Introduction
Acute respiratory distress syndrome (ARDS) is caused
by a variety of insults, such as sepsis, major trauma, pneumonia,
transfusions and aspiration of gastric contents. It is associated
with a high rate of mortality according to the most recent report
of the ARDS Network clinical trials (1). Sepsis is the most common predisposing
factor of ARDS and is characterized by systemic inflammation in
response to microbial toxins, such as lipopolysaccharide (LPS), a
component of the cell wall of gram-negative bacteria. Cellular
characteristics of ARDS include loss of alveolar-capillary membrane
integrity, excessive trans-epithelial neutrophil migration and
release of pro-inflammatory cytotoxic mediators (2). Fibroblasts are important in normal
and pathological repair (3).
Fibroblasts are also involved in the inflammatory reaction and
exhibit a critical role in the switch from acute inflammation to
tissue repair (3). In addition,
lung fibroblast migration and proliferation occur early after lung
injury and are required for ongoing lung healing (4,5).
Therefore, protecting lung fibroblasts and ameliorating their
inflammatory reactions has been suggested to be a promising method
for alleviating lung injury.
Brain natriuretic peptide (BNP) is a member of the
atria natriuretic peptide (ANP) family, and was first isolated from
porcine brains (6). As a cardiac
hormone predominantly produced by ventricular myocytes, BNP has
been used as a biomarker for heart failure (7,8). It
has vasodilatory and natriuretic functions that counteract
vasoconstriction and the fluid-retaining effect of the
rennin-angiotensin system. As a man-made peptide developed through
gene engineering, recombinant human brain natriuretic peptide
(rhBNP) is widely used clinically for the treatment of
decompensated heart failure (9).
Most recently, rhBNP has also been administered intravenously for
use in critical care units such as guiding fluid therapy,
predicting clinical outcomes of critical illness including sepsis.
BNP level may be a good indicator of cardiac preload in patients
with high volume load, and serve as a powerful predictor of
mortality in patients with sepsis (10–12).
In our previous studies, it was identified that rhBNP exhibited
protective effects on certain organs, including lung (13–15).
However, the underlying mechanism remains unclear, and little
information is available regarding the effect of rhBNP on
inflammatory responses in HFL-1 human fetal lung fibroblasts.
Therefore, the present study investigated the effects of rhBNP on
inflammatory molecules and signaling pathways in HFL-1 cells
challenged by LPS.
Materials and methods
Materials
rhBNP was obtained from Nuodikang Biological
Pharmaceutical Company Ltd. (Chengdu, China). LPS and fetal calf
serum (FCS) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Tissue culture supplements and medium were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). All other materials and
antibodies were purchased from Takara Biotechnology Co., Ltd.
(Dalian, China). Experiments were conducted according to the
National Institutes of Health (NIH) guidelines and approved by the
ethics committee of the General Hospital of Shenyang Military
District (Shenyang, China).
Cell culture
HFL-1 cells were obtained from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in 100-mm tissue culture dishes
with Dulbecco's modified Eagle's medium (DMEM) supplemented with
10% FCS, 50 mg/ml penicillin, 50 mg/ml streptomycin, and 0.25 mg/ml
Fungizone. The cells were passaged every 3 to 5 days. Fibroblasts
used in these studies were between cell passages 10 and 20.
Groups and treatments
Cells were divided into the following groups:
Control group, rhBNP (0.1 µM) group, LPS (1 µg/ml)
group, LPS (1 µg/ml) + rhBNP (0.1 µM) group, and LPS
(1 µg/ml) + rhBNP (0.1 µM) + inhibitor group. For all
experiments, cells were plated in 6-well plates and grown to 80%
confluence. For the control and control + rhBNP groups, HFL-1 cells
were not exposed to LPS. The cells were serum deprived for 24 h in
DMEM medium containing 10% FBS prior to the addition of LPS and/or
0.1 µM. SB203580 (p38 inhibitor, 20 µM), SP600125
[c-Jun NH2-terminal kinase (JNK) inhibitor, 25 µM], PD098059
[extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor, 5
µM) or pyrrolidine dithiocarbamate [PDTC; nuclear factor
(NF)-κB inhibitor, 10 µM) was added 30 min prior to LPS
exposure, respectively. The cells were then incubated with LPS (1
µg/ml) for 0, 2, 4, 6, 8, 12, and 24 h.
Lactate dehydrogenase (LDH) release
assays
Following treatment, LDH release in the medium was
measured using a previously described method (16). The absorbance values were read
using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA)
at 440 nm, and the results of the absorbance from the test wells
were expressed as a percentage of the control wells. Results from a
single experiment are reported. Similar data were obtained in six
independent experiments.
Measurement of interleukin (IL)-1β
secretion
The level of IL-1β secretion in culture media was
determined by an enzyme-linked immunosorbent assay kit (R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's instructions. Concentrations were calculated with
reference to the standard curve.
