Introduction
Iron contributes in multiple ways to the
physiological processes of the human body, both as a part of
cytochrome heme, as well as a key component molecule in hemoglobin
and myoglobin heme, which binds oxygen in red blood cells. Due to
the absence of an effective excretory mechanism, the maintenance of
normal iron levels is critical and is mainly succeeded by
regulating intestinal absorption and by continuously recycling and
reusing cellular iron (1,2).
The ability of iron to release free oxygen radicals
can be a potential health hazard, while excessive iron levels may
promote oxidative stress by increasing the steady-state
concentration of intermediate oxygen radicals. This may lead to
fibrosis and carcinogenicity, and may also deactivate essential
metabolic enzymes (1,3).
Body iron is found mainly in the form of heme iron
(organic iron) and non-heme iron (inorganic iron). Different
absorption mechanisms are implemented for these two types of
dietary iron. The total iron absorption is low, ~10% of the 10-20
mg obtained from diet. Heme iron is absorbed more efficiently than
non-heme iron (1).
Heme iron enters the cell either by binding to a
carrier protein of the cell membrane, or through an endocytotic
mechanism. Inside the cell, the enzymatic decomposition of the heme
molecule is mediated by heme oxygenase, resulting in the release of
Fe3+, CO and biliverdin. Fe3+ is then reduced
to Fe2+.
Iron absorption depends on the amount of stored iron
in the body, which is reflected by transferrin saturation (TS)
levels (3). Transferrin is
expressed by immature enterocytes and binds to them, serving as an
intestinal 'iron probe'. Immature enterocytes are not actively
involved in iron absorption, but they express transferrin receptors
(TFRs) and the HFE protein. The HFE gene is responsible for
the production of HFE protein, which is found on the cell surface,
primarily in the liver and intestines, as well as in macrophages of
the immune system. It activates the divalent metal transporter 1
(DMT-1)/ferroportin (FPN) system, which serves as a detector of
iron levels in the body and prepares immature enterocytes for
diversification to their mature absorptive form (3). HFE regulates the production of
another major protein known as hepcidin, which is considered to be
a specific regulatory hormone of iron levels. HFE also interacts
with TFRs. However, the role of these interactions in iron
regulation is still unclear (4).
Normally, the saturation of plasma transferrin
regulates the expression of hepatic hepcidin via the HFE, TFR2 and
hemojuvelin (HJV) signaling pathway. Hepcidin is secreted into the
blood, binds with FPN in the intestines and macrophages, and
induces the degradation of FPN, thus reducing intestinal absorption
and the recycling of iron from macrophages, to maintain the
saturation of plasma transferrin. In the case of hereditary
hemochromatosis (HH), mutations in the HFE, HJV and
TFR2 genes prevent the synthesis of hepcidin, increasing FPN
and iron release levels in intestinal cells and macrophages, thus
leading to an increase in plasma TS levels, and ultimately causing
iron deposition in the liver and other tissues.
A heterogeneous group of hereditary and idiopathic
causes represent diseases characterized by iron overload (5). HH is an autosomal recessive genetic
disease linked to iron metabolism and is a commonly inherited
disorder in which iron obtained through food cannot be eliminated
after absorption, as the body does not have an effective excretory
mechanism to eliminate the excess iron. Over time, this excess iron
leads to an accumulation state, which is toxic to cells. The
clinical significance of this autosomal recessive disease is mainly
observed in homozygotes for the mutated gene. If not treated in
time, HH may prove to be fatal. In Northwestern Europe, 3–5
individuals in 1,000 are homozygous carriers (http://www.irondisorders.org/hemochromatosis). Of
note, abnormalities in the regulation of iron levels, which may
result in iron accumulation, occur gradually and patients remain
asymptomatic up to the forth decade of life, although total body
iron may have already accumulated, reaching levels of 10–20 g,
mainly deposited in the liver, heart and endocrine glands as
hemosiderin (6).
HH occurs 2 to 10-fold more frequently in adult
males than in females. Possibly in women, due to menstruation,
monthly iron loss delays the accumulation of iron for approximately
one decade and symptoms usually begin to appear after menopause. In
men, clinical symptoms appear earlier, although rarely before the
age of 40 or 50 (7).
The genotyping of HFE gene mutations may
reveal individual excretory mechanism disorders, and may thus lead
to the early initiation of treatment, long before the development
of clinical symptoms. This would contribute significantly to the
prevention of hemochromatosis-induced cirrhosis and to a normal
life expectancy.
The most frequent polymorphism of the HFE
gene is located in the short arm of chromosome 6 (6p), at position
845, where guanine (G) is replaced by adenine (A), resulting in the
replacement of a cysteine by tyrosine at position 282 of the HFE
protein sequence (C282Y), which leads to a non-functional protein
(8). The C282Y polymorphism
prevents HFE protein from reaching the cell surface, thus
preventing the interaction with hepcidin and TFRs (4).
The second most frequent HFE gene
polymorphism consists of a histidine to aspartic acid replacement
at position 63 of the HFE protein sequence (H63D), due to a
cytosine (C) to guanine (G) replacement at position 187. This
polymorphism may disrupt iron homeostasis and cause iron
accumulation when it occurs simultaneously with the C282Y
polymorphism (8).
A further association that seems to play a
significant role in the development of hemochromatosis is the S65C
polymorphism, where serine is replaced by a cysteine at position 65
of the HFE protein sequence (9).
