Introduction
Postoperative pain is a common clinical symptom
predominantly resulting from peripheral and central sensitization
due to the persistent excitement of nociceptors. Current methods
for analgesia lack efficacy; thus, research is required to
elucidate how to reduce and ultimately eliminate postoperative
pain. It has been demonstrated that intracellular signal
transduction pathways serve important roles in the induction of
peripheral and central sensitization. Nuclear factor κB (NF-κB) is
a ubiquitous transcription factor that regulates the expression of
numerous genes, resulting in mediation of the production of
cytokines, chemokines and iNOS, which serve crucial roles in the
development of inflammatory and neuropathic pain (1). Evidence suggests that persistent
activation of c-Jun N-terminal kinase (JNK) in spinal astrocytes
resulting from nerve injury and inflammation can induce central
sensitization. JNK signaling serves an important role in regulating
the pain threshold. The JNK cascade is a critical signaling pathway
for the initiation and the maintenance of neuropathic pain
(2).
Nociceptive responses to noxious stimuli are
initiated at the peripheral nociceptor terminals (3). Ion channels serve a vital role in the
onset and conduction of pain signals. Increased excitability of
peripheral nociceptive sensory fibers resulting from the action of
inflammatory mediators can alter the activity of ion channels,
subsequently inducing inflammatory pain (4). The KATP channels, which
consist of pore-forming (Kir6.1 or Kir6.2) and regulatory (SUR1 or
SUR2) subunits, couple the intracellular metabolic state to
membrane excitability. Recombinant KATP channels
consisting of Kir6.1 and SUR2B subunits predominantly exist in
vascular smooth muscle cells and endothelial cells (5). In addition, vascular dysfunction and
vascular endothelial cells serve a contributory role in mechanical
pain (6). It has been previously
reported that SUR2 may be used as a therapeutic target of pinacidil
(7). It has been demonstrated that
the KATP channels are involved in the metabolism after a
stress response, mediate analgesia and also participate in
neuroprotection under metabolic stress (8,9).
Thus, KATP is an important adaptive regulator for
autoprotection. Activation of KATP channels has been
implicated in mediating the antinociceptive effects subsequent to
ventricular, intrathecal or epidural injection of KATP
activators in various animal models (10,11).
However, the effect of activated peripheral KATP on
peripheral and central sensitization remains to be fully
elucidated.
In the present study, the KATP opener,
pinacidil, was used to precondition rats following skin/muscle
incision and retraction (SMIR) (12), in order to establish the effects of
direct activation of peripheral KATP on pain sensation.
The role of the NF-κB/JNK signaling pathway in postoperative
peripheral and central sensitization was investigated, and the
possible molecular targets for preoperative activation of
KATP for preventive analgesia were discussed.
Materials and methods
Animal grouping
Male Sprague-Dawley rats (n=30) weighing 200–250 g
were obtained from the Experimental Animal Center at Nantong
University (Nantong, China), and housed in temperature-controlled
rooms and received water and food, ad libitum. The current
study was approved by the Experimental Animal Protection and Ethics
Committee of Nantong University (Jiangsu, China).
The rats were randomly assigned to the following
five groups (six rats per group): Control group, incision (sham
surgery) group; incision plus retraction (SMIR) group; SMIR plus
pinacidil (pinacidil) group; and the SMIR plus pyrrolidine
dithiocarbamate (PDTC) group. The rats in the control group did not
receive any treatment. The rats in the sham surgery group had an
incision made through the skin and muscle. The rats in the SMIR
group underwent 1-h retraction subsequent to skin/muscle incision.
The rats in the pinacidil group received an intraperitoneal
injection with pinacidil (25 µg/kg; D9035-250MG;
Sigma-Aldrich, St. Louis, MO, USA) 30 min prior to the SMIR
procedure. The rats in the PDTC group received an intraperitoneal
injection of PDTC (100 mg/kg; P-8765; Sigma-Aldrich) 30 min prior
to the SMIR procedure.
Behavioral assessments
Prior to the initiation of the experiment, all rats
were adapted to the testing conditions for a minimum of 2 days. The
room temperature and humidity remained stable for all experiments.
To quantify mechanical allodynia, the mechanical withdrawal
threshold (MWT) was determined using von Frey filaments (range,
1.4–26 g; North Coast Medical, Inc., Morgan Hill, CA, USA)
(13). Briefly, each rat was
placed in a Plexiglas® box (Xiyangyang, Inc., Shenzhen,
China) with a wire mesh floor. Following habituation for 30 min to
this environment, the von Frey filament was pressed perpendicular
to the plantar surface of both hind paws and held for no more than
4 sec. A positive response was noted if the rats exhibited paw
withdrawal, flinches or licking. If there was no response
(negative), the next heavier filament was tested. Each trial was
repeated five times. At each 30-sec interval the 50% threshold was
determined by the 'up and down' method (13).
