Introduction
Lung cancer (LC) is the leading cause of
cancer-associated mortality worldwide. Despite continuous efforts
to improve therapeutic outcomes, the overall 5-year survival rate
remains <16% (1,2). The definite mechanism leading to the
metastasis and relapse of primary tumors remains to be elucidated;
however, the cancer stem cell (CSC) hypothesis states that a
subpopulation of tumor cells with distinct stem-like properties is
responsible for tumor initiation, differentiation, possibly distant
metastasis and resistance to conventional chemoradiotherapy.
Members of this subpopulation are defined as CSCs or
tumor-initiating cells (TICs) (3).
The isolation and identification of CSCs is an
essential premise. Currently, CSCs are isolated primarily using
four methods: Detection of CSC-specific biomarkers by flow
cytometry, assessment of side population (SP) phenotypes by Hoechst
33342 excretion, floating sphere culturing in serum-free conditions
and determination of aldehyde dehydrogenase (ALDH) activity
(4). None of the methods mentioned
above are exclusively used to isolate CSCs. Previously, tumorsphere
models have been widely described and used in investigations of
cancer (5). Increasing evidence
has indicated that the serum-free sphere culture protocol is a
reasonable approach to enrich CSCs in vitro, including
neurospheres from glioblastoma (6,7).
Simultaneously, several studies have supported the
existence of CSCs in LC, and lung CSCs with enhanced tumorigenicity
have been reported as a subset of cells, which exclude Hoechst
33342 dye (SP phenotype), including drug-resistant,
CD133+, ALDHhigh and CD44+
(8). Although no universal marker
or set of markers has been fully established for lung CSCs, studies
have identified lung CSCs by the detection of CD133+, SP
phenotype (ABCG2+) and/or ALDHhigh (9,10).
CD133 is a transmembrane glycoprotein; this protein
was initially described as a specific marker of human hematopoietic
stem cells, and has been used as the primary marker of putative
CSCs (11). Various types of solid
malignant tumor that express CD133 have been reported to have
aggressive biological behavior, poor prognosis and high rates of
recurrence (12–17). Breast cancer resistance protein
(ABCG2), is a multidrug resistance efflux pump and maintains the SP
phenotype based on its excretion of Hoechst 33342. The
overexpression of ABCG2 is associated with resistance to a wide
range of anticancer agents (18,19).
ABCG2 has also been reported as a potential marker of TICs
(20). To date, CD133 and ABCG2
appear to be the most reproducible markers of lung CSCs.
The present study attempted to generate lung CSCs
using a sphere-forming assay, and to analyze the features of lung
tumorospheres to examine the correlation between the expression
levels of two primary candidate CSC markers, CD133 and ABCG2. The
experimental results suggested that the stem-like properties were
enriched in the lung tumorospheres, with high expression levels of
CD133 and ABCG2. Although the present study did not demonstrate
that all the sphere-derived cells were CSCs, the results suggested
that CD133+ and ABCG2+ cells may be a small
subpopulation, which contributes to the determination of stem-like
features in lung tumorospheres.
Materials and methods
Reagents
Mouse monoclonal antibody against ABCG2 BXP-21 (cat.
no. ab3380) was purchased from Abcam (Cambridge, MA, USA).
Allophycocyanin (APC)-conjugated anti-CD133/1 (cat. no.
130-090-826) antibody and rabbit anti-CD133 (cat. no. MAB4399)
antibody were obtained from Miltenyi Biotec (Bergisch Gladbach,
Germany) and EMD Millipore (Billerica, MA, USA), respectively.
Rabbit monoclonal antibody directed against β-actin (cat. no. 4970)
and horseradish-peroxidase-conjugated horse anti-rabbit and
anti-mouse (cat. nos. 7074 and 7076) IgG secondary antibodies were
purchased from Cell Signalling Technology, Inc. (Danvers, MA, USA).
Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG
antibody (cat. no. #E031210-01) was purchased from EarthOx, LLC
(San Francisco, CA, USA). Alexa Fluor® 568-conjugated
goat anti-rabbit IgG antibody (cat. no. #A-11011) was purchased
from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Reverse transcription (RT) and quantitative polymerase chain
reaction (qPCR) kits were purchased from Takara Biotechnology Co.,
Ltd. (Dalian, China). Cisplatin was obtained from Sigma-Aldrich
(St. Louis, MO, USA), and dissolved in a 0.15 M NaCl solution.
Aliquots were stored at −20°C for up to 3 months and thawed
immediately prior to use. All other chemicals and solvents were of
the highest analytical grade available.
Cell culture
The A549 human LC cell line was obtained from
American Type Culture Collection (Rockville, MD, USA) and cultured
in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin at
an atmosphere of 5% CO2 at 37°C. Cells in the
logarithmic phase of growth were used for all experiments.
LC tumorosphere culture, harvest and
differentiation
Suspended cells were collected from confluent A549
cells and centrifuged at 800 × g for 5 min at room temperature. The
pellets were gently dissociated with a pipette and resuspended in
serum-free Dulbecco's modified Eagle's medium (DMEM)-F12 medium
(Gibco; Thermo Fisher Scientific, Inc.). The cells were plated at a
density of 1×103 cells/ml in ultra-low attachment plates
(Corning Inc. Acton, MA, USA) supplemented with 20 ng/ml basic
fibroblast growth factor (bFGF; PeproTech, Inc., Rocky Hill, NJ,
USA), 20 ng/ml epidermal growth factor (PeproTech, Inc.) and 2% B27
(Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2
humidified incubator at 37°C. The medium was replaced every 2–3
days by carefully removing half the upper layer of medium and
replacing it with an equal volume of fresh medium. It has been
reported that the more serial passages in the spheres, the more
CSCs present (4). The spheres were
collected by gentle centrifugation at 600 × g for 6 min at room
temperature, and mechanically dissociated into single cell
suspensions until they were of a sufficient number (>200 cells)
for passaging. The third passage of LC spheres was used in the
present study to ensure the reliability of the results. The spheres
were filtered using a cell strainer, and those with a diameter
>40 μm were selected for use in the subsequent
experiments. To determine the serum-induced differentiation of
sphere-forming cells, spheroids were isolated and cultured in DMEM
supplemented with 10% FBS without growth factors at 37°C in a 5%
CO2 humidified incubator.
