Introduction
Neuregulin 1 (Nrg1) is a member of the epidermal
growth factor family and functions through the activation of the
tyrosine kinase domain of ErbB receptors (1). The bioactivity of Nrg1 is primarily
mediated via homodimers consisting of its cognate receptor ErbB4
(2) or via heterodimeric complexes
of ErbB3⁄Neu and ErbB4⁄Neu (3).
These receptors, including Neu and ErbB4, have a high affinity for
Nrg1 and contain an active tyrosine kinase domain (4). The interaction between ligands and
receptors regulates a wide spectrum of biological processes via the
initiation of a complicated cascade of intracellular signaling
pathways, in which extracellular signal-regulated kinases (Erk),
Akt, mitogen-activated protein kinase (MAPK), PI3γ, protein kinase
C (PKC) and Jak-STAT are involved (5–8).
Moreover, the activation of these signaling pathways may lead to
cell cycle arrest, cell proliferation, differentiation, tumorigenic
development and anti-apoptotic processes (3,9,10). A
previous study demonstrated that the Nrg1-ErbB signaling pathway is
essential in organ development, cell differentiation and
tumorigenesis (7).
Expression and functions of Nrg1 have also been
systematically described in the nervous system, particularly in the
spinal cord, cortex and hippocampus. Moreover, recent data suggest
that Nrg1-ErbB4 signaling is key in the pathogenesis of a diverse
range of neurodegenerative and neurological diseases, including
Alzheimer's disease (11,12), multiple sclerosis (13), schizophrenia (14), neurospongioma (15) and brain damage (5). Perturbed Nrg1 signaling in these
diseases extends our understanding of the biological functions of
Nrg1.
Neuroinflammation is essential in diseases affecting
the central nervous system (CNS). Neuronal apoptosis occurs in
response to multiple cytokines, chemokines and toxic factors
secreted by glial cells during this process (16). Inflammatory signals, such as
prostaglandins, proinflammatory cytokines or lipopolysaccharide
(LPS), can cross the intact or ruptured blood-brain barrier (BBB)
of the CNS (17), and controlled
and balanced inflammation contribute to the repair of damaged
tissues following brain injury (18).
Although multiple factors have been determined to be
associated with the neuroinflammatory process, the involvement of
Nrg1-ErbB signaling in this condition remains largely unknown.
Nrg1, like other specific members of the cytokine family, such as
interleukin-1 and epidermal growth factor (EGF), was affected
following brain infection and injury; thus, Nrg1 is hypothesized to
partially contribute to the development of schizophrenia (19). Nrg1 mRNA and intracellular protein
levels increased in response to the duration of LPS exposure in
human umbilical venous endothelial cells, supporting its potential
role as a systemic endogenous inhibitor of perinatal inflammatory
brain damage during fetal development (20).
It was hypothesized that inflammation can affect the
expression of Nrg1 and result in intracellular signaling changes,
thus contributing to the pathology of neuroinflammation-based
neurodegenerative diseases. LPS is a component of the outer cell
wall of Gram-negative bacteria, and it has been widely used to
induce inflammation in the brain (11,21).
Therefore, the effects of LPS-induced neuroinflammation on the
expression of Nrg1 and its related cell signaling molecules were
analyzed in several major brain structures in vivo using a
mouse model treated with intraperitoneal injection of LPS.
It was observed that LPS induced differential
changes in Nrg1 and its receptors Neu and ErbB4 in the cortex,
striatum, hippocampus and hypothalamus. Notably, a profound
reduction of Nrg1 expression was found in the hypothalamus and
striatum, suggesting that the Nrg1 signaling in these locations is
more prone to be influenced by LPS-induced neuroinflammation. These
findings may provide greater insight into the function of Nrg1 in
inflammation-related neuronal diseases, including AD, PD and SCI,
and may further aid in the development of novel clinical
therapies.
Materials and methods
Animals
Eleven female C57BL/6 mice (age, 3 months; weight,
~25 g) were purchased from the Guangdong Medical Laboratory Animal
Center (Guangzhou, China) and were maintained at 25°C under a 12-h
dark/light cycle, with access to food and water ad libitum.
All experimental procedures conducted on animals were approved by
the Animal Ethics Committees of Shantou University Medical College
(Shantou, China) and the authorities of Guangdong Province.
LPS treatment
A total of 1.0 mg LPS (Sigma-Aldrich Israel, Ltd.,
Rehovot, Israel) was dissolved in 10 ml normal saline (pH 7.4),
such that the final concentration of LPS solution was 0.01%. Six
mice were intraperitoneally injected with LPS at a dose of 1.0
mg/kg (10 ml/kg), and 5 mice in the control group were injected
with normal saline at 10 ml/kg.
Tissue preparation
Mice were then sacrificed by decapitation 24 h after
LPS or saline injection. For morphological studies, 2 mice from
each group were subjected to intracardiac perfusion of
phosphate-buffered saline-buffered paraformaldehyde for fixation.
For molecular studies, seven brains, three from the normal
saline-treated mice and four from LPS-treated mice, were collected.
The right and left hemisphere from each brain was separated.
Moreover, the frontoparietal cortex, hippocampus, striatum and
hypothalamus from each side were dissected and stored at −80°C for
either reverse transcription-polymerase chain reaction (RT-PCR) or
western blot analysis (22).
