Introduction
Sirtuin (Sirt)1, a mammalian homologue of silent
mating type information regulation 2 (1), is an NAD+-dependent
histone/protein deacetylase, which regulates caloric
restriction-mediated longevity in model organisms (2). It is also an important regulatory
factor of cell defense (3) and is
involved in a wide variety of cellular processes, including
differentiation, inflammation, energy metabolism, DNA repair and
cancer (4). Resveratrol, which is
a naturally occurring polyphenol found in grapes and red wine
(5), has been found to activate
Sirt1. Jackson et al (6)
and Sauve et al (7) noted
that NAM can inhibit Sirt1 activity effectively both in vivo
and in vitro.
Previously a series of researches have demonstrated
that Sirt1 may promote cell proliferation (8,9).
Treatment with resveratrol can increase muscle mass of mdx mice,
which is often used as a disease model for human muscular dystrophy
(10). Consistent with this,
overexpression of Sirt1 can promote muscle precursor cell
proliferation and cell cycle progression (11). Additionally, associated with the
Sirt1-mediated proliferation of C2C12 cells were the bidirectional
decreases and increases in the expression levels of the
cyclin-dependent kinase inhibitors p21Waf1/Cip1 and
p27Kip1, respectively. However, the underlying mechanism
of Sirt1 in regulating the expression of p21Waf1/Cip1
and p27Kip1 remains to be determined. Another mechanism
for Sirt1-mediated cell proliferation is that Sirt1 may prolong
cell survival date and inhibit the rate of cell death. For example,
Sirt1 can induce the expression of superoxide dismutase 2
(Sod2/Mn-Sod), which may decrease the levels of reactive oxygen
species and reduce oxidative stress-induced cell death in C2C12
myoblast cells (12).
Additionally, Sirt1 can notably affect the expression of
transcription factors, including FOXO, E2F1 and p53 (13,14),
which are important factors that regulate cell differentiation and
apoptosis. As well as normal cells, Sirt1 can also promote the
proliferation of tumor cells through inhibiting the differentiation
of tumor cells (15). Although
Sirt1 can improve myoblast proliferation, the specific mechanism(s)
remain unknown.
Myostatin, a member of the transforming growth
factor (TGF)-β superfamily, has been shown to negatively regulate
skeletal muscle myogenesis (16).
Myostatin predominantly affects myoblast cells in the G1 phase and
subsequently interferes with the progression into S phase to slow
down myoblast proliferation (17).
However, whether myostatin is involved in the Sirt1-mediated
regulation of C2C12 myoblast cell proliferation remains to be
reported. Previous research found that the expression level of
Sirt1 increased in myostatin-deficient mice (18). Futhermore, myostatin may inhibit
myoblast proliferation by upregulating the expression of
p21Waf1/Cip1. The evidence suggested that certain
correlation exists between Sirt1 and myostatin in the regulation of
cell proliferation (17). The
present study co-incubated C2C12 cells with Srit1 activator, Sirt1
inhibitor and myostatin inhibitor, and then assessed the cell
growth and the expression of the myostatin pathway. These results
indicated that myostatin is notably involved in Sirt1-mediated
regulation of C2C12 myoblast cell proliferation. These results may
assist with the design of novel methods or targets for the therapy
of muscular dystrophy.
Materials and methods
Cell culture
C2C12 myoblast cells were obtained from the Lab of
College of Animal Science and Technology of Huazhong Agricultural
University. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare Life Sciences,. Logan, UT,
USA) supplemented with 1% antibiotics (Gino Biomedical Technology
Co., Ltd., Hangzhou, China) and 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences) in a humidified air of 5% CO2
at 37°C.
Reagents and antibodies
Resveratrol was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Immediately prior to use, a 0.1 M stock solution
stored at −20°C was diluted to the desired concentration with
culture medium, and the final concentration of DMSO for all
treatments was maintained at 0.2%. SB431542 was purchased from
Selleck Chemicals (Houston, TX, USA). NAM was from Beyotime
Institute of Biotechnology (Hangzhou, China) and the Cell Counting
kit (CCK)-8 was from Dojindo Laboratories (Kumamoto, Japan). The
antibodies used were anti-myostatin rabbit polyclonal (Abcam,
Cambridge, MA, USA; cat. no. ab98337), anti-P107 rabbit polyclonal
(Abcam; cat. no. ab2451), anti-p-P107 rabbit polyclonal (Bioss,
Inc., Woburn, MA, USA; cat. no. bs-5696R), anti-β-actin mouse
monoclonal (Anbo, Changzhou, China; cat. no. E0012) and anti-rabbit
secondary antibody (Abcam; cat. no. ab6721). Other reagents were
from Goodbio Technology Co., Ltd. (Wuhan, China) or HyClone.
Cell proliferation assay
Exponentially growing C2C12 myoblast cells
(8×103 cells/well; 100 µl) were seeded into
96-well plates 20–24 h prior to replacing fresh serum free medium
containing the indicated concentrations of drug. SB431542 was added
first, followed by NAM after 30 min in the group of the SB431542
combined with NAM. After incubation for 24 h, culture medium was
replaced with drug-free medium (100 µl), and the effect of
drug was examined by a CCK-8 assay, according to the manufacturer's
protocol. Briefly, 10 µl of CCK-8 was added to each well and
the cells were further incubated away from light at 37°C for 1–3 h.
