Introduction
Sevoflurane inhalation is a popular general
anesthetic option for pediatric patients (1). It has non-hepatic, non-renal
dependent elimination features and low solubility, thus providing
faster induction and emergence qualities than the majority of other
commonly used general anesthetics (2). However, results of recent animal
research regarding the neural safety of sevoflurane are
paradoxical. Sevoflurane is considered to be neuroprotective in
certain preconditioning situations (3,4), but
it has also been observed to induce widespread neuronal apoptosis
in the mouse brain at certain concentrations (5,6).
Behavioral investigations have demonstrated that
subanesthetic concentrations of volatile anesthetics may enhance
learning and memory in mice (7).
Learning and memory involve synaptic plasticity, which is
exemplified by long-term potentiation of the excitatory
postsynaptic potential (8).
Tight-seal whole-cell recordings have demonstrated that sevoflurane
at subanesthetic concentrations [0.05–0.07 minimum alveolar
concentration (MAC)] increases excitatory synaptic transmission in
the region I of hippocampus proper (CA1) area, however, at 0.36 MAC
it inhibits this action (9).
These observations suggest a low level of
sevoflurane anesthesia may enhance learning and memory, possibly
associated with neuroprotective regulation in hippocampal apoptosis
signaling pathways. As various agents that induce apoptosis are
either oxidants or stimulators of cellular oxidative metabolism
(10), sevoflurane may affect
oxidative stress in the hippocampus. To investigate this
hypothesis, systemic and hippocampal oxidative status, neuronal
apoptosis and recognition memory were examined following
sevoflurane exposure at three different concentrations:
Subanesthetic (0.3%, 0.1 MAC; LS) (11,12),
subclinical (1.3%, 0.3 MAC; MS) (13), and the highest tolerated
concentration (2.3%, 0.7 MAC; HS) (14). The highest tolerated concentration
was based on previous experiments that confirmed a substantial
apoptosis-inducing effect under an established sevoflurane exposure
system (14,15).
Materials and methods
Animals
Animal use in the present study was authorized by
the Institutional Animal Care and Use Committee at Sun Yat-sen
University (Guangzhou, China). All attempts were made to use a
smaller number of animals and decrease their suffering. Male
Sprague-Dawley (SD) rats were acquired from the Experimental Animal
Center of Sun Yat-sen University. They were accommodated in a room
with a 12-h light-dark cycle (light from 07:00 to 19:00), and the
room temperature was maintained at 21±1°C. Food and water were
provided ad libitum. The rats were housed in the same room
in different cages. Each cage contained a different litter. Rats of
different group allocation were marked by ear punching.
Earlier studies described litter variability in the
rate of apoptosis among neonate mice (16). Therefore, an equal number of
control and experimental rats were taken from the same litters so
that each experiment had its own group of littermate controls. Only
male rats were used including 96 pups from 16 litters.
Sevoflurane exposure
SD rats of postnatal day 7 (P7; weight, 16–17 g)
were randomly allocated into 4 groups: An air-treated control group
and 0.3, 1.3 and 2.3% sevoflurane (Abbott Laboratories, Abott Park,
IL, USA) treatment groups, with 21 rats in each treatment group.
Rats in the sevoflurane treatment groups were placed in a plastic
chamber and exposed to 0.3, 1.3 or 2.3% sevoflurane for 6 h with
air as a carrier at a gas flow of 2 l/min. During sevoflurane
exposure, the chamber was heated to 38°C using a warming device
(NPS-A3; Midea Group Co. Ltd., Foshan, China). Sevoflurane, oxygen
and carbon dioxide levels in the chamber were calibrated by a gas
monitor (Datex-Ohmeda, Inc., Madison, WI, USA). After 6 h,
sevoflurane delivery ceased, and the animals were exposed to air
again. When these rats moved freely, they were placed back into the
maternal cage. During anesthesia, the respiratory frequency and
skin color of the rats were monitored by an investigator. If signs
of apnea or hypoxia were observed, the rat was exposed to air
immediately and excluded from the experiment. Rats in the control
group were placed into the same container as those in the
sevoflurane groups but exposed to air for 6 h.
Arterial blood gas analysis
P7–P8 rats from the sevoflurane-treated groups and
the air-treated group underwent arterial blood gas analysis
according to a procedure described previously (17). Samples were obtained immediately
prior to (0 h, n=3 in each subgroup) and following anesthesia (6 h,
n=3 in each subgroup) from a total of 12 rats. Briefly, the rats
underwent a quick arterial blood sampling from the left cardiac
ventricle via trans-thoracic puncture, and the samples were
transferred to heparinized glass capillary tubes (Tianhong Glass
Co., Taixin, China). A single sample (100 µl) was analyzed
immediately following blood collection by a blood gas analyzer (GEM
Premier 3000; Instrumentation Laboratory, Barcelona, Spain). The
pH, arterial carbon dioxide tension, arterial oxygen tension, and
blood glucose levels of arterial blood were analyzed. At the time
of blood sampling, the experiments were terminated when the rats
were sacrificed by decapitation. The analysis of each sample was
repeated independently a minimum of three times.