RT-PCR analysis
Total RNA from brain tissue was extracted using the
DNAiso Reagent (Tiangen Biotech (Beijing) Co., Ltd., Beijing,
China) according to the manufacturer's protocol. Reverse
transcription was performed using StarScript II First-strand cDNA
Synthesis mix (Genestar Biosolutions Co., Ltd., Beijing, China),
and RT-PCR was performed using 2X Taq PCR StarMix (Genstar
Solutions). Sequences of primers are listed in Table I. PCR amplification was conducted
with an initial denaturing step at 94°C for 2 min, then 35 cycles
at 94°C for 30 sec, at 60°C for 30 sec, and at 72°C for 30 sec, and
a further extension at 72°C for 10 min. For glyceraldehyde
3-phosphate dehydrogenase (GAPDH) cDNA amplification, 28 reaction
cycles were employed. Then, 5 µl of the PCR products were
used to perform gel electrophoresis using a 2.0% agarose gel
(GeneChoice, Inc., Frederick, MD, USA) containing Gelred (1:10,000;
Biotium, Inc., Hayward, CA, USA). The bands were identified under
ultraviolet light.
 | Table ISequences of primers for reverse
transcription-polymerase chain reaction. |
Table I
Sequences of primers for reverse
transcription-polymerase chain reaction.
| Gene | Domain | Primers | Accession no. | Annealing
temperature (°C) | Expected length
(bp) |
|---|
| ErbB4 | 5′ terminus |
5′-GCCAAAAATGAAGCTGGCGA-3′ | NM_010154.1 | 60 | 219 |
| 3′ terminus |
5′-TTGTGCTCGATGCTGGTGAT-3′ | | | |
| Neu | 5′ terminus |
5′-TAGCTGGGCATTCACCCTAC-3′ | BC053078.1 | 55 | 358 |
| 3′ terminus |
5′-AGGGCATAAGCTGTGTCACC-3′ | | | |
| Nrg1 | 5′ terminus |
5′-GTCTCAGAGGGTGCCTCAAC-3′ | NM_178591.2 | 60 | 332/230 |
| 3′ terminus |
5′-GTTCTTCCGGGTGGGTACTG-3′ | | | |
| GAPDH | 5′ terminus |
5′-GTGGAGTCATACTGGAACATGTAG-3′ | | 60 | 150 |
| 3′ terminus |
5′-AATGGTGAAGGTCGGTGTG-3′ | | | |
Western blot analysis
For western blot analysis, tissue was dissolved in
200 µl radioimmunoprecipitation buffer containing PMSF (1%)
(Solarbio Biotech Corp, Beijing, China) and homogenized using a
motor-driven micro tissue grinder (Kimble Chase, Vineland, NJ,
USA). The supernatants were collected following centrifugation at
14,000 × g for 15 min at 4°C. Protein concentrations were
determined by the bicinchoninic acid assay (Solarbio Biotech Corp).
Equivalent quantities (20 µg) of tissue lysates were heated
at 95°C in 20% sample loading buffer consisting of 0.125 mol/l
Tris-HCl, pH 1.8; 20% glycerol, 10% sodium dodecyl sulfate, 0.1%
bromophenol blue and 5% β-mercaptoethanol, and were then resolved
using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and electroblotted onto a polyvinylidene difluoride membrane
(Millipore, Billerica, MA, USA). Non-specific protein-binding sites
were blocked with 5% bovine serum albumin (Amresco, LLC, Solon, OH,
USA) or non-fat milk diluted in Tris-buffered saline buffer
containing 0.1% Tween-20 (TBST, pH 7.4). The membranes were
incubated with the following anti-mouse primary antibodies:
Polyclonal rabbit p-ErbB-4 (Tyr 1056; 1:500; #sc-33040); polyclonal
rabbit ErbB-4 (C-18; 1:500; #sc-283); polyclonal mouse
p-ErbB-2/p-Neu (7F8; 1:500; #sc-81508); monoclonal mouse Neu (3B5;
1:500; #sc-33684); polyclonal rabbit p-c-Src (Tyr 419; 1:500;
#sc-101802); monoclonal mouse Src-1 (1135/H4; 1:500; #sc-32789);
monoclonal mouse anti-p-Akt1/2/3 (11E6; 1:1,000; #sc-81433);
monoclonal mouse Akt1 (G-5; 1:1,000; #sc-55523); monoclonal mouse
p-Erk (E-4; 1:1,000; #sc-7383); monoclonal mouse Erk1/2 (MK1;
1:1,000; #sc-135900); monoclonal mouse GAPDH (G-9; 1:1,000;
#sc-3650620) (all 1:1,000 Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and monoclonal mouse Nrg1 Ab-1 (7D5; 1:500; MS-272-P1;
LabVision; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
overnight at 4°C. After 3 washes for 5 min each, horseradish
peroxidase-conjugated goat anti-mouse (#BA1051) and rabbit
anti-goat (#BA1055) secondary antibodies (1:1,000; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) diluted in 3% non-fat
milk in TBST were applied, followed by 3 washes with TBST for 5 min
each at room temperature. The antigens were visualized using an
enhanced chemiluminescence (ECL) solution (Beyotime Institute of
Biotechnology, Haimen, China). The signal intensity was quantified
using Image J software, version 1.48 (rsb.info.nih.gov/ij/) as the average densitometry
multiplied by the area (measured as the number of pixels).
Immunohistochemical staining
Mice were perfused with a 4% paraformaldehyde
solution followed by decapitation and then cryoprotected in 20%
sucrose. Brain sections (8 µm thick) were prepared using a
cryostat microtome (Leica CM1860, Leica Biosystems, Germany).
Antigen retrieval was performed using 10-mM citrate buffer (pH 6.0)
for 40 min at 99°C. Then, the tissue sections were incubated in 3%
H2O2 to clear endogenous peroxidase and were
then saturated with 10% normal goat serum for 10 min. Next,
sections were incubated with the following primary antibodies:
Rabbit polyclonal anti-pNeu and pErbB4 antibodies (1:100; Santa
Cruz Biotechnology, Inc.). After being washed in PBS 3 times for 5
min each, the biotinylated secondary antibody and the
streptavidin-peroxidase conjugate were applied. The enzyme activity
was developed using an AEC kit (ZLI-9036; ZSGB Biotechnology Co.,
Ltd., Beijing, China). the antigen-antibody complexes were
visualized using the AEC method, according to the manufacturer's
protocols.