The HFE gene affects iron absorption by
altering the expression of hepcidin (10). The C282Y polymorphism of the
HFE gene is responsible, alone or in combination with H63D
and/or S65C, for almost 90% of hemochromatosis cases among Northern
European populations (11). All
three HFE gene polymorphisms are associated with the disease
(10). In this study, we performed
a population-based frequency distribution analysis of the
HFE gene polymorphisms, rs1800562 (C282Y), rs1799945 (H63D)
and rs1800730 (S65C), and their susceptibility to developing HH. We
examined samples obtained from 1,446 individuals of Greek
ethnicity.
Subjects and methods
In this study, samples from 1,446 healthy Greek
(Caucasian) individuals, 670 men and 776 women of Greek origin were
collected and analyzed. Samples were obtained from buccal swabs.
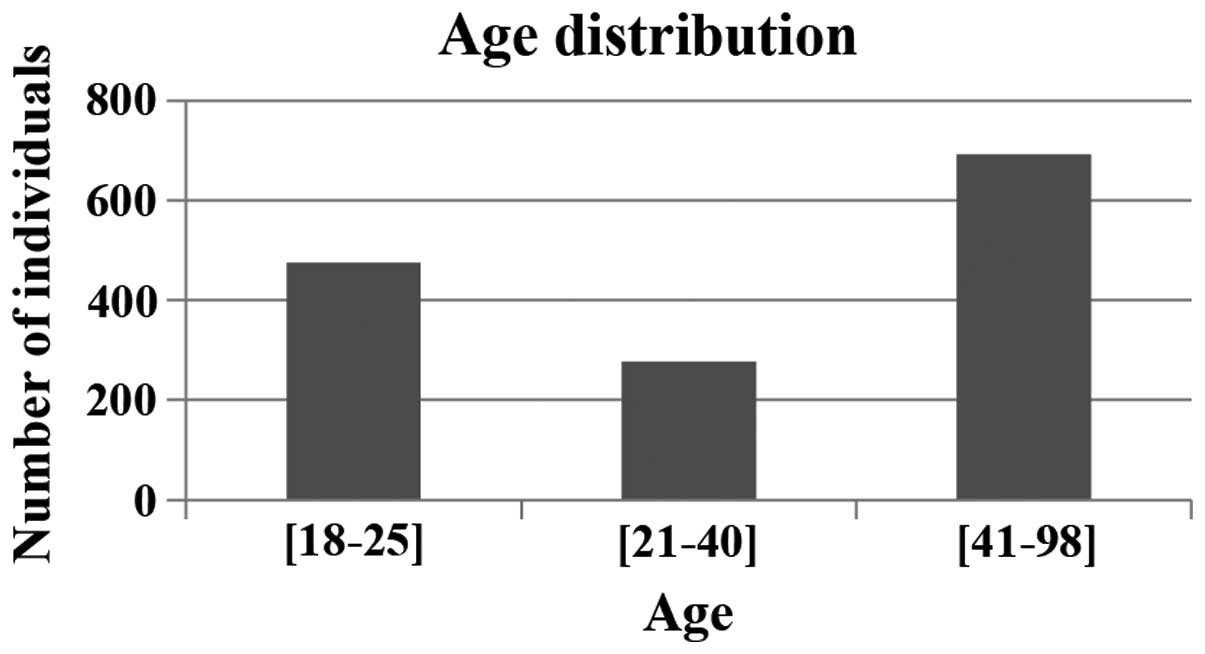
The median age of the participants was 38 years their ages ranged
from 18 to 98 years (Fig. 1). All
volunteers provided written and signed informed consent. Following
anonymization, genomic DNA was isolated from epithelial cells
collected from the oral cavity with swabs, using nucleic acid
isolation columns (Tissue Nucleospin; Machery-Nagel GmbH & Co.
KG, Düren, Germany). Genotypes of HFE polymorphisms were
determined by real-time polymerase chain reaction (PCR) using the
Simple Probes commercial LightSnip kit and the LightCycler
FastStart DNA Master HybProbe Kit (Roche Diagnostics, Penzberg,
Germany). The reactions were performed on a LightCycler 480
Real-Time PCR system (Roche Applied Science, Mannheim, Germany) in
accordance with the manufacturer's recommendations. Hybridization
was analyzed using melting curve analysis software provided with
the instrument. The genotypes were classified as homozygous for the
wild-type allele, and heterozygote and homozygous for the
polymorphism allele.
Contingency tables 2×2 (1 degree of freedom) were
designed and odds ratios (ORs), as well as the corresponding
confidence intervals were calculated. Statistical analysis was
performed at a significance level of a=0.05 and a statistically
significance P-value was calculated by Fisher's exact test. All
genotypes were tested for Hardy-Weinberg equilibrium (HWE) using
web-based software (http://scienceprimer.com/hardy-weinberg-equilibrium-calculator).
