Introduction
Enterotoxigenic Escherichia coli (ETEC) is
one of the leading causes of bacterial diarrhea in humans and pigs.
ETEC colonizes the intestine by fimbrial adhesins (K88/F4, K99/F5,
987P/F6, F41/F7 and F18), and then produces enterotoxins, such as
heat labile (LT), heat-stable (STa and STb), and entero-aggregative
Escherichia coli heat-stable enterotoxin-1, which leads to
excessive loss of fluids and electrolytes resulting in profuse
diarrhea, frequently without histological lesions (1,2). The
alterations of intestinal function following ETEC colonization are
widely investigated, including autophagy (3), immune responses (4–7),
nutrient absorption (8), barrier
integrity (9–11) and apoptosis (12,13).
However, investigations regarding the intestinal
immune response have remained controversial. Finamore et al
(4) identified that ETEC (K88)
infection activates the toll-like receptor 4 (TLR4)-nuclear factor
(NF)-κB signaling pathway by increasing the expression levels of
TLR4 and myeloid differentiation primary response 88. The
phosphorylation of the conserved helix-loop-helix ubiquitous kinase
[CHUK; also termed inhibitor of κB (IκB) kinase(IKK)α], IKKβ, IκBα)
and NF-κB subunit p65, resulted in the overproduction of
inflammatory cytokines interleukin (IL)-8 and IL-1β in Caco-2/TC7
cells and pig explants (5 week-old crossbreed
Pietrain/Duroc/Large-White piglets) (4). McLamb et al (6) also reported that ETEC (F18) infection
significantly promoted intestinal immune responses, identified
elevation in IL-6 and -8 expression, neutrophil recruitment and
mast cell activation in weaned pigs. However, Wang and Hardwidge
(7) identified that ETEC blocks
NF-κB signaling, which is commonly induced by tumor necrosis factor
(TNF), IL-1β, and flagellin by secreting a heat-stable,
proteinaceous factor. A previous study identified that ETEC
supernatant significantly blocks the degradation of the NF-κB
inhibitor IκBα by preventing IκBα polyubiquitination, without
affecting IκBα phosphorylation (7). A previous study indicated that ETEC
infection inhibits the intestinal immune responses due to the fact
that NF-κB is the principal pathway associated with the induction
of host pro-inflammatory responses following infection (14,15).
Thus, the present study was conducted to investigate
the function of ETEC infection on intestinal immunity in a mouse
model infected with a porcine isolated ETEC strain.
Materials and methods
Bacterial strains and antibodies
The current study used ETEC298 (serotype O107;
oqxAB; F18; STa, STb, SLT-IIe), which was a gift from Dr Wenkai Ren
(Chinese Academy of Sciences, Changsha, China). It was isolated
from piglets with diarrhea. ETEC298 was cultured in Luria-Bertani
broth and on agar at 37°C, as previously described (3,16).
Antibodies against mitogen-activated protein kinase 8 [MAPK8; also
termed c-Jun N-terminal kinase (JNK)] (1:200; sc-571),
phosphorylated-Jnk (1:200; sc-12882) and TLR4 (1:200; sc-10741),
proliferating cell nuclear antigen (PCNA; 1:200; sc-56) and actin
(1:200; SC-1616) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Antibodies against MAPK3 [also termed
extracellular signal-related kinase 1/2 (ERK1/2)] (1:500; CST
4695), phosphorylated-ERK1/2 (1:500; CST 4370), p38 (1:500; CST
8690), p-p38 (1:500; CST 4511) and p65 (1:500; CST 6956) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Animal model
The current study was performed according to the
guidelines of the Laboratory Animal Ethical Commission of the
Institute of Subtropical Agriculture, Chinese Academy of Sciences
(Changsha, China). The ETEC infection model was established
according to the method described by Allen et al (17). Female ICR mice were purchased from
Shanghai Laboratory Animal Center (Shanghai, China). The mice were
housed in a pathogen-free mouse colony at 20–30°C, 45–60% humidity
with a 12-h light/dark cycle. At six weeks of age, mice (24–26 g,
n=42 for ETEC infection; n=15 for control) were randomly assigned
into either the ETEC infection or control groups and inoculated by
oral gavage with either 1×109 colony forming units
ETEC298 or with sterile phosphate-buffered saline (PBS, Hunan World
Well-being Bio-tech Co., Ltd., Hunan, China). Mice were sacrificed
by cervical dislocation after 24 h, and the jejunum and ileum was
collected. Segments (2 cm) of the jejunum and ileum (middle part)
were dissected after washing the intestinal contents with PBS.
Tissue samples were shock cooled with liquid nitrogen. After shock
freezing the samples were kept at −80°C until further processing.