Statistical analysis
Statistical analyses were performed with SPSS17.0
(SPSS software Inc., Chicago, IL, USA). Data were analyzed using
Student's t-test for independent samples. P<0.05 was considered
to indicate a statistically significant difference.
Results
Neuroinflammatory response to LPS
administration in major mouse brain regions
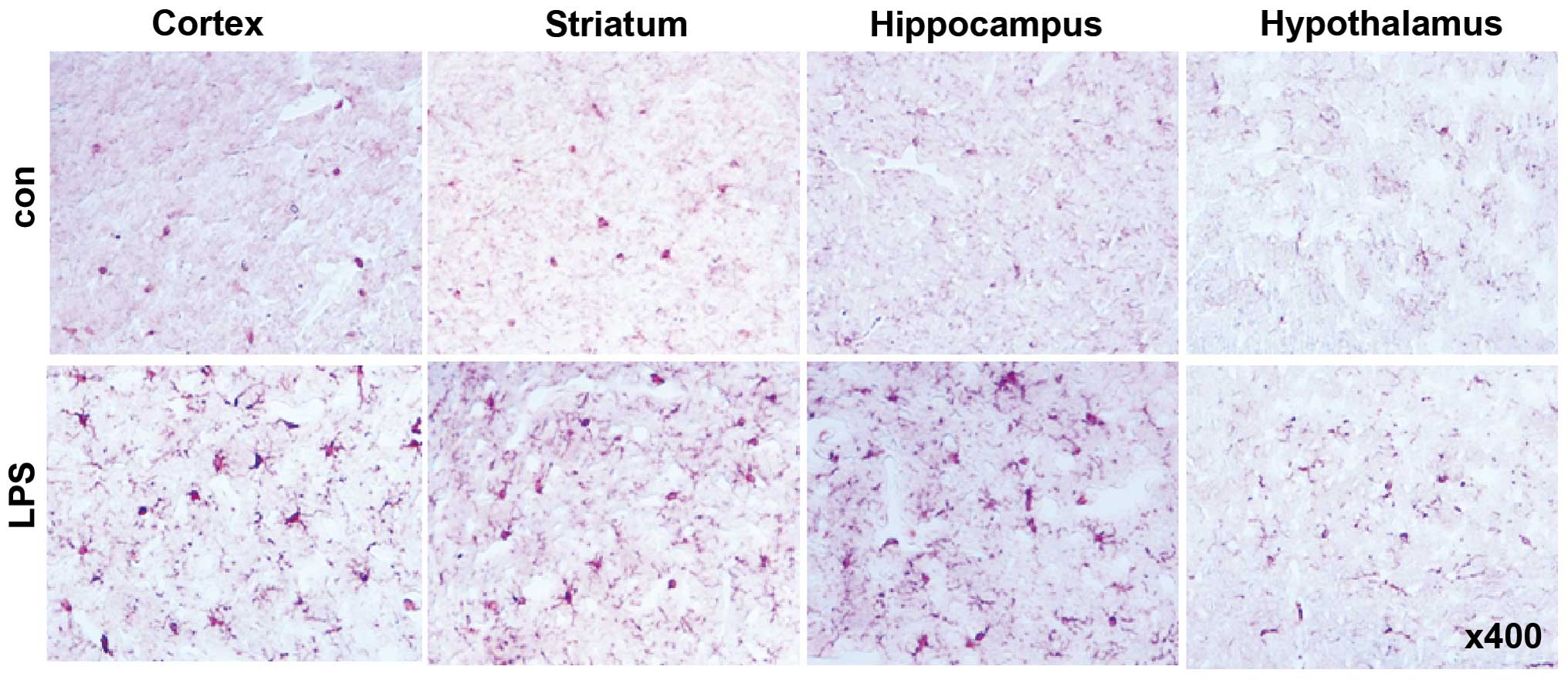
Intraperitoneal administration of LPS induced an
apparent increase in the number of activated microglial cells
positive for Iba-1 in the cortex, striatum, hippocampus and
hypothalamus (Fig. 1), indicating
the successful induction of neuroinflammation in our model.
Determination of Nrg1, Neu and ErbB4
expression in different brain regions in response to LPS
RT-PCR was used to investigate the expression
changes of Nrg1, Neu and ErbB4 at the mRNA level in major brain
regions in response to LPS administration for 24 h. It was
demonstrated that LPS induced an apparent increase in the Neu mRNA
level in the cortex, striatum and hippocampus, but a profound
reduction in the Neu mRNA level was observed in the hypothalamus
(Fig. 2A). LPS showed no effect on
the mRNA levels of ErbB4 in the cortex and hypothalamus (Fig. 2A). However, the mRNA levels of
ErbB4 were reduced in the striatum but were increased in the
hippocampus in the LPS-treated mice compared with that of the
controls (Fig. 2A). In addition,
LPS reduced the mRNA levels of type I Nrg1 in the striatum and
hypothalamus (Fig. 2A). In
accordance with these findings, in these 2 regions reduced protein
levels of Nrg1 were observed, the molecular weights of which were
70, 50, 45 and 36 kDa. By contrast, the mRNA level of type II Nrg1
was reduced in the hippocampus, which also showed reduced 45 and 36
kDa Nrg1 protein levels (Fig. 2B).
Notably, the LPS-treated mice showed no apparent changes in the
mRNA levels of both types of Nrg1 in the cortex compared with the
vehicle control mice (Fig. 2B).
This was further confirmed by western blot analysis, which revealed
no profound changes in the different Nrg1 isoforms at the protein
level (Fig. 2B).