Results
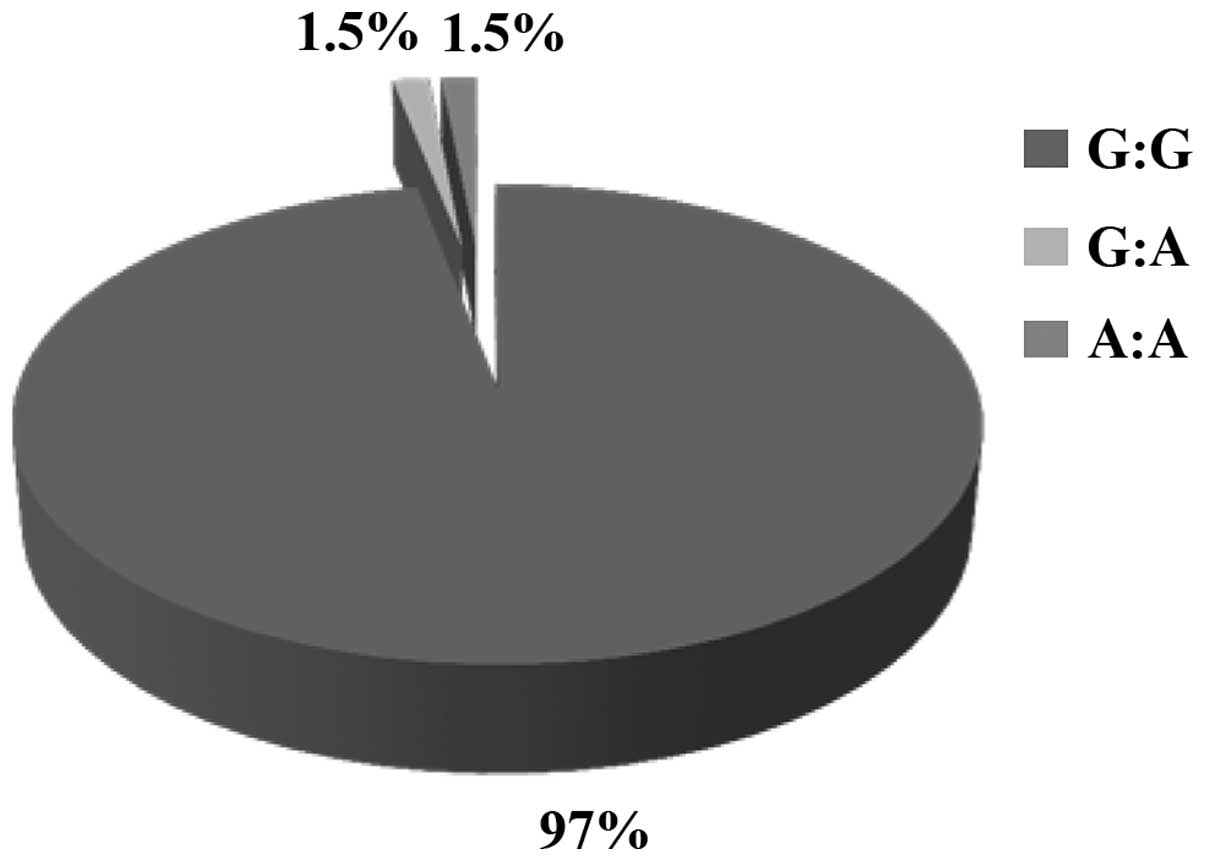
The majority (97%) of the 1,446 Greek volunteers
analyzed for polymorphism rs1800562 was homozygous wild-type (G:G).
Of the volunteers, 1.52% were heterozygous (G:A), and only 1.48%
were homozygous for the mutant allele (A:A). The frequency of the
wild-type G alleles was 2,828 (97.7%) and that of the mutated A
allele was only 64 (2.3%). A Hardy-Weinberg disequilibrium was
detected for polymorphism rs1800562 in the volunteer Greek
population in our study (χ2=608.06) (Table I and Fig. 2).
 | Table IGenotype and allele distribution of
the rs1800562 in polymorphism in 1,446 volunteers. |
Table I
Genotype and allele distribution of
the rs1800562 in polymorphism in 1,446 volunteers.
| rs1800562 | No. (%) |
|---|
| Genotype
frequency | |
| G:G | 1,403 (97.0) |
| G:A | 22 (1.5) |
| A:A | 21 (1.5) |
| Total | 1,446 (100.0) |
| Allele frequency | |
| G | 2,828 (97.7) |
| A | 64 (2.3) |
| Total | 2,892 (100.0) |
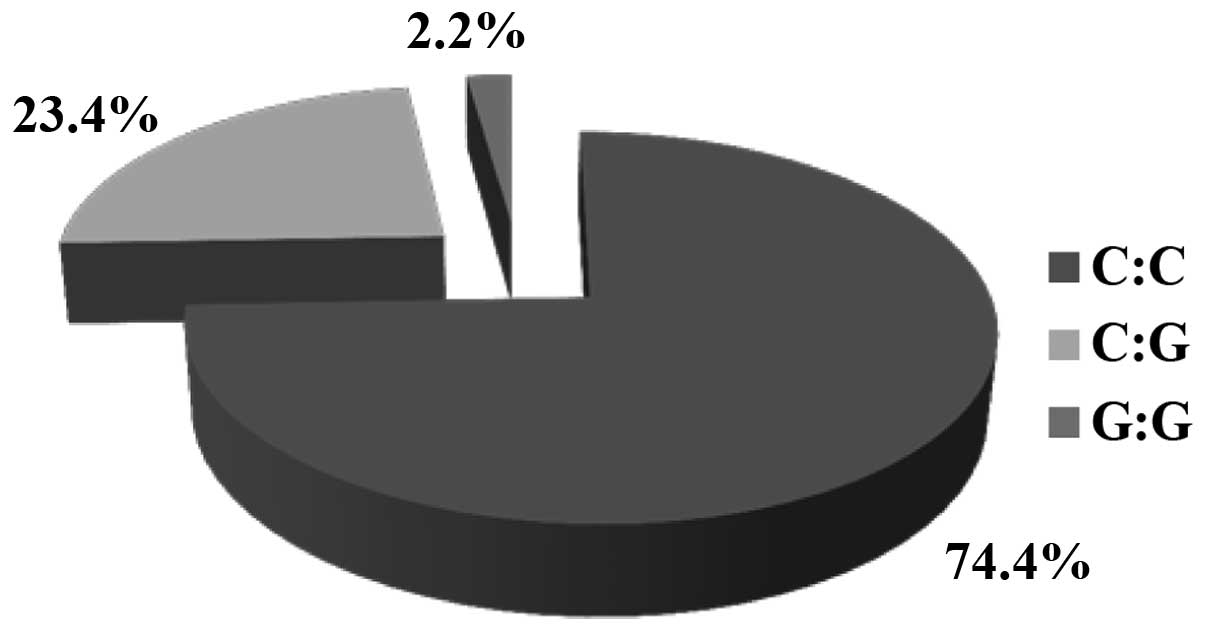
As regards polymorphism rs1799945, data were
obtained only from 1,429 volunteers. Of these volunteers, 74.4%
were homozygous for the wild-type genotype (C:C). In addition,
23.4% were heterozygous (C:G) and 2.2% were homozygous for the
mutant allele (G:G). The frequency of the wild-type allele was
2,462 (86.1%) and that of the mutated allele was 396 (13.9%). HWE
was detected for polymorphism rs1799945 in the volunteer Greek
population of our study (χ2=0.62) (Table II and Fig. 3).