Quantitative analysis of the IL-1β mRNA
expression
Total RNA extraction was isolated using TRIzol
Reagent (Invitrogen; Thermo Fischer Scientific, Inc.) according to
the manufacturer's instructions. An RNA denaturation mix consisting
of isolated RNA, nuclease-free water and oligo dT primers was used.
cDNA was synthesized using a reverse transcription kit (TransGen
Biotech, Inc., Beijing, China) according to the manufacturer's
instructions. Following reverse transcription, quantitative
analysis of the IL-1β mRNA expression was analyzed by the real-time
polymerase chain reaction (PCR) method. The following primers
(Takara Biotechnology Co., Ltd.) were used: Forward,
5′-ATGCCTCGTGCTGTCTGACC-3′ and reverse,
5′-CCATCTTTAGGAAGACACGGGTT-3′ for IL-1β; and forward,
5′-ATGTGCCGGACCTTGGAAG-3′ and reverse, 5′-CCTCGGGTTAGCTGAGAGATCA-3′
for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). PCR assays
were performed in duplicate on the 7500 real-time PCR machine
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling
conditions were as follows: Incubation for 2 min at 50°C followed
by another incubation step at 95°C for 10 min, 15 sec at 95°C and 1
min at 60°C for 40 cycles. Reaction specificity was evaluated by
melting curve analysis, which was performed by heating the plate
from 55°C to 95°C and measuring SYBR Green I (Takara Biotechnology
Co., Ltd.) dissociation from the amplicons. The calculation of
quantification cycles (Cq values) and further analysis of these
data were performed by the Sequence Detector software (version
1.2.3; Syngene, Cambridge, UK). The relative expression of mRNA in
each sample was quantified and normalized to the GAPDH mRNA levels
by the 2−ΔΔCq method (17).
Western blot analysis
HFL-1 cells were collected in lysis buffer and
centrifuged at 1,500 × g for 5 min at 5°C. Total cellular protein
in the supernatant was determined using the bicinchoninic acid
(Takara Biotechnology Co., Ltd.) method. Equal quantities of
protein (40 µg per lane) were separated on 10% sodium
dodecyl sulfate-polyacrylamide gels (Takara Biotechnology Co.,
Ltd.) and electrically transferred to nitrocellulose membranes
(Takara Biotechnology Co., Ltd.) at 80 V at 4°C for 2 h. The
nitrocellulose membranes were blocked with 5% non-fat dry milk, and
then blots were incubated overnight at 4°C with the following
primary monoclonal antibodies: Rabbit anti-p-p38 (cat no. 10359),
anti-p38 (cat no. 10360), anti-p-JNK (cat no. 18295), anti-JNK (cat
no. 18296), anti-ERK1/2 (cat no. 10621), anti-p-ERK1/2 (cat no.
10620), NF-κB p65 (cat no. 18667), anti-p-IκB (cat no. 18669) or
anti-β-actin antibody (cat no. 21765) (all 1:1,000; Cell Signaling
Technology, Danvers, MA, USA). The following day, the membranes
were washed in phosphate-buffered saline with Tween-20 (Takara
Biotechnology Co., Ltd.), incubated with
horseradish-peroxidase-conjugated goat anti-rabbit second antibody
Takara Biotechnology Co., Ltd.) and washed again. Signals were
visualized by enhanced chemiluminescence (Takara Biotechnology Co.,
Ltd.). The intensity of each band was measured and analyzed with
the Quantity One software (version 4.1; Bio-Rad Laboratories).
Statistical analysis
All quantitative data are expressed as the mean ±
standard error of the mean. Numerical data were performed using
one-way analysis of variance with Tukey's post hoc test.
Statistical analysis was performed using SPSS (version 15; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. Each experiment was performed
at least three times.
Results
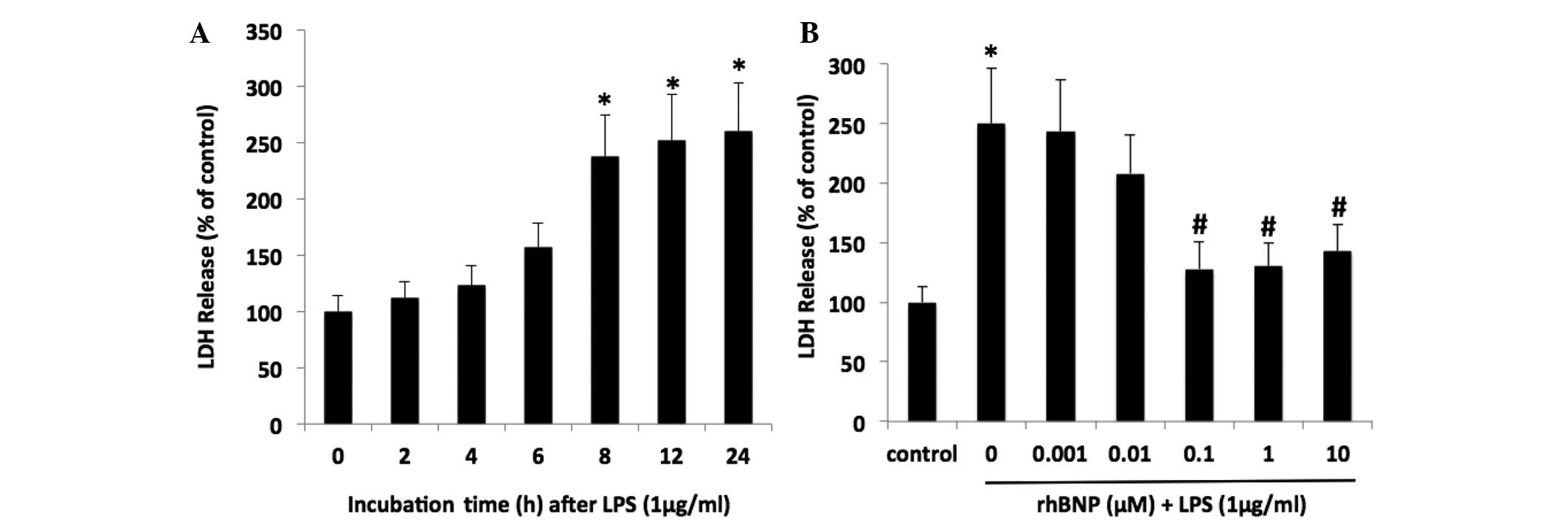
rhBNP attenuates LPS-induced cell
injury
To evaluate LPS-induced cell injury, the release of
LDH into the medium was measured. The LDH leakage was increased in
a time-dependent manner following treatment with LPS (P<0.05 vs.