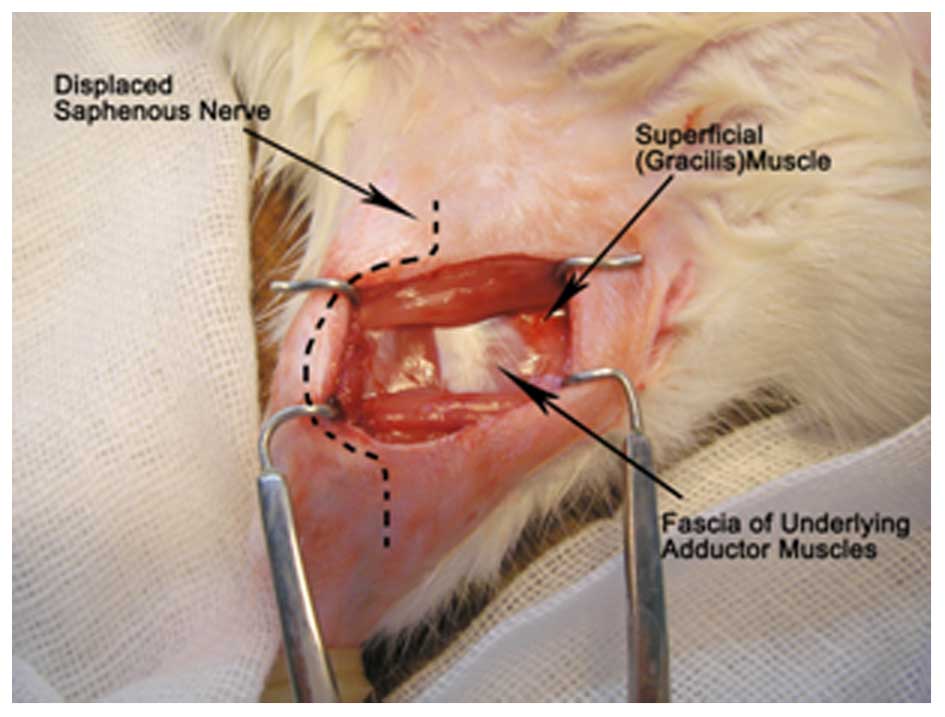
Establishment of the SMIR model
Rats were anesthetized by an intraperitoneal
injection of Nembutal (40 mg/kg; P3761; Sigma-Aldrich), and laid
supine under sterilized conditions. A 1.5–2-cm incision was made in
the medial side of the right hind limb approximately 4 mm medial to
the saphenous vein to reveal the muscle of the thigh. An incision
(7–10 mm long) was then made in the superficial muscle layer of the
thigh. The superficial muscle was then retracted 2 cm by spreading
blunt scissors within the muscle incision site. This retraction was
maintained for 1 h. During the retraction period, the incision site
was covered with gauze moistened with sterile saline to prevent
dehydration of the surgical site. Following the SMIR procedure, the
incision was covered with gauze coated with gentamycin (Yantai
Justaware Pharmaceutical Co., Ltd., Yantai, China) to avoid
infection. The establishment of the injury site during the 1 h
retraction period of the SMIR surgery is presented in Fig. 1 (14).
Western blotting analysis
A total of three days subsequent to SMIR surgery,
the rats in each group were anesthetized as described above;
peripheral muscle and L3-5 segments of the spinal cord were removed
and homogenized on ice in sodium dodecyl sulphate sample buffer (10
ml/mg tissue), containing a cocktail of proteinase and phosphatase
inhibitors (Sigma-Aldrich), using a hand-held pestle. The protease
inhibitor cocktail (cat no. P2714) contained AEBSF, E-64, bestatin,
leupeptin, aprotinin and sodium EDTA, and the phosphatase cocktail
(cat no. P5726; all Sigma-Aldrich) contained sodium orthovanadate,
sodium molybdate, sodium tartrate and imidazole. The cell lysates
were collected and transferred to a 1.5-ml centrifuge tube.
Subsequent to centrifugation at 10,000 × g for 18 min at 4°C, the
protein was extracted, boiled and denatured for 5 min and stored at
4°C. Subsequently, equal amounts (40 µg per lane) of total
protein from each sample were fractionated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis on 5 and 10% separating
gels (Beyotime Institute of Biotechnology, Jiangsu, China),
sequentially, and transferred to polyvinylidene difluoride
membranes (Merck Millipore, Shanghai, China). The required protein
volume per lane was calculated by dividing the total protein amount
loaded per lane by the protein concentrations. Thereafter, the
membranes were incubated overnight at 4°C with one of the following
primary monoclonal antibodies against NF-κB (rabbit; diluted
1:1,000; cat no. sc-372; Cell Signaling Technology), p-JNK [goat;
diluted 1:200; cat no. sc-12882; Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA)], Kir6.1 (rabbit; diluted 1:50; cat no. sc-20808;
SUR2 (rabbit; diluted 1:50; cat no. sc-25684) and GAPDH (mouse;