 | Table IIGenotype and allele distribution of
the rs1799945 polymorphism in 1,429 volunteers. |
Table II
Genotype and allele distribution of
the rs1799945 polymorphism in 1,429 volunteers.
| rs1799945 | No. (%) |
|---|
| Genotype
frequency | |
| C:C | 1,064 (74.4) |
| C:G | 334 (23.4) |
| G:G | 31 (2.2) |
| Total | 1,429 (100.0) |
| Allele frequency | |
| C | 2,462 (86.1) |
| G | 396 (13.9) |
| Total | 2,858 (100.0) |
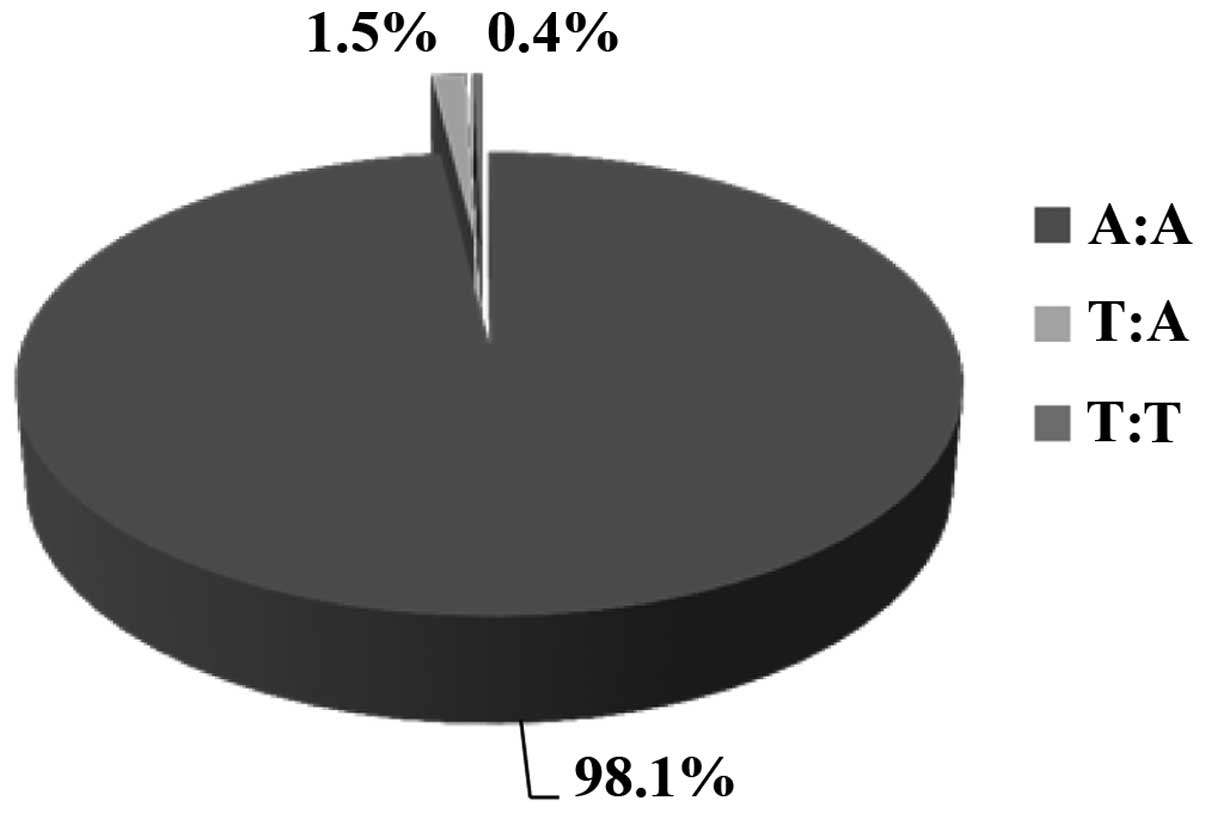
Finally, we obtained data for polymorphism rs1800730
from 1,219 volunteers. The vast majority (98.1%) of the volunteers
was homozygous for the wild-type genotype (A:A). Of these
volunteers, 1.5% were heterozygous for the mutant allele (A:T) and
only 0.4% were homozygous (T:T). The frequency of the wild-type
allele was 2,410 (98.8%) and that of the mutated allele was only
28, which represented only 1.2% of the examined volunteers.