RT-PCR and qPCR analyses
The total RNA of the A549 attached cells and
corresponding spheres were extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). First-strand cDNA was
synthesized from 1 μg total RNA using M-MuLV Reverse
Transcriptase (Applied Biosystems, Foster City, CA, USA) to a final
volume of 20 μl. For RT-PCR, samples were assayed in a 12.5
μl reaction mixture containing 2.5 μl cDNA, 1.25
μl of 10× Ex Taq Buffer, 1 μl dNTP mixture, 1
μl forward primer, 1 μl reverse primer, 0.05
μl TaKaRa Ex Tag HS and 5.7 μl RNase Free
dH2O. The RT reaction was performed using a DNA Engine
Peltier Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The semi-quantitative PCR conditions were as follows: Initial
denaturation at 95°C for 2 min, followed by 32 cycles of
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, and
extension at 72°C for 30 sec, with extension at 72°C for 3 min in
the final cycle. For qPCR, samples were assayed in a 10 μl
reaction mixture containing 5 μl of 2× One Step SYBR RT-PCR
Buffer, 0.45 μl of PrimeScript 1 Step Enzyme mix, 0.4
μl PCR forward primer (10 μM), 0.4 μl PCR
reverse primer (10 μM), 2 μl cDNA and 1.8 μl
RNase Free dH2O using the One-Step SYBR®
PrimeScript™ qPCR kit (Takara Biotechnology Co., Ltd.). The qPCR
was performed using an ABI 7500 Real-Time PCR system (Applied
Biosystems) for a total of 40 cycles with an annealing temperature
of 95°C for 5 sec and 60°C for 20 sec. The primer pairs encoding
products of 113, 69, 126, 281, 206 and 225 bp, respectively, were
as follows: CD133, forward 5′-GAGTCGGAAACTGGCAGATAGCA-3′ and
reverse 5′-ACGCCTTGTCCTTGGTAGTGTTG-3′; ABCG2, forward
5′-CACAAGGAAACACCAATGGCT-3′ and reverse
5′-ACAGCTCCTTCAGTAAATGCCTTC-3′; octamer-binding transcription
factor 4 (OCT4), forward 5′-ACATCAAAGCTCTGCAGAAAGAAC-3′ and reverse
5′-CTGAATACCTTCCCAAATAGAACCC-3′; Nanog, forward
5′-AGAAGGCCTCAGCACCTA-3′ and reverse 5′-GGCCTGATTGTTCCAGGATT-3′;
sex-determining region Y-box 2 (Sox2), forward
5′-AGCAACGGCAGCTACAGCA-3′ and reverse 5′-TGGGAGGAAGAGGTAACCACAG-3′
and GAPDH, forward 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse
5′-GAAGATGGTGATGGGATTTC-3′. All primers were synthesized by BGI
Tech Solutions Co., Ltd. (Beijing, China). The thermal cycling
conditions were as follows: 95°C for 30 sec, followed by 5 sec at
95°C, and 1 min at 60°C for 40 cycles. Gene expression was
normalized to GAPDH, and the fold change was calculated using the
following formula: Fold change in gene expression =
2−(ΔΔdCq) (21).
Flow cytometric analysis of the
expression levels of CD133 and ABCG2
In total, 1.0×106 viable attached cells
and sphere-forming cells were collected. The cells were wasHed
twice in phosphate-buffered saline (PBS), following which they were
incubated with mouse monoclonal anti-ABCG2 (1:200 dilution) for 10
min at 4°C and then incubated with FITC-conjugated goat anti-mouse
IgG (1:200 dilution) for another 10 min at 4°C in the dark.
Subsequently, the cells were washed in PBS, resuspended, fixed in
100 μl 1% (m/v) paraformaldehyde and subjected to
fluorescence analysis using flow cytometry. Dead cells were
eliminated using 1 mg/ml propidium iodide (PI) staining
(Sigma-Aldrich). To detect the expression of CD133, the cells were
incubated with anti-CD133-APC for 15 min at 4°C in the dark, and
subsequent procedures were performed, according to the protocol
described above. The data were processed using CellQuest software
(BD Biosciences, San Jose, CA, USA).
Immunofluorescence staining
The A549 attached cells and sphere-forming cells
were seeded on glass coverslips in at a density of
5.0×103 cells/well six-well plates, and cultured in a 5%
CO2 incubator at 37°C overnight. The cells were fixed by
immersion in ice-cold methanol for 15 min. Following washing of the
coverslips twice in PBS and blocking for 30 min in blocking buffer
(10% normal goat serum and 0.5% Triton X-100 in PBS), the
coverslips were incubated with mouse anti-ABCG2 and rabbit
anti-CD133 primary antibodies at a 1:200 dilution for 12 h at 4°C.
The coverslips were then washed three times in PBS for 5 min each
and incubated with FITC-conjugated goat anti-mouse IgG or Alexa
Fluor® 568-conjugated goat anti-rabbit IgG secondary
antibody at a 1:200 dilution for 30 min at 37°C. Subsequently, the
cells were counterstained with 4′,6-diamidino-2-phenylindole and
visualized using an inverted fluorescence microscope (Leica,
Mannheim, Germany).
Cell viability analysis
The A549 adherent cells and sphere-derived cells
were plated in triplicate at a density of 4,000 cells/well in
standard coated 96-well plates and allowed to adhere overnight at
37°C in a 5% CO2 humidified incubator. Wells containing
medium only were used as a background negative control. Cell
proliferation was measured during the following 4 days using a Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan),
according to the manufacturer's protocol. The spectrometric
absorbance was measured at 450 nm using a microplate reader. The
growth curves were constructed according to the mean value of
absorbance relative to the background. To examine drug sensitivity,
the cells were first seeded in a 96-well plate at a density of
5,000 cells/well and cultured in medium containing increasing
concentrations of cisplatin (0–25.6 μg/ml) for 48 h at 37°C
in a 5% CO2 humidified incubator. At the end of the drug
exposure duration, cell viability was measured using the CCK-8
assay, and the growth inhibition rate and the half maximal
inhibitory concentration (IC50) values were calculated
using GraphPad Prism 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA).