diluted 1:20,000; cat no. sc-365062), all Santa Cruz Biotechnology,
and then with the corresponding horseradish peroxidase-conjugated
secondary antibodies (goat anti-rabbit IgG, cat no. RPN4301; donkey
anti-goat IgG, cat no. RPN510; goat anti-mouse IgG, cat no. RPN998,
all diluted 1:3,000; GE Healthcare Life Sciences, Chalfont, UK) at
room temperature for 2 h. All antibodies used for western blotting
were purchased from Santa Cruz Biotechnology, Inc, unless otherwise
stated. Following three washes for 10 min with TBS-T (Beijing
Biosntech Biotechnology Co., Ltd., Beijing, China) at room
temperature, the intensity of the visualization signal was detected
using an enhanced chemiluminescence substrate kit (cat no. WP20005;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), and the protein
levels were quantified using Image J software (version 1.40;
National Institutes of Health, Bethesda, MD, USA). Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) served as an endogenous internal
reference gene. The relative expression of each target protein was
calculated as the ratio of the intensity of the target protein band
to that of GAPDH. Each determination was performed 3 times with
tissues from different rats.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA), and all data
were expressed as the mean ± standard deviation. Pairwise
differences between the two groups were compared by Student's
t-test and one-way analysis of variance to compare the differences
among a minimum of three groups. Graphs were drawn using Excel
(Microsoft Corporation, Redmond, WA, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
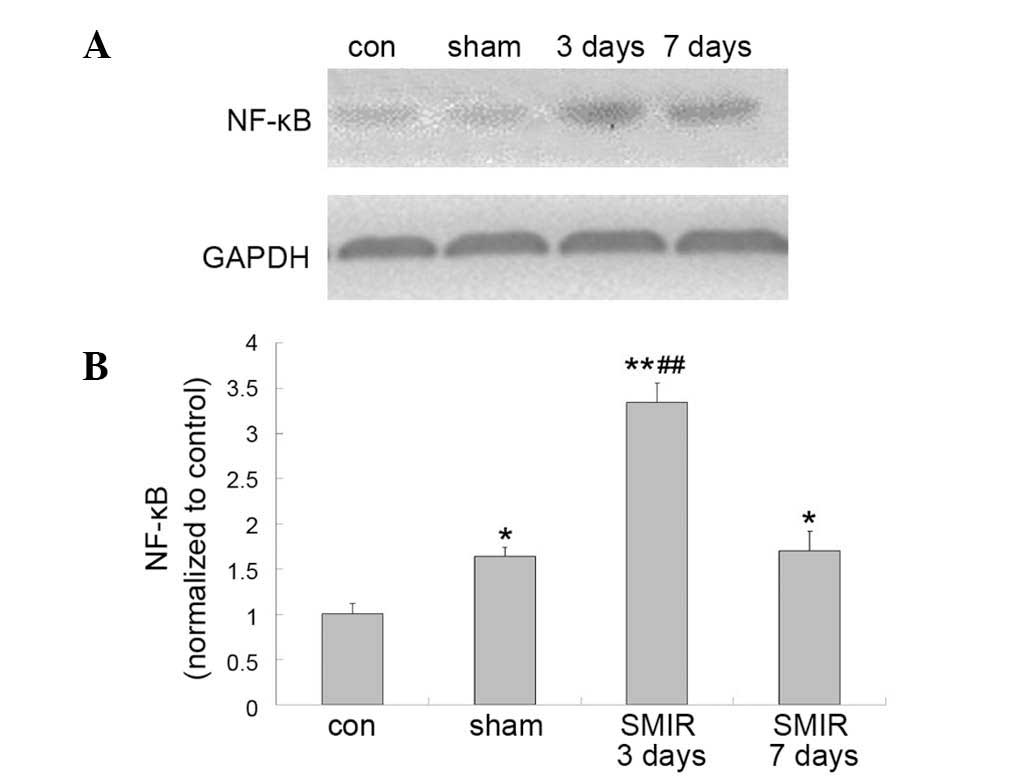
Alterations in NF-κB p65 levels around
the incision site in the control, sham and SMIR groups
In comparison with the NF-κB p65 level in the
control group, the sham surgery and SMIR groups were significantly
increased following surgery (P<0.05 and P<0.01,
respectively). In addition, the NF-κB p65 level in the SMIR group 3
days subsequent to surgery was significantly greater than that of
the sham group (P<0.01) (Fig.
2).
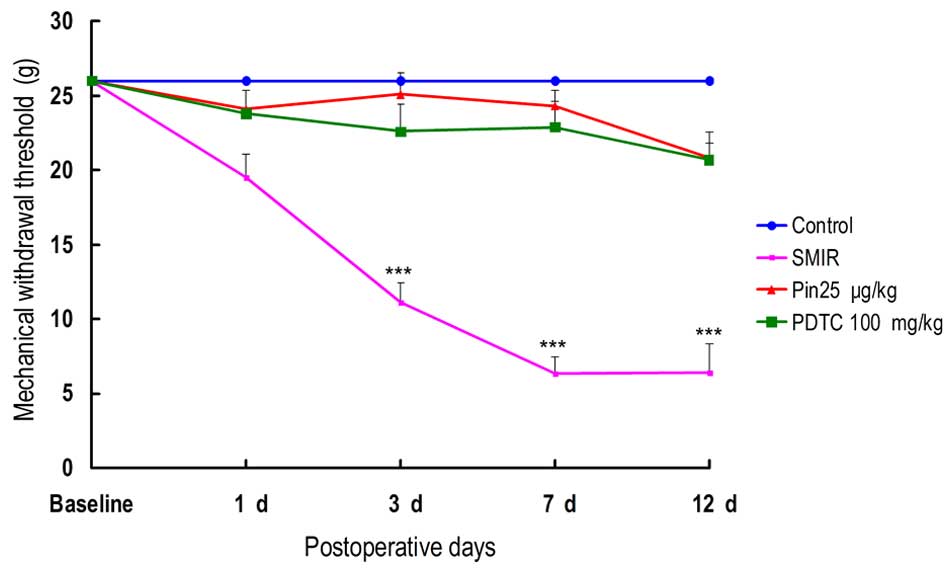
Alterations in MWT
It has been previously reported that SMIR is able to
induce mechanical allodynia in rats, however it was previously
reported to have no effects on hot or cold hyperalgesia (12) As presented in Fig. 3, compared with the control group
SMIR induced no significant alterations in the MWT of rats on the
first day subsequent to surgery. However, the MWT was significantly
reduced 3, 7 and 12 days subsequent to SMIR surgery. No significant
reduction in MWT was observed in the rats from the pinacidil group
or PDTC group in comparison with the rats in the control group
(P>0.05). Administration of pinacidil and PDTC reversed the
SMIR-induced reduction in MWT following SMIR surgery (Fig. 3).