Hardy-Weinberg disequilibrium was detected for polymorphism
rs1800730 in the volunteer Greek population of our study
(χ2=149.05) [Table
III and Fig. 4].
 | Table IIIGenotype and allele distribution of
the rs1800730 polymorphism in 1,219 volunteers. |
Table III
Genotype and allele distribution of
the rs1800730 polymorphism in 1,219 volunteers.
| rs1800730 | No. (%) |
|---|
| Genotype
frequency | |
| A:A | 1,196 (98.1) |
| A:T | 18 (1.5) |
| T:T | 5 (0.4) |
| Total | 1,429 (100.0) |
| Allele frequency | |
| A | 2,410 (98.8) |
| T | 28 (1.2) |
| Total | 2,438 (100.0) |
No association between the HFE polymorphisms
rs1800562, rs1799945 and rs1800730 and gender could be established.
The OR of the male and female volunteers exhibited no statistically
significant difference (Table
IV).
 | Table IVCorrelation between genders regarding
the frequency distribution of the HFE gene polymorphisms,
rs1800562, rs1800562 and rs1800562. |
Table IV
Correlation between genders regarding
the frequency distribution of the HFE gene polymorphisms,
rs1800562, rs1800562 and rs1800562.
| Gene
polymorphism | Allele | Total | Men | Women | Odds ratio | Confidence
interval |
|---|
| rs1800562 | A | 53 | 26 | 27 | 1.12 | 0.65–1.3 |
| G | 2,817 | 1,302 | 1,515 | | |
| rs1800562 | G | 229 | 95 | 135 | 0.77 | 0.59–1.02 |
| C | 2,295 | 1,093 | 1,203 | | |
| rs1800562 | T | 20 | 11 | 9 | 0.74 | 0.31–1.79 |
| A | 2,402 | 1,141 | 1,261 | | |
Data for all three polymorphisms of the HFE
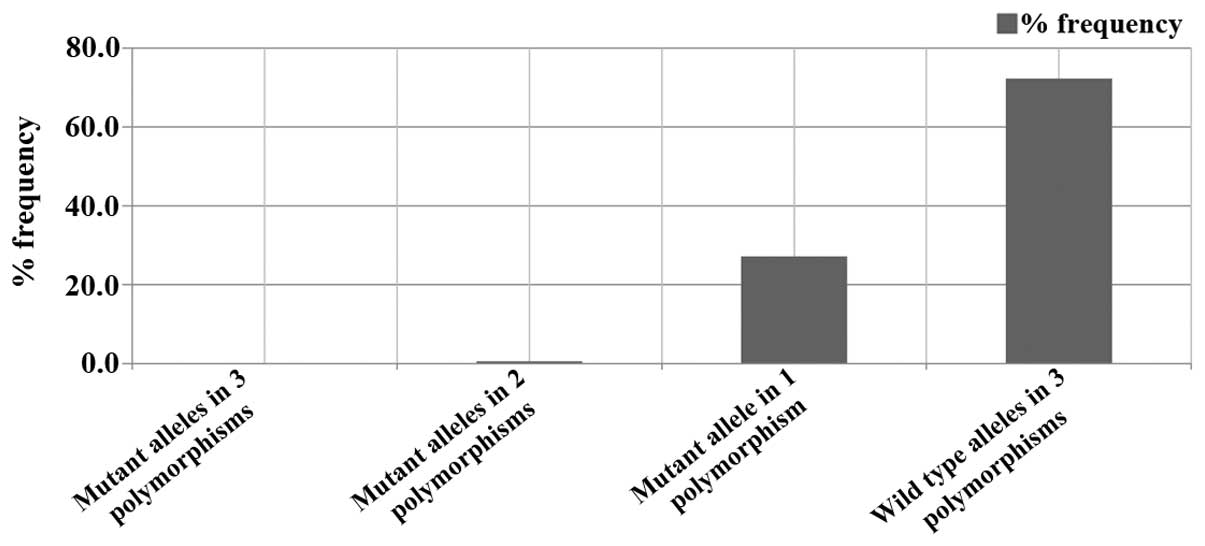
gene were from 1,205 volunteers. No volunteer carried all three
polymorphisms simultaneously. Moreover, 871 (73%) individuals
carried the wild-type alleles in all three polymorphisms, and only
six volunteers (0.5%) were heterozygous for two polymorphisms and
homozygous for the wild-type allele of the third polymorphism. Over
a quarter of the 1,205 volunteers (327 individuals, 27.2%) carried
a single mutant allele of one of the investigated polymorphisms,
while the other two were wild-type (Fig. 5).
Discussion
HH is an autosomal recessive genetic disorder that
is associated with iron metabolism and is among the most common
genetic disorders observed in individuals of European descent,
characterized by excessively increased dietary iron absorption
(12).
Non-heme iron exists either in a trivalent
(Fe3+) or in a divalent (Fe2+) form. The
Fe3+ ion is poorly absorbed as it tends to form salt
complexes with anions and at a pH value >3 is insoluble. On the
contrary, Fe2+ hardly forms complexes and is soluble at
high pH values ≤8 (1). Non-heme
iron is absorbed almost exclusively as Fe2+.
Fe2+ can enter the cell either by binding with an
intraluminal form of transferrin followed by endocytosis or via the
co-transport of Fe2+ and H+ through DMT-1
(3). Regardless of the route
followed by ferrous iron (Fe2+) into the cell,
cytoplasmic Fe2+ binds to mobilferrin to reach the
basolateral membrane and then enters the plasma through FPN, an
exporter of divalent metals. Once in the circulation,
Fe2+ is once again reduced to Fe3+ and is
transferred to other organs bound to plasma transferrin, the major
iron transport protein (1).