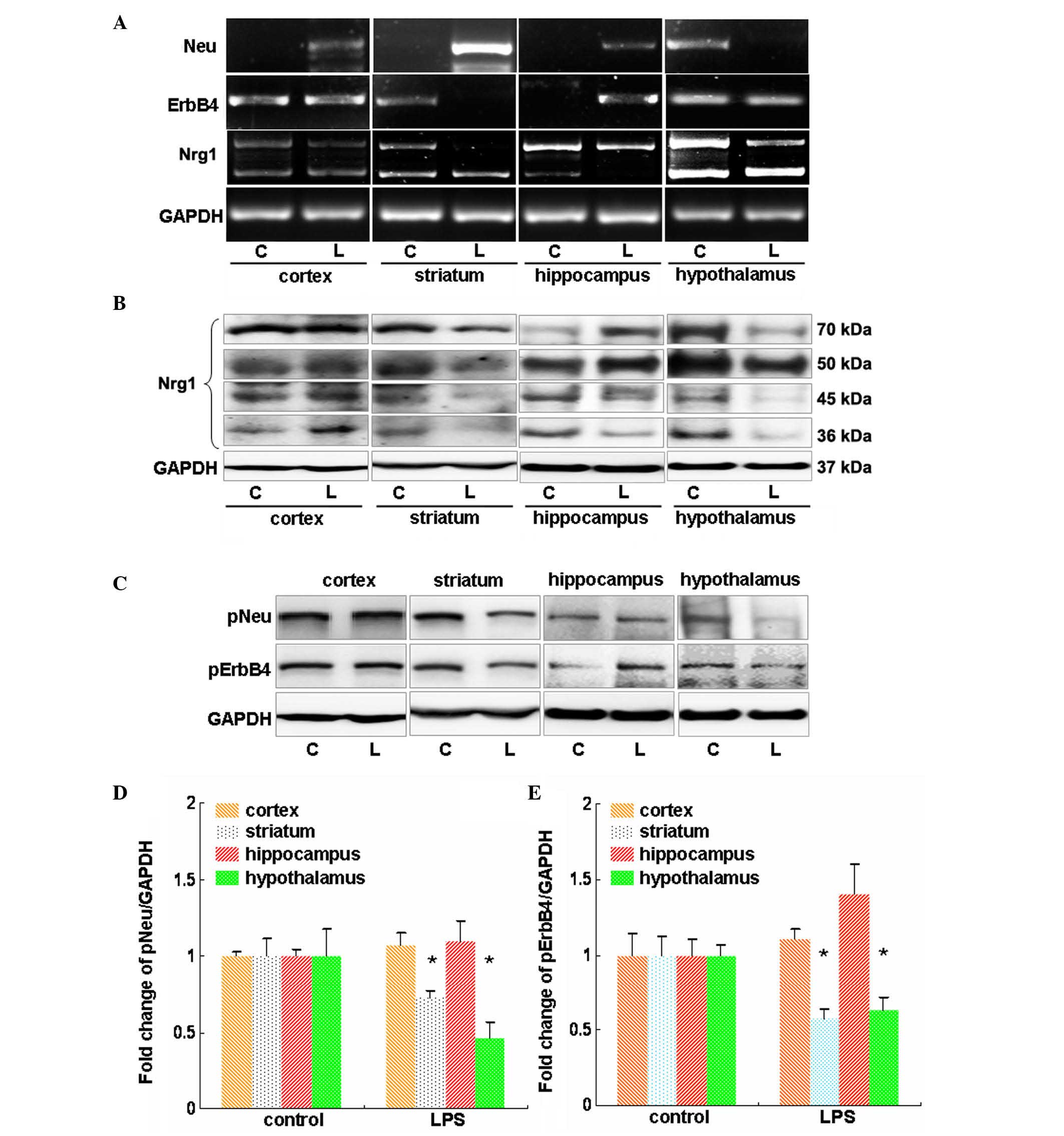 | Figure 2Evaluation of the effects of LPS on
Nrg1 expression and Neu and ErbB4 expression and activation in
mouse cortex, striatum, hippocampus and hypothalamus. (A) Reverse
transcription-polymerase chain reaction analysis of the influence
of LPS on the mRNA levels of Nrg1, Neu and ErbB4. (B) Western blot
analysis of the effect of LPS on the protein levels of multiple
isoforms of Nrg1. (C) Western blot analysis of the effect of LPS on
the phosphorylation-induced activation of Neu and ErbB4.
Quantification of the (D) Neu and (E) ErbB4 westernblot results.
Independent Student's t-test was applied. *P<0.05 vs.