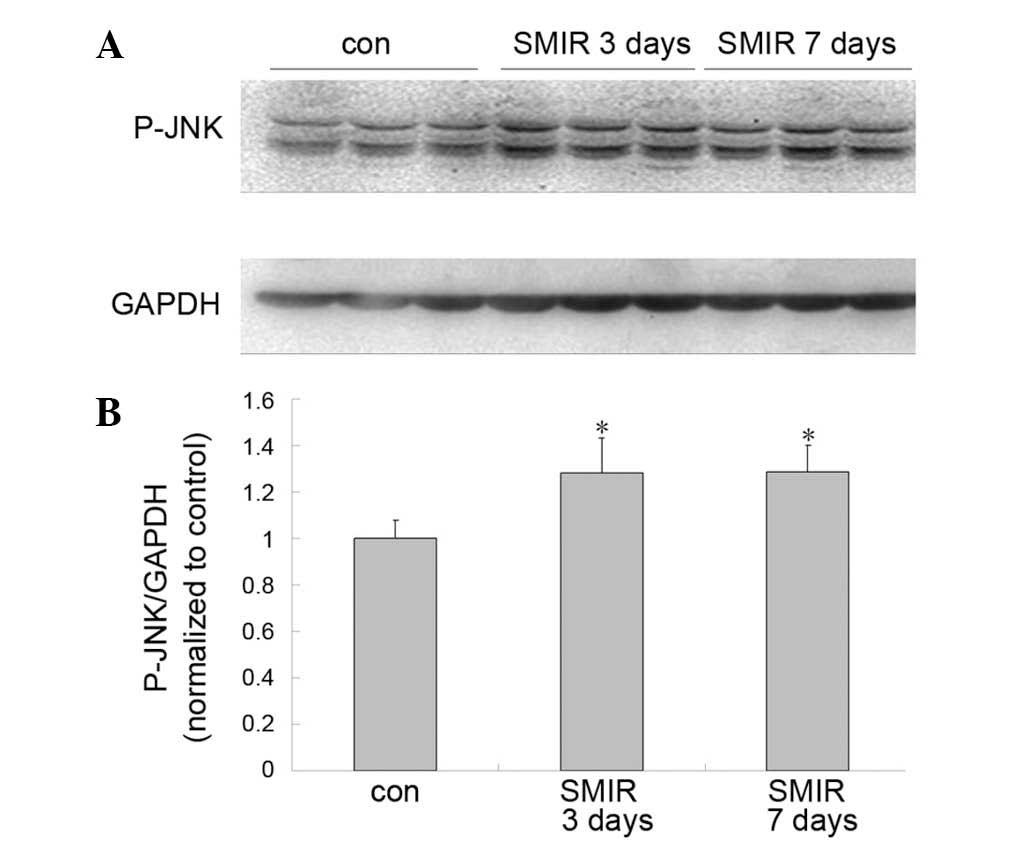
Alterations in spinal levels of JNK
Compared with the control group, the JNK level was
observed to be significantly increased in the SMIR group on days 3
and 7 after surgery (P<0.05) (Fig.
4).
Alterations in NF-κB p65, Kir6.1 and SUR2
levels around the incision site
Compared with the control group, the NF-κB p65 level
was observed to be significantly increased (P<0.01) on day 3
following surgery; whereas the Kir6.1 and SUR2 levels were
significantly reduced in the SMIR group (P<0.01). No significant
alterations were observed in the levels of NF-κB p65, Kir6.1 and
SUR2 between the pinacidil and PDTC groups. The NF-κB p65 level in
the pinacidil group and PDTC group was significantly reduced
compared with that of the SMIR group (P<0.01). The Kir6.1 and
SUR2 levels were significantly increased in the pinacidil group and
PDTC groups in comparison with the SMIR group (P<0.05,
P<0.01) (Fig. 5).
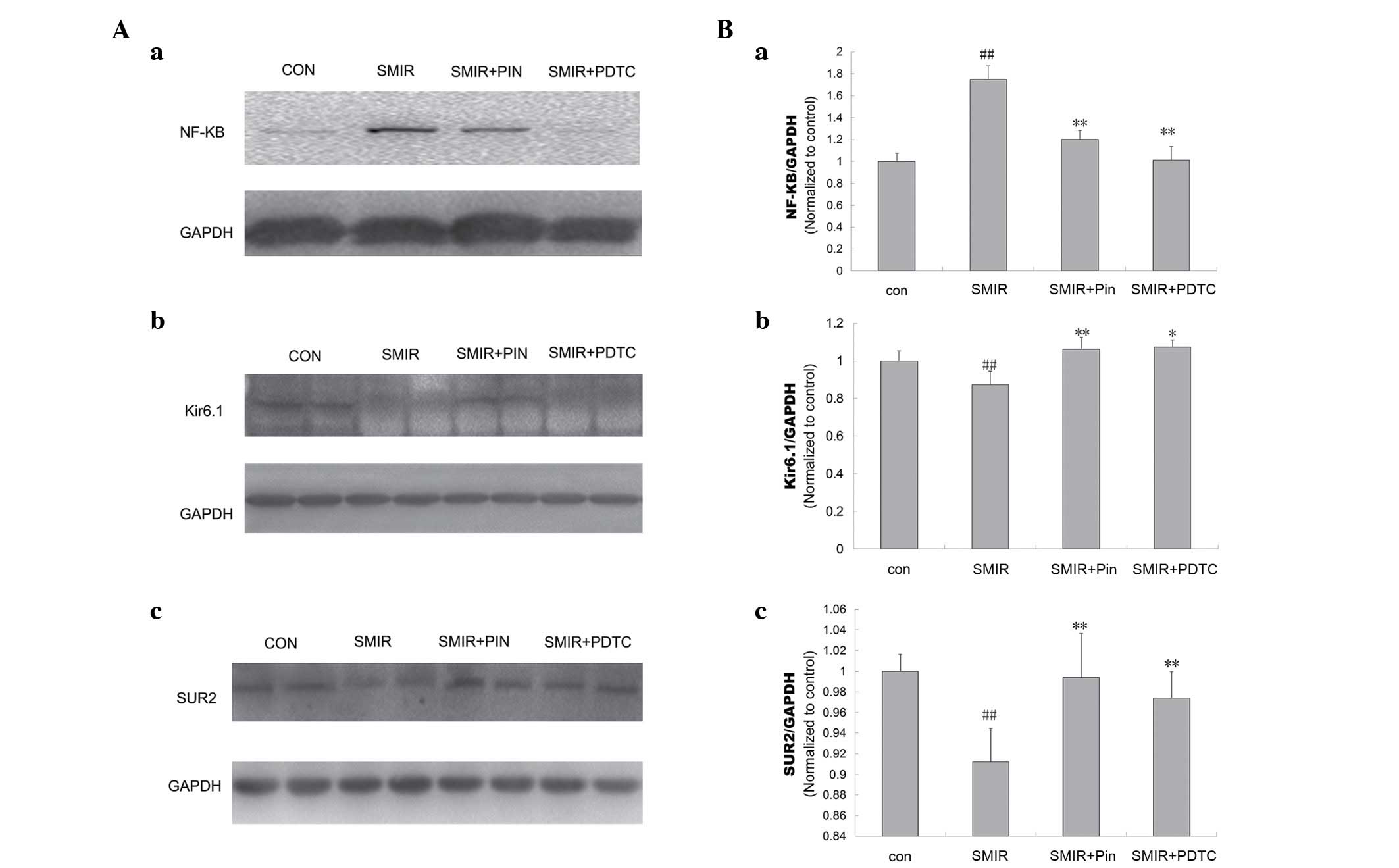 | Figure 5Comparison of NF-κB p65, Kir6.1 and
SUR2 protein level around the incision site in control, SMIR, Pin
and PDTC rats. (A) Western blotting of (a) NF-κB p65, (b) Kir6.1
and (c) SUR2 levels in different groups; (B) normalized (a) NF-κB
p65, (b) Kir6.1 and (c) SUR2 levels determined by western blotting.
