of an angiotensin
converting-enzyme inhibitor, ramipril, on cardiovascular events in
high-risk patients. The heart outcomes prevention evaluation study
investigators. N Engl J Med. 342:145–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yusuf S, Sleight P, Pogue J, Bosch J,
Davies R and Dagenais G: Cardiac rehabilitation and secondary
prevention of coronary heart disease. Circulation. 111:369–376.
2005. View Article : Google Scholar
|
|
48
|
Jeffery TK and Wanstall JC: Pulmonary
vascular remodeling: A target for therapeutic intervention in
pulmonary hypertension. Pharmacol Ther. 92:1–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Budhiraja R, Tuder RM and Hassoun PM:
Endothelial dysfunction in pulmonary hypertension. Circulation.
109:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rich S and McLaughlin VV: Endothelin
receptor blockers in cardiovascular disease. Circulation.
108:2184–2190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Minamino T, Kurihara H, Takahashi M,
Shimada K, Maemura K, Oda H, Ishikawa T, Uchiyama T, Tanzawa K and
Yazaki Y: Endothelin-converting enzyme expression in the rat
vascular injury model and human coronary atherosclerosis.
Circulation. 95:221–230. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stewart DJ, Kubac G, Costello KB and
Cernacek P: Increased plasma endothelin-1 in the early hours of
acute myocardial infarction. J Am Coll Cardiol. 18:38–43. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Reriani M, Raichlin E, Prasad A, Mathew V,
Pumper GM, Nelson RE, Lennon R, Rihal C, Lerman LO and Lerman A:
Long-term administration of endothelin receptor antagonist improves
coronary endothelial function in patients with early
atherosclerosis. Circulation. 122:958–966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Blanco-Colio LM: TWEAK/Fn14 Axis: A
promising target for the treatment of cardiovascular diseases.
Front Immunol. 5:32014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang S, Fantozzi I, Tigno DD, Yi ES,
Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ and
Yuan JX: Bone morphogenetic proteins induce apoptosis in human
pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol
Physiol. 285:L740–L754. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Merklinger SL, Jones PL, Martinez EC and
Rabinovitch M: Epidermal growth factor receptor blockade mediates
smooth muscle cell apoptosis and improves survival in rats with
pulmonary hypertension. Circulation. 112:423–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Strauer BE, Brehm M, Zeus T, Bartsch T,
Schannwell C, Antke C, Sorg RV, Kögler G, Wernet P, Müller HW and
Köstering M: Regeneration of human infarcted heart muscle by
intracoronary autologous bone marrow cell transplantation in
chronic coronary heart disease: The IACT-study. J Am Coll Cardiol.
46:1651–1658. 2005. View Article : Google Scholar : PubMed/NCBI
|