control, Fig. 1A). In addition,
the effect of rhBNP on LDH release in the HFL-1 cells was greatest
at concentrations of 0.1, 1 and 10 µM (Fig. 1B). rhBNP at a dose of 0.1 µM
was administered in the following experiments. These results
demonstrated that rhBNP could attenuate LPS-induced cell
injury.
rhBNP reduces the IL-1β level in HFL-1
cells
Compared with the control group, the level of IL-1β
in the culture medium was significantly increased after 24 h
exposure to LPS (P<0.05). As shown in Fig. 2A, LPS-induced IL-1β secretion was
significantly inhibited by 0.1 µM rhBNP (P<0.05 vs. LPS).
Moreover, pretreatment with the p38 inhibitor SB203580, JNK
inhibitor SP600125, ERK1/2 inhibitor PD098059, or NF-κB inhibitor
PDTC inhibited the LPS-induced increase in the IL-1β level
(P<0.05 vs. LPS). For IL-1β mRNA expression, the IL-1β level was
significantly induced by 0.1 µM rhBNP when compared with the
LPS group (P<0.05, Fig.
2B).
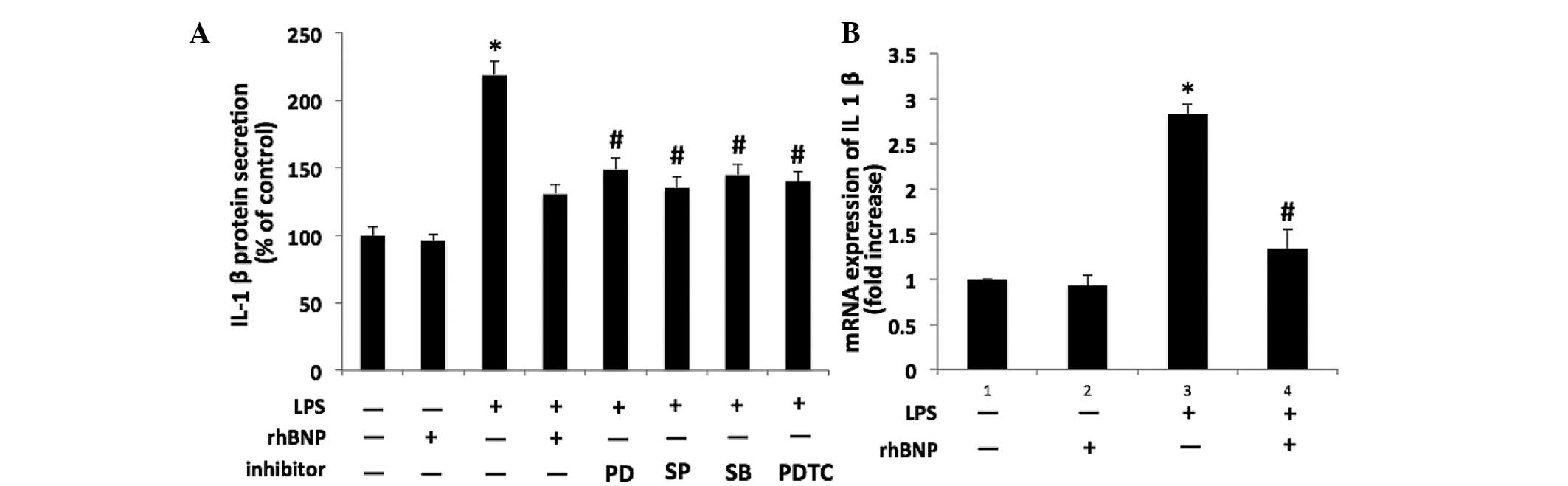 | Figure 2Effects of LPS, rhBNP and
mitogen-activated protein kinase pathway inhibitors on secretion of
IL-1β in HFL-1 cells. (A) IL-1β protein levels in the culture media
of HFL-1 cells with or without treatment with LPS, rhBNP, p38
inhibitor (SB203580, 20 µM), c-Jun NH2-terminal kinase
inhibitor (SP600125, 25 µM), extracellular signal-regulated
kinase 1/2 inhibitor (PD098059, 5 µM) and nuclear factor-κB
inhibitor (PDTC, 10 µM) for 24 h were analyzed by
enzyme-linked immunosorbent assay. (B) Effects of LPS and rhBNP on
IL-1β mRNA expression in HFL-1 cells were determined by reverse
transcription-quantitative polymerase chain reaction.