Cell invasion analysis
Cell invasion assays were performed using 24-well
Transwell plates (8 μm pore size; Corning, Inc.) coated with
1 mg/ml Matrigel (BD Biosciences). In total, 1.0×105
cells/well were suspended in 300 μl 0.1% bovine serum
albumin (Invitrogen; Thermo Fisher Scientific, Inc.)/serum-free
medium and added to the upper compartment of the Transwell plates.
Subsequently, 500 μl complete medium containing 10% FBS was
added to the lower wells of each plate, and the cells were
incubated in a 5% CO2 incubator for 24 h at 37°C. After
24 h, the upper surface of the chambers were scraped using cotton
swabs, and the cells on the lower surface of the membrane were
considered invasive cells. These invasive cells were fixed for 15
min in methanol, stained with Giemsa solution and examined using a
TE2000 inverted micro scope (Nikon, Tokyo, Japan).
Cell cycle analysis
For cell cycle analysis, the adherent A549 cells,
spheres and sphere-forming cells following serum-induced culture
were harvested by trypsinization and fixed with ice-cold 70%
ethanol overnight at 4°C. The cells were then washed twice in PBS,
resuspended in PBS and stained with PI/RNase staining buffer (BD
Biosciences) at 37°C for 30 min. The cell cycle profiles were
obtained using flow cytometry at 488 nm, and the data were analyzed
using CellQuest software (BD Biosciences).
Western blot analysis
Cell lysates of A549 attached cells and spheres were
prepared according to manufacturer's details of the protein extract
kit (KEYGEN Biotech, Beijing, China). Protein concentrations were
determined using the bicinchoninic acid method. Equal quantities of
protein (50 μg/lane of total extract) were separated by 12%
sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then
electrophoretically transferred onto PVDF membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked in 5% skim milk
powder dissolved in Tris-buffered saline with Tween 20 (TBST) for 2
h at room temperature, following which they were incubated with the
following primary antibodies at 4°C overnight: ABCG2, CD133 and
β-actin. The membranes were then washed three times in TBST buffer,
and were subsequently incubated with secondary antibody for 1.5 h
at room temperature. The bands were visualized using a molecular
imaging system. Densitometric analysis was performed using
Image-ProPlus software (Media Cybernetics, Inc., Rockville MD,
USA), and β-actin was used as a control.
Tumorigenicity experiments
Male (n=20) and female (n=200 athymic BALB/c nude
mice (4–6 weeks old) with body weights of 18–22 g were provided by
the Experimental Animal Centre of Chongqing Medical University
(Chongqing, China). All mice were housed in microinsolator cages in
a specific homothermic pathogen-free environment with a 12-h
light/dark cycle and access to food and water ad libitum. To
generate subcutaneous xenograft models, different numbers
(1×106, 2×105, 1×105,
4×104 and 1×104) of freshly dissociated
attached cells and sphere-derived cells were mixed with 100
μl PBS and subcutaneously inoculated into the left and right
flank of each BALB/c nude mouse to detect their tumorigenic
capacity. The tumorigenicity was measured primarily by tumor size,
tumor weight and latency, the latter determined as the duration
between injection and the detection of palpable tumors. The primary
tumor sizes were measured with a calliper on a weekly basis, and
approximate tumor weights were determined using the following
formula: V = 1/2 × a × b2, where b is the smaller of the
two perpendicular diameters and V is tumor volume. When the average
tumor size reached 1,000–2,000 mm3, the animals were
sacrificed by cervical dislocation. The grafts were then removed
and fixed in formalin, and paraffin sections were prepared for
hematoxylin and eosin (H&E) staining and analyzed under a light
microscope. All of the in vivo experimental procedures were
approved by the Committee on the Ethical Use of Animals of the
First Affiliated Hospital of Chongqing Medical University.
Statistical analysis
All experiments were performed in triplicate. The
data are graphically represented as the mean ± standard error of
the mean. The values were compared with controls using either
Student's t-test or two-way analysis of variance using Prism
GraphPad 5.0 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
LC cells form floating spheres
The A549 attached cells were cultured in serum-free
medium, as described above. Under ultra-low attached conditions,
the cells grew into floating, tridimensional clusters, termed
spheres. The spheres began to form on day 3 or 4, and formation was
more substantial on day 7. Between days 9 and 12, the spheres had
completely formed. By days 15–16, the spheres had become
well-rounded structures, which were composed of abundant, cohesive
cells. Fig. 1A shows the
generation of a sphere from a single LC A549 cell. After 48 h of
serum-driven culture, floating spheres were found to have adhered
and migrated to the wall of the culture flasks, and gradually
differentiated into adherent cells.