Enzyme-linked immunosorbent assay
(ELISA)
Plasma and hippocampal homogenate were collected at
6 h following sevoflurane exposure. For plasma collection, blood
samples were obtained from the left ventricle and transferred into
heparinized tubes. The samples were centrifuged for 30 min at 3,000
× g at 4°C within 30 min of collection. Supernatants of the blood
samples were stored at −80°C for later use in the plasma ELISA. For
hippocampal homogenates, brain tissue was removed following rapid
decapitation. Bilateral hippocampi were dissected in 0°C
phosphate-buffered saline (PBS) on ice under an XTX-4A microscope
(Xindiweiye Medical Instrument Co., Ltd., Wuhan, China).
Hippocampal tissue was homogenized in 1 ml/g of 0°C PBS, and the
homogenate was stored at −80°C for later use in ELISA. ELISA assays
were performed according to the protocols of the commercial kits
for rat superoxide dismutase (SOD), malondialdehyde (MDA) and
glutathione peroxidase (GSH-px; Kaysam Bio-Technology Co., Ltd,
Guangzhou, China). The results were obtained by using a microplate
reader (Thermo MK3, Thermo Fisher Scientific Inc., Waltham, MA,
USA).
Histopathological examination
Rats in the three sevoflurane-treated groups and the
air-treated control group were sacrificed 6 h after the 6-h
exposure to sevoflurane/air for terminal
deoxynucleotidyl-transferase dUTP nick end labeling (TUNEL)
staining. The animals were anesthetized with a fatal dose of 10%
chloral hydrate (Huakai Resin Co., Ltd., Jining, China) and
perfused transcardially with normal saline until the liver and
lungs were clear of blood, a fixative of 4% paraformaldehyde (Tange
Biotechnology Co., Ltd., Shanghai, China) in 0.1 M phosphate buffer
(pH 7.4) was then perfused. The perfusion continued for 15–25 min,
the brains were subsequently detached and preserved in the same
fixative overnight. Paraffin blocks of the brain tissue (0.5 mm
thick) included different levels of the hippocampus along the
septotemporal axis and associated areas (18). Paraffin coronal sections of the
hippocampus (5-µm thick) underwent TUNEL staining (Roche,
Basel, Switzerland) and were stained with hematoxylin. The results
were examined in detail under a light microscope (Nikon ECLIPSE
50i; Nikon Corporation, Tokyo, Japan) to observe the morphological
changes of the pyramidal neurons in CA1 and region III of
hippocampus proper. TUNEL-positive neuronal cell numbers in the
bilateral CA1 of the hippocampal pyramidal cell layer (3 sections
per animal, n=3 for each group) were counted by two individuals
blinded to the groupings (14).
Cells were counted at magnification ×400 only when neuronal
structures of appropriate size and shape were demonstrated clearly
with a TUNEL-positive nucleus. Uncertain structures were examined
under magnification ×1,000 and were not counted if the
identification remained unclear (19).
Behavioral studies
Object exploration tests for
recognition memory
Following postnatal day 28, the rats underwent an
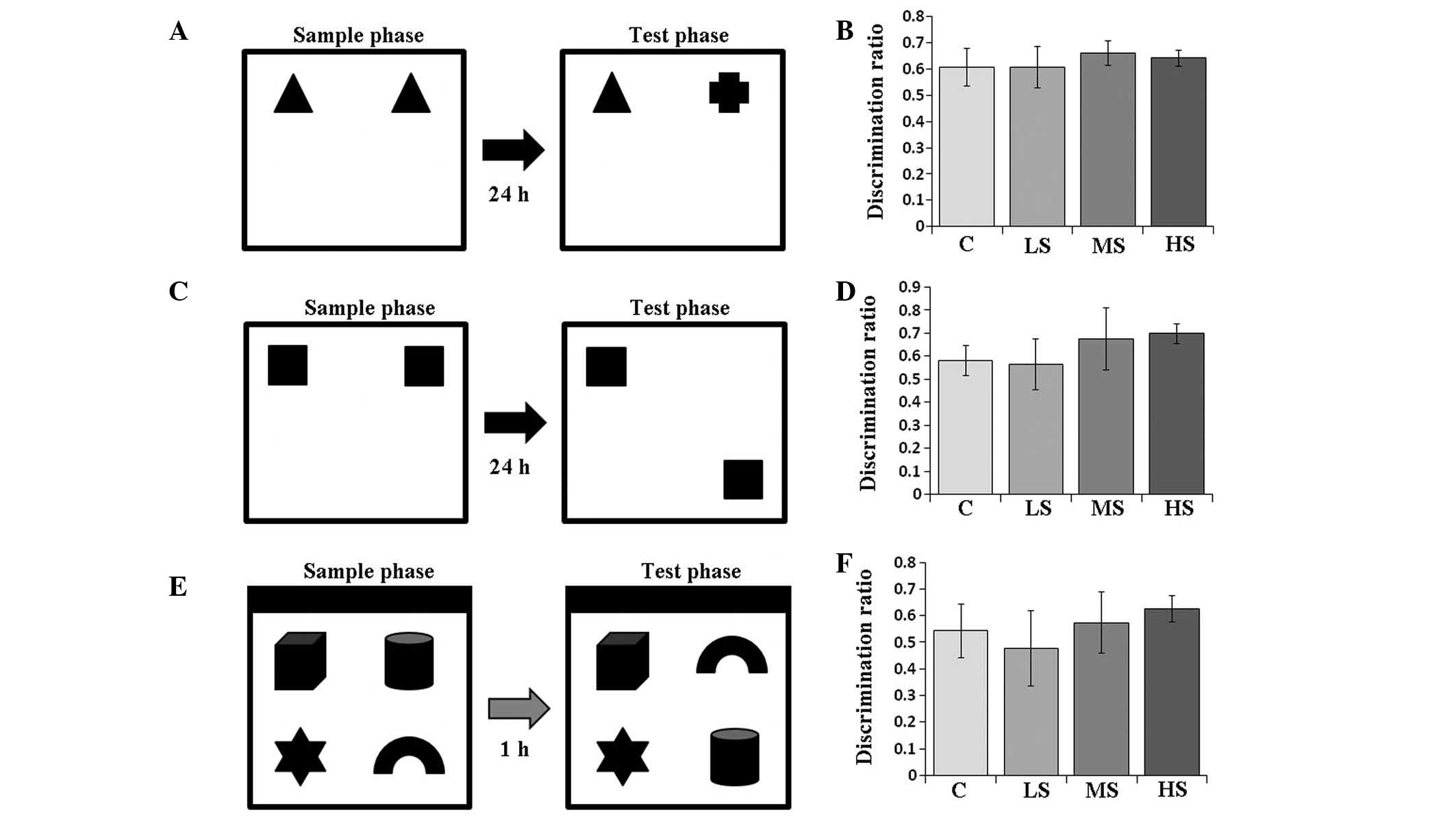
object exploration test (Fig. 1).