*P<0.05 vs. the control group, #P<0.05
vs. the LPS group. LPS, lipopolysaccharide; rhBNP, recombinant
human brain natriuretic peptide; LDH, lactate dehydrogenase; IL,
interleukin. |
rhBNP suppresses the LPS-induced
phosphorylation of p38, JNK, and ERK1/2 MAP kinases
Protein expression of key molecules in the
intracellular mitogen-activated protein kinases signaling pathways,
p-p38, p-JNK, and p-ERK1/2 were detected using western blot
analysis. The results showed that the expression of p-p38, p-JNK
and p-ERK1/2 was significantly increased in the LPS group
(P<0.05 vs. control), whereas treatment with rhBNP significantly
suppressed the expression of p-p38, p-JNK and p-ERK1/2 when
compared with the LPS group (P<0.05, Fig. 3).
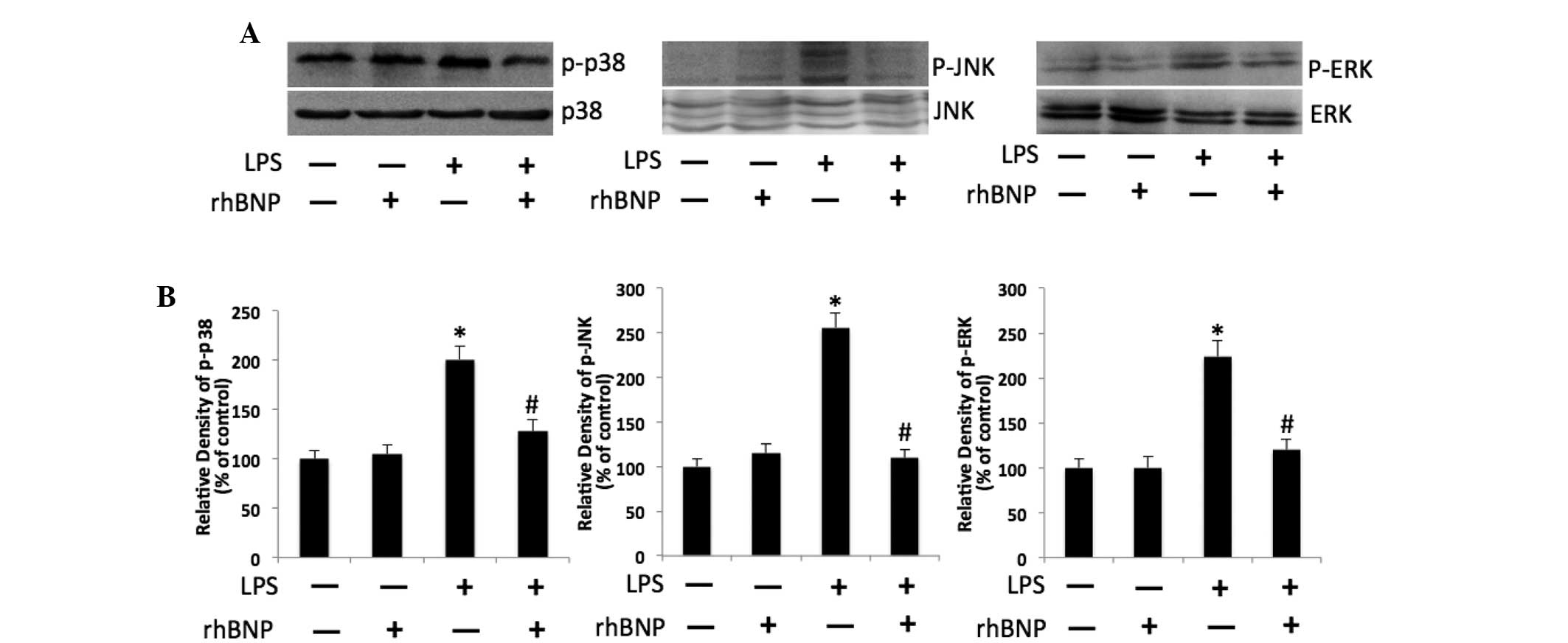 | Figure 3Effects of rhBNP and LPS on
phosphorylation of p38, JNK and ERK1/2 in HFL-1. (A) Expression of
p-p38, p38, p-JNK, JNK, p-ERK1/2 and ERK1/2 was detected by western
blot analysis. Immunostaining of p38, JNK, and ERK1/2 served as
respective controls. (B) Bar graphs represents semi-quantitative
densitometry from western blot analysis. *P<0.05 vs.