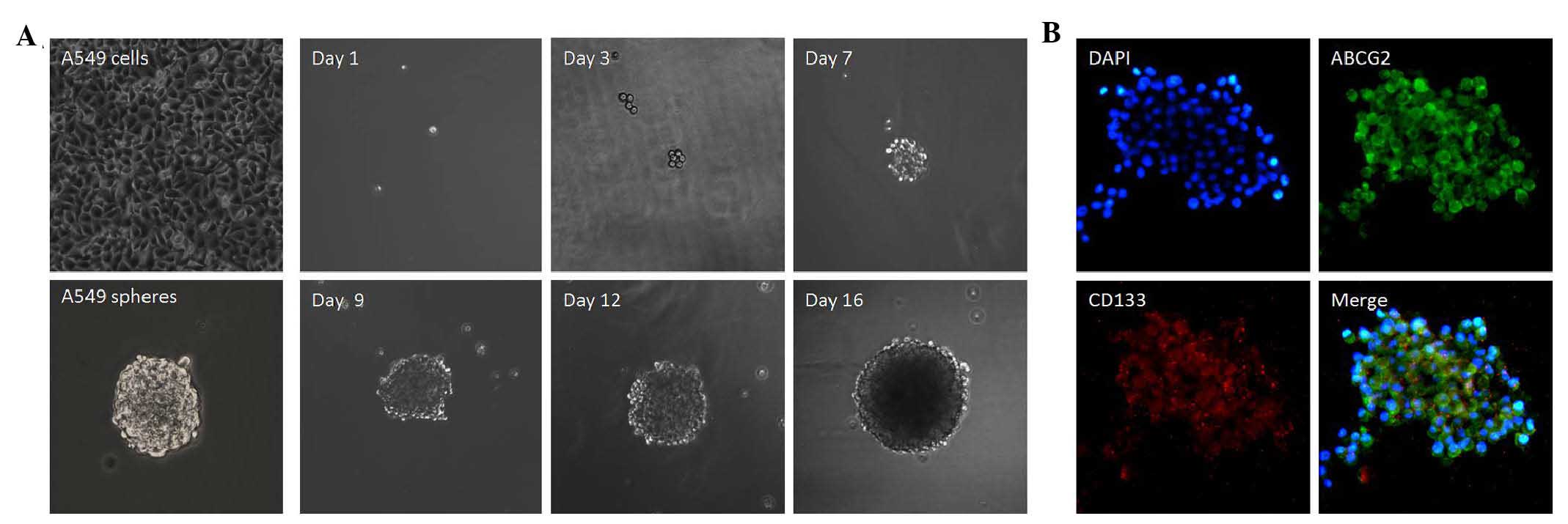 | Figure 1Phase images of sphere-forming assay
in A549 cells. (A) Growth of a single cell was recorded separately
on days 1, 3, 7, 9, 12 and 16 (original magnification, ×100). (B)
Intracellular localization of CD133 and ABCG2. Immunostaining
showed upregulation of CD133 and ABCG2 in the lung cancer spheres
(original magnification, ×400). Chromatin was stained with DAPI
(blue), CD133, with Alexa Fluor® 568 (red) and ABCG2
with fluorescein isothiocyanate (green). The images shown are
representative of three independent experiments. ABCG2, breast
cancer resistance protein; DAPI, 4′,6-diamidino-2-phenylindole. |
Intracellular localization of CD133 and
ABCG2 in LC tumorospheres
To examine the subcellular localization of CD133 and
ABCG2 in the sphere-forming cells, immunofluorescence staining of
CD133 and ABCG2 was performed. Positive staining was observed, with
CD133 and ABCG2 present primarily in the membranes of the spheres.
Dual staining for CD133 and ABCG2 indicated that the two candidate
CSC markers were colocalized in the spheres (Fig. 1B).
LC spheres overexpress the CD133 and
ABCG2 candidate CSC markers
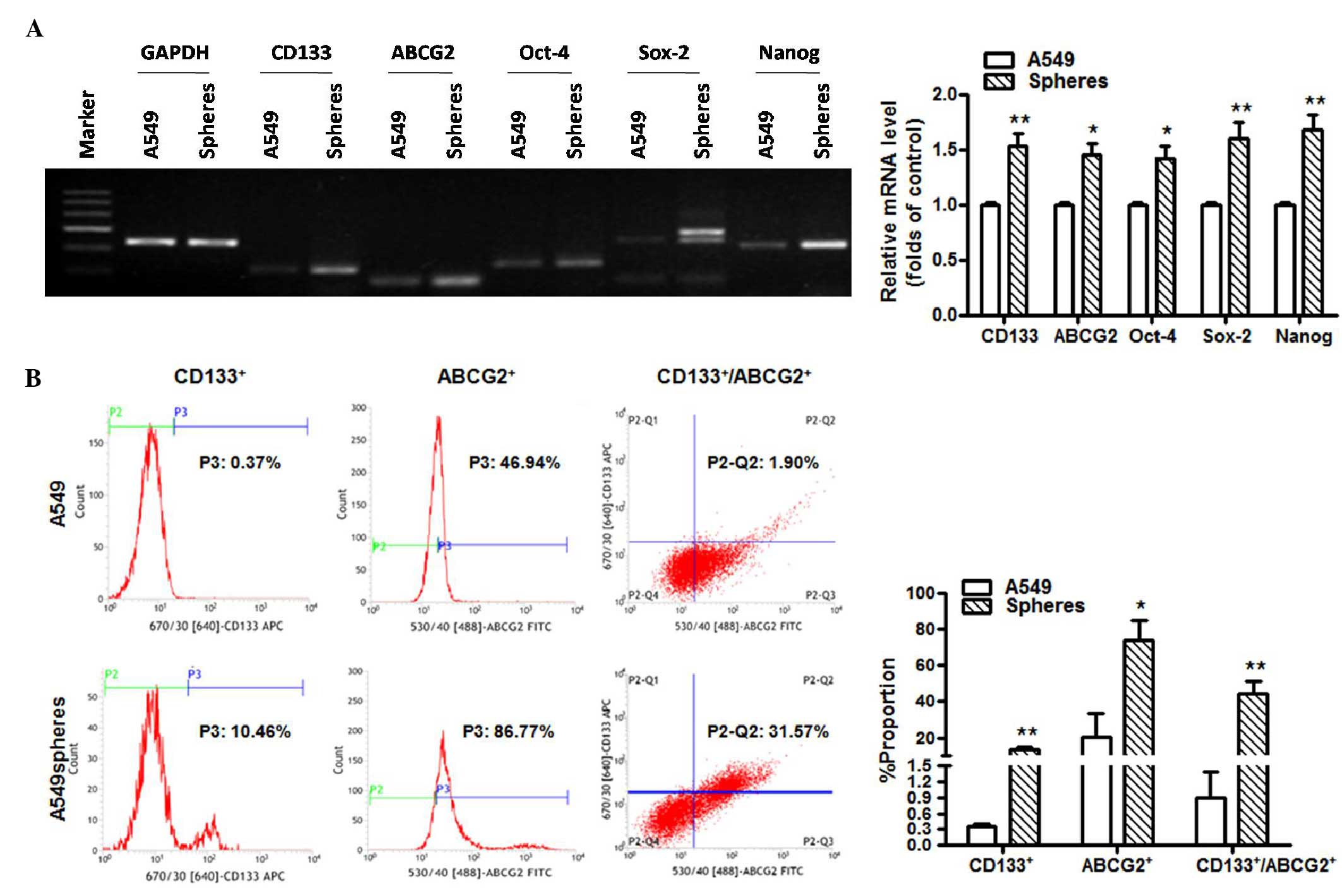
To clarify the differential gene expression profiles
between the spheres and the adherent A549 cells, RT-PCR and qPCR
analyses were performed (Fig. 2A and
B). As expected, cancer cells cultured in serum-free medium
caused a shift in CSC markers, including marked upregulation of the
following CSC markers: CD133, ABCG2, Oct-4, Sox-2 and Nanog.