Iron can accumulate and become toxic to the body,
due to the inability of the body to effectively eliminate this
excess. If left untreated, HH can lead to morbidity, including
liver cirrhosis, hepatocellular carcinoma (HCC), diabetes, heart
disease and even death (13). Iron
overload leads to the development of histological lesions in many
vital organs. In the liver, iron is deposited inside hepatocytes,
Kupffer cells and biliary ductal cells, and iron accumulation leads
to fibrosis, which gradually progresses to cirrhosis. HCC manifests
in 20–30% of patients with cirrhosis. Iron deposition in the
myocardium causes progressive heart failure, dilation of the heart
and disturbances in the cardiac conduction system. Hemosiderin
accumulation in the pancreas can induce damage to the islets of
Langerhans, which may lead to a subsequent development of diabetes
mellitus, particularly in patients with genetic predisposition and
a family history of diabetes. Other endocrine glands may also be
affected, such as the thyroid and gonads. Almost all patients who
develop cirrhosis develop bronze skin pigmentation due to
hemosiderin accumulation in skin macrophages and increased melanin
in the epidermis. Finally, the deposition of hemosiderin in the
joints may cause damage due to advanced synovial calcification and
secondary lesions of the adjacent bone (3).
The diagnosis of hemochromatosis is based on a
combination of clinical, laboratory and genetic findings. Diagnosis
requires confirmation of elevated serum ferritin and TS levels,
with or without symptoms. The measurement of serum ferritin levels
is a more useful prognostic indicator of the severity of the
disease. Liver biopsy can be performed either to determine the
stage and degree of fibrosis accompanied by severe increased
ferritin or transaminase levels or to diagnose non-classical HH in
patients with other genetic abnormalities (13). Genetic testing confirms the
diagnosis (3).
Since the diagnostic strategies for this disease are
not yet standardized, genetic predisposition analysis for
hemochromatosis has become crucial (14). Genotypic analysis of HFE
gene frequent mutations can lead to early detection, thus resulting
in the initiation of treatment before the development of clinical
symptoms, liver cirrhosis in particular, contributing significantly
to a normal life expectancy (15).
In the present study, we examined how three single
nucleotide polymorphisms of the HFE gene, related to
hemochromatosis (16), are
distributed in a Greek population sample. To the best of our
knowledge, this is the largest population-based study that has
taken place in Greece to date, analyzing these specific mutations.
A comparison of the study population frequencies for all three
polymorphisms versus other populations was also performed and
possible gender specific differences were investigated.
The rs1800562 (C282Y) polymorphism of the HFE
gene leads to a defect in the mechanisms regulating iron levels and
in the malfunction of hepcidin, resulting in an increased
probability of developing type 1 HH. The mutant allele (A) occurs
in 1% of the global population, and in 4% of the European
population. This study revealed the A allele in 2% of the Greek
study population. The mutant allele appeared twice as often in our
study population as compared to the global population. Furthermore,
the rs1800562 (C282Y) polymorphism in the Greek study population
appeared half as often as in the European population (Table V).
 | Table VComparative frequency and allelic
distribution rates of the rs1800562 polymorphism in the Greek,
European and global populations. |
Table V
Comparative frequency and allelic
distribution rates of the rs1800562 polymorphism in the Greek,
European and global populations.
| Population | Allele
|
|---|
| A | G |
|---|
| Global | 1% | 99% |
| European | 4% | 96% |
| Greek | 2% | 98% |
As regards the rs1800562 (C282Y) polymorphism, the
genetic predisposition of developing HH was higher for individuals
homozygous for the mutant A:A genotype, who are more prone to
develop symptoms. These symptoms seem to be more severe in men or
women after menopause, and appear in 0.1% of the global population
and in 0.2% of the Northern European population. In the Greek
population in this study, the results revealed an increased
prevalence of the homozygous mutant genotype A:A, which was present
in 1% of the volunteers. Individuals heterozygous for this
mutation, i.e., those with the G:A genotype, are usually not
affected unless carrying the H63D mutation simultaneously
(http://browser.1000genomes.org/Homo_sapiens/Variation/Population?db=core;r=6:26092641-26093641;v=rs1800562;vdb=variation;vf=1229831)
(Table VI).
 | Table VIComparative frequency and genotype
distribution rates of the rs1800562 polymorphism in the Greek,
European and global population. |
Table VI
Comparative frequency and genotype
distribution rates of the rs1800562 polymorphism in the Greek,
European and global population.
| Population | % Genotype
|
|---|
| G:G | G:A | A:A |
|---|
| Global | 97.6 | 2.4 | 0.1 |
| European | 91.7 | 8.2 | 0.2 |
| Greek | 97.0 | 2.0 | 1.0 |
The HFE gene polymorphism rs1799945 (H63D)
also causes a malfunction in the homeostatic mechanisms regulating
iron and hepcidin, although to a lesser extent, when compared to
the rs1800562 (C282Y) polymorphism. The mutant allele (G) occurs
worldwide at a rate of 7% and at a rat of 17% in Europe. The
frequency of the G allele in the Greek study population was 9%,
similar to that in the global population, but much lower than that
in the Northern European population (Table VII).
 | Table VIIComparative frequency and allelic
distribution rates of the rs1799945 polymorphism in the Greek,
European and global populations. |
Table VII
Comparative frequency and allelic
distribution rates of the rs1799945 polymorphism in the Greek,
European and global populations.