##P<0.01 vs. control; *P<0.05,
**P<0.01 vs. SMIR. NF-κB, nuclear factor κB; SMIR,
skin/muscle incision and retraction; Pin, pinacidil; PDTC,
pyrrolidine dithiocarbamate; con, control; GADPH, glyceraldehyde
3-phosphate dehydrogenase. |
Alterations in spinal levels of NF-κB p65
and JNK
Compared with the control group, the levels of NF-κB
and JNK were significantly increased in the SMIR group (P<0.01).
In addition, the levels of NF-κB and JNK in the pinacidil and PDTC
groups were significantly reduced, compared to those in the SMIR
group (NF-κB, P<0.01; JNK, P<0.05) (Fig. 6).
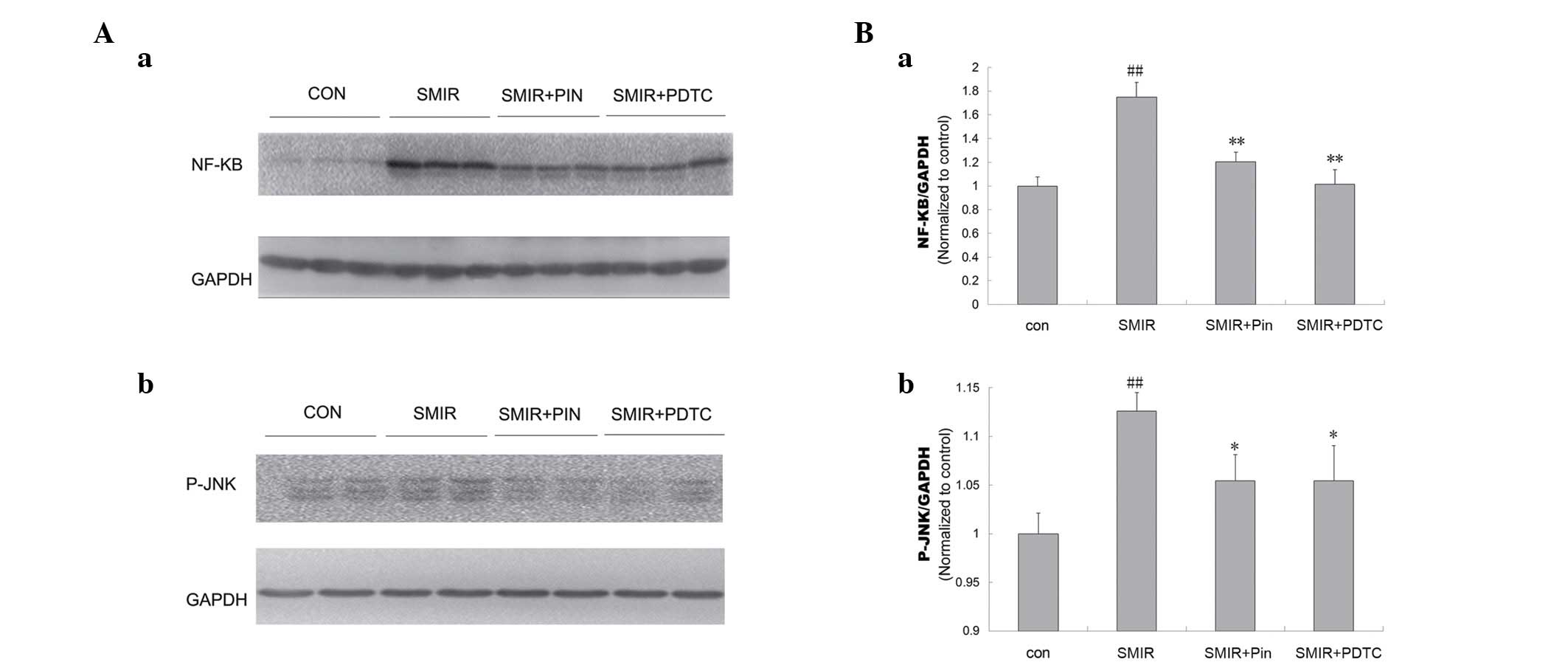 | Figure 6Comparison of the NF-κB and JNK
expression levels in the spinal cord in control, SMIR, Pin and PDTC
rats. (A) Western blotting analysis of (a) NF-κB and (b) JNK levels
in the different groups; (B) normalized (a) NF-κB and (b) JNK
levels determined by western blotting. ##P<0.01 vs.
control; *P<0.05, **P<0.01 vs. SMIR.