Compared with the attached cells, the flow cytometry data (Fig. 2C) showed significantly increased
expression levels of CD133 (0.35±0.06, vs. 13.37±1.66%) and ABCG2
(20.63±13.18, vs. 73.72±11.57%) on the spheres.
LC tumorospheres harbor cells with CSC
characteristics
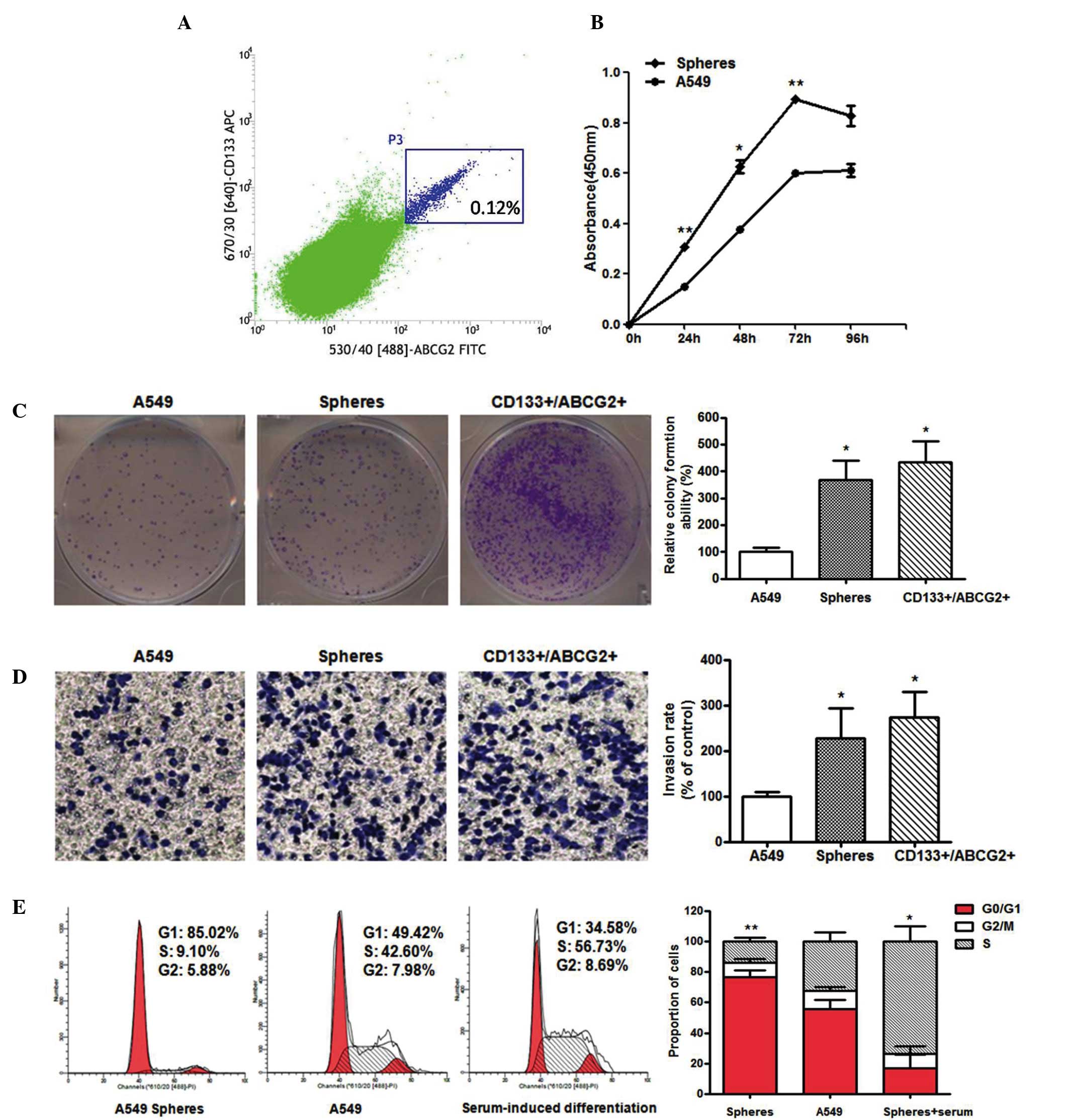
The results of the flow cytometric analysis revealed
the existence of distinct subpopulations with differential
expression of CD133 and ABCG2 in the tumorospheres; a small
subpopulation of CD133+/ABGC2+ cells was
identified in the A549 cells, whereas the primary cell population
was negative for the two CSC markers. To investigate possible
functional differences between these subpopulations, the
CD133+/ABCG2+ cells were subjected to FACS
(Fig. 3A) and assessed using
several functional assays. The CCK-8 assays revealed that the A549
spheres had markedly higher proliferation potential, as shown in
Fig. 3B. Consistently, the results
from the colony formation assay suggested that the
CD133+/ABCG2+ sphere-derived cells had higher
cloning efficiency (3–4-fold; 368.569±70.96, vs. 100±17.321)
leading to the formation of larger colonies at earlier time points,
with more cells having the ability to initiate a clone (Fig. 3C). In addition, the sphere-derived
cells appeared to penetrate the Matrigel more readily (Fig. 3D), and the number of stained
invasive cells was greater, compared with the attached cells
(227.293±66.781, vs. 100±8.66). Therefore, the LC tumorospheres
were found to possess augmented proliferation and invasion
abilities.
It is well known that there is a close association
between cell cycle and cell function, including the ability of
proliferation and differentiation, and chemosensitivity (22). In order to clarify the difference
of cell cycle distribution between the A549 adherent cells and
spheres, the cell cycle was analyzed using flow cytometry. The
results (Fig. 3E) revealed that
the A549 spheres formed in serum-free conditions exhibited the
following cell cycle distribution: 76.69±4.31% in the G0/G1 phase,
14.02±2.65% in The S phase and 9.29±2.70% in the G2/M phase.