Absorbance was measured with excitation at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
5-Bromo-2-deoxyuridine (BrdU) assay
The cells were cultured in growth media for 12 h,
followed by BrdU treatment (BrdU 10 mg/ml stock solution in saline
was diluted 1:1,000 in the culture medium) for 1 h prior to
harvesting. After washing with phosphate-buffered saline (PBS),
cells were then fixed for 20 min in 4% paraformaldehyde and
permeabilized with 0.3% Triton X-100 for 10 min. Following blocking
with 10% goat serum in PBS for 1 h, the cells were incubated with
anti-BrdU antibody (1:200; BD Biosciences, San Jose, CA, USA; cat.
no. 552598) at 4°C for 18 h, followed by incubation with and the
secondary goat anti-mouse immunoglobulin G antibody conjugated with
Alexa Fluor 594 (1:1,000 dilution; Abcam; cat. no. ab150116) for 2
h at room temperature in the dark. The nuclei were visualized by
4′,6-diamidino-2-phenylindole and images were captured using an
Olympus microscope (IX71; Olympus, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After treatment for 6 h, the total RNA from C2C12
myoblast cells was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. First-strand cDNA was synthesized using a
PrimeScript™ RT reagent kit (Takara Bio, Inc., Dalian, China; cat.
no. RR037A). DNA amplification was performed in a StepOne system
(Applied BioSystems; Thermo Fisher Scientific, Inc.) using SYBR
Green PCR kit (Takara Bio, Inc.; cat. no. RR420A) for Sirt1, MyoD
and β-actin. The primer sequences are listed in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) | Accession no. |
|---|
| Sirtuin1 | | |
| Forward |
CCTTGGAGACTGCGATGTTA | NM_019812.2 |
| Reverse |
ATGAAGAGGTGTTGGTGGC | |
| MyoD | | |
| Forward |
GCCGCCTGAGCAAAGTGAATG | M84918.1 |
| Reverse |
CAGCGGTCCAGGTGCGTAGAAG | |
| β-Actin | | |
| Forward |
TGGTGGGAATGGGTCAGAAG | EF095208.1 |
| Reverse |
GTAGAAGGTGTGGTGCCAGA | |
| Myostatin | | |
| Forward |
GATTATCACGCTACGACGGA | NM_010834 |
| Reverse |
CCTGGGTTCATGTCAAGTTTC | |
Western blotting
Following treatment for 24 h, the C2C12 myoblast
cells were harvested in phosphate-buffered saline, centrifuged at
5,000 g for 10 min at 4°C and the supernatant was then removed. The
samples were homogenized in ice-cold radioimmunoprecipitation lysis
buffer (Beyotime Institute of Biotechnology) with 1% protease
inhibitor cocktail (Sangon Biotech, Shanghai, China) and 1%
phosphatase inhibitors (Goodbio Technology Co., Ltd.), and were
incubated on ice for 20–30 min. The samples were centrifuged at
10,000 g for 10 min at 4°C. The protein concentration of the
supernatant was measured using the bicinchoninic acid Protein
Quantification kit (BestBio, Shanghai, China). Supernatant
fractions of equal protein concentration (50 µg) were
subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene membranes (EMD
Millipore, Bedford, MA, USA). Blocking was performed in
Tris-buffered saline with 5% (w/v) non-fat dry milk for 2 h at room
temperature. Following blocking, the membranes were probed with
primary antibodies at 4°C overnight. The antibodies used were
anti-p-P107 (1:500), anti-P107 (1:250), anti-myostatin (1:500) and
anti-β-actin (1:5,000). The membranes were washed (6×5 min) with
Tris-buffered saline containing Tween-20, and incubated with the
appropriate secondary antibody (1:5,000) for 2 h at room
temperature. The membranes were washed as before and horseradish
peroxidase activity was detected using Chemiluminescence reagent
(Beyotime Institute of Biotechnology) and exposure to G:BOX Chemi
xT4 Gel imaging system instrument (Syngene UK,
Cambridge, UK). The blots were quantified by densitometric analysis
using the ImageJ software (version 1.46; imagej.nih.gov/ij/).