NF-κB, nuclear factor κB; JNK, c-Jun N-terminal kinase; SMIR,
skin/muscle incision and retraction; Pin, pinacidil; PDTC,
pyrrolidine dithiocarbamate; con, control; GADPH, glyceraldehyde
3-phosphate dehydrogenase. |
Discussion
It has been identified that formalin-induced animal
models of inflammatory nociception are unable to simulate the
microenvironment around the incision site (15). However, SMIR has been previously
reported to be able to evoke mechanical allodynia in rats, however
produced no effects on hot or cold hyperalgesia (12). NF-κB is a ubiquitous transcription
factor that is important in inflammatory pain and neuropathic pain
(1). As presented in Fig. 2, the NF-κB levels around the
incision site were significantly increased following surgery
compared with control group. In addition, the NF-κB levels around
the incision site were increased in the SMIR group on day 3 after
surgery compared with those in the sham group. It was suggested
that these effects were due to the extent of the alterations to the
microenvironment resulting from surgery. This observation indicated
that the incision and retraction procedure was able to
significantly upregulate the NF-κB levels compared with the
incision alone. As presented in Fig.
3, compared with the control group, there were no significant
alterations in the MWT of rats undergoing SMIR on the first day
after surgery. However, the MWT was significantly reduced on days
3, 7 and 12 subsequent to SMIR surgery. This indicates that it
requires time for alterations in the microenvironment around the
incision to activate central sensitization. SMIR surgery was able
to result in the microenvironmental deterioration of peripheral
nociceptor terminals around the incision site, inducing an increase
in excitability of the nociceptors, resulting in peripheral and
central sensitization. Thus, the SMIR model can provide a more
accurate reflection of the microenvironment around the incision
site.
In the current study, it was identified that
deterioration of the microenvironment resulting from SMIR surgery
upregulated the levels of NF-κB around the incision and NF-κB and
JNK expression in the spinal cord, which inhibited the expression
of Kir6.1 and SUR2. The results indicated that NF-κB, JNK and
KATP participate in peripheral and central sensitization
induced by SMIR surgery. These results thus indicate that the
deterioration of the microenvironment at peripheral nociceptor
terminals may act as a trigger for NF-κB and JNK signaling and
KATP activity, which is an adaptive mechanism for
autoprotection.
Preventive analgesia inhibits peripheral and central
sensitization using multimodal analgesic techniques, including
non-steroidal anti-inflammatory drugs, α2 agonists, local
anesthetics, ketamine and α2δ ligands and opioids, which can reduce
the occurrence of opioid-adverse events (16). It has been demonstrated that
functional KATP channels are present in nociceptors
(17). The opening of the
KATP channels may inhibit the nociceptive responses
induced by noxious stimuli by dampening the hyper-excitability of
the nociceptors, and it has been identified that KATP
has no significant effect on the resting membrane conductance of
dorsal root ganglion neurons (18). Pinacidil is a sulfonylurea receptor
agonist that opens the SUR2 potassium-sensitive ATP channel
(7). It has been demonstrated that
pinacidil does not readily cross the blood brain barrier under
normal physiological conditions in the rat (19). To assess the effect of activated
peripheral KATP on peripheral and central sensitization
resulting from deteriorations to the microenvironment of peripheral
nociceptor terminals around the incision site, the following
experiments were conducted. The minimum effective dose of 25
µg/kg pinacidil was injected intraperitoneally into the rats
30 min prior to SMIR, according to the pre-experiment results. This
resulted in activation of peripheral KATP, and then the
levels of Kir6.1, SUR2 and MWT was analyzed. It was identified that
pinacidil exerted an inhibitory effect on the SMIR-induced
reduction of MWT, Kir6.1 and SUR2 levels around the incision,
increases in NF-κB levels around the incision and increases in
NF-κB and JNK levels in the spinal cord. These results suggested
that pinacidil activated peripheral KATP and blocked the
subsequent microenvironmental deterioration around the incision
site induced by the SMIR procedure, thus preventing peripheral and
central sensitization. The present study indicated that
microenvironmental deterioration of peripheral nociceptor terminals
around the incision site is an initial step of peripheral and
central sensitization. Activation of peripheral KATP
prior to central sensitization inhibits NF-κB expression around the
incision; thus, an inflammatory microenvironment that motivates
central excitatory is unable to be formed, thus NF-κB and JNK
expression in the spinal cord is inhibited to prevent hyperalgesia.
It has been demonstrated that activation of KATP can
regulate cell metabolism and electrical activity, increase ATP
synthesis, reduce oxyradicals, prevent calcium overload and
maintain mitochondrial function, thus ameliorating the effects on
the microenvironment (14,20–23).
It is hypothesized that activated KATP preconditioned by
pinacidil exerts a protective effect on the microenvironment by
regulating cell activity, increasing ATP synthesis, reducing
oxyradicals and preventing calcium overload to suppress
microenvironmental deterioration resulting from SMIR surgery.
Kir6.1/SUR2B is the major isoform of KATP channels in
vascular smooth muscles, and vascular dysfunction and vascular
endothelial cells serve a key role in mechanical pain (6,24).
Thus the functional KATP channels in vascular
endothelial cells serve a vital role in peripheral and central
sensitization resulting from postoperative microenvironmental
deterioration. Preconditioning of KATP may be an
effective method of preventive analgesia.