However, the A549 attached cells exhibited the following cell cycle
distribution: 55.61±5.88% in the G0/G1 phase, 32.38±6.24% in the S
phase and 12.01±2.47% in the G2/M phase. Therefore, the cell cycle
of the spheres was arrested in the G0/G1 phase, with a
corresponding reduction in the numbers of cells in the S and G2/M
phases. However, in response to the serum stimulus, the spheres
acquired the following cell cycle phase distribution: 16.77±8.92%
in the G0/G1 phase, 73.64±10.06% in the S phase and 9.59±5.04% in
the G2/M phase. This distribution indicated that the A549 spheres
were steadily maintained in a quiescent state in serum-free
conditions, which may partly contribute to the refractoriness to
cancer therapy. However, the cell cycle progress was promoted with
a significantly increased ratio of sub S cells following treatment
with serum, suggesting that the spheres had the capacity for
unlimited self-renewal and extensive proliferation, and to generate
differentiated progeny.
LC tumorospheres exhibit increased
resistance to cisplatin
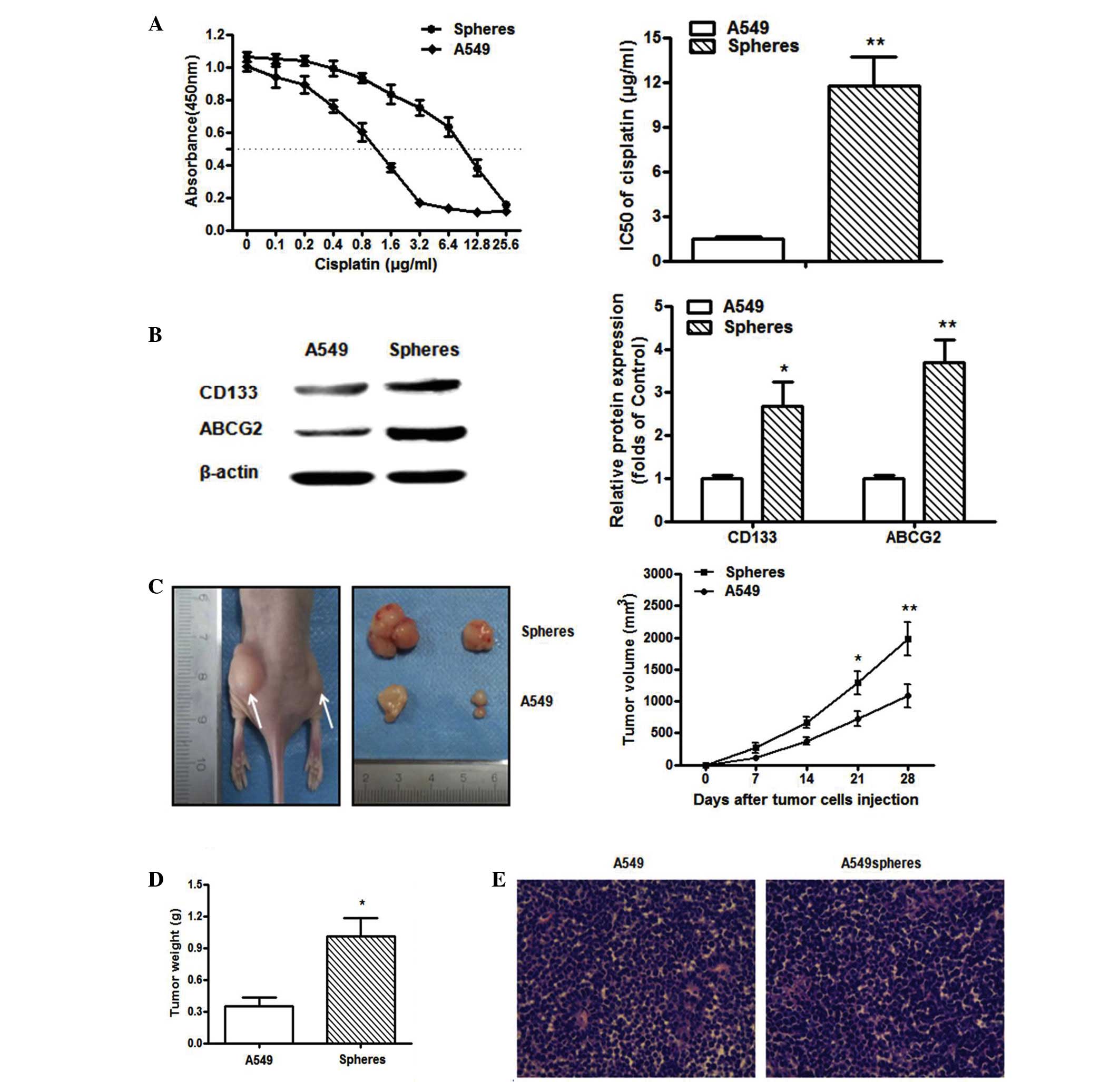
To examine whether LC tumorospheres possess the
hypothesized chemoresistant phenotype of CSCs, the present study
assessed the sensi tivity of the sphere-forming cells and
differentiated cells to cisplatin, which is commonly used in
chemotherapy. The LC sphere-forming cells exhibited an increased
IC50 value (11.752±1.937, vs. 1.485±0.121 μg/ml),
as shown in Fig. 4A. In addition,
treatment with 5 μg/ml cisplatin did not affect the number
or the size of the tumorospheres, suggesting enhanced
chemoresistance of the sphere-forming cells (data not shown). The
chemoresistance of tumor cells occurs, in part, due to the
overexpression of ATP-binding cassette family members, including
ABCG2, P-glycoprotein and multidrug resistance protein 1 (23,24).
CD133 has also been reported to be partially associated to
multidrug resistance. In the present study, the expression levels
of CD133 and ABCG2 were further confirmed at the protein level by
immunoblotting. The data indicated that the protein expression
levels of CD133 and ABCG2 were substantially increased in
tumorospheres, compared with adherent cells (Fig. 4B).