Statistical analysis
The data are presented as the mean ± standard error
of the mean for at least three individual experiments. All analyses
were performed using SPSS 19.0 software (IBM SPSS, Chicago, IL,
USA). The results were analyzed by analysis of variance, least
significant differences test for comparisons between two groups,
and correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sirt1 regulates the proliferation of
C2C12 myoblast cells
To elucidate the role of Sirt1 in the control of
C2C12 myoblast cell growth, C2C12 cells were treated with
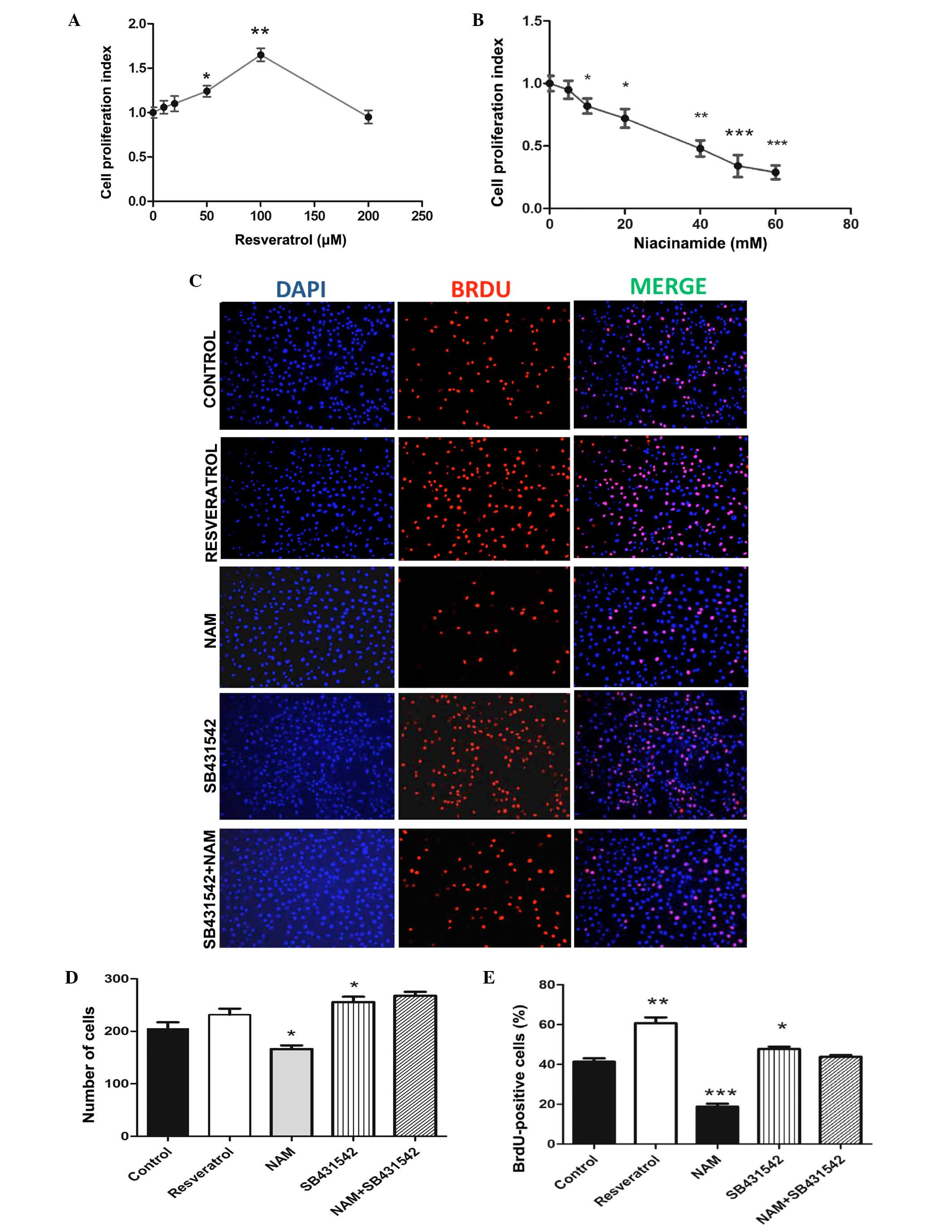
resveratrol and NAM (Fig. 1). The
present study administered different concentrations of resveratrol
on C2C12 myoblast cells and measured the cells growth after 24 h.
The results showed that following treatment of C2C12 cells with the
resveratrol at different concentrations of 10, 20, 50, 100 or 200
µM, the cell proliferation index was significantly higher
compared with that of the untreated control group (P<0.05;
Fig. 1A). Additionally, the
results demonstrated that the cell proliferation index of C2C12
myoblast cells increased with the growth following treatment with
resveratrol in a dose-dependent manner when the resveratrol
concentration ranged between 0 and 100 µM (Pearson
correlation coefficient, 0.993). However, when the concentration of
resveratrol reached >100 µM, the cell proliferation index
decreased and the proliferation of cells was inhibited. C2C12
myoblast cell proliferation stabilized at increased doses of
resveratrol to 165±7.2% (Fig. 1A)
of the control group at 100 µM and the BrdU positive rate
significantly increased by 18.75% (Fig. 1E).
Subsequently, the effect of different concentrations
of NAM on cell growth of C2C12 myoblast cells was detected. It was
revealed that following treatment of C2C12 myoblast cells with NAM
at different concentrations of 10, 20, 40, 50 and 60 mM, the cell
proliferation index was significantly lower compared with that of
the untreated control group (P<0.05; Fig. 1B). In addition, the results
revealed that the survival rate of C2C12 myoblast cells decreased
with the increase of NAM concentration in a dose-dependent manner
in the NAM concentration range of 0–60 mM. C2C12 myoblast cell
viability reached 34±8.8% of the untreated cells (Fig. 1B) at 50 mM and the BrdU positive
rate was 18.75% (Fig. 1E).