The mice were used as a model to investigate the alterations of
intestinal immunity following ETEC infection due to the fact that
previous studies demonstrated that human or porcine isolated ETEC
affects the intestinal functions of mice, although no diarrhea was
observed subsequent to ETEC inoculation (17,18).
The dosage of ETEC for infection, and the time points for tissue
collection were based on previous studies (17,18).
As the jejunum is the main target for ETEC in the host (18), thus, the current study also focused
on the alterations of intestinal immunity in mouse jejunum.
Morphological analyses
For light microscopic observation (DM6M, Leica
Microsystems, Wetzlar, Germany), jejunum tissues were fixed with
10% formalin (Hunan World Well-being Bio-tech Co., Ltd.) and PBS at
4°C, dehydrated in a graded series of ethanol, then embedded in
paraffin wax (Hunan World Well-being Bio-tech Co., Ltd.). Tissue
sections (5 µm, cut by SYD-S2020; YuDe, Shengyang, China)
were mounted on slides, dewaxed (in xylene, Sangon Biotech, Co.,
Ltd., Shanghai, China), hydrated and then stained with hematoxylin
& eosin (Hunan World Well-being Bio-tech Co., Ltd.).
Immunoblotting
Equal amounts of proteins (100 µg) obtained
from cytoplasmic or nuclear fractions were separated by sodium
dodecyl sulfate-polyacrylimide gel electrophoresis, transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA), and blocked with 5% non-fat milk in Tris-buffered saline with
Tween-20 (20 mM Tris, pH 7.5, 150 mM NaCl and 0.1% Tween-20 (Sangon
Biotech, Co., Ltd.) for 3 h. Membranes were incubated with primary
antibodies overnight at 4°C and then with horseradish peroxidase
conjugated-goat anti-rabbit IgG (1:5,000; cat. no. sc-2030; Santa
Cruz Biotechnology, Inc. for 1 h at room temperature prior to
development and analysis using 3.2 Alpha Imager 2200 software
(Alpha Innotech Corporation, San Leandro, CA, USA). The resultant
signals were digitally quantified and the data was normalized to
PCNA or actin abundance. PCNA or actin was used as an internal
loading control for nuclear and cytoplasmic protein fractions,
respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from liquid nitrogen frozen
jejunum or ileum samples with TRIzol regent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and then treated with
DNase I (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Total RNA (1 µg) was
reverse-transcribed to cDNA using the iScript cDNA synthesis kit
(Bio-Rad, Hercules, CA, USA). Expression of target genes was
assayed by RT-qPCR using SYBR Green mix (Takara Biotechnology
(Dalian) Co., Ltd., Dalian, China). The primers used are as
described in previous studies (15,19).
β-actin was used as an internal control to normalize target gene
transcript levels. RT-qPCR was performed according to a previous
study (20). Briefly, 1.0
µl cDNA template was added to a total volume of 10 µl
containing 5 µl SYBR Green mix (Takara Biotechnology
(Dalian) Co., Ltd.), 0.2 µl Rox (Takara Biotechnology
(Dalian) Co., Ltd.), 3.0 µl ddH2O, and 0.4
µl each of forward and reverse primers (Sangon Biotech, Co.,
Ltd.). The following cycling protocol was used with an ABI7900
thermocycler (Applied Biosysterms, Thermo Fisher Scientific, Inc.):
i) Pre-denaturation (10 sec at 95°C); ii) amplification and
quantification (40 cycles of 5 sec at 95°C and 20 sec at 60°C);
iii) melting (60–99°C with a heating rate of 0.1°C/sec and
fluorescence measurement). Relative expression was normalized and
expressed as a ratio to the expression in the control group.