This was based on a partial modification of the recognition memory
test protocol (20). It was
performed in a white plastic cubical box (60×60×60 cm) and between
each trial, the box was wiped with 40% ethanol. In the
object-in-place test, the north wall of the box was painted black
(Fig. 1E). Subjects underwent
three habituation periods prior to the first test (object
recognition test). During each habituation period, rats were
brought individually into the box to stay for 10 min. Each period
was separated by a 24 h interval. The object recognition test began
24–48 h after the last habituation period. For subsequent object
location and object-in-place tests, tests began 24–48 h after one
habituation period. The objects used in the tests were made of
glass and were of a similar size (10×10×10 cm) but different shapes
(Fig. 1). Novel objects were
placed in a counterbalanced position to eliminate any side
preference of the rats. Each test consisted of a sample and a test
phase, and the objects were placed 10 cm away from the walls in the
box. A rat was released into the box facing the wall. Between each
rat release, the box was wiped again with 40% ethanol.
Object recognition
In the sample phase (4 min), two identical objects
were placed for the subject rat to explore (Fig. 1A). Then, the subject was placed
back into its colony cage. After 24 h, the test phase for
evaluating object memory began. In the test phase (4 min), one of
the two previously identical objects was replaced by a novel object
with a different shape. The subject was again released into the box
for exploration.
Object location recognition
This test was performed one week after the object
recognition test. In the sample phase (4 min), another two
identical objects were placed for the subject rat to explore
(Fig. 1C). Then, the subject was
placed back into its colony cage. After 24 h, the test phase for
evaluating object location memory began. In the test phase (4 min),
one of the two previously identical objects was moved to another
corner of the box. The subject was then released into the box for
exploration.
Object-in-place recognition
This test was performed one week after the object
location test. In the sample phase (5 min), four different objects
were placed for the subject rat to explore (Fig. 1E). Then, the subject was placed
back into its colony cage. After 1 h, the test phase for evaluating
object-in-place memory began. In the test phase (5 min), the two
previous objects were interchanged in position (Fig. 1E). The subject was then released
into the box for exploration. The longer time spent investigating
the pair of objects that switched positions, the stronger the
object-in-place memory of the subject.
Recognition memory
One investigator blinded to the group allocation of
the rats recorded the exploring time (21). The time when the subject rat
directed its nose to explore an object within 2 cm was considered
active exploring behavior. As previous studies have indicated,
recognition memory is most sensitive in the first 1–2 min of the
test phase (22–24), the discrimination ratio (DR) was
calculated only from data obtained during the first 2 min of the
test phase. DR was defined as the time the subject rat spent
exploring the novel object(s) or position(s) divided by the total
exploration time in the test phase. Rats that scored a higher DR
exhibited stronger recognition memory and discrimination
ability.
Statistical analysis
Statistical analysis was conducted using SPSS,
version 20 for Windows (IBM SPSS, Armonk, NY, USA) and P<0.05
was considered to indicate a statistically significant difference.
The standard error of the mean is presented as error bars on all
figures. The arterial blood gas analysis data (pH,
PaCO2, PaO2 and glucose levels) prior to and
following exposure to sevoflurane/air were compared using Student's
t-test. The ELISA, TUNEL staining and behavioral studies data were
analyzed using one-way analysis of variance (ANOVA). The Bonferroni
method was employed for multiple comparisons among the four
groups.
Results
Arterial blood gas following sevoflurane
treatment
Arterial blood gas analyses (arterial blood pH,
PaCO2, PaO2 and glucose levels) prior to and
following sevoflurane/air expo sure were not significantly
different as compared by Student's t-test (Table I), indicating the absence of severe
apnea and hypoxemia during anesthesia.
 | Table IArterial blood gas analysis prior to
and following sevoflurane exposure. |
Table I
Arterial blood gas analysis prior to
and following sevoflurane exposure.