Tumorigenic potential of LC spheres
The cancer stem-like properties were further
confirmed in the LC spheres through in vivo tumorigenicity
experiments. The data showed that the A549 sphere-derived cells
generated subcutaneous tumors with larger volumes in a shorter
duration, compared with those from the attached cells. The tumor
growth curves were compared for the two groups (Fig. 4C and D). In addition, as few as
4×104 cells from the A549 sphere-forming cells were able
to form tumors at earlier time points when subcutaneously injected
into nude mice, whereas 2×106 attached cells were
required for the same effect; this value was 50-fold higher than
that for the sphere-forming cells. No differences in the overall
health or conditions of vital organs were observed between the mice
in the two groups (data not shown). The xenografts were confirmed
as lung adenocarcinoma using H&E staining (Fig. 4E), and the transplanted tumors
derived from the A549 spheroid-forming cells were histologically
similar to those from A549 adherent cells, predominantly containing
differentiated cells. These data suggested that the LC
sphere-derived cells showed an increased capacity for xenograft
tumor formation in vivo.
Discussion
The CSC theory has received substantial attention in
previous years. Determining whether this theory is applicable to LC
may shed light on the initiation and metastasis formation of LC,
and assist in developing specific therapies to target them, as
current therapeutic strategies are developed to target the bulk of
cancer cells and not the rarer CSCs. The first challenge lies in
the specific isolation and identification of CSCs from LC.
The culture of CSCs as floating spheres was first
described in brain tumors by Singh et al (25). In the following years,
tumorospheres were continually developed from a wide range of solid
tumors, including breast (26),
ovarian (12,27), pancreatic (28), oesophageal (29), liver (30), colonic (31), stomach (32) and prostate (33) cancer. To varying degrees, all these
tumorospheres possess stem cell characteristics.
The present study provided a systematic evaluation
of sphere-forming cells derived from the A549 LC cell line. The
successful culture and isolation of lung tumorospheres in
serum-free and anchorage-independent conditions were performed
(Fig. 1A) and the stem-like
properties of the tumorospheres were further investigated.
The expression of stemness genes is often used to
identify CSCs (4). CD133, ABCG2
and other transcription factors, including OCT4, Sox2 and Nanog
were analyzed. They are essential for the maintenance of stem
cells, and the overexpression of these genes was observed in
sphere-derived cells compared with the attached cells. The
upregulation in the expression levels of the stemness genes in
sphere-forming cells was closely associated with the self-renewal
potential, clonality, chemoresistance, invasion and metastasis, and
the ability to form spheres and initiate xenografts. Of note, the
CD133+ sphere-derived cells colocalized with ABCG2,
which may represent a subpopulation of putative CSCs. Therefore
FACS was used in the present study to select
CD133+/ABCG2+ cells from the A549 spheres,
which were only a small proportion of the tumorospheres, but
exhibited higher cloning efficiency and more robust
proliferation.
Chemoresistance remains the major cause of cancer
associated-mortality and is also considered a hallmark of CSCs
(23). To clarify whether
sphere-derived cells possess chemoresistant properties, the present
study analyzed the dose-response curves for the response to
cisplatin of A549 adherent cells and sphere-derived cells. As
expected, the sphere-derived cells exhibited increased resistance
to treatment, with a higher percentage of survival, compared with
the control cells. These data supported the effect of
sphere-derived cells in chemoresistance, which may further reveal
why conventional chemotherapy treatment fails to eradicate cancer
cells, and leads to tumor metastasis and recurrence.
As chemotherapeutic drugs are generally more toxic
towards rapidly proliferating cells, CSCs may resist the toxicity
of cisplatin by remaining quiescent (4). To further examine whether their
resistance is dependent on quiescence or the expression of
drug-resistance proteins, including ABCG2, cell cycle and western
blot analyses were performed. It was observed that the cell cycle
of the A549 tumorospheres was arrested in the G0/G1 phase under
serum-free conditions to maintain the quiescent and
undifferentiated state. Following the withdrawal of growth factors
and the addition of 10% FBS to the medium, floating sphere-derived
cells were able to adhere and differentiate. The upregulation of
drug-resistance proteins was further confirmed by immunoblotting.
Therefore the overexpression of CD133 and ABCG2 at the
translational level may be closely associated with the
chemoresistance of A549 tumorospheres.
However, tumorospheres are not homogeneous
structures enriched with only undifferentiated cells; they also
contain more differentiated cells. Until now, no reports have
accurately determined how many cells within tumorospheres are
actually CSCs. The gold standard for evaluating the presence of
CSCs is the transplantation of a small number of cells into an
immunocompromised mouse and evaluating of the capacity of
tumorigenesis in vivo (34). In the present study, A549 attached
cells and a 50-fold lower number of cells from A549 spheres were
harvested and used to generate subcutaneous xenograft models. The
sphere-derived cells exhibited the capacity to generate xenografts
with larger volumes and faster growth rates, compared with the
attached cells. These results demonstrated that the sphere-forming
cells predominantly represented CSCs, which possessed potent
proliferation capacity and tumorigenicity.
In conclusion, the present study demonstrated that
non-adherent tumorospheres of an LC cell line cultured in a defined
serum-free medium exhibited the characteristics of multipotent stem
cells. The genetic composition of the sphere-derived cells, in
terms of the co-expression of CD133 and ABCG2, may represent the
determining factor for the stem-like features and is likely to be
valuable for future gene and stem cell therapy for LC. However,
further investigations are required to elucidate the underlying
mechanisms of lung CSCs in detail.
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
TICs
|
tumor initiating cells
|
|
SP
|
side population
|
|
LC
|
lung cancer
|
|
ABCG2/BCRP
|
breast cancer resistance protein
|
|
FITC
|
fluorescein isothiocyanate
|
|
FACS
|
fluorescence activated cell
sorting
|
|
PI
|
propidium iodide
|
|
H&E
|
hematoxylin and eosin
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81302018) and the Oncology
National Clinical Key Specialty Construction Project [The Medical
Letter of National Health and Family Planning Commission Office
(2013) 544].