the control group (control group, n=3; LPS group, n=4). LPS,
lipopolysaccharide; Nrg1, neuregulin-1; C, control group; L, LPS
group; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; p-,
phosphorylated. |
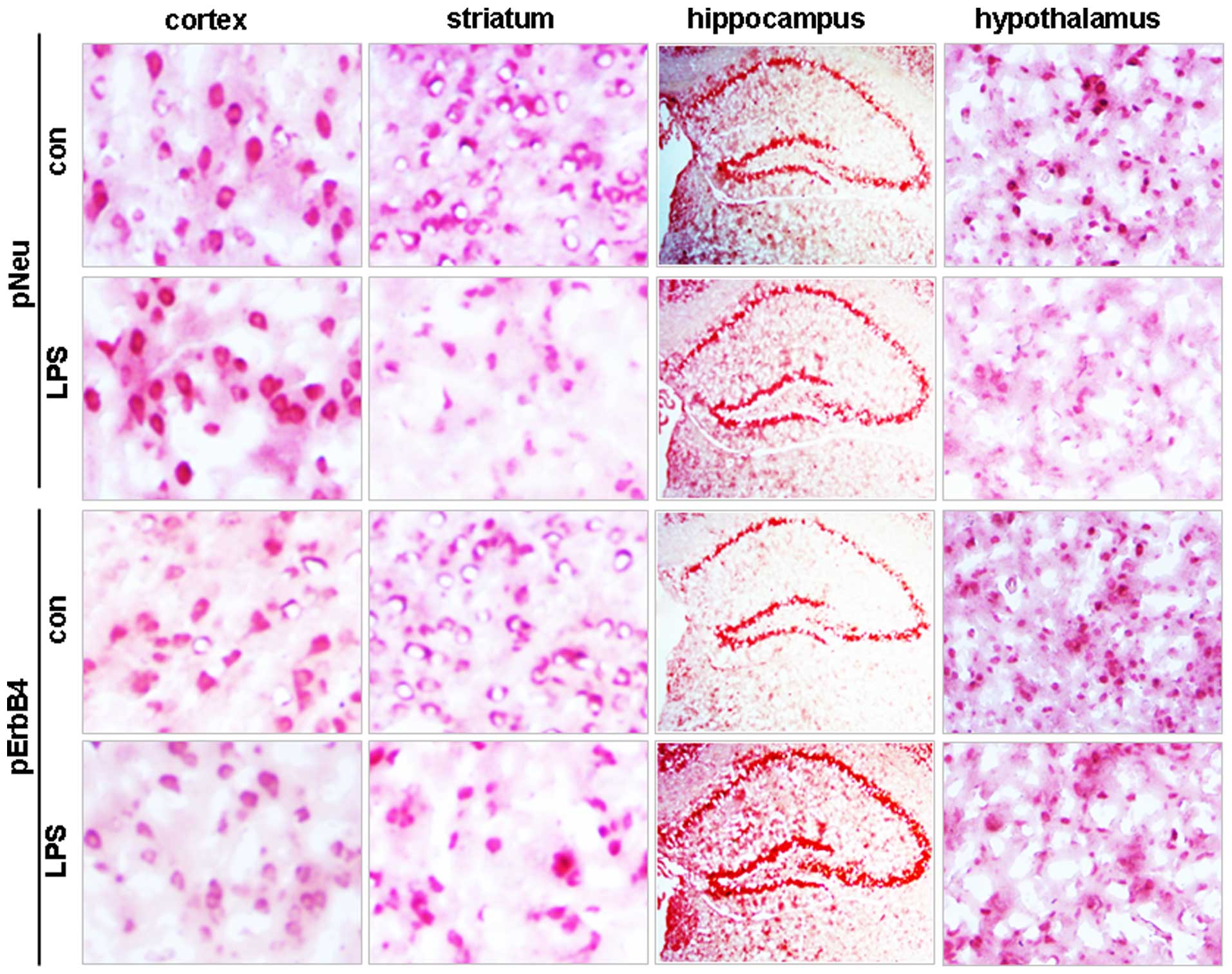
The effect of LPS on Neu and ErbB4 phosphorylation
was then investigated in major brain regions. As shown in Fig. 2C and D, administration of LPS
showed no apparent effect on the phosphorylation of Neu and ErbB4
in the cortex (P>0.05 vs. the vehicle control); however, it
induced a significant reduction in the phosphorylation of molecules
in the striatum and hypothalamus (P<0.05 vs. the vehicle
control; Fig. 2C and D). In the
hippocampus, LPS showed no effect on pNeu level, but it increased
the pErbB4 level (Fig. 2C–E).
Effect of LPS on the
phosphorylation-induced activation of Neu and ErbB4 in major brain
regions in mice
In accordance with the western blot data,
immunohistochemical staining demonstrated no changes in either pNeu
or pErbB4 levels in cortical brain tissue, but it reduced the
stained signals for the two molecules in the striatum and
hypothalamus (Fig. 3). None of the
hippocampal areas, including the CA1, CA2 and CA3 zones and the
dentate gyrus, showed changes in pNeu levels, whereas increased
pErbB4 levels were observed in these areas in LPS-treated mice
compared with control (Fig.
3).
Western blot analysis of the effects of
LPS on signaling pathways in different parts of the brain
To gain additional insight into the influence of LPS
on the downstream signaling pathways of Nrg1, the phosphorylation
levels of Src, Akt1 and Erk were measured using western blot
analysis (Fig. 4). The
phosphorylation of c-Src was significantly upregulated in the
cortical tissue (P<0.05 vs. the vehicle control). By contrast,
it was down-regulated in the hypothalamus (P<0.01 vs. the
vehicle control) (Fig. 4A and B).
In addition, the Akt1 phosphorylation level, as indicated by the
pAkt1/Akt1 ratio, was significantly increased in the striatum
(P<0.05 vs. the vehicle control), with no significant changes
identified in the cortex and hypothalamus. The hippocampus also
showed an increased average pAkt1/Akt1 ratio, although no
significant difference was found when compared with the control
mice (Fig. 4A and C). As indexed
by pErk/Erk ratio, LPS resulted in marginally reduced Erk
phosphorylation in the cortex but increased it in the striatum,
although no significance was found when compared with the vehicle
controls. No obvious changes in Erk phosphorylation were observed
in either the hippocampus or hypothalamus (Fig. 4A and D).