Statistical analysis
Data are expressed as the mean ± standard error. All
statistical analyses were performed using SPSS software (version
16.0; SPSS, Inc., Chicago, IL, USA). Data were analyzed using
Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical results
For the mice, there was a mortality rate of 11 out
of 42 (26%) within 24 h of infection with ETEC298 strain, whereas
there was a mortality rate of 0 out of 42 following treatment with
PBS. Microscopic analyses demonstrated that the most remarkable
alterations following ETEC298-infection were the loss of microvilli
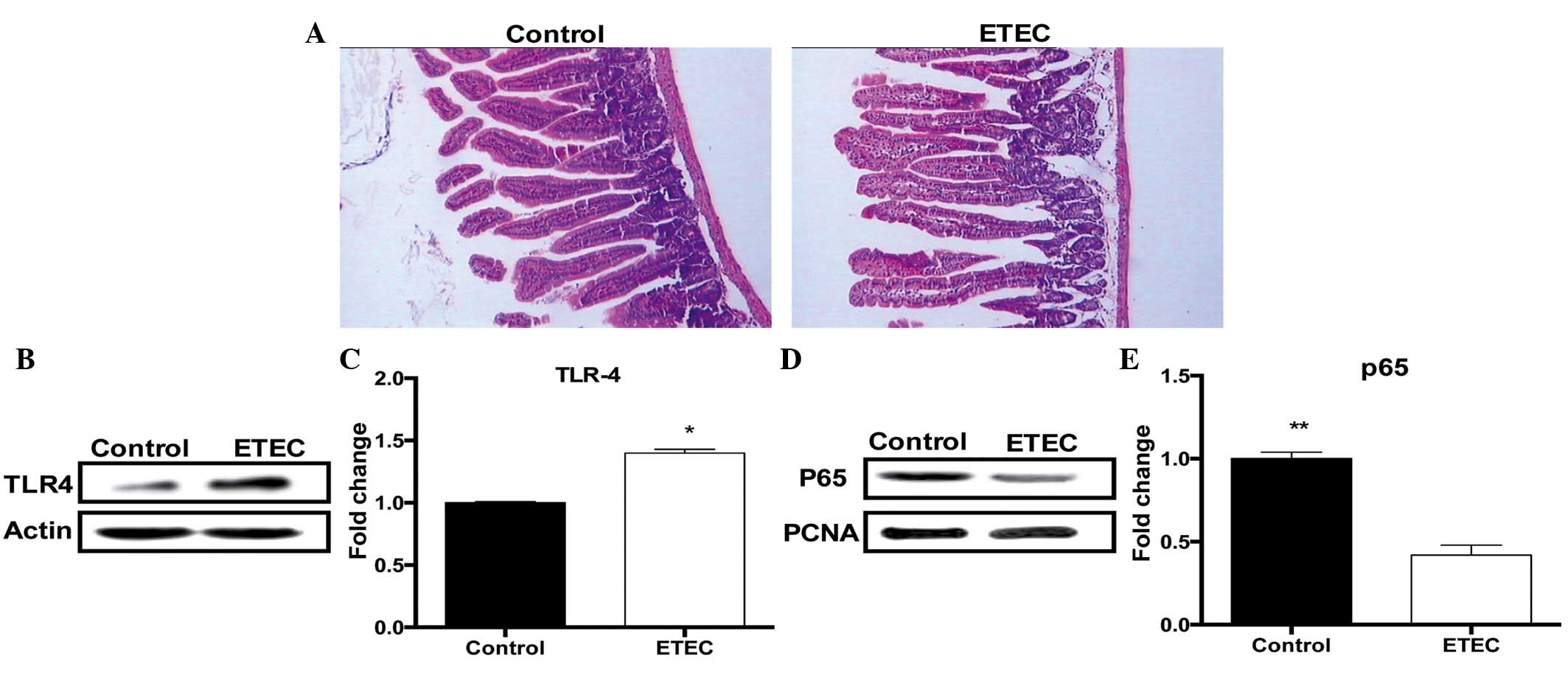
in the jejunum (Fig. 1A).
ETEC298 infection increases TLR4
expression and inhibits NF-κB signaling
Infection with ETEC298 significantly (P<0.05)
increased TLR4 protein expression in the jejunum 24 h
post-infection (Fig. 1B and C), in
agreement with a previous study that demonstrated that ETEC
infection significantly enhanced TLR4 gene expression in a piglet
infection model (21). However,
ETEC298 infection significantly (P<0.01) reduced p65 protein
abundance in the nucleus (Fig. 1D and
E), suggesting that ETEC infection inhibits the NF-κB signaling
pathway.