However, when the concentration of NAM reached >20 mM, the cell
proliferation rate was rather lower and the cell condition steadily
deteriorated (Fig. 1B).
SB431542 promotes the proliferation of
C2C12 myoblast cells
It has been demonstrated that SB431542 stimulates
proliferation and differentiation, and inhibits the activity of
TGF-β1 and activin receptor-like kinases (ALKs). It is a selective
and potent inhibitor of the phylogenetically related subset of
ALK-4 (activin type I receptor) and ALK-5 (TGFβ type I receptor).
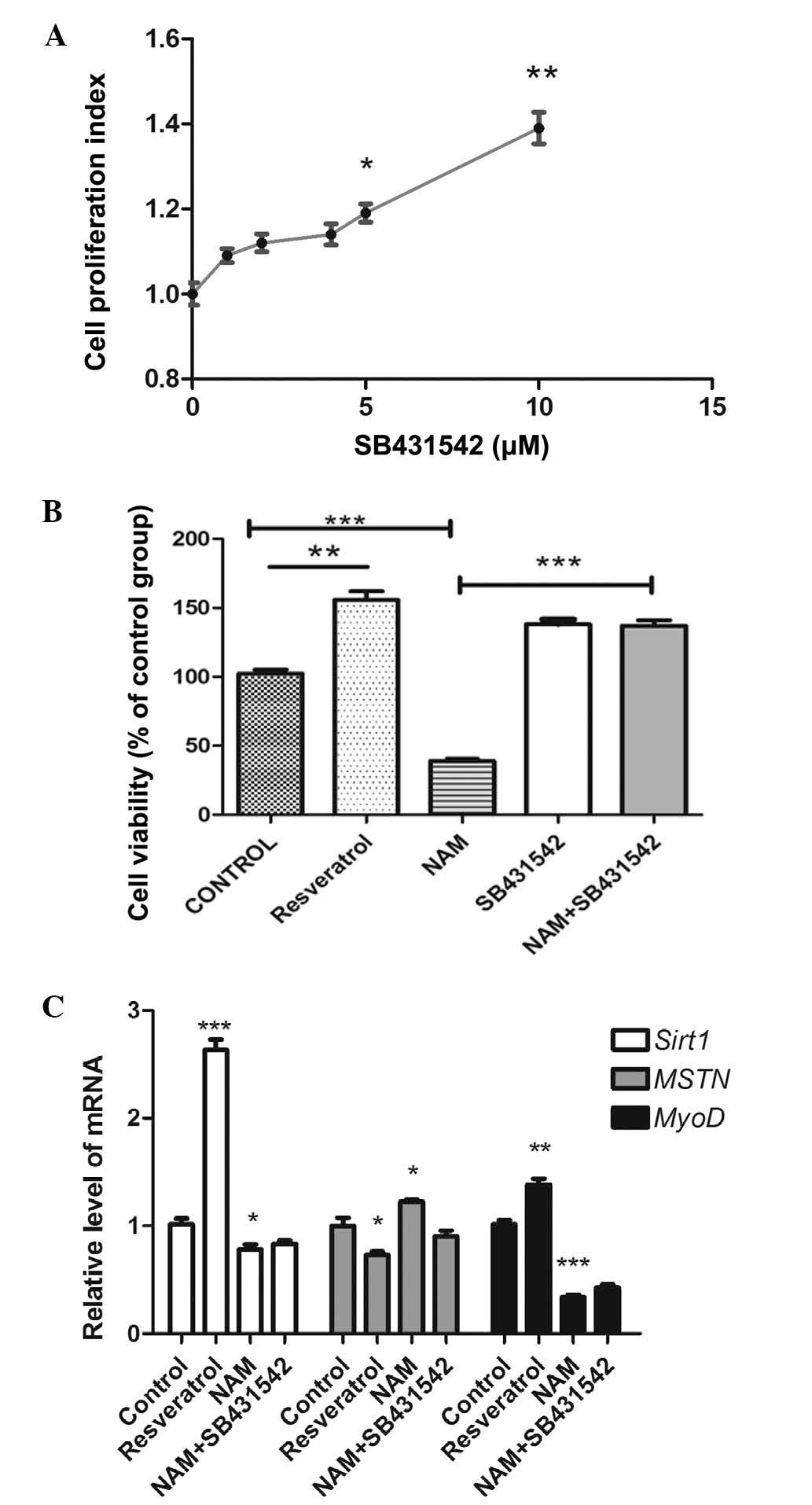
The results showed that following treatment with SB431542 at
different concentrations of 2, 4, 5 and 10 µM, the cell
proliferation index was significantly higher compared with that of
the control group (P<0.05; Fig.
2A). The total number of cells (255±10.27; Fig. 1D) and the BrdU positive rate of
47.73±1.26% were both significant compared with the control group
(Fig. 1E).