References
|
1
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, La Noce M, Laino L, De Francesco F and Papaccio G:
Cancer stem cells in solid tumors: An overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar
|
|
5
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamuro S, Okamoto Y, Sano E, Ochiai Y,
Ogino A, Ohta T, Hara H, Ueda T, Nakayama T, Yoshino A and Katayama
Y: Characterization of glioma stem-like cells from human
glioblastomas. Int J Oncol. 47:91–96. 2015.PubMed/NCBI
|
|
7
|
Iacopino F, Angelucci C, Piacentini R,
Biamonte F, Mangiola A, Maira G, Grassi C and Sica G: Isolation of
cancer stem cells from three human glioblastoma cell lines:
Characterization of two selected clones. PLoS One. 9:e1051662014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zakaria N, Yusoff NM, Zakaria Z, Lim MN,
Baharuddin PJ, Fakiruddin KS and Yahaya B: Human non-small cell
lung cancer expresses putative cancer stem cell markers and
exhibits the transcriptomic profile of multipotent cells. BMC
Cancer. 15:842015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alamgeer M, Peacock CD, Matsui W, Ganju V
and Watkins DN: Cancer stem cells in lung cancer: Evidence and
controversies. Respirology. 18:757–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
11
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
Garbe Y, Alison MR, Corbeil D and Kunz-Schughart LA: CD133 as a
biomarker for putative cancer stem cells in solid tumors:
Limitations, problems and challenges. J Pathol. 229:355–378. 2013.
View Article : Google Scholar
|
|
12
|
Cioffi M, D'Alterio C, Camerlingo R,
Tirino V, Consales C, Riccio A, Ieranò C, Cecere SC, Losito NS,
Greggi S, et al: Identification of a distinct population of
CD133(+)CXCR4(+) cancer stem cells in ovarian cancer. Sci Rep.
5:103572015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kashihara H, Shimada M, Kurita N, Iwata T,
Sato H, Kozo Yoshikawa, Higashijima J, Chikakiyo M, Nishi M and
Matsumoto N: CD133 expression is correlated with poor prognosis in
colorectal cancer. Hepatogastroenterology. 61:1563–1567.
2014.PubMed/NCBI
|
|
14
|
Nomura A, Banerjee S, Chugh R, Dudeja V,
Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors,
induces epithelial-mesenchymal transition and increases metastasis
in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nosrati A, Naghshvar F and Khanari S:
Cancer stem cell markers CD44, CD133 in primary gastric
adenocarcinoma. Int J Mol Cell Med. 3:279–286. 2014.
|
|
16
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
Jiang G, Ge C, Xie H, Wan D, et al: CD133 positive hepatocellular
carcinoma cells possess high capacity for tumorigenicity. Int J
Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng BH, Liu AG, Gu WG, Deng L, Cheng XG,
Tong TJ and Zhang HZ: CD133+ subpopulation of the HT1080 human
fibrosarcoma cell line exhibits cancer stem-like characteristics.
Oncol Rep. 30:815–823. 2013.PubMed/NCBI
|
|
18
|
Nakanishi T and Ross DD: Breast cancer
resistance protein (BCRP/ABCG2): Its role in multidrug resistance
and regulation of its gene expression. Chin J Cancer. 31:73–99.
2012. View Article : Google Scholar
|
|
19
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: A perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sicchieri RD, da Silveira WA, Mandarano
LR, Gonçalves de Oliveira TM, Carrara HH, Muglia VF, de Andrade JM
and Tiezzi DG: ABCG2 is a potential marker of tumor-initiating
cells in breast cancer. Tumor Biol. 36:9233–9243. 2015. View Article : Google Scholar
|
|
21
|
Ballester M1, Castelló A, Ibáñez E,
Sánchez A and Folch JM: Real-time quantitative PCR-based system for
determining transgene copy number in transgenic animals.
Biotechniques. 37:610–613. 2004.PubMed/NCBI
|
|
22
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar
|
|
23
|
Abdullah LN and Chow EK: Mechanisms of
chemoresistance in cancer stem cells. Clin Transl Med. 2:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
26
|
Piscitelli E, Cocola C, Thaden FR,
Pelucchi P, Gray B, Bertalot G, Albertini A, Reinbold R and Zucchi
I: Culture and characterization of mammary cancer stem cells in
mammospheres. Methods Mol Biol. 1235:243–262. 2015. View Article : Google Scholar
|
|
27
|
House CD, Hernandez L and Annunziata CM:
In vitro enrichment of ovarian cancer tumor-initiating cells. J Vis
Exp. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fredebohm J, Boettcher M, Eisen C, Gaida
MM, Heller A, Keleg S, Tost J, Greulich-Bode KM, Hotz-Wagenblatt A,
Lathrop M, et al: Establishment and characterization of a highly
tumorigenic and cancer stem cell enriched pancreatic cancer cell
line as a well defined model system. PLoS One. 7:e485032012.
View Article : Google Scholar
|
|
29
|
Fujiwara D, Kato K, Nohara S, Iwanuma Y
and Kajiyama Y: The usefulness of three-dimensional cell culture in
induction of cancer stem cells from esophageal squamous cell
carcinoma cell lines. Biochem Biophys Res Commun. 434:773–778.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu L, Zhang W, Wang J and Liu R: Evidence
of CD90+CXCR4+ cells as circulating tumor stem cells in
hepatocellular carcinoma. Tumor Biol. 36:5353–5360. 2015.
View Article : Google Scholar
|
|
31
|
Saha A, Shree Padhi S, Roy S and Banerjee
B: HCT116 colonospheres shows elevated expression of hTERT and
β-catenin protein-a short report. J Stem Cells. 9:243–251.
2014.
|
|
32
|
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J,
Chen R and Zhou Y: Sphere-forming body-forming cells in the human
gastric cancer cell line MKN-45 possess cancer stem cell
properties. Int J Oncol. 42:453–459. 2013.
|
|
33
|
Castillo V, Valenzuela R, Huidobro C,
Contreras HR and Castellon EA: Functional characteristics of cancer
stem cells and their role in drug resistance of prostate cancer.
Int J Oncol. 45:985–994. 2014.PubMed/NCBI
|
|
34
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|