NF-κB is a widely expressed transcription factor for
genes involved in cell survival, inflammation, differentiation and
growth (25–29), and is necessary for the
upregulation of the KATP channel (30). JNK is a stress-activated member of
the mitogen-activated protein kinase family (31). Previous studies have demonstrated
that hyperalgesia and allodynia are induced by tissue or nerve
injury via the JNK pathway in primary sensory neurons and the
spinal cord. Furthermore, this activation can maintain central
sensitization (32,33). There are numerous common upstream
molecules involved in the NF-κB/JNK signaling pathway, which
participate in mediation of inflammation (34,35).
In the current study, it was identified that deterioration of the
microenvironment resulting from SMIR surgery upregulated the levels
of NF-κB around the incision and NF-κB and JNK expression in the
spinal cord, providing a basis for the activation of
KATP, inhibiting the expression of Kir6.1 and SUR2. This
indicated that the SMIR procedure was able to activate NF-κB/JNK
signaling and inhibit KATP activity, inducing
postoperative allodynia. PDTC, an antioxidant and a specific
inhibitor of NF-κB, reversibly inhibits the nuclear translocation
of NF-κB (36). In the current
study, an 100 mg/kg intraperitoneal injection of PDTC was selected,
administered 30 min prior to SMIR surgery according to a method
described previously (37). It was
identified that the rats in the PDTC group exhibited a
significantly reduced NF-κB level, higher Kir6.1 and SUR2 levels
around the incision and reduced spinal JNK levels compared with
those in the SMIR group. The rats pretreated with PDTC additionally
displayed significantly reduced MWT compared with those in the SMIR
group. This observation suggests that the deteriorative
microenvironment at peripheral nociceptor terminals inhibits
peripheral KATP and activates JNK signaling via the
NF-κB signaling pathway. In addition, activation of peripheral
KATP exerting a preventative analgesic effect is
suggested to be dependent on the NF-κB signaling pathway. Thus, the
NF-κB/JNK signaling pathway is a potential molecular target for
ameliorating the microenvironmental deterioration around the
incision and preventing peripheral and central sensitization.
Taking the results of the current study and those of
previous studies into account, it is suggested that alterations in
the microenvironment provide a basis for the effect of activated
KATP. Furthermore, preconditioning KATP can
result in the amelioration of the microenvironment deteriorations
to prevent peripheral and central sensitization via the NF-κB/JNK
signaling pathway, thus providing a novel approach to preventive
analgesia.
In summary, it was identified that the activation of
peripheral KATP by pinacidil prior to surgery was able
to ameliorate the microenvironmental deteriorations resulting from
the SMIR procedure, thus preventing peripheral and central
sensitization via the NF-κB/JNK signaling pathway. Thus,
KATP/NF-κB/JNK may serve as an important molecular
target for preventive analgesia.
References
|
1
|
Fu ES, Zhang YP, Sagen J, Candiotti KA,
Morton PD, Liebl DJ, Bethea JR and Brambilla R: Transgenic
inhibition of glial NF-kappa B reduces pain behavior and
inflammation after peripheral nerve injury. Pain. 148:509–518.
2010. View Article : Google Scholar :
|
|
2
|
Manassero G, Repetto IE, Cobianchi S,
Valsecchi V, Bonny C, Rossi F and Vercelli A: Role of JNK isoforms
in the development of neuropathic pain following sciatic nerve
transection in the mouse. Mol Pain. 8:392012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Julius D and Basbaum AI: Molecular
mechanisms of nociception. Nature. 413:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linley JE, Rose K, Ooi L and Gamper N:
Understanding inflammatory pain: Ion channels contributing to acute
and chronic nociception. Pflugers Arch. 459:657–669. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inagaki N, Gonoi T, Clement JP, Wang CZ,
Aguilar-Bryan L, Bryan J and Seino S: A family of sulfonylurea
receptors determines the pharmacological properties of
ATP-sensitive K+ channels. Neuron. 16:1011–1017. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coderre TJ and Bennett GJ: A hypothesis
for the cause of complex regional pain syndrome-type I (reflex
sympathetic dystrophy): Pain due to deep-tissue microvascular
pathology. Pain Med. 11:1224–1238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan LH, Tian HY, Wang J, Huo JH, Hu Z, Ma
AQ and Cao YX: Downregulation of Kir6.1/SUR2B channels in the obese
rat aorta. Nutrition. 25:359–363. 2009. View Article : Google Scholar
|
|
8
|
Roper J and Ashcroft FM: Metabolic
inhibition and low internal ATP activate K-ATP channels in rat
dopaminergic substantia nigra neurones. Pflügers Arch. 430:44–54.
1995. View Article : Google Scholar
|
|
9
|
Yamada K and Inagaki N: Neuroprotection by
KATP channels. J Mol Cell Cardiol. 38:945–949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zoga V, Kawano T, Liang MY, Bienengraeber
M, Weihrauch D, McCallum B, Gemes G, Hogan Q and Sarantopoulos C:
KATP channel subunits in rat dorsal root ganglia: Alterations by
painful axotomy. Mol Pain. 6:62010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perimal EK, Akhtar MN, Mohamad AS, Khalid
MH, Ming OH, Khalid S, Tatt LM, Kamaldin MN, Zakaria ZA, Israf DA,
et al: Zerumbone-induced antinociception: Involvement of the
L-arginine-nitric oxide-cGMP-PKC-K+ATP channel pathways. Basic Clin
Pharmacol Toxicol. 108:155–162. 2011. View Article : Google Scholar
|
|
12
|
Flatters SJ: Characterization of a model
of persistent postoperative pain evoked by skin/muscle incision and
retraction (SMIR). Pain. 135:119–130. 2008. View Article : Google Scholar
|
|
13
|
Dixon WJ: Staircase bioassay: The
up-and-down method. Neurosci Biobehav Rev. 15:47–50. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao S, Qin Y, Chen J and Shen S: Effects
of pinacidil on changes to the microenvironment around the incision
site, of a skin/muscle incision and retraction, in a rat model of
postoperative pain. Mol Med Rep. 12:829–836. 2015.PubMed/NCBI
|
|
15
|
Brennan TJ, Zahn PK and Pogatazki-Zahn EM:
Mechanisms of incisional pain. Anesthesiol Clin North America.