SB431542 relieves the inhibition of C2C12
myoblast cell proliferation by NAM
In order to investigate the role of the myostatin
signaling pathway in Sirt1-mediated regulation of C2C12 myoblast
cell proliferation, the present study divided C2C12 myoblast cells
into four groups and treated with SB431542 (10 µM),
resveratrol (100 µM), NAM (50 mM) and a combination of
SB431542 (10 µM) and NAM (50 mM). The cell growth of C2C12
myoblast cells was then assessed after 24 h treatment. Consistent
with previous studies, the present results showed that following
treatment with SB431542, the cell proliferation index (Fig. 2A) and BrdU positive rate (Fig. 1E) were significantly higher
compared with that of the control group (P<0.05). Following
treatment with SB431542 and NAM, the BrdU positive rate and cell
viability were significantly higher compared with that of NAM alone
(P<0.05; Figs. 1E and 2B, respectively). This indicated that
Srit1 inhibited the expression of myostatin. By contrast, the
inhibition of Sirt1 with NAM may promote the expression of
myostatin, therefore, the inhibition of myostatin by SB431542 may
release the effect of Sirt1 inhibition.
Myostatin signaling pathway is involved
in Sirt1-mediated regulation of C2C12 myoblast cell
proliferation
To confirm the role of Sirt1 in C2C12 cell
proliferation, the present study measured the mRNA expression of
Sirt1. As shown in Fig. 2C, the
mRNA expression of Sirt1 was upregulated by resveratrol treatment
(P<0.05), and was significantly downregulated by NAM treatment
(P<0.05). Notably, the mRNA expression of Sirt1 was
significantly higher in cells treated with SB431542 and NAM
compared with in cells treated with NAM alone (P<0.05); however,
no significant difference was observed in the mRNA expression of
Sirt1 between control cells and cells treated with SB431542 and NAM
(P<0.05). These data demonstrated that resveratrol, NAM and
SB431542 combined with NAM induced cell proliferation by the
activation or inhibition of Sirt1 expression.
MyoD is a myogenic regulatory factor critical for
myogenic specification. Previous studies (19,20)
reported that myostatin downregulates the expression of MyoD, which
indicates that MyoD is the downstream target of myostatin. The
members of the retinoblastoma (RB) family include
RB1/P105, RB2/P130 and RBL1/P107 (21–23),
and these genes encode retinoblastoma protein (pRb). P107 belongs
to a subline of pRb and has numerous common functions with pRb
(24). Additionally, certain
research has shown that myostatin may also be involved in the
inhibition of myoblast by the RB1 gene, which can be
regarded as an indicator of Sirt1 improving C2C12 proliferation via
myostatin. Myostatin can inhibit the proliferation of myoblast
cells by controlling the transition of G1 to S via the
phosphorylation and inactivation of pRb (17). To determine whether Sirt1 mediated
the regulation of C2C12 myoblast cell proliferation via the
myostatin signaling pathway, the present study measured the mRNA
expression levels of MyoD and myostatin following treatment with
resveratrol, NAM or the combination of SB431542 and NAM. Treatment
with resveratrol significantly upregulated the mRNA expression of
MyoD (P<0.05), while the treatment of NAM significantly
downregulated the expression of MyoD (P<0.05), and the
expression of MyoD of C2C12 cells treated with SB431542 and NAM was
higher compared with those treated with NAM alone (P<0.05)
(Fig. 2C). However, the mRNA
expression of MyoD in the C2C12 cells treated with SB431542 and NAM
showed no notable change compared with the NAM group. Treatment
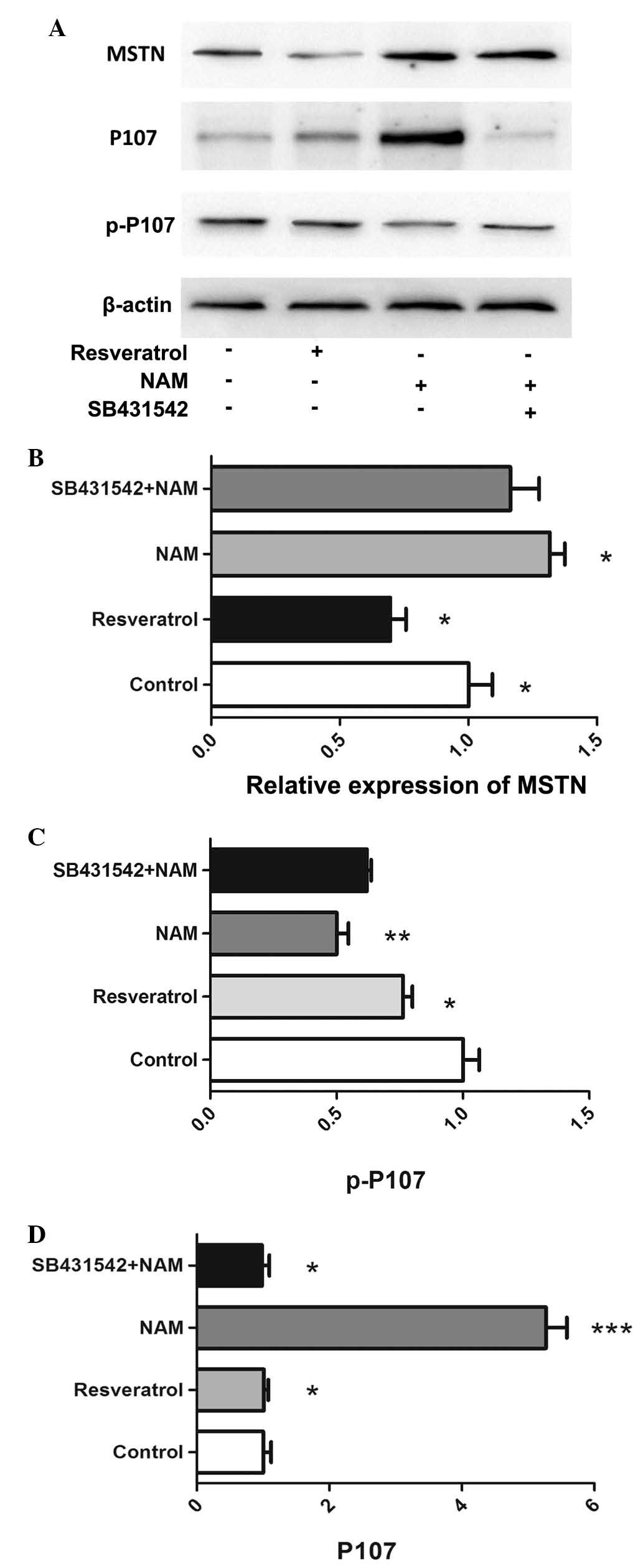
with resveratrol significantly downregulated the mRNA and protein
expression levels of myostatin (P<0.05; Figs. 2C, 3A
and B). Treatment with NAM significantly downregulated the
protein expression of p-P107 (P<0.05; Fig. 3A and C), and significantly
upregulated the protein expression levels of myostatin and P107