| Population | Allele
|
|---|
| G | C |
|---|
| Global | 7% | 93% |
| European | 17% | 83% |
| Greek | 9% | 91% |
As regards the rs1799945 (H63D) polymorphism,
genetic predisposition for developing HH is higher for those
carrying the homozygous mutant G:G genotype. Individuals carrying
the homozygous mutant G:G genotype are predisposed to developing
mild symptoms of hemochromatosis, particularly if they also carry
the mutant allele polymorphism rs1800562; the mutant G:G genotype
is found in 1.2% of the global population and in 3.6% of the
European population. In our study population, the results revealed
an increased frequency of rs1799945 (H63D) compared with the global
population, which occured in 2.2% of the population. Individuals
heterozygous for this mutation, i.e., those carrying the C:G
genotype, may not be affected unless they carry the C282Y mutation
as well (http://www.snpedia.com/index.php/Rs1800562) (Table VIII).
 | Table VIIIComparative frequency and genotype
distribution rates of the rs1799945 polymorphism in the Greek,
European and global populations. |
Table VIII
Comparative frequency and genotype
distribution rates of the rs1799945 polymorphism in the Greek,
European and global populations.
| Population | % Genotype
|
|---|
| C:C | C:G | G:G |
|---|
| Global | 86.6 | 12.2 | 1.2 |
| European | 69.2 | 27.2 | 3.6 |
| Greek | 74.4 | 23.4 | 2.2 |
The rs1800730 (S65D) polymorphism of the HFE
gene has the same effect on hepcidin as the above-mentioned
mutations; however, the effects appear in less than the mutations
rs1799945 and rs18000562. The mutant allele (T), which causes the
mutation, appears in <1% of the global population, while in the
European population, it is found at a rate of 2%. In the Greek
population it occurs at almost the same rate to that of the global
and the European, namely 0.8% (Table
IX).
 | Table IXComparative frequency and allelic
distribution rates of the rs1800730 polymorphism in the Greek,
European and global populations. |
Table IX
Comparative frequency and allelic
distribution rates of the rs1800730 polymorphism in the Greek,
European and global populations.
| Population | Allele
|
|---|
| T | A |
|---|
| Global | <1% | 100.0% |
| European | 2.0% |
98.0% |
| Greek | 0.8% |
99.2% |
The proportion of homozygous carriers relative to
this mutation, i.e., those carrying the T:T genotype in the global
population is <1%, in the European population it is 0.2% and in
the Greek population it is 0.4%. These individuals are predisposed
to present with very mild symptoms of hemochromatosis, particularly
if they also carry the rs1800562 polymorphism. Heterozygous
individuals, i.e., those carrying the T:A genotype are found in
0.7% of the global population, in 2.8% of the European population
(17) and in 1.5% of the Greek
population (Table X).
 | Table XComparative frequency and genotype
distribution rates of the rs1800730 polymorphism in the Greek,
European and global populations. |
Table X
Comparative frequency and genotype
distribution rates of the rs1800730 polymorphism in the Greek,
European and global populations.
| Population | % Genotype
|
|---|
| A:A | A:T | T:T |
|---|
| Global | 99.2 |
0.7 | <1% |
| European | 97.0 |
2.8 | 0.2 |
| Greek | 98.1 | 10.5 | 0.4 |
According to the literature, the rs1799945 (H63D)
and rs1800730 (S65D) polymorphisms, in order to be held responsible
for any abnormal iron levels, they should 'coexist' with at least
one mutant allele of the rs1800562 (C282Y) polymorphism. There are
indications that these polymorphisms, even in homozygosity, are not
sufficient to cause HH (http://browser.1000genomes.org/Homo_sapiens/Variation/Population?v=rs1799945;%20vdb=variation,
http://browser.1000genomes.org/Homo_sapiens/Variation/Population?r=6:26090685-26091685;v=rs1800730;vdb=variation;vf=1229981).
In this study, from 1,460 samples genotyped for both
the C282Y and H63D polymorphisms, only two (2) were found to be compound heterozygotes
(G:A/C:G), that is only 0.1% of the sample population. In addition,
among the 1,256 volunteers analyzed for both the C282Y and the S65D
polymorphisms, none of them were compound heterozygotes (G:A/A:T)
i.e., 0% of the sample population. Based on these results we can
conclude that the levels of compound heterozygosity in the Greek
population are infinitesimal (zero).
According to the HWE law, genotype frequencies are
functions of allele frequencies and a large random mating
population is at equilibrium given that there is no migration,
natural selection, or genetic drift (18). The absence of such conditions can
cause changes in the gene pool frequencies, namely evolution.
Violation of the HWE conditions may lead to population
stratification, and genotyping errors could be reflected as
significant deviations from HWE predictions (19). In the present study, HWE was
detected only for the rs1799945 (H63D) polymorphism. This could be
interpreted as a stability of this particular polymorphism
regarding the mechanisms of evolution mentioned above, which means
that evolution did not occur, and theoretically the gene pool
frequencies remained unaltered. However, we cannot safely assume
that the next generations will also follow the HWE because
evolution is an inevitable result. The rs1800562 (C282Y) and
rs1800730 (S65D) polymorphisms are in disequilibrium which means
that, as regards these polymorphisms, the population studied has
evolved due to several factors.
The results indicated that the investigated Greek
population had a greater similarity to the global population. It
seems that, in relation to the Northern European population, the
Greek population varies considerably: all three investigated
polymorphisms occur more frequently in individuals of Northern
European descent. The susceptibility to develop hemochromatosis
seems to be enhanced in the Northern European population as
compared to the Southern European population (http://www.irondisorders.org/hemochromatosis). The
geographical position of Greece, on the crossroad between three
continents, may genetically influence the Greek population. Despite
the fact that the clinical manifestation of HH appears 2–10-fold
more frequently in adult men than in women, no statistically
significant difference could be demonstrated in the distribution of
the HH-associated polymorphisms analyzed between the two genders.