23:1–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reuben SS and Buvanendran A: Preventing
the development of chronic pain after orthopaedic surgery with
preventive multimodal analgesic techniques. J Bone Joint Surg Am.
89:1343–1358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawano T, Zoga V, McCallum JB, Wu HE,
Gemes G, Liang MY, Abram S, Kwok WM, Hogan QH and Sarantopoulos CD:
ATP-sensitive potassium currents in rat primary afferent neurons:
Biophysical, pharmacological properties and alterations by painful
nerve injury. Neuroscience. 162:431–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ploug KB, Amrutkar DV, Baun M,
Ramachandran R, Iversen A, Lund TM, Gupta S, Hay-Schmidt A, Olesen
J and Jansen-Olesen I: K(ATP) channel openers in the
trigemino-vascular system. Cephalalgia. 32:55–65. 2012. View Article : Google Scholar
|
|
19
|
Du X, Wang C and Zhang H: Activation of
ATP-sensitive potassium channels antagonize nociceptive behavior
and hyper-excitability of DRG neurons from rats. Mol Pain.
7:352011. View Article : Google Scholar
|
|
20
|
Ko EA, Han J, Jung ID and Park WS:
Physiological roles of K+ channels in vascular smooth muscle cells.
J Smooth Muscle Res. 44:65–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flagg TP, Enkvetchakul D and Koster JC:
Muscle KATP channels: Recent insights to energy sensing and
myoprotection. Physiol Rev. 90:799–829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matejíková J, Kucharská J, Pintérová M,
Pancza D and Ravingerová T: Protection against ischemia-induced
ventricular arrhythmias and myocardial dysfunction conferred by
preconditioning in the rat heart: Involvement of mitochondrial
K(ATP) channels and reactive oxygen species. Physiol Res. 58:9–19.
2009.
|
|
23
|
Batchu SN, Chaudhary KR, El-Sikhry H, Yang
W, Light PE, Oudit GY and Seubert JM: Role of PI3Kα and sarcolemmal
ATP-sensitive potassium channels in epoxyeicosatrienoic acid
mediated cardioprotection. J Mol Cardiol. 53:43–52. 2012.
View Article : Google Scholar
|
|
24
|
Nesic O, Sundberg LM, Herrera JJ,
Mokkapati VU, Lee J and Narayana PA: Vascular endothelial growth
factor and spinal cord injury pain. J Neurotrauma. 27:1793–1803.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freudenthal R, Boccia MM, Acosta GB, Blake
MG, Merlo E, Baratti CM and Romano A: NF-kappaB transcription
factor is required for inhibitory avoidance long-term memory in
mice. Eur J Neurosci. 21:2845–2852. 2005. View Article : Google Scholar
|
|
26
|
Guo Q, Robinson N and Mattson MP: Secreted
beta-amyloid precursor protein counteracts the proapoptotic action
of mutant presenilin-1 by activation of NF-kappaB and stabilization
of calcium homeostasis. J Biol Chem. 273:12341–12351. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mattson MP and Meffert MK: Roles for
NF-kappaB in nerve cell survival, plasticity and disease. Cell
Death Differ. 13:852–860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mattson MP: NF-kappaB in the survival and
plasticity of neurons. Neurochem Res. 30:883–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi W, Cui N, Wu Z, Yang Y, Zhang S, Gai
H, Zhu D and Jiang C: Lipopolysaccharides up-regulate Kir6.1/SUR2B
channel expression and enhance vascular KATP channel activity via
NF-kappaB-dependent signaling. J Biol Chem. 285:3021–3029. 2010.
View Article : Google Scholar
|
|
31
|
Bonny C, Borsello T and Zine A: Targeting
the JNK pathway as a therapeutic protective strategy for nervous
system diseases. Rev Neurosci. 16:57–67. 2005.PubMed/NCBI
|
|
32
|
Zhang Q, Wang J, Duan MT, Han SP, Zeng XY
and Wang JY: NF-κB, ERK, p38 MAPK and JNK contribute to the
initiation and/or maintenance of mechanical allodynia induced by
tumor necrosis factor-alpha in the red nucleus. Brain Res Bull.
99:132–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao YJ and Ji RR: Activation of JNK
pathway in persistent pain. Neurosci Lett. 437:180–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Benedetti G, Fredriksson L, Herpers B,
Meerman J, van de Water B and de Graauw M: TNF-α-mediated NF-κB
survival signaling impairment by cisplatin enhances JNK activation
allowing synergistic apoptosis of renal proximal tubular cells.
Biochem Pharmacol. 85:274–286. 2013. View Article : Google Scholar
|
|
35
|
Li J, Yang L, Qin W, Zhang G, Yuan J and
Wang F: Adaptive induction of growth differentiation factor 15
attenuates endothelial cell apoptosis in response to high glucose
stimulus. PLoS One. 8:e655492013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu SF, Ye X and Malik AB: Inhibition of
NF-kappaB activation by pyrrolidine dithiocarbamate prevents in
vivo expression of proinflammatory genes. Circulation.
100:1330–1337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao S, Zhang H, Cao D, Liu Y and Li X:
Lipopolysaccharide exposure during pregnancy leads to aortic
dysfunction in offspring rats. PloS One. 9:e1022732014. View Article : Google Scholar : PubMed/NCBI
|