| Group | Time (h) | n | Arterial blood
|
|---|
| pH | PaCO2
(kPa) | PaO2
(kPa) | Glucose (mmol
l−1) |
|---|
| Control | 0 | 3 | 7.38±0.05 | 3.52±0.09 | 13.38±0.05 | 4.97±0.55 |
| 6 | 3 | 7.38±0.06 | 3.53±0.07 | 13.37±0.05 | 5.40±0.60 |
| LS | 0 | 3 | 7.40±0.05 | 3.56±0.05 | 13.38±0.07 | 4.90±0.78 |
| 6 | 3 | 7.40±0.08 | 3.54±0.06 | 13.37±0.06 | 4.77±0.61 |
| MS | 0 | 3 | 7.36±0.06 | 3.55±0.04 | 13.38±0.05 | 5.07±0.47 |
| 6 | 3 | 7.42±0.07 | 3.57±0.08 | 13.37±0.07 | 5.37±0.59 |
| HS | 0 | 3 | 7.39±0.05 | 3.51±0.08 | 13.40±0.04 | 5.40±0.66 |
| 6 | 3 | 7.39±0.05 | 3.59±0.07 | 13.38±0.06 | 5.30±0.46 |
Antioxidant levels following sevoflurane
treatment
No significant difference to the levels of SOD were
identified between each group. SOD concentrations in the plasma of
the control, LS, MS and HS groups were 8.37±0.42, 8.55±0.38,
8.40±0.42 and 8.42±0.39 U/ml, respectively (F=0.471, P=0.704). SOD
concentrations in the hippocampal homogenate of the control, LS, MS
and HS groups were 11.11±1.47, 10.67±1.45, 11.57±1.33 and
12.43±1.83 U/ml, respectively (F=2.907, P=0.045). Plasma and
hippocampal GSH-px levels decreased when the concentration was
>1.3%. GSH-px concentrations in the plasma of the control, LS,
MS and HS groups were 4.66±0.38, 4.53±0.38, 4.07±0.26 and 4.25±0.22
U/l, respectively (F=8.492, P<0.001). GSH-px concentrations in
the hippocampal homogenate of the control, LS, MS and HS groups
were 7.75±0.72, 7.02±0.50, 5.33±0.44 and 5.87±0.66 U/l,
respectively (F=40.880, P<0.001). MDA levels increased following
treatment with sevoflurane, however, a paradoxical decrease of MDA
was observed when a subclinical concentration was administered