ETEC298 infection activates the MAPK
pathway
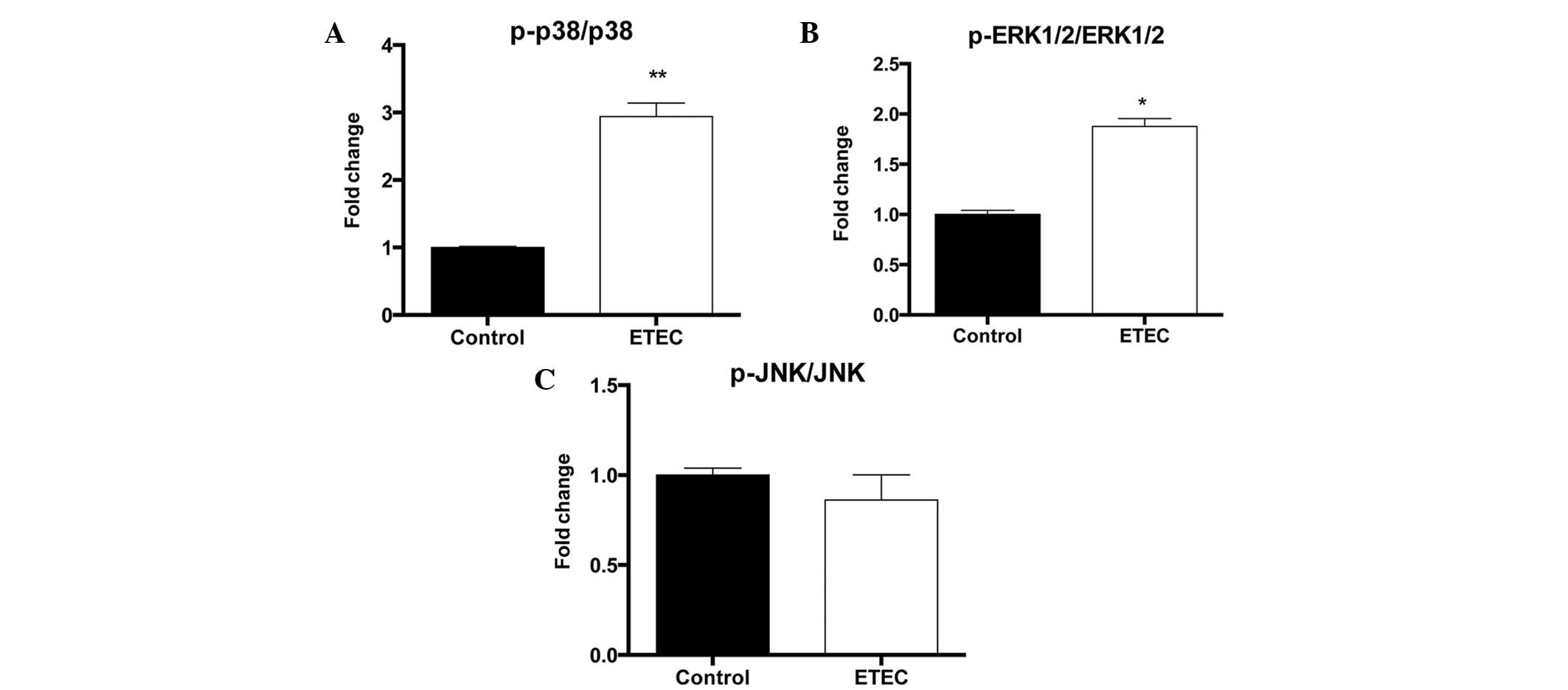
To determine the effect of ETEC on p38 MAPK, ERK1/2
and JNK activation, the activation-associated phosphorylation of
these kinases was quantified using phosphorylation-specific
antibodies. While ETEC infection was observed to have no
significant effect on total p38, ERK1/2 and JNK protein levels, it
induced significant phosphorylation of p38 (P<0.01) and ERK1/2
(P<0.05), without significant impact on JNK phosphorylation
(Fig. 2).
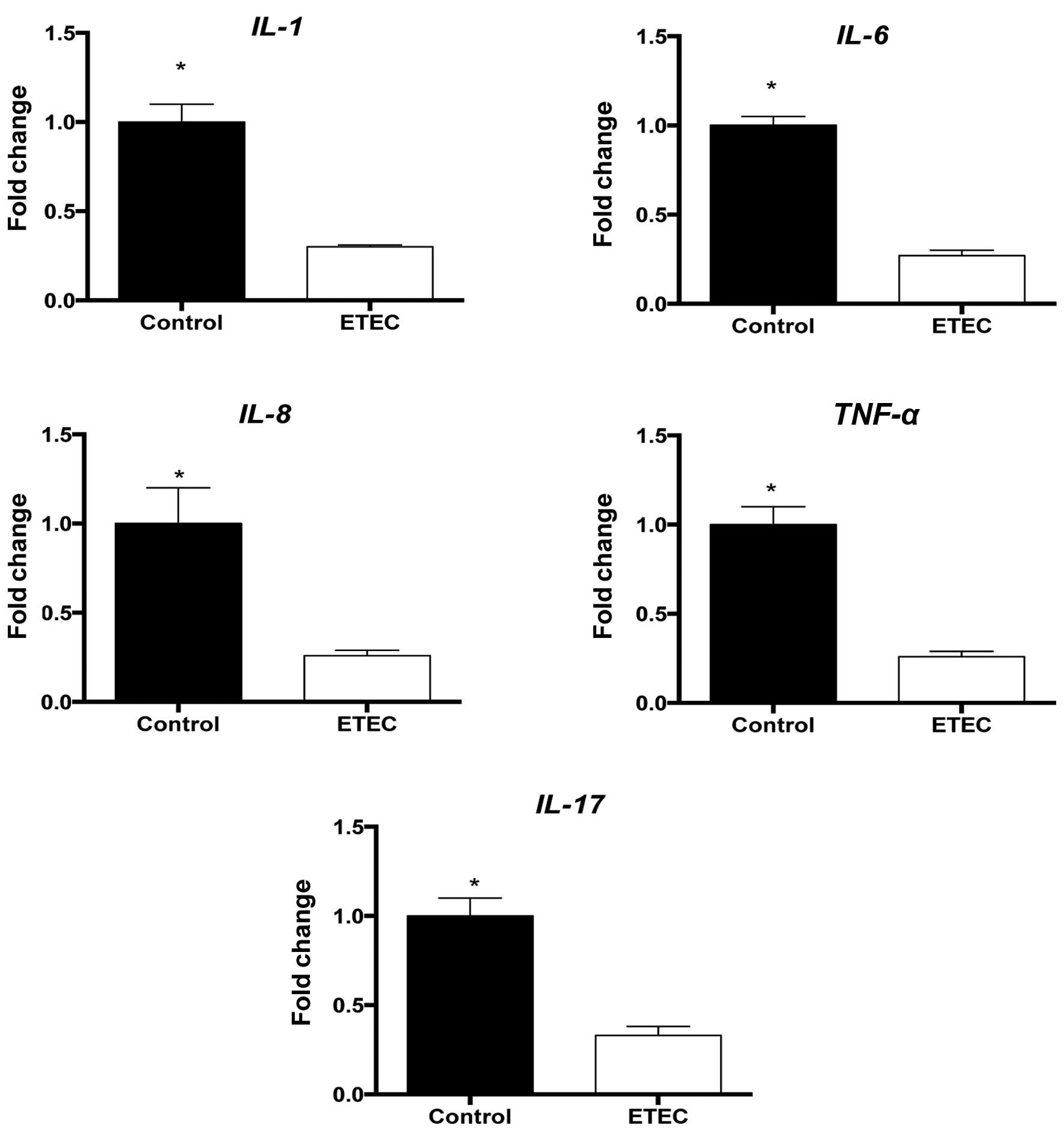
ETEC infection promotes inflammation
Inflammatory responses are intended to disarm or
destroy invading microorganisms, remove irritants and prepare for
tissue repair or wound healing. The expression levels of the
pro-inflammatory cytokines IL-1β, IL-6, IL-8
and TNF-α following ETEC infection were measured.
IL-17A expression was also measured due to the fact that it
also serves a vital regulatory role in intestinal function. ETEC
infection was observed to significantly (P<0.05) reduce the
expression of all measured cytokines in the jejunum (Fig. 3).
ETEC affects intestinal immunity
Intestinal immune regulators serve an essential role
in protection against bacterial invasion and maintenance of mucosal
homeostasis (22–24). These regulators contain J-chain,
the polymeric immunoglobulin receptor (pIgR), mucin 2 and 4,
α-defensins, cryptdin-related sequence (CRS) peptides, lysozyme C,
secretory group IIA phospholipase A2 (sPLA2), regenerating
islet-derived 3γ (REG3γ) and RNase angiogenin 4 (ANG4) in mice