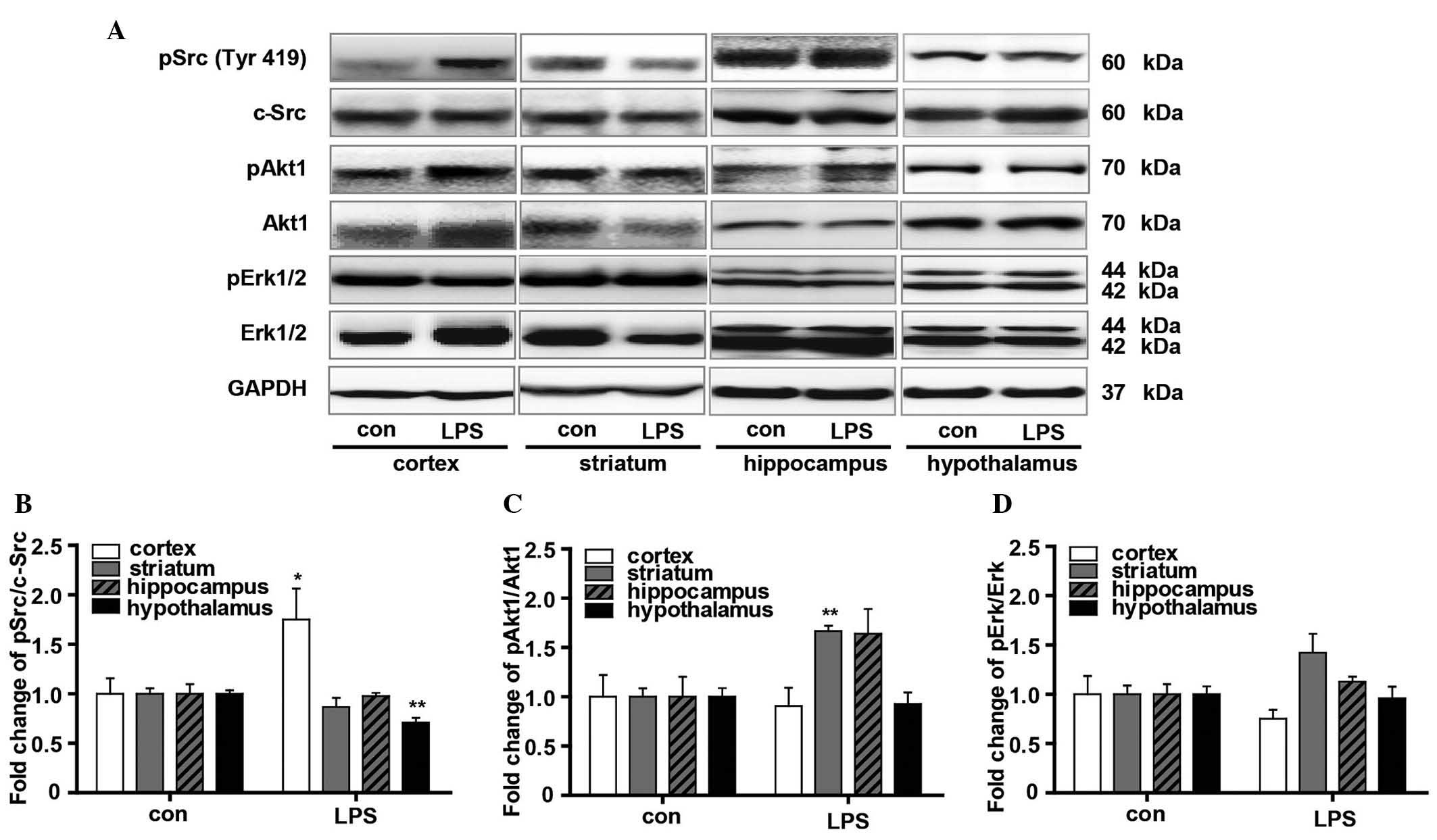 | Figure 4Evaluation of the effects of LPS on
the signaling pathway downstream of Nrg1. (A) Western blot analysis
of the effect of LPS on the phosphorylation of Src, Akt1 and Erk in
the cortex, striatum, hippocampus and hypothalamus in mice. Graphs
presenting quantification of (B) Src, (C) Akt1 and (D) Erk western
blot results. Independent Student's t-test was applied.
*P<0.05 and **P<0.01 vs. the control
group (control group, n=3; LPS group, n=4). LPS,
lipopolysaccharide; Nrg1, neuregulin 1; Erk, extracellular
signal-regulated kinase; p-, phosphorylated; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Discussion
Traditionally, transforming growth factor
α-transactivated ErbB1/epidermal growth factor receptor is required
for the LPS response, and neuregulins are required to transactivate
ErbB4 during the acid-stress response (23). Stability enhancement of neuregulin
receptor degradation protein-1 inhibits the production of
proinflammatory cytokines in macrophages triggered by toll-like
receptors. This process may also reduce the production of
LPS-induced proinflammatory mediators, such as inducible nitric
oxide synthase, nitric oxide, cyclooxygenase-2, and prostaglandin
E2 (24). A loss of
ADAM17-mediated growth factor shedding was reported to abrogate
cytokine release of primary SMC stimulated by LPS or the
supernatant of acid-exposed epithelial cells (23). Previous studies have revealed that
Nrg1 functions by blocking the delayed neuronal death and
proinflammatory responses in rats subjected to focal ischemic
stroke (25). Neuregulins can
modulate a diverse array of biological effects based on the
expression of the receptors and cell types that are associated with
the survival and function of neuronal cells (26–30).
To the best of our knowledge, the present study observed for the
first time the differential changes in Nrg1 expression and the
phosphorylation of its receptors Neu and ErbB4, as well as of its
downstream signaling molecules, in different brain regions of the
mouse following neuroinflammation induced by LPS. Either type I or
type II Nrg1 mRNA levels were reduced by LPS in the hippocampus,
striatum and hypothalamus, whereas the mRNA level of its receptor
Neu was reduced only in the hypothalamus. Using western blotting,
it was demonstrated that the expression of several isoforms of Nrg1
was upregulated in the cortex and hippocampus in response to LPS.
However, their expression was downregulated in the LPS-affected
striatum and hypothalamus, in which the phosphorylation levels of
Neu and ErbB4 were notably downregulated. These combined results
suggest that LPS can differentially modulate the expression of Nrg1
signaling, and that Nrg1 may differentially function in different
brain regions upon neuroinflammation.