(Fig. 2). MDA concentrations in
the plasma of the control, LS, MS and HS groups were 0.71±0.05,
0.87±0.07, 0.71±0.04 and 0.93±0.09 nmol/ml, respectively (F=37.226,
P<0.001). MDA concentrations in the hippocampal homogenate of
the control, LS, MS and HS groups were 1.14±0.14, 1.65±0.18,
1.21±0.20 and 1.71±0.20 nmol/ml, respectively (F=31.974,
P<0.001).
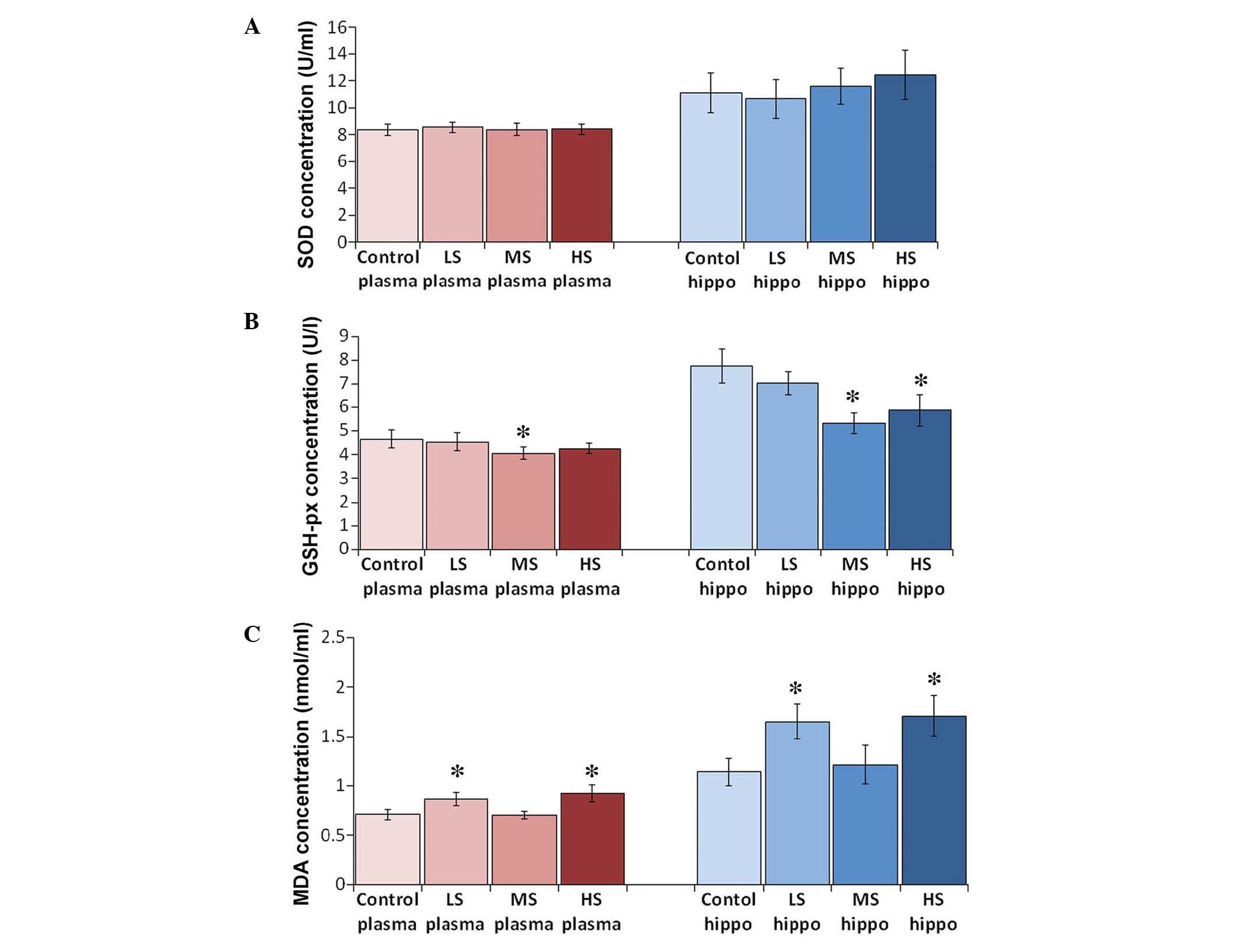 | Figure 2Levels of SOD, GSH-px, and MDA in
plasma and hippocampus by ELISA. Levels of (A) SOD, (B) GSH-px and
(C) MDA in the plasma and the hippocampus by ELISA. All results are
presented as the mean ± standard deviation, *P<0.01
vs. the control group. LS, subanesthetic concentration, 0.1 MAC;
MS, subclinical concentration, 0.3 MAC; HS, highest tolerated
concentration, 0.7 MAC; SOD, superoxide dismutase; GSH-px,
glutathione peroxidase; MDA, malondialdehyde; ELISA, enzyme-linked
immunosorbent assay. |
Neuronal apoptosis following sevoflurane
treatment
Sevoflurane induced hippocampal neuronal apoptosis
in a concentration-dependent manner. The number of TUNEL-positive
neurons increased as the concentration of sevoflurane increased
(Fig. 3). The number of TUNEL
positive neurons in the control, LS, MS and HS groups were
17.4±5.0, 26.8±5.76, 39.5±6.4 and 54.0±6.7 cells/100
µm2 CA3 area, respectively (F=14.069,
P<0.01).
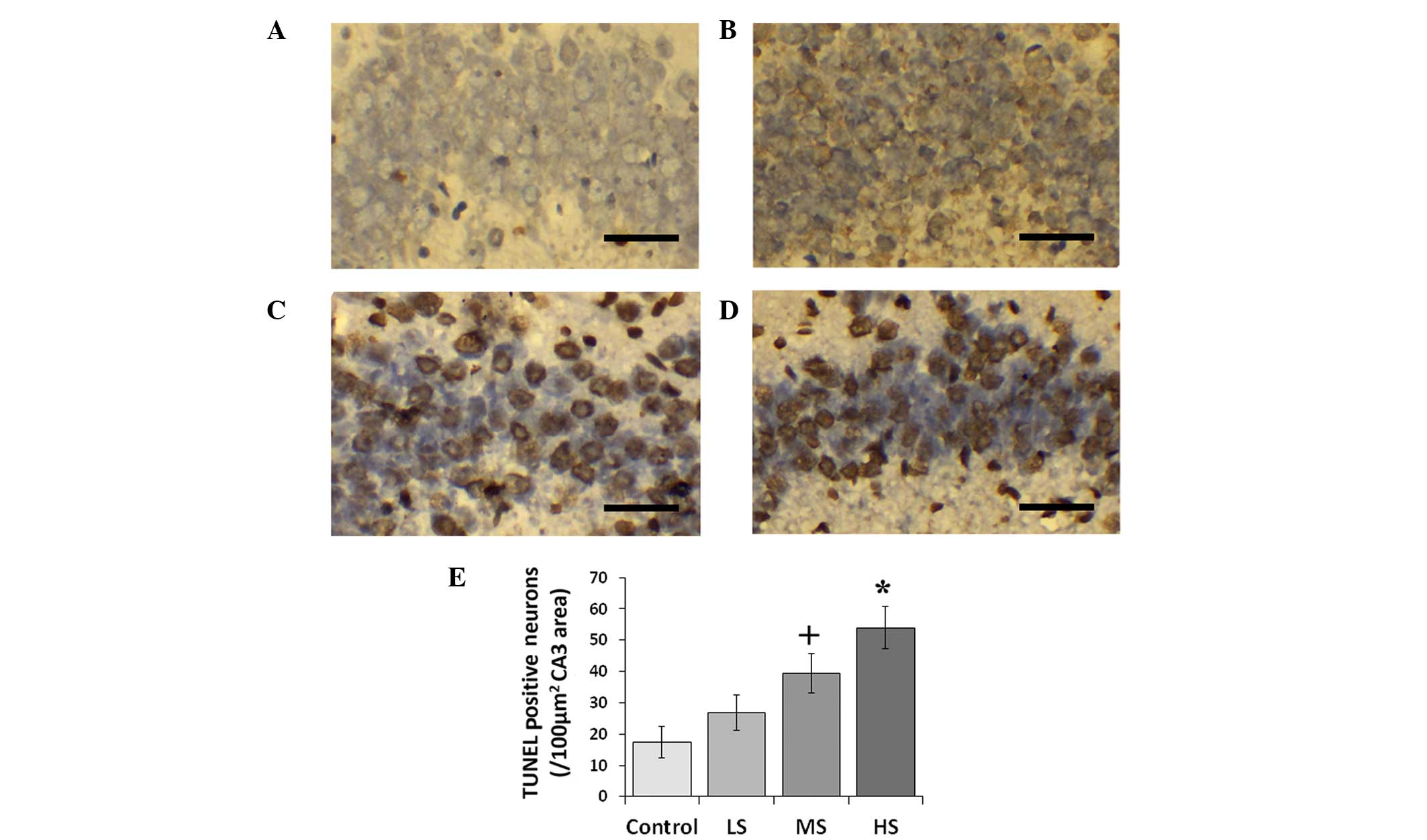 | Figure 3TUNEL staining in the hippocampus
(CA3) and TUNEL-positive neuron count. Representative
photomicrographs of TUNEL staining of coronal sections of the
hippocampus of (A) air control post-natal day 7 rats and rats
treated with (B) LS, (C) MS and (D) HS). Scale bar, 50 µm.