(15,25). As presented in Table I, ETEC298 infection significantly
reduced the expression of pIgR, J-chain and
Lyz2 (lysozyme) (P<0.05), however significantly increased
CRS4C (cysteine-rich sequence 4C) and CRS1C
expression levels (P<0.05), and had little effect on other genes
in the jejunum. In the ileum, the ETEC298 group had significantly
reduced (P<0.05) expression of pIgR and CRS1C,
however increased (P<0.05) the expression of mucin 2,
Lyz and cryptdin-5 compared with the control group,
while no significant difference between the infected and control
groups was observed for the remaining factors (Table II).
 | Table IRelative expression of intestinal
innate immune factors in the jejunum in the ETEC298 and control
groups. |
Table I
Relative expression of intestinal
innate immune factors in the jejunum in the ETEC298 and control
groups.
| Factor | ETEC298 | Control group |
|---|
| J-chain | 0.34±0.06a | 1.00±0.09 |
| pIgR | 0.49±0.06a | 1.00±0.21 |
| Mucin 2 | 0.22±0.02 | 1.00±0.15 |
| Mucin 4 | 2.68±1.46 | 1.00±0.34 |
|
Cryptdin-1 | 1.70±0.36 | 1.00±0.12 |
|
Cryptdin-4 | 0.98±0.29 | 1.00±0.33 |
|
Cryptdin-5 | 1.54±0.36 | 1.00±0.12 |
| CRS-1C | 4.45±1.15a | 1.00±0.15 |
| CRS-4C | 3.84±0.83a | 1.00±0.12 |
| sPLA2 | 0.75±0.21 | 1.00±0.16 |
| ANG4 | 1.10±0.23 | 1.00±0.11 |
| REG3γ | 0.98±0.14 | 1.00±0.09 |
| Lyz2 | 0.25±0.04a | 1.00±0.18 |
 | Table IIRelative expression of intestinal
innate immune factors in the ileum in the ETEC298 and control
groups. |
Table II
Relative expression of intestinal
innate immune factors in the ileum in the ETEC298 and control
groups.