the control group, #P<0.05 vs. the LPS group. rhBNP,
recombinant human brain natriuretic peptide; LPS,
lipopolysaccharide; LDH, lactate dehydrogenase; p-, phosphorylate;
JNK, c-Jun NH2-terminal kinase; ERK1/2 extracellular
signal-regulated kinase 1/2. |
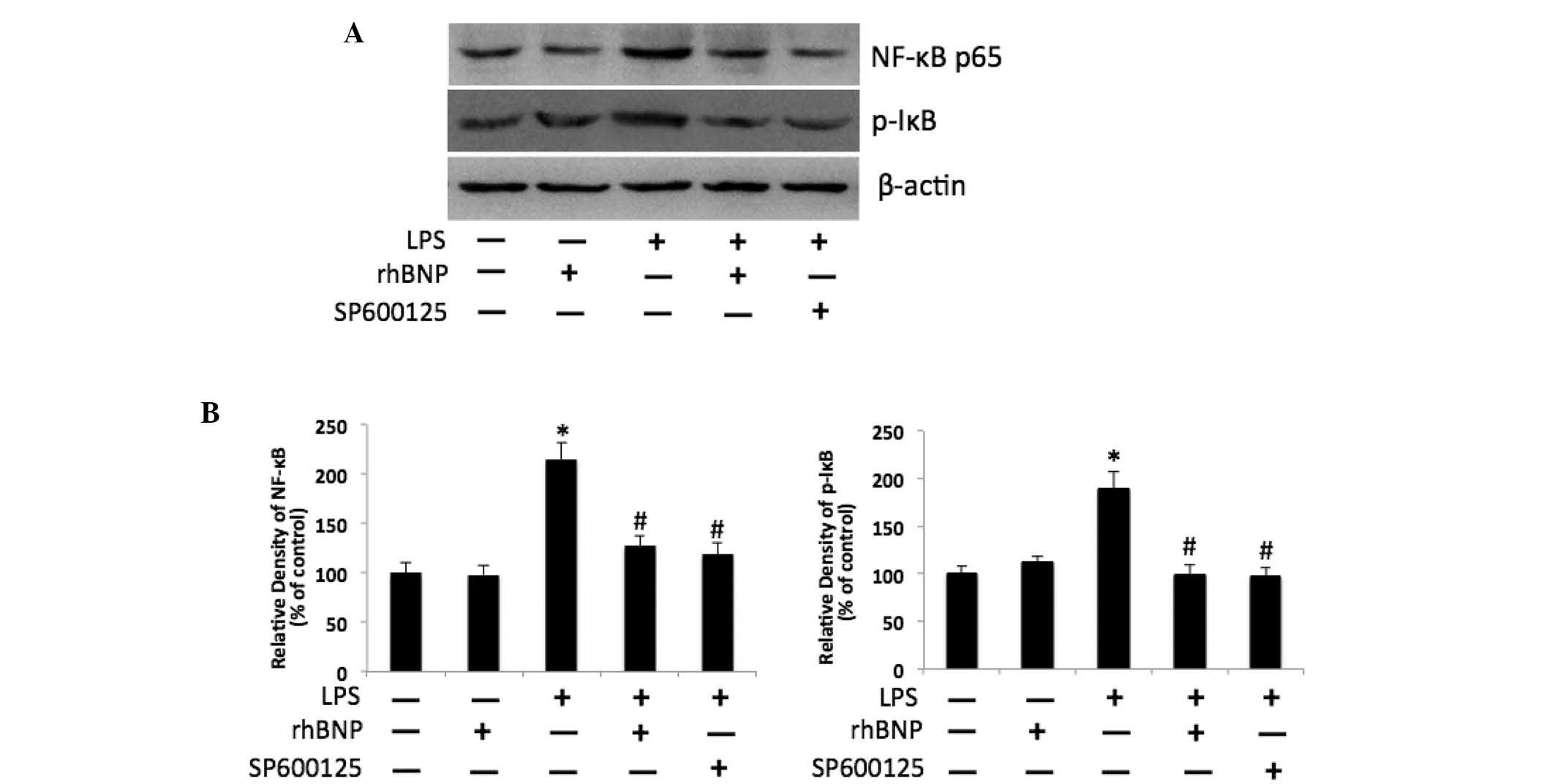
rhBNP suppresses LPS-induced NF-κB
activation via inhibition of JNK phosphorylation
The activity of the molecules in the NF-κB signaling
pathway was determined by measuring the levels of the NF-κB p65 and
p-IκB proteins in the cellular extract of HFL-1 cells. The
expression of NF-κB p65 and p-IκB were increased significantly in
the LPS group (P<0.05 vs. control); treatment with rhBNP
significantly inhibited the LPS-induced increase of NF-κB p65 and
p-IκB (P<0.05 vs. LPS); furthermore, pretreatment with the JNK
inhibitor SP600125 attenuated the increase of NF-κB p65 and p-IκB
(P<0.05 vs. LPS) (Fig. 4).