(E) Comparison of TUNEL-positive neuron numbers between the air
control and sevoflurane-treated groups. All data are presented as
the mean ± standard deviation. +P<0.05 and
*P<0.01 vs. the control group. CA3, region III of
hippocampus proper; LS, subanesthetic concentration, 0.1 MAC; MS,
subclinical concentration, 0.3 MAC; HS, highest tolerated
concentration, 0.7 MAC; TUNEL, terminal
deoxynucleotidyl-transferase dUTP nick end labeling. |
Total exploration times in the
behavioural studies
Total exploration times in the object recognition
test are listed in Table II. The
discrimination ratios of the control, LS, MS and HS groups in the
object recognition test were 0.608±0.071, 0.607±0.080, 0.662±0.047,
0.642±0.031, respectively. The discrimination ratio was analyzed by
one-way ANOVA, and the result indicated no significant difference
among the groups (F=0.978, P=0.423; Fig. 1). Treatment with different
concentrations of sevoflurane did not influence the discrimination
ability that depends on object recognition memory.
 | Table IITotal exploration time (sec, mean ±
standard deviation) in the sample phase and test phase. |
Table II
Total exploration time (sec, mean ±
standard deviation) in the sample phase and test phase.
| Treatment | Object recognition
| Object location
| Object-in-place
|
|---|
| Sample phase | Test phase | Sample phase | Test phase | Sample phase | Test phase |
|---|
| Control | 21.0±2.2 | 24.0±3.3 | 20.0±9.9 | 51.8±8.3 | 21.0±2.2 | 24.0±3.3 |
| LS | 21.8±4.3 | 20.7±3.6 | 18.7±13.0 | 50.0±9.5 | 21.8±4.3 | 20.7±3.6 |
| MS | 22.8±3.9 | 21.8±10.3 | 35.0±4.4 | 20.2±5.9 | 22.8±3.9 | 21.8±10.3 |
| HS | 26.2±1.9 | 23.2±1.9 | 55.3±7.7 | 37.5±7.1 | 26.2±1.9 | 23.2±1.9 |
Total exploration times in the object location
recognition test are also listed in Table II. The discrimination ratios of
control, LS, MS and HS groups in the object location recognition
test were 0.582±0.066, 0.565±0.109, 0.677±0.134 and 0.698±0.042,
respectively. The discrimination ratio was analyzed with one-way
ANOVA, and the result demonstrated no significant difference among
the groups (F=2.485, P=0.090; Fig.
1). Treatment with different concentrations of sevoflurane did
not influence discrimination ability that depends on object
location recognition memory.
Total exploration times in the object-in-place
recognition test are listed in Table
II. The discrimination ratio of control, LS, MS and HS groups
in the object-in-place recognition test were 0.543±0.100,
0.477±0.142, 0.573±0.115 and 0.625±0.051, respectively. The
discrimination ratio was analyzed with one-way ANOVA, and the
result demonstrated no significant difference among the groups
(F=1.670, P=0.205; Fig. 1).
Treatment with different concentrations of sevoflurane did not
influence the discrimination ability that depends on
object-in-place recognition memory.
Discussion
SOD catalyzes the dismutation of superoxide into
oxygen and hydrogen peroxide, thereby serving as an essential
antioxidant in the body. It exerts anti-inflammatory effects and
protects cells from damage. GSH-px is another enzyme that serves to
reduce oxidative damage and protect the structural integrity and
function of cell membranes (25).
SOD and GSH-px are major antioxidant enzymes that eliminate free
radicals and have marked anti-oxidative stress effects, maintaining
an equilibrium between oxidants and antioxidants in the body
(26). By contrast, MDA, the
direct product of lipid peroxidation, serves as an indicator of the
extent of cell damage. MDA may disrupt cell membrane structure,
lead to DNA fragmentation and hasten apoptosis (27). Therefore, determination of MDA, SOD
and GSH-px levels in the plasma and the hippocampus indicate the
general state of free radical metabolism in the body and the extent
of hippocampal damage during sevoflurane anesthesia.
Free radicals destroy the structure of the cell
membrane structure and attack DNA, fracturing it and increasing the
rate of apoptosis. They are considered to be closely correlated
with inflammatory processes and tumorigenesis (28). As TUNEL staining detects DNA
fragmentation in apoptotic cells (29), the increased production of MDA
demonstrated in the plasma and the hippocampus possibly accelerates
the process of apoptosis in the sevoflurane-treated animals.
Mammalian cells require an appropriate balance of
oxidants and antioxidants (10).