| Factor | ETEC298 | Control group |
|---|
| J-chain | 0.72±0.10 | 1.00±0.10 |
| pIgR | 0.50±0.10a | 1.00±0.13 |
| Mucin 2 | 2.00±0.75a | 1.00±0.08 |
| Mucin 4 | 0.90±0.36 | 1.00±0.27 |
|
Cryptdin-1 | 1.10±0.14 | 1.00±0.09 |
|
Cryptdin-4 | 1.54±0.27 | 1.00±0.26 |
|
Cryptdin-5 | 1.85±0.30a | 1.00±0.11 |
| CRS-1C | 0.47±0.10a | 1.00±0.28 |
| CRS-4C | 1.26±0.21 | 1.00±0.16 |
| sPLA2 | 0.91±0.17 | 1.00±0.14 |
| ANG4 | 1.01±0.25 | 1.00±0.17 |
| REG3γ | 0.25±0.11 | 1.00±0.79 |
| Lyz2 | 1.69±0.14a | 1.00±0.21 |
Discussion
In present study, ETEC infection was observed to
reduce the expression levels of pro-inflammatory cytokines,
including IL-1β, IL-6, IL-8,
TNF-α and IL-17A, indicating ETEC infection
represses the intestinal inflammatory responses in mice. However,
Roselli et al (26)
observed that ETEC (K88) infection promotes neutrophil
transmigration, the expression of chemokines essential for
neutrophil migration, such as IL-8, growth-related oncogene-α and
epithelial neutrophil-activating peptide-78, and the expression of
IL-1β and TNF-α, which are regulators of chemokine expression, in
Caco-2 cells. In addition, similar results have been observed in
intestinal epithelial IPI-2I cells (27), differentiated porcine intestinal
epithelial IPEC-1 cells (28),
IPEC J2 cells (29) and in
vivo with piglets (30). An
additional study demonstrated that ETEC (K88) induces the secretion
of IL-6 and IL-8 in intestinal epithelial cells associated with F4
fimbriae and flagellin (31).
Furthermore, Ren et al (18) observed that ETEC infection promotes
the expression of pro-inflammatory cytokines through the activation
of the NF-κB and MAPK pathways. A possible explanation for these
discrepancies may be the fact that different infectious models and
bacterial strains were used. The strain used in the current study
lacks LT, unlike the ETEC strain previously used (18). Other clinical isolated strains,
which lack LT, have additionally been observed to fail to promote
the expression of pro-inflammatory cytokines in mouse jejunum
subsequent to 24 h infection (Liu et al, unpublished
data).
The inhibition of the expression of pro-inflammatory
cytokines may be via the inactivation of the NF-κB pathway
subsequent to ETEC infection. In line with the results of the
current study, Wang and Hardwidge (7) reported that ETEC blocks NF-κB
signaling by secreting a heat-stable, proteinaceous factor. The
ETEC supernatant has been observed to significantly block the
degradation of the NF-κB inhibitor IκBα by preventing IκBα
polyubiquitination, without affecting IκBα phosphorylation
(7). However, numerous studies
have suggested that ETEC infection may promote the activation of
the NF-κB pathway in intestinal epithelial cells (HCT-8 cells)
(32) and porcine intestinal
epitheliocyte (PIE) cells (33).
Thus, it is notable to investigate the underling reason for this
discrepancy. In the current study, ETEC infection was observed to
activate the MAPK pathway via the increased phosphorylation of p38
and ERK1/2. In agreement with the results of the current study,
ETEC was observed to be able to activate MAPK signaling pathway in
intestinal epithelial cells (HCT-8 cells) through mechanisms that
are primarily dependent upon LT (32). Shimazu et al (33) also demonstrated that ETEC infection
promotes MAPK activation in PIE cells. A possible explanation for
MAPK activation following ETEC infection is the activation of TLR
signaling. It has been widely reported that ETEC infection
activates TLR signaling in PIE cells (33), human intestinal Caco-2/TC7 cells
and intestinal explants isolated from 5-week-old crossbreed
Pietrain/Duroc/Large-White piglets (4), and weaned piglets (34).
ETEC infection affects intestinal innate immunity,
including secretory immunoglobulin A (sIgA, based on the mRNA
expression of J-chain and pIgR), goblet cells (based on the mRNA
expression of Mucin 2) and Paneth cells (based on the mRNA
expression of CRS-1C, CRS-4C, Lyz2 and Cryptdin-5). Numerous
studies have indicated that ETEC infection affects the secretion of
sIgA in piglets (34,35). However, little literatures have
reported the effect of ETEC infection on the intestinal goblet and
Paneth cell function in vivo. Considering that ETEC
infection affects the expression of antibacterial substances
expressed by goblet and Paneth cell, thus it is interesting to
explore the effect of ETEC infection on Paneth cell and goblet
cell.