Discussion
Our previous studies have demonstrated that rhBNP
prevents LPS-induced acute lung injury in a dog model and may be
associated with adjusting the levels of endogenous antioxidant
enzymes (14). However, the
effects of rhBNP on HFL-1 cells in terms of inflammation have not
yet been described. rhBNP is known to exert its anti-inflammatory
activity in various organs (18–21).
Therefore, further investigation of the mechanism by which rhBNP
influences LPS-induced human lung fibroblasts has important
clinical implications. Consistently, the results of the present
study indicate that rhBNP may have anti-inflammatory effects on
LPS-induced HFL-1 cells via inhibiting MAPK and NF-κB pathways.
It is generally known that fibroblasts are important
in inflammation resolution following cell injury (22). Lung fibroblasts are important
responsive and regulatory components of lung inflammation induced
by LPS and have also been shown to generate inflammatory cytokines,
such as IL-1β, IL-8, and tumor necrosis factor-α, which have been
demonstrated to mediate lung injury (23,24).
Moreover, IL-1β is an important mediator of inflammation and
activates the NF-κB pathway in cells (25). LDH is normally retained within
cells; however, once cells are damaged LDH is released into the
medium. Thus, LDH release is an indicator of the integrity of the
cell membrane. This study demonstrated that treatment with LPS
resulted in a significant increase in the levels of LDH in the
culture medium. The current study aimed to demonstrate the
therapeutic effect of rhBNP on LPS-induced cell injury in
vitro. The results indicated that rhBNP significantly
ameliorated the LDH release after LPS treatment, showing its
protective effects on cell injury. The effect of rhBNP on the
secretion of the key inflammatory cytokine IL-1β was then detected.
The results showed that the increased protein and mRNA expression
of IL-1β induced by LPS was significantly reduced following
treatment with rhBNP.
BNP is a type of neuroendocrine hormone and can
dilate blood vessels selectively, and aid sodium and urine
excretion. RhBNP is a freeze-dried peptide produced by genetic
recombination. The amino acid sequence of rhBNP is the same as
endogenous BNP obtained from humans (26). rhBNP can be combined with
natriuretic peptide receptor embedded in effector cell membrane and
activate the combination of sweet bird cyclase which is connected
to it. Furthermore, it enhances the levels of cyclic guanosine
monophosphate (cGMP) in effector cells. The elevated cGMP acts on
protein kinase G on the capillary endothelial cell membrane, which
results in dephosphorylation of myosin light chains, vascular
smooth muscle contraction and relaxation and extended vessels
(27). Therefore, our recent
studies indicated that rhBNP exhibited anti-inflammatory effects on
the kidney and intestinal tissues in animal models of
endotoxin-induced injuries (13,15).
MAPK is vital in cell growth, differentiation,
proliferation and apoptosis as an essential component of signal
transduction (28). Certain
studies have indicated that members of the MAPK signaling pathway,
including ERK, p38 MAPK and JNK, are important in signal
transduction pathways activated by stimuli and mediate a number of
physiological and pathological changes in cell function (29). The MAPK pathway is also the signal
cascade pathway of pro-inflammatory molecules and regulates the
inflammatory response through NF-κB activation (30,31).
In order to determine the effect of rhBNP in lung fibroblasts under
LPS stimulation, the NF-κB pathway was investigated as it is
involved in numerous cellular processes, particularly inflammation
(32,33). Moreover, activation of NF-κB by
cytokines, such as IL-1β, can promote inflammatory mediator
production (32). Our previous
study and certain recent studies have shown that inhibition of the
NF-κB pathway ameliorated organ injury in animal models, which
probably occurred via the inactivation of NF-κB p65 and further
inhibition of inflammation (13,34,35).
In the present study, activation of p38, ERK1/2 and JNK was
detected in HFL-1 cells following treatment with LPS, and rhBNP
treatment was shown to ameliorate this effect. In addition, the JNK
inhibitor decreased IL-1β secretion in LPS-induced HFL-1 cells.
Thus, these results indicate that the inhibition of the MAPK
signaling pathway is the mechanism underlying the inhibition of the
secretion of the inflammatory cytokines following treatment with
rhBNP.
NF-κB is primarily found in the cytoplasm in an
inactive non-DNA-binding form that is associated with the inhibitor
protein IκB in unstimulated cells. Following simulation, NF-κB
translocates into the nucleus and regulates the transcription of
genes, including those coding for the inflammatory molecules
(36). Previous studies have shown
that sustained NF-κB activation is correlated with lung injury, and
rhBNP was also involved in inhibiting inflammatory processes
(17,37). The results of the present study
indicated that LPS-induced IL-1β elevation was significantly
inhibited by the NF-κB specific inhibitor PDTC, which demonstrated
that an increase in LPS-induced IL-1β may depend on the NF-κB
signaling pathway. Furthermore, NF-κB was significantly activated
in LPS-induced HFL-1 cells as shown by increased expression of
NF-κB p65 and p-IκB. However, rhBNP treatment significantly
inhibited the activation of NF-κB that was induced by LPS. These
results showed that the inhibitory effect of rhBNP on the
LPS-induced IL-1β elevation was also dependent on the NF-κB
activation. Furthermore, the JNK inhibitor attenuated the NF-κB
activation that was induced by LPS, which suggested that
LPS-induced NF-κB activation may be dependent on JNK
activation.