The elevation of MDA in the plasma of sevoflurane-treated rats
suggests that a large number of oxygen free radicals accumulate in
the body, and an imbalance occurs between free radical production
and detoxification. Such an imbalance decreases the activity of SOD
and GSH-px and increases the levels of MDA, driving the
anti-oxidation system into a low energy state. More lipid
peroxidation occurs than the antioxidant defense systems process,
thus generating MDA, the end product of lipid peroxidation.
The relatively low level of plasma and hippocampal
MDA that paradoxically appeared in the MS group suggests that
sevoflurane may reduce oxidative stress at the subclinical
concentration of 0.3 MAC (1.3%). However, this benign effect did
not appear to ameliorate the apoptotic process in the hippocampus,
indicating that an unknown pathway rather than the oxidative system
affects sevoflurane-induced apoptosis.
Sevoflurane was demonstrated to impact the
antioxidative status of erythrocytes characterized by decreased SOD
and increased GSH-px (30). The
preservation of erythrocytes was observed to be compromised in
surgery with sevoflurane instead of propofol anesthesia, which
indicates that sevoflurane may result in oxidative stress (31). By contrast, Allaouchiche et
al (32) investigated the
oxidative status of the circulation and lungs during sevoflurane
anesthesia at 1 MAC and observed relatively low levels of MDA and
GSH-px in the plasma and bronchoalveolar lavage fluid compared with
propofol and desflurane anesthesia, indicating that antioxidant
properties of sevoflurane may exist. Similarly, Turkan et al
(33) investigated oxidative
stress in the liver, brain, kidney and lung tissue of rats exposed
to 8% sevoflurane for 4 h and observed MDA to be significantly
decreased. However, in the majority of clinical scenarios,
sevoflurane is co-administered with narcotics, such as an opioid,
during surgery, thus a concentration >1 MAC is rarely employed
for maintenance of anesthesia (34).
These findings suggest that the proper application
of sevoflurane at a subclinical concentration may lower general
oxidative stress levels in the body.
Novel object recognition tasks are a method of
assessing recognition memory in rodents (35). The hippocampus is involved in
recognition memory when such memory involves remembering a
particular stimulus that occurred in a particular place or when the
memory contains a temporal or object proximity component (36). Lesions to the hippocampus produce
moderate and reliable recognition memory impairments (35). Despite the fact that sevoflurane
reliably induces neuronal apoptosis in the postnatal brain, no
long-term impact on recognition memory was observed in the present
study.
In conclusion, although early exposure to a
subclinical concentration of sevoflurane reduces oxidative stress,
it does not prevent the process of sevoflurane-induced hippocampal
apoptosis. These changes do not affect subsequent recognition
memory in juvenile rats. The current study suggested collection of
human data regarding anti-oxidation measures and their clinical
outcome is required, particularly for the developing brain.
Acknowledgments
This study was supported by funding from the B.
Braun Research Fund for Anesthesiology (grant no. BBDF-2014-016);
the Guangdong Science and Technology Planning Project (grant no.
2013B051000045); and the Guangzhou International Science and
Technology Cooperation Project (grant no. 2012J5100019). The
sources of funding had no role in the design and conduct of this
project or in the preparation of the manuscript.
Abbreviations:
|
SOD
|
superoxide dismutase
|
|
GSH-px
|
glutathione peroxidase
|
|
MDA
|
malondialdehyde
|
|
ELISA
|
enzyme linked immunosorbent assay
|
References
|
1
|
Mellon RD, Simone AF and Rappaport BA: Use
of anesthetic agents in neonates and young children. Anesth Analg.
104:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarner JB, Levine M, Davis PJ, Lerman J,
Cook DR and Motoyama EK: Clinical characteristics of sevoflurane in
children. A comparison with halothane. Anesthesiology. 82:38–46.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Payne RS, Akca O, Roewer N, Schurr A and
Kehl F: Sevoflurane-induced preconditioning protects against
cerebral ischemic neuronal damage in rats. Brain Res. 1034:147–152.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuo Z: A novel mechanism for sevoflurane
preconditioning-induced neuroprotection. Anesthesiology.
117:942–944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Xue Z and Sun A: Subclinical
concentration of sevoflurane potentiates neuronal apoptosis in the
developing C57BL/6 mouse brain. Neuroscience letters. 447:109–114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficits in fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komatsu H, Ohara T, Nogaya J, Yokono S and
Ogli K: Subanesthetic concentrations of volatile anesthetics may
enhance acquired avoidance training in ddN mice. Anesth Analg.
73:295–299. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komatsu H, Nogaya J, Kuratani N, Ueki M,
Yokono S and Ogli K: Psychomotor performance during initial stage
of exposure to halothane, enflurane, isoflurane and sevoflurane in
mice. Clin Exp Pharmacol Physiol. 24:706–709. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otsubo T, Maekawa M, Nagai T, Sakio H and
Hori Y: Facilitatory effects of subanesthetic sevoflurane on
excitatory synaptic transmission and synaptic plasticity in the
mouse hippocampal CA1 area. Brain Res. 1197:32–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buttke TM and Sandstrom PA: Oxidative
stress as a mediator of apoptosis. Immunol Today. 15:7–10. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orliaguet G, Vivien B, Langeron O,
Bouhemad B, Coriat P and Riou B: Minimum alveolar concentration of
volatile anesthetics in rats during postnatal maturation.