In conclusion, ETEC infection inhibits the
expression in mouse jejunum of pro-inflammatory cytokines
associated with the inhibition of NF-κB. ETEC infection promotes
the activation of the MAPK pathway in the mouse model. Furthermore,
ETEC infection affects the intestinal sIgA levels, and the function
of goblet and Paneth cells.
References
|
1
|
Field M: Intestinal ion transport and the
pathophysiology of diarrhea. J Clin Invest. 111:931–943. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moeser AJ and Blikslager AT: Mechanisms of
porcine diarrheal disease. J Am Vet Med Assoc. 231:56–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang Y, Li F, Tan B, Liu G, Kong X,
Hardwidge PR and Yin Y: Enterotoxigenic Escherichia coli infection
induces intestinal epithelial cell autophagy. Vet Microbiol.
171:160–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finamore A, Roselli M, Imbinto A, Seeboth
J, Oswald IP and Mengheri E: Lactobacillus amylovorus inhibits the
TLR4 inflammatory signaling triggered by enterotoxigenic
Escherichia coli via modulation of the negative regulators and
involvement of TLR2 in intestinal Caco-2 cells and pig explants.
PLoS One. 9:e948912014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu YH, Li XQ, Zhang W, Zhou D, Liu HY and
Wang JF: Dose-dependent effects of Lactobacillus rhamnosus on serum
interleukin-17 production and intestinal T-cell responses in pigs
challenged with Escherichia coli. Appl Environ Microbiol.
80:1787–1798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLamb BL, Gibson AJ, Overman EL, Stahl C
and Moeser AJ: Early weaning stress in pigs impairs innate mucosal
immune responses to enterotoxigenic E. coli challenge and
exacerbates intestinal injury and clinical disease. PLoS One.
8:e598382013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X and Hardwidge PR: Enterotoxigenic
Escherichia coli prevents host NF-κB activation by targeting IκBα
polyubiquitination. Infect Immun. 80:4417–4425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosal A, Chatterjee NS, Chou T and Said
HM: Enterotoxigenic Escherichia coli infection and intestinal
thiamin uptake: Studies with intestinal epithelial Caco-2
monolayers. Am J Physiol Cell Physiol. 305:C1185–C1191. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakashima R, Kamata Y and Nishikawa Y:
Effects of Escherichia coli heat-s enterotoxin and guanylin on the
barrier integrity of intestinal epithelial T84 cells. Vet Immunol
Immunopathol. 152:78–81. 2013. View Article : Google Scholar
|
|
10
|
Johnson AM, Kaushik RS and Hardwidge PR:
Disruption of transepithelial resistance by enterotoxigenic
Escherichia coli. Vet Microbiol. 141:115–119. 2010. View Article : Google Scholar
|
|
11
|
Egberts HJ, de Groot EC, van Dijk JE,
Vellenga L and Mouwen JM: Tight junctional structure and
permeability of porcine jejunum after enterotoxic Escherichia coli
infection. Res Vet Sci. 55:10–14. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Syed HC and Dubreuil JD: Escherichia coli
STb toxin induces apoptosis in intestinal epithelial cell lines.
Microb Pathog. 53:147–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson AM, Kaushik RS, Rotella NJ and
Hardwidge PR: Enterotoxigenic Escherichia coli modulates host
intestinal cell membrane asymmetry and metabolic activity. Infect
Immun. 77:341–347. 2009. View Article : Google Scholar :
|
|
14
|
Ren WK, Yin J, Zhu XP, Liu G, Li NZ, Peng
YY and Yin YL: Glutamine on intestinal inflammation: A mechanistic
perspective. Eur J Inflamm. 11:315–326. 2013.
|
|
15
|
Ren W, Chen S, Yin J, Duan J, Li T, Liu G,
Feng Z, Tan B, Yin Y and Wu G: Dietary arginine supplementation of
mice alters the microbial population and activates intestinal
innate immunity. J Nutr. 144:988–995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Huan H, Wan T, Wang L, Gao S and
Jiao X: Antigenic determinants analysis and detection of virulence
factors in F18 fimbriae Escherichia coli strains isolated from
pigs. Wei Sheng Wu Xue Bao. 54:236–242. 2014.In Chinese. PubMed/NCBI
|
|
17
|
Allen KP, Randolph MM and Fleckenstein JM:
Importance of heat-labile enterotoxin in colonization of the adult
mouse small intestine by human enterotoxigenic Escherichia coli
strains. Infect Immun. 74:869–875. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren W, Yin J, Duan J, Liu G, Zhu X, Chen
S, Li T, Wang S, Tang Y and Hardwidge PR: Mouse jejunum innate
immune responses altered by enterotoxigenic Escherichia coli (ETEC)
infection. Microbes Infect. 16:954–961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren WK, Liu SP, Chen S, Zhang F, Li N, Yin
J, Peng Y, Wu L, Liu G, Yin Y and Wu G: Dietary L-glutamine
supplementation increases Pasteurella multocida burden and the
expression of its major virulence factors in mice. Amino Acids.