Inflammation is considered to activate transcription
factors, including nuclear factor (NF)-κB and Jak-STAT, which can
regulate proinflammatory gene expression. Nrg1 and its receptors
ErbB3 and ErbB4 are highly expressed not only in the developing
nervous system but also in the mature nervous system (31–33).
Following brain lesion development, the levels of Nrg and the ErbB4
receptor are increased (34,35),
suggesting that Nrg/ErbB4 signaling may function in synaptic
plasticity and neuroprotection. Nrg1 is crucial in the development
of the brain (36), laying the
foundation for its roles in inflammation-related processes in the
disease setting. It is considered that Nrg1 functions upstream of
NF-κB-mediated cell signaling in response to certain inflammatory
conditions (37).
The mechanisms underlying the role of Nrg1 in
neuroinflammation are unknown. It has been reported that Nrg1β can
exert neuroprotective effects on cell survival via the activation
of the phosphatidyl-inositol-3-kinase (PI3K)/Akt signaling pathway
(38,39). It was suggested that the potential
neuroprotective effects of Nrg1 are due to its ability to modulate
multiple biological processes during neuroinflammation. To test
this, Nrg1 and the phosphorylation levels of certain signaling
pathway proteins, including Src, Akt1 and Erk were examined. Using
western blotting techniques, it was demonstrated that the
phosphorylation level of Src was increased in the cortex but
decreased in the hypothalamus. However, the phosphorylation levels
of Akt1 and Erk were upregulated in the striatum and
hippocampus.
In conclusion, the present results show differential
changes in Nrg1 signaling in major mouse brain regions in response
to LPS, suggesting a differential role for Nrg1 in these brain
regions during the development of neuroinflammation. Modulating
Nrg1 signaling may assist in the treatment of neurotraumatic and
neurodegenerative diseases that involve neuroinflammation.
Acknowledgments
The authors would like to thank the National Natural
Science Foundation of China (grant nos. 81171138 and 81471279 to Dr
Wei-Jiang Zhao). This study was also supported by the Talent
Support Grant from SUMC (grant no. 250122 0118 to Dr Wei-Jiang
Zhao).
References
|
1
|
Woo RS, Li XM, Tao Y, Carpenter-Hyland E,
Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al:
Neuregulin-1 enhances depolarization-induced GABA release. Neuron.
54:599–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hancock ML, Canetta SE, Role LW and
Talmage DA: Presynaptic type III neuregulin1-ErbB signaling targets
alpha7 nicotinic acetylcholine receptors to axons. J Gen Physiol.
131:i42008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J and Kern JA: Neuregulin-1 activates
the JAK-STAT pathway and regulates lung epithelial cell
proliferation. Am J Respir Cell Mol Biol. 27:306–313. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shamir A and Buonanno A: Molecular and
cellular characterization of Neuregulin-1 type IV isoforms. J
Neurochem. 113:1163–1176. 2010.PubMed/NCBI
|
|
5
|
Shyu WC, Lin SZ, Chiang MF, Yang HI,
Thajeb P and Li H: Neuregulin-1 reduces ischemia-induced brain
damage in rats. Neurobiol Aging. 25:935–944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao W and Ren SG: Neuregulin-1 (Nrg1) is
mainly expressed in rat pituitary gonadotroph cells and possibly
regulates prolactin (PRL) secretion in a juxtacrine manner. J
Neuroendocrinol. 23:1252–1262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao W, Shen Y and Ren S: Endogenous
expression of Neuregulin-1 (Nrg1) as a potential modulator of
prolactin (PRL) secretion in GH3 cells. Cell Tissue Res.
344:313–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao WJ and Ren SG: Endogenous
neuregulin-1 expression in the anterior pituitary of female
Wistar-Furth rats during the estrous cycle. Nan Fang Yi Ke Da Xue
Xue Bao. 31:921–927. 2011.In Chinese. PubMed/NCBI
|
|
9
|
Peles E, Ben-Levy R, Tzahar E, Liu N, Wen
D and Yarden Y: Cell-type specific interaction of Neu
differentiation factor (NDF/heregulin) with Neu/HER-2 suggests
complex ligand-receptor relationships. EMBO J. 12:961–971.
1993.PubMed/NCBI
|
|
10
|
Puricelli L, Proietti CJ, Labriola L,
Salatino M, Balañá ME, Aguirre Ghiso J, Lupu R, Pignataro OP,
Charreau EH, Bal de Kier Joffé E and Elizalde PV: Heregulin
inhibits proliferation via ERKs and phosphatidyl-inositol 3-kinase
activation but regulates urokinase plasminogen activator
independently of these pathways in metastatic mammary tumor cells.
Int J Cancer. 100:642–653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rebola N, Simões AP, Canas PM, Tomó AR,
Andrade GM, Barry CE, Agostinho PM, Lynch MA and Cunha RA:
Adenosine A2A receptors control neuroinflammation and consequent
hippocampal neuronal dysfunction. J Neurochem. 117:100–111. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaudhury AR, Gerecke KM, Wyss JM, Morgan
DG, Gordon MN and Carroll SL: Neuregulin-1 and erbB4
immunoreactivity is associated with neuritic plaques in Alzheimer
disease brain and in a transgenic model of Alzheimer disease. J
Neuropathol Exp Neurol. 62:42–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Viehover A, Miller RH, Park SK, Fischbach
G and Vartanian T: Neuregulin: An oligodendrocyte growth factor
absent in active multiple sclerosis lesions. Dev Neurosci.
23:377–386. 2001. View Article : Google Scholar
|
|
14
|
Corfas G, Roy K and Buxbaum JD: Neuregulin
1-erbB signaling and the molecular/cellular basis of schizophrenia.