Anesthesiology. 95:734–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alkire MT, Nathan SV and McReynolds JR:
Memory enhancing effect of low-dose sevoflurane does not occur in
basolateral amygdala-lesioned rats. Anesthesiology. 103:1167–1173.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alkire MT and Gorski LA: Relative amnesic
potency of five inhalational anesthetics follows the Meyer-Overton
rule. Anesthesiology. 101:417–429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng X, Liu JJ, Zhou X, Song FH, Yang XY,
Chen XS, Huang WQ, Zhou LH and Ye JH: Single sevoflurane exposure
decreases neuronal nitric oxide synthase levels in the hippocampus
of developing rats. Br J Anaesth. 109:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Song FH, He W, Yang XY, Zhou ZB,
Feng X and Zhou LH: Neonatal exposure to sevoflurane causes
apoptosis and reduces nNOS protein expression in rat hippocampus.
Mol Med Rep. 6:543–546. 2012.PubMed/NCBI
|
|
16
|
Young C, Jevtovic-Todorovic V, Qin YQ,
Tenkova T, Wang H, Labruyere J and Olney JW: Potential of ketamine
and midazolam, individually or in combination, to induce apoptotic
neurodegeneration in the infant mouse brain. Br J Pharmacol.
146:189–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu LX, Yon JH, Carter LB and
Jevtovic-Todorovic V: General anesthesia activates BDNF-dependent
neuroapoptosis in the developing rat brain. Apoptosis.
11:1603–1615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Yan L, Zhao X, Wu W and Zhou LH:
The diversity of nNOS gene expression in avulsion-injured spinal
motoneurons among laboratory rodents. Nitric Oxide. 22:37–42. 2010.
View Article : Google Scholar
|
|
19
|
Ananth C, Dheen ST, Gopalakrishnakone P
and Kaur C: Distribution of NADPH-diaphorase and expression of
nNOS, N-methyl-D-aspartate receptor (NMDAR1) and non-NMDA glutamate
receptor (GlutR2) genes in the neurons of the hippocampus after
domoic acid-induced lesions in adult rats. Hippocampus. 13:260–272.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Howland JG, Cazakoff BN and Zhang Y:
Altered object-in-place recognition memory, prepulse inhibition and
locomotor activity in the offspring of rats exposed to a viral
mimetic during pregnancy. Neuroscience. 201:184–198. 2012.
View Article : Google Scholar
|
|
21
|
Howland JG and Cazakoff BN: Effects of
acute stress and GluN2B-containing NMDA receptor antagonism on
object and object-place recognition memory. Neurobiol Learn Mem.
93:261–267. 2010. View Article : Google Scholar
|
|
22
|
Dix SL and Aggleton JP: Extending the
spontaneous preference test of recognition: Evidence of
object-location and object-context recognition. Behav Brain Res.
99:191–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clark RE, Zola SM and Squire LR: Impaired
recognition memory in rats after damage to the hippocampus. J
Neurosci. 20:8853–8860. 2000.PubMed/NCBI
|
|
24
|
Barker GR and Warburton EC: NMDA receptor
plasticity in the perirhinal and prefrontal cortices is crucial for
the acquisition of long-term object-in-place associative memory. J
Neurosci. 28:2837–2844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drabko K, Bojarska-Junak A and Kowalczyk
J: Activity of superoxide dismutase and glutathione peroxidase and
concentrations of malonyldialdehyde, vitamin E, total antioxidant
status and extracellular cytokines concentrations in children with
acute lymphoblastic leukaemia (ALL). Med Wieku Rozwoj. 10:861–868.
2006.In Polish.
|
|
26
|
Aguilar A, Alvarez-Vijande R, Capdevila S,
Alcoberro J and Alcaraz A: Antioxidant patterns (superoxide
dismutase, glutathione reductase, and glutathione peroxidase) in
kidneys from non-heart-beating-donors: Experimental study.
Transplant Proc. 39:249–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Surapaneni KM and Venkataramana G: Status
of lipid peroxidation, glutathione, ascorbic acid, vitamin E and
antioxidant enzymes in patients with osteoarthritis. Indian J Med
Sci. 61:9–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Djordjevic VB: Free radicals in cell
biology. Int Rev Cytol. 237:57–89. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turkan H, Aydin A, Sayal A and Karahalil
B: The effect of sevoflurane and desflurane on markers of oxidative
status in erythrocyte. Toxicol Ind Health. 27:181–186. 2011.
View Article : Google Scholar
|
|
31
|
Tsuchiya M, Asada A, Kasahara E, Sato EF,
Shindo M and Inoue M: Antioxidant protection of propofol and its
recycling in erythrocyte membranes. Am J Respir Crit Care Med.
165:54–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allaouchiche B, Debon R, Goudable J,
Chassard D and Duflo F: Oxidative stress status during exposure to
propofol, sevoflurane and desflurane. Anesth Analg. 93:981–985.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turkan H, Aydin A, Sayal A, Eken A, Akay C
and Karahalil B: Oxidative and antioxidative effects of desflurane
and sevoflurane on rat tissue in vivo. Arh Hig Rada Toksikol.
62:113–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang HL, Yang L, Guo XY, Zhang LP, Bi SS
and Lu W: Response surface analysis of sevoflurane-remifentanil
interactions on consciousness during anesthesia. Chinese Med J
(Engl). 125:2682–2687. 2012.
|
|
35
|
Broadbent NJ, Gaskin S, Squire LR and
Clark RE: Object recognition memory and the rodent hippocampus.
Learn Mem. 17:5–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barker GR and Warburton EC: When is the
hippocampus involved in recognition memory? J Neurosci.
31:10721–10731. 2011. View Article : Google Scholar : PubMed/NCBI
|