In conclusion, the results of this study
demonstrated that rhBNP could inhibit HFL-1 cell injury induced by
LPS by inhibiting the MAPK and NF-κB signaling pathways. These
findings indicate that rhBNP protects HFL-1 cell injury in response
to endotoxin insult, and that rhBNP may be used not only as a
diagnostic or prognostic biomarker in critical care units, but also
as a novel therapeutic agent to ameliorate lung injury.
Acknowledgments
This study was supported by the Postdoctoral Science
Foundation, China (grant no. 2014M552693), and Science and
Technology Project of Liaoning, China (grant no. 2013225089)
awarded to Dr Zhi Song.
References
|
1
|
No authors listed. Ventilation with lower
tidal volumes as compared with traditional tidal volumes for acute
lung injury and the acute respiratory distress syndrome. The Acute
Respiratory Distress Syndrome Network. N Engl J Mes. 342:1301–1308.
2000. View Article : Google Scholar
|
|
2
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: Four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buckley CD, Pilling D, Lord JM, Akbar AN,
Scheel-Toellner D and Salmon M: Fibroblasts regulate the switch
from acute resolving to chronic persistent inflammation. Trends
Immunol. 22:199–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horowitz JC, Cui Z, Moore TA, Meier TR,
Reddy RC, Toews GB, Standiford TJ and Thannickal VJ: Constitutive
activation of prosurvival signaling in alveolar mesenchymal cells
isolated from patients with nonresolving acute respiratory distress
syndrome. Am J Physiol Lung Cell Mol Physiol. 290:L415–L425. 2006.
View Article : Google Scholar
|
|
5
|
Marshall RP, Bellingan G, Webb S,
Puddicombe A, Goldsack N, McAnulty RJ and Laurent GJ:
Fibroproliferation occurs early in the acute respiratory distress
syndrome and impacts on outcome. Am J Respir Crit Care Med.
162:1783–1788. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maekawa K, Sudoh T, Furusawa M, Minamino
N, Kangawa K, Ohkubo H, Nakanishi S and Matsuo H: Cloning and
sequence analysis of cDNA encoding a precursor for porcine brain
natri-uretic peptide. Biochem Biophys Res Commun. 157:410–416.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mueller C, Scholer A, Laule-Kilian K,
Martina B, Schindler C, Buser P, Pfisterer M and Perruchoud AP: Use
of B-type natriuretic peptide in the evaluation and management of
acute dyspnea. N Engl J Med. 350:647–654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lehr HA, Guhlmann A, Nolte D, Keppler D
and Messmer K: Leukotrienes as mediators in ischemia-reperfusion
injury in a microcirculation model in the hamster. J Clin Invest.
87:2036–2041. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burger MR and Burger AJ: BNP in
decompensated heart failure: Diagnostic, prognostic and therapeutic
potential. Curr Opin Investig Drugs. 2:929–935. 2001.
|
|
10
|
Yamanouchi S, Kudo D, Endo T, Kitano Y and
Shinozawa Y: Blood N-terminal proBNP as a potential indicator of
cardiac preload in patients with high volume load. Tohoku J Exp
Med. 221:175–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Wu Y, Tang L, Zhu W, Chen F, Xu T,
Bo L, Li J and Deng X: Brain natriuretic peptide for prediction of
mortality in patients with sepsis: A systematic review and
meta-analysis. Crit Care. 16:R742012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li N, Zhang Y, Fan S, Xing J and Liu H:
BNP and NT-proBNP levels in patients with sepsis. Front Biosci
(Landmark Ed). 18:1237–1243. 2013. View
Article : Google Scholar
|
|
13
|
Yang H, Song Z, Jin H, Cui Y, Hou M and
Gao Y: Protective effect of rhBNP on intestinal injury in the
canine models of sepsis. Int Immunopharmacol. 19:262–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song Z, Cui Y, Ding MZ, Jin HX and Gao Y:
Protective effects of recombinant human brain natriuretic peptide
against LPS-Induced acute lung injury in dogs. Int Immunopharmacol.
17:508–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li N, Jin HX, Song Z, Bai CZ, Cui Y and
Gao Y: Protective effect of recombinant human brain natriuretic
peptide on acute renal injury induced by endotoxin in canines. Cell
Biochem Biophys. 70:1317–1324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Xu ZF and Deng Y: Effect of
manganese exposure on intracellular Ca2+ homeostasis and
expression of NMDA receptor subunits in primary cultured neurons.