(Fig. 3B and D). The expression of
p-P107 was significantly higher in cells treated with SB431542 and
NAM compared with those treated with NAM alone. By contrast, the
expression of P107 was significantly lower in cells treated with
SB431542 and NAM compared with those treated with NAM alone
(P<0.05).
Discussion
Sirt1 serves a critical role in cell proliferation;
however, the mechanism remains to be fully understood. Kabra et
al (25) found that Sirt1
inhibits cell proliferation in colon cancer, while Pardo et
al (26) conversely reported
that Sirt1 promoted the proliferation and suppressed the
differentiation of myoblast precursors. Rathbone et al
(11) found that Sirt1
overexpression promoted the proliferation of skeletal muscle
precursor cells, which may be due to the different cell type. In
the present study, the Sirt1 activator resveratrol promoted the
proliferation of C2C12 myoblast cells (Fig. 1A and E), while its inhibitor NAM
decreased the proliferation of C2C12 myoblast cells (Fig. 1B and E).
The present data showed that Sirt1 promotes the
proliferation of C2C12 myoblast cells only when resveratrol
concentration was ≤100 µM. When resveratrol concentration
was >100 µM, the survival rate of C2C12 myoblast cells
decreased. This may be due to the toxic effect of the solvent DMSO
on the cells. By contrast, resveratrol can not only activate Sirt1,
but also inhibits lipoxygenase (27), cyclooxygenase (28) and various protein kinases,
therefore, as a result resveratrol may be toxic to cells when its
concentration is too high.
Myostatin, a member of the TGF-β signaling pathway,
is a negative regulator of skeletal muscle mass (16). Myostatin acts on muscle tissue by
binding a cell-bound receptor called activin type II receptor, for
example ALK4 and 5, which effect the phosphorylation of receptor
Smad proteins 2/3 (29). SB431542
is a small molecule inhibitor of ALK4 and 5, and it can inhibit the
myostatin signaling pathway. Certain previous studies found that
myostatin inhibits the proliferation of C2C12 myoblast cells
(30,31). Another previous study found that
SB431542 also promotes the enlargement of C2C12 myotube diameter,
and thus promotes the differentiation of C2C12 myoblast cells.
Consistent with the previous studies, the present study found that
SB431542 promotes the proliferation of C2C12 myoblast cells in a
concentration-dependent manner (Fig.
2A). Myostatin can inhibit G1 phase of the myoblast cell cycle
and subsequently interfere with the progression into S phase to
inhibit myoblast proliferation by increasing p21 expression and
hypophosphorylated pRb (17). It
has also been reported that when cells are close to the end of G1
phase, pRb is phosphorylated by cyclin-dependent kinase (CDK) and
acetylated, and Sirt1 can inhibit Rb activity by deacetylation
(32). The intersection between
myostatin and Sirt1 on cell phase regulation suggested that certain
interaction between myostatin and Sirt1 signaling exists. Indeed,
the present results indicated that the myostatin signaling pathway
is involved in the regulation of cell proliferation by Sirt1. The
present data showed that inhibition of the myostatin signaling
pathway can effectively alleviate the inhibition effect of NAM on
the proliferation of C2C12 myoblast cells (Figs. 1E and 2B). To confirm the interaction between
myostatin and Sirt1, the mRNA expression of Sirt1 was determined
following treatment with resveratrol, NAM or a combination of NAM
and SB431542. As shown in Fig. 2C,
resveratrol and NAM decreased and increased Sirt1 expression in
C2C12 myoblast cells, respectively. Our previous results indicated
that the survival rate was higher in cells treated with SB431542
and NAM compared with in cells treated with NAM alone. Consistent
with this, the mRNA expression of Sirt1 was significantly higher in
cells treated with SB431542 and NAM compared with those treated
with NAM alone. This demonstrated that myostatin can inversely
affect Sirt1 expression.