45:947–955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren W, Luo W, Wu M, Liu G, Yu X, Fang J,
Li T, Yin Y and Wu G: Dietary L-glutamine supplementation improves
pregnancy outcome in mice infected with type-2 porcine circovirus.
Amino Acids. 45:479–488. 2013. View Article : Google Scholar
|
|
21
|
Hermes RG, Manzanilla EG, Martín-Orúe SM,
Pérez JF and Klasing KC: Influence of dietary ingredients on in
vitro inflammatory response of intestinal porcine epithelial cells
challenged by an enterotoxigenic Escherichia coli (K88). Comp
Immunol Microbiol Infect Dis. 34:479–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pabst O: New concepts in the generation
and functions of IgA. Nat Rev Immunol. 12:821–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vereecke L, Beyaert R and van Loo G:
Enterocyte death and intestinal barrier maintenance in homeostasis
and disease. Trends Mol Med. 17:584–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bevins CL and Salzman NH: Paneth cells,
antimicrobial peptides and maintenance of intestinal homeostasis.
Nat Rev Microbiol. 9:356–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren W, Duan J, Yin J, Liu G, Cao Z, Xiong
X, Chen S, Li T, Yin Y, Hou Y and Wu G: Dietary L-glutamine
supplementation modulates microbial community and activates innate
immunity in the mouse intestine. Amino Acids. 46:2403–2413. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roselli M, Finamore A, Britti MS and
Mengheri E: Probiotic bacteria Bifidobacterium animalis MB5 and
Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the
inflammation-associated response induced by enterotoxigenic
Escherichia coli K88. Br J Nutr. 95:1177–1184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zanello G, Meurens F, Berri M, Chevaleyre
C, Melo S, Auclair E and Salmon H: Saccharomyces cerevisiae
decreases inflammatory responses induced by F4+ enterotoxigenic
Escherichia coli in porcine intestinal epithelial cells. Vet
Immunol Immunopathol. 141:133–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zanello G, Berri M, Dupont J, Sizaret PY,
D'Inca R, Salmon H and Meurens F: Saccharomyces cerevisiae
modulates immune gene expressions and inhibits ETEC-mediated ERK1/2
and p38 signaling pathways in intestinal epithelial cells. PLoS
One. 6:e185732011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sargeant HR, Miller HM and Shaw MA:
Inflammatory response of porcine epithelial IPEC J2 cells to
enterotoxigenic E. coli infection is modulated by zinc
supplementation. Mol Immunol. 48:2113–2121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sargeant HR, McDowall KJ, Miller HM and
Shaw MA: Dietary zinc oxide affects the expression of genes
associated with inflammation: Transcriptome analysis in piglets
challenged with ETEC K88. Vet Immunol Immunopathol. 137:120–129.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Devriendt B, Stuyven E, Verdonck F,
Goddeeris BM and Cox E: Enterotoxigenic Escherichia coli (K88)
induce proinflammatory responses in porcine intestinal epithelial
cells. Dev Comp Immunol. 34:1175–1182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Gao X and Hardwidge PR:
Heat-labile enterotoxin-induced activation of NF-κB and MAPK
pathways in intestinal epithelial cells impacts enterotoxigenic
Escherichia coli (ETEC) adherence. Cell Microbiol. 14:1231–1241.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimazu T, Villena J, Tohno M, Fujie H,
Hosoya S, Shimosato T, Aso H, Suda Y, Kawai Y, Saito T, et al:
Immunobiotic Lactobacillus ensenii elicits anti-inflammatory
activity in porcine intestinal epithelial cells by modulating
negative regulators of the Toll-like receptor signaling pathway.
Infect Immun. 80:276–288. 2012. View Article : Google Scholar :
|
|
34
|
Xiao D, Tang Z, Yin Y, Zhang B, Hu X, Feng
Z and Wang J: Effects of dietary administering chitosan on growth
performance, jejunal morphology, jejunal mucosal sIgA, occluding,
claudin-1 and TLR4 expression in weaned piglets challenged by
enterotoxigenic Escherichia coli. Int Immunopharmacol. 17:670–676.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao Y, Han F, Huang X, Rong Y, Yi H and
Wang Y: Changes in gut microbial populations, intestinal
morphology, expression of tight junction proteins and cytokine
production between two pig breeds after challenge with Escherichia
coli K88: A comparative study. J Anim Sci. 91:5614–5625. 2013.
View Article : Google Scholar : PubMed/NCBI
|