Neurotoxicology. 30:941–949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Chiurchiù V, Izzi V, D'Aquilio F,
Carotenuto F, Di Nardo P and Baldini PM: Brain natriuretic peptide
(BNP) regulates the production of inflammatory mediators in human
THP-1 macrophages. Regul Pept. 148:26–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
James ML, Wang H, Venkatraman T, Song P,
Lascola CD and Laskowitz DT: Brain natriuretic peptide improves
long-term functional recovery after acute CNS injury in mice. J
Neurotrauma. 27:217–228. 2010. View Article : Google Scholar
|
|
20
|
Kiemer AK and Vollmar AM: The atrial
natriuretic peptide regulates the production of inflammatory
mediators in macrophages. Ann Rheum Dis. 60(Suppl 3): iii68–iii70.
2001.
|
|
21
|
Moro C, Klimcakova E, Lolmède K, Berlan M,
Lafontan M, Stich V, Bouloumié A, Galitzky J, Arner P and Langin D:
Atrial natriuretic peptide inhibits the production of adipokines
and cytokines linked to inflammation and insulin resistance in
human subcutaneous adipose tissue. Diabetologia. 50:1038–1047.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Serhan CN, Brain SD, Buckley CD, Gilroy
DW, Haslett C, O'Neill LA, Perretti M, Rossi AG and Wallace JL:
Resolution of inflammation: State of the art, definitions and
terms. FASEB J. 21:325–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Zhou X and Rong L: Analysis of
mechanical ventilation and lipopolysaccharide-induced acute lung
injury using DNA microarray analysis. Mol Med Rep. 11:4239–4245.
2015.PubMed/NCBI
|
|
24
|
Lu Z, Ma Y, Zhang S, Liu F, Wan M and Luo
J: Transforming growth factor-beta1 small interfering RNA inhibits
growth of human embryonic lung fibroblast HFL-I cells in vitro and
defends against radiation-induced lung injury in vivo. Mol Med Rep.
11:2055–2061. 2015.
|
|
25
|
Wang Z and Tai HH: Interleukin-1 beta and
dexamethasone regulate gene expression of prostaglandin H
synthase-2 via the NF-κB pathway in human amnion derived WISH
cells. Prostaglandins Leukot Essent Fatty Acids. 59:63–69. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen HH, Grantham JA, Schirger JA,
Jougasaki M, Redfield MM and Burnett JC Jr: Subcutaneous
administration of brain natriuretic peptide in experimental heart
failure. J Am Coll Cardiol. 36:1706–1712. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burger AJ: A review of the renal and
neurohormonal effects of B-type natriuretic peptide. Congest Heart
Fail. 11:30–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Craig EA, Stevens MV, Vaillancourt RR and
Camenisch TD: MAP3Ks as central regulators of cell fate during
development. Dev Dyn. 237:3102–3114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song HY, Lee JA, Ju SM, Yoo KY, Won MH,
Kwon HJ, Eum WS, Jang SH, Choi SY and Park J: Topical transduction
of superoxide dismutase mediated by HIV-1 Tat protein transduction
domain ameliorates 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced inflammation in mice. Biochem Pharmacol.
75:1348–1357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Q, Huang Y, Yang Y and Qiu H:
Acid-induced cell injury and death in lung epithelial cells is
associated with the activation of mitogen-activated protein
kinases. Mol Med Rep. 8:565–570. 2013.PubMed/NCBI
|
|
31
|
Yoon WJ, Moon JY, Song G, Lee YK, Han MS,
Lee JS, Ihm BS, Lee WJ, Lee NH and Hyun CG: Artemisia fukudo
essential oil attenuates LPS-induced inflammation by suppressing
NF-kappaB and MAPK activation in RAW 264.7 macrophages. Food Chem
Toxicol. 48:1222–1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Beinke S and Ley SC: Functions of
NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J.
382:393–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beinke S, Robinson MJ, Hugunin M and Ley
SC: Lipopolysaccharide activation of the TPL-2/MEK/extracellular
signal-regulated kinase mitogen-activated protein kinase cascade is
regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105.
Mol Cell Biol. 24:9658–9667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Zhang X, Tan M, Zheng R and Zhao
L: The effect of NF-κB antisense oligonucleotide on
transdifferentiation of fibroblast in lung tissue of mice injured
by bleomycin. Mol Biol Rep. 41:4043–4051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu T, Zhang W, Xiao M, Chen H and Jin H:
Protective role of andrographolide in bleomycin-induced pulmonary
fibrosis in mice. Int J Mol Sci. 14:23581–23596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dogra C, Changotra H, Mohan S and Kumar A:
Tumor necrosis factor-like weak inducer of apoptosis inhibits
skeletal myogenesis through sustained activation of nuclear
factor-kappaB and degradation of MyoD protein. J Biol Chem.
281:10327–10336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Everhart MB, Han W, Sherrill TP, Arutiunov
M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE and
Blackwell TS: Duration and intensity of NF-kappaB activity
determine the severity of endotoxin-induced acute lung injury. J
Immunol. 176:4995–5005. 2006. View Article : Google Scholar : PubMed/NCBI
|