To further confirm this, the expression levels of
myostatin and its downstream proteins were assessed. Certain
previous studies found that myostatin overexpression can reduce
MyoD protein levels during cell proliferation and differentiation
(19,33). P107 is involved in the cell cycle
and belongs to a subline of pRb, which are downstream molecules of
myostatin. Western blot analysis was used to assess the protein
expression levels of myostatin and P107. It was demonstrated that
myostain expression was reduced following treatment with
resveratrol for 24 h (Fig. 3A).
However, for C2C12 myoblast cells treated with NAM, the protien
expression levels of myostatin and P107 were significantly
upregulated, while the protein expression of p-P107 was
downregulated (Fig 3A–D). Notably,
treatment with NAM significantly reduced the mRNA expression levels
of MyoD. When the C2C12 myoblast cells were co-incubated with
SB431542 and NAM, the mRNA expression of MyoD and the protein
expression of p-P107 were higher, and P107 expression was
significantly lower compared with those cells incubated with NAM
alone. This suggested that inhibition of the myostatin pathway
attenuated the inhibitory effect of NAM by P107 protein
phosphorylation. Previous studies have demonstrated that myostatin
may regulate the proliferation of cells via p21, which has been
demonstrated to be an inhibitor of cyclin D-CDK4/6 activity.
Additionally Thomas et al (17) found that myostatin can upregulate
the expression of p21Waf1/Cip1 and decrease the
expression and activity of CDK2 protein, which may be due to the
accumulation of hypo-phosphorylated pRb, thus leading to the arrest
of myoblasts in G1 phase of the cell cycle, subsequently inhibiting
cell proliferation (17).
Additionally, the overexpression of Sirt1 caused adverse effects,
for example, Sirt1 overexpression induced a decrease of
p21Waf1/Cip1 expression (34). Since the present study found that
Sirt1 activation downregulated the myostatin signaling pathway, it
was speculated that Sirt1 promoted cell proliferation by inhibiting
the myostatin signaling pathway.
Taken together, these data indicated that myostatin
is involved in the regulation of C2C12 myoblast cell proliferation
via Srit1 and that myostatin can affect Srit1 expression. Further
studies are warranted to elucidate the underlying mechanisms of how
Sirt1 affects myostatin signaling.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81271943).
References
|
1
|
Cheng HL, Mostoslavsky R, Saito S, Manis
JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW and Chua KF:
Developmental defects and p53 hyperacetylation in Sir2 homolog
(SIRT1)-deficient mice. Proc Natl Acad Sci USA. 100:10794–1099.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McBurney MW, Yang X, Jardine K, Hixon M,
Boekelheide K, Webb JR, Lansdorp PM and Lemieux M: The mammalian
SIR2alpha protein has a role in embryogenesis and gametogenesis.
Mol Cell Biol. 23:38–54. 2003. View Article : Google Scholar :
|
|
3
|
Horio Y, Hayashi T, Kuno A and Kunimoto R:
Cellular and molecular effects of sirtuins in health and disease.
Clin Sci (Lond). 121:191–203. 2011. View Article : Google Scholar
|
|
4
|
Ferrara N, Rinaldi B, Corbi G, Conti V,
Stiuso P, Boccuti S, Rengo G, Rossi F and Filippelli A: Exercise
training promotes SIRT1 activity in aged rats. Rejuvenation Res.
11:139–150. 2008. View Article : Google Scholar
|
|
5
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jackson MD, Schmidt MT, Oppenheimer NJ and
Denu JM: Mechanism of nicotinamide inhibition and
transglycosidation by Sir2 histone/protein deacetylases. J Biol
Chem. 278:50985–50998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sauve AA, Moir RD, Schramm VL and Willis
IM: Chemical activation of Sir2-dependent silencing by relief of
nicotinamide inhibition. Mol Cell. 17:595–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohanna M, Bonet C, Bille K, Allegra M,
Davidson I, Bahadoran P, Lacour JP, Ballotti R and Bertolotto C:
SIRT1 promotes proliferation and inhibits the senescence-like
phenotype in human melanoma cells. Oncotarget. 5:2085–2095. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan
F, Meng S, Wang Y, Yuan Z and Bi W: SIRT1 regulates YAP2-mediated
cell proliferation and chemoresistance in hepatocellular carcinoma.