Other biological functions, such as menstruation, may contribute to
regular iron loss, which delays iron accumulation for approximately
a decade. HH symptoms usually appear after menopause.
Therapeutic phlebotomy is the primary treatment for
hemochromatosis (20). It seems to
stabilize the situation and contribute to the prevention of the
progression to cirrhosis, which adversely affects the long-term
survival of patients (http://www.snpedia.com/index.php/Rs1800730).
Orthotopic liver transplantation is performed in patients with
advanced cirrhosis. Moreover, the administration of chelating
agents, such as deferoxamine helps in iron elimination. A series of
hydroxypyridinone dendrimers are believed to possess a high
affinity and selectivity for Fe3+, which reduces
absorption from the duodenum (21). A recent publication demonstrated
that proton pump inhibitors may inhibit iron absorption,
restricting its accumulation by increasing gastric pH. This
prevents iron absorption, which mainly exists in the divalent form
(17).
The three HFE gene polymorphisms investigated
in the present study may lead to disturbances in the iron level
adjustment mechanisms, as well as in the impairment of hepcidin,
resulting in the increased probability of developing type 1 HH.
The genetic status of the HFE gene
polymorphisms rs1800562, rs1799945 and rs1800730 seems to be an
important preventive tool for delaying the development of the
clinical symptoms of HH. In particular, for individuals with a
family history of HH, genetic analyses and frequent iron and
transferrin blood level measurements will prove beneficial and may
help maintain a better quality of life and increase the average
life expectancy.
References
|
1
|
Boron W and Boulpaep EL: Blood physiology.
Medical Physiology: A Cellular and Molecular Approach. Koutsileiris
M: 3. Greek Edition. Paschalidis Medical Editions; Athens; pp.
p16532006
|
|
2
|
Vander A, Sherman J, Luciano D and
Tsakopoulos M: Human Physiology: The Mechanisms of Body Function.
2. 8th edition. Paschalidis Medical Editions; Athens: pp. p516pp.
p7502011
|
|
3
|
Damjanov I: Pathophysiology. Greek
Edition. Parisianos Scientific Editions; Athens: pp. 448–449. pp.
512–515. 2011
|
|
4
|
Genetics Home Reference. http://ghr.nlm.nih.gov/gene/HFE.
Accessed April 1, 2016.
|
|
5
|
Tandara L and Salamunic I: Iron
metabolism: Current facts and future directions. Biochem Med
Zagreb. 22:311–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yen AW, Fancher TL and Bowlus CL:
Revisiting hereditary hemochromatosis: Current concepts and
progress. Am J Med. 119:391–399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puntarulo S: Iron, oxidative stress and
human health. Mol Aspects Med. 26:299–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Recklinghausen FD: Uber
haemochromatose. Tageblatt Versammlung Dtsche. Naturforscher Arzte
Heidelberg. 62:324–325. 1889.
|
|
9
|
Mura C, Raguenes O and Férec C: HFE
mutations analysis in 711 hemochromatosis probands: Evidence for
S65C implication in mild form of hemochromatosis. Blood.
93:2502–2505. 1999.PubMed/NCBI
|
|
10
|
Merryweather-Clarke AT, Cadet E, Bomford
A, Capron D, Viprakasit V, Miller A, McHugh PJ, Chapman RW, Pointon
JJ, Wimhurst VL, et al: Digenic inheritance of mutations in HAMP
and HFE results in different types of haemochromatosis. Hum Mol
Genet. 12:2241–2247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lyon E and Frank EL: Hereditary
hemochromatosis since discovery of the HFE gene. Clin Chem.
47:1147–1156. 2001.PubMed/NCBI
|
|
12
|
Gallego CJ, Burt A, Sundaresan AS, Ye Z,
Shaw C, Crosslin DR, Crane PK, Fullerton SM, Hansen K, Carrell D,
et al: Penetrance of Hemochromatosis in HFE Genotypes Resulting in
p.Cys282Tyr and p.[Cys282Tyr];[His63Asp] in the eMERGE Network. Am
J Hum Genet. 97:512–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crownover BK and Covey CJ: Hereditary
hemochromatosis. Am Fam Physician. 87:183–190. 2013.PubMed/NCBI
|
|
14
|
Hentze MW, Muckenthaler MU, Galy B and
Camaschella C: Two to tango: Regulation of mammalian iron
metabolism. Cell. 142:24–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ganz T: Hepcidin, a key regulator of iron
metabolism and mediator of anemia of inflammation. Blood.
102:783–788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanson EH, Imperatore G and Burke W: HFE
gene and hereditary hemochromatosis: a HuGE review. Human Genome
Epidemiology. Am J Epidemiol. 154:193–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hutchinson C, Geissler CA, Powell JJ and
Bomford A: Proton pump inhibitors suppress absorption of dietary
non-haem iron in hereditary haemochromatosis. Gut. 56:1291–1295.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Namipashaki A, Razaghi-Moghadam Z and
Ansari-Pour N: The Essentiality of Reporting Hardy-Weinberg
Equilibrium Calculations in Population-Based Genetic Association
Studies. Cell J. 17:187–192. 2015.PubMed/NCBI
|
|
19
|
Wigginton JE, Cutler DJ and Abecasis GR: A
note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet.
76:887–893. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franchini M and Veneri D: Recent advances
in hereditary hemochromatosis. Ann Hematol. 84:347–352. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou T, Neubert H, Liu DY, Liu ZD, Ma YM,
Kong XL, Luo W, Mark S and Hider RC: Iron binding dendrimers: a
novel approach for the treatment of haemochromatosis. J Med Chem.
49:4171–4182. 2006. View Article : Google Scholar : PubMed/NCBI
|