Nat Neurosci. 7:575–580. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ritch PA, Carroll SL and Sontheimer H:
Neuregulin-1 enhances motility and migration of human astrocytic
glioma cells. J Biol Chem. 278:20971–20978. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirsch EC and Hunot S: Neuroinflammation
in Parkinson's disease: A target for neuroprotection? Lancet
Neurol. 8:382–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaillard PJ, de Boer AB and Breimer DD:
Pharmacological investigations on lipopolysaccharide-induced
permeability changes in the blood-brain barrier in vitro. Microvasc
Res. 65:24–31. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glezer I, Simard AR and Rivest S:
Neuroprotective role of the innate immune system by microglia.
Neuroscience. 147:867–883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nawa H and Takei N: Recent progress in
animal modeling of immune inflammatory processes in schizophrenia:
Implication of specific cytokines. Neurosci Res. 56:2–13. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoffmann I, Bueter W, Zscheppang K,
Brinkhaus MJ, Liese A, Riemke S, Dörk T, Dammann O and Dammann CE:
Neuregulin-1, the fetal endothelium, and brain damage in preterm
newborns. Brain Behav Immun. 24:784–791. 2010. View Article : Google Scholar :
|
|
21
|
Cao M, Zheng H, Tan X, Xu W, Rui Y, Li L,
Liu X, Xu G, Cui G, Xu J, et al: Upregulation of CBLL1 in rat brain
cortex after lipopolysaccharide treated. J Mol Histol. 44:135–145.
2013. View Article : Google Scholar
|
|
22
|
Chiu K, Lau WM, Lau HT, So KF and Chang
RC: Micro-dissection of rat brain for RNA or protein extraction
from specific brain region. J Vis Exp. 269:2007.
|
|
23
|
Dreymueller D, Martin C, Schumacher J,
Groth E, Boehm JK, Reiss LK, Uhlig S and Ludwig A: Smooth muscle
cells relay acute pulmonary inflammation via distinct ADAM17/ErbB
axes. J Immunol. 192:722–731. 2014. View Article : Google Scholar
|
|
24
|
Zhu L, Bi W and Lu D, Zhang C, Shu X, Wang
H, Qi R, Shi Q and Lu D: Regulation of ubiquitin-specific
processing protease 8 suppresses neuroinflammation. Mol Cell
Neurosci. 64:74–83. 2015. View Article : Google Scholar
|
|
25
|
Xu Z, Jiang J, Ford G and Ford BD:
Neuregulin-1 is neuro-protective and attenuates inflammatory
responses induced by ischemic stroke. Biochem Biophys Res Commun.
322:440–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bermingham-McDonogh O, McCabe KL and Reh
TA: Effects of GGF/neuregulins on neuronal survival and neurite
outgrowth correlate with erbB2/neu expression in developing rat
retina. Development. 122:1427–1438. 1996.PubMed/NCBI
|
|
27
|
Verdi JM, Groves AK, Farinas I, Jones K,
Marchionni MA, Reichardt LF and Anderson DJ: A reciprocal cell-cell
interaction mediated by NT-3 and neuregulins controls the early
survival and development of sympathetic neuroblasts. Neuron.
16:515–527. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaskovsky A, Lupowitz Z, Erlich S and
Pinkas-Kramarski R: ErbB-4 activation promotes neurite outgrowth in
PC12 cells. J Neurochem. 74:979–987. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Erlich S, Goldshmit Y, Lupowitz Z and
Pinkas-Kramarski R: ErbB-4 activation inhibits apoptosis in PC12
cells. Neuroscience. 107:353–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldshmit Y, Erlich S and Pinkas-Kramarski
R: Neuregulin rescues PC12-ErbB4 cells from cell death induced by
H2O2. Regulation of reactive oxygen species
levels by phosphatidylinositol 3-kinase. J Biol Chem.
276:46379–46385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marchionni MA, Goodearl AD, Chen MS,
Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D,
Sudhalter J, Kobayashi K, et al: Glial growth factors are
alternatively spliced erbB2 ligands expressed in the nervous
system. Nature. 362:312–318. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pinkas-Kramarski R, Eilam R, Spiegler O,
Lavi S, Liu N, Chang D, Wen D, Schwartz M and Yarden Y: Brain
neurons and glial cells express Neu differentiation
factor/heregulin: A survival factor for astrocytes. Proc Natl Acad
Sci USA. 91:9387–9391. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pinkas-Kramarski R, Eilam R, Alroy I,
Levkowitz G, Lonai P and Yarden Y: Differential expression of
NDF/neuregulin receptors ErbB-3 and ErbB-4 and involvement in
inhibition of neuronal differentiation. Oncogene. 15:2803–2815.
1997. View Article : Google Scholar
|
|
34
|
Eilam R, Pinkas-Kramarski R, Ratzkin BJ,
Segal M and Yarden Y: Activity-dependent regulation of Neu
differentiation factor/neuregulin expression in rat brain. Proc
Natl Acad Sci USA. 95:1888–1893. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Erlich S, Shohami E and Pinkas-Kramarski
R: Closed head injury induces up-regulation of ErbB-4 receptor at
the site of injury. Mol Cell Neurosci. 16:597–608. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Esper RM, Pankonin MS and Loeb JA:
Neuregulins: Versatile growth and differentiation factors in
nervous system development and human disease. Brain Res Rev.
51:161–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Buonanno A and Fischbach GD: Neuregulin
and ErbB receptor signaling pathways in the nervous system. Curr
Opin Neurobiol. 11:287–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li BS, Ma W, Jaffe H, Zheng Y, Takahashi
S, Zhang L, Kulkarni AB and Pant HC: Cyclin-dependent kinase-5 is
involved in neuregulin-dependent activation of phosphatidylinositol
3-kinase and Akt activity mediating neuronal survival. J Biol Chem.
278:35702–35709. 2003. View Article : Google Scholar : PubMed/NCBI
|