Oncogene. 33:1468–1474. 2014. View Article : Google Scholar
|
|
10
|
Hori YS, Kuno A, Hosoda R, Tanno M, Miura
T, Shimamoto K and Horio Y: Resveratrol ameliorates muscular
pathology in the dystrophic mdx mouse, a model for duchenne
muscular dystrophy. J Pharmacol Exp Ther. 338:784–794. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rathbone CR, Booth FW and Lees SJ: Sirt1
increases skeletal muscle precursor cell proliferation. Eur J Cell
Biol. 88:35–44. 2009. View Article : Google Scholar :
|
|
12
|
Tanno M, Sakamoto J, Miura T, Shimamoto K
and Horio Y: Nucleocytoplasmic shuttling of the NAD+-dependent
histone deacetylase SIRT1. J Biol Chem. 282:6823–6832. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ripoli M, Barbano R, Balsamo T, Piccoli C,
Brunetti V, Coco M, Mazzoccoli G, Vinciguerra M and Pazienza V:
Hypermethylated levels of E-cadherin promoter in Huh-7 cells
expressing the HCV core protein. Virus Res. 160:74–81. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haigis MC and Sinclair DA: Mammalian
sirtuins: Biological insights and disease relevance. Annu Rev
Pathol. 5:253–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ota H, Tokunaga E, Chang K, Hikasa M,
Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y and Kaneki M: Sirt1
inhibitor, Sirtinol, induces senescence-like growth arrest with
attenuated Ras-MAPK signaling in human cancer cells. Oncogene.
25:176–185. 2006.
|
|
16
|
McPherron AC, Lawler AM and Lee SJ:
Regulation of skeletal muscle mass in mice by a new TGF-beta
superfamily member. Nature. 387:83–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas M, Langley B, Berry C, Sharma M,
Kirk S, Bass J and Kambadur R: Myostatin, a negative regulator of
muscle growth, functions by inhibiting myoblast proliferation. J
Biol Chem. 275:40235–40243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Mcfarlane C, Lokireddy S, Bonala
S, Ge X, Masuda S, Gluckman PD, Sharma M and Kambadur R:
Myostatin-deficient mice exhibit reduced insulin resistance through
activating the AMP-activated protein kinase signalling pathway.
Diabetologia. 54:1491–1501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Langley B, Thomas M, Bishop A, Sharma M,
Gilmour S and Kambadur R: Myostatin inhibits myoblast
differentiation by down-regulating MyoD expression. J Biol Chem.
277:49831–49840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spiller MP, Kambadur R, Jeanplong F,
Thomas M, Martyn JK, Bass JJ and Sharma M: The myostatin gene is a
downstream target gene of basic helix-loop-helix transcription
factor MyoD. Mol Cell Biol. 22:7066–7082. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ewen ME, Xing YG, Lawrence JB and
Livingston DM: Molecular cloning, chromosomal mapping and
expression of the cDNA for p107, a retinoblastoma gene
product-related protein. Cell. 66:1155–1164. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hannon GJ, Demetrick D and Beach D:
Isolation of the Rb-related p130 through its interaction with CDK2
and cyclins. Genes Dev. 7:2378–2391. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mayol X, Graña X, Baldi A, Sang N, Hu Q
and Giordano A: Cloning of a new member of the retinoblastoma gene
family (pRb2) which binds to the E1A transforming domain. Oncogene.
8:2561–2566. 1993.PubMed/NCBI
|
|
24
|
Assoian RK and Yung Y: A reciprocal
relationship between Rb and Skp2: Implications for restriction
point control, signal transduction to the cell cycle and cancer.
Cell Cycle. 7:24–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kabra N, Li Z, Chen L, Li B, Zhang X, Wang
C, Yeatman T, Coppola D and Chen J: SirT1 is an inhibitor of
proliferation and tumor formation in colon cancer. J Biol Chem.
284:18210–18217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pardo PS and Boriek AM: The physiological
roles of Sirt1 in skeletal muscle. Aging (Albany NY). 3:430–437.
2011. View Article : Google Scholar
|
|
27
|
Olas B and Wachowicz B: Resveratrol, a
phenolic antioxidant with effects on blood platelet functions.
Platelets. 16:251–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banerjee S, Bueso-Ramos C and Aggarwal BB:
Suppression of 7,12-dimethylbenz (a)anthracene-induced mammary
carcinogenesis in rats by resveratrol: Role of nuclear
factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9.
Cancer Res. 62:4945–4954. 2002.PubMed/NCBI
|
|
29
|
Lee SJ: Regulation of muscle mass by
myostatin. Annu Rev Cell Dev Biol. 20:61–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taylor WE, Bhasin S, Artaza J, Byhower F,
Azam M, Willard DH Jr, Kull FC Jr and Gonzalez-Cadavid N: Myostatin
inhibits cell proliferation and protein synthesis in C2C12 muscle
cells. Am J Physiol Endocrinol Metab. 280:E221–E228.
2001.PubMed/NCBI
|
|
31
|
Rios R, Carneiro I, Arce VM and Devesa J:
Myostatin regulates cell survival during C2C12 myogenesis. Biochem
Biophys Res Commun. 280:561–566. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong S and Weber JD: Deacetylation of the
retinoblastoma tumour suppressor protein by SIRT1. Biochem J.
407:451–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joulia D, Bernardi H, Garandel V,
Rabenoelina F, Vernus B and Cabello G: Mechanisms involved in the
inhibition of myoblast proliferation and differentiation by
myostatin. Exp Cell Res. 286:263–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rathbone CR, Lees SJ and Booth F: Sirt1
increases satellite cell cycle progression. FASEB. 212007.
|