Introduction
With the rapid development of the global economy and
the rising quality of life, the morbidity rate of atherosclerosis
(AS) is increasing. Major cardiovascular and cerebrovascular
diseases, including coronary heart disease (CHD), stroke and sudden
death have become the leading cause of mortality worldwide. The
progression of AS has been frequently investigated and various
hypotheses on its pathogenesis have been proposed. However, the
exact etiology and mechanism of this disease has not been fully
elucidated. The hypothesis of 'inside-out' inflammation has been
proposed by Ross, which suggests that AS is an 'inside-out'
vascular inflammatory response (1–3).
However, this theory fails to fully clarify the pathogenesis of AS.
Furthermore, the anti-inflammatory and lipid-reducing statin-based
treatments, suggested therapy based on this theory may achieve an
efficacy rate of 30–40% in the treatment of atherosclerotic
cardio-cerebral vascular diseases (4).
Previous studies have demonstrated that tunica
adventitia contributes to the formation of the intimal lesion of
AS. According to a previous study, angiogenesis in the arterial
wall adventitia occurs at the stage of hyperlipidemia, prior to
endothelial dysfunction in great vessels (5). Additionally, clinical and
experimental studies have demonstrated that neovascularization is
closely associated with the development of AS and that the degree
of proliferation of the vasa vasorum in adventitia was positively
associated with microvascular network expansion in the plaques
(6,7). A previous study, which investigated
the effect of the local application of pro-angiogenic, fibroblast
growth factor 2 in the abdominal aortic adventitia of
ApoE−/− mice, determined that the adventitia
angiogenesis occurred prior to the development of AS and the
quantity of vasa vasorum was proportional to the size of the plaque
(8). In a pig model of
hyperlipidemia, the use of thalidomide, an inhibitor of
angiogenesis, effectively reduced the expression of the
pro-angiogenic vascular endothelial growth factor (VEGF) protein,
inhibited adventia angiogenesis in the coronary artery and delayed
neointima formation (9).
Additionally, previous studies have also suggested that inhibiting
angiogenesis in the vascular wall may effectively impede AS
(10–12). Thus, inhibiting neovascularization
may represent a novel treatment for AS.
Tongxinluo (TXL) is a traditional Chinese medicine
(TCM), which is composed of 12 Chinese medicines and herbs,
including ginseng, ground beetle (usually rich in chitin), leech,
scorpion, centipede, cicada slough, red peony root, spina date
seed, dalbergia wood and borneol. Its primary active ingredients
include paeoniflorin, ginsenoside Rg1, ginsenoside Rb1 and
jujubosides A and B (13).
Approved by registration at the State Food and Drug Administration
of China, in 1996, TXL has been used widely for the clinical
treatment of CHD, angina and ischemic stroke. Evidence has
previously demonstrated that TXL protects the cardiovascular system
and may reduce blood lipid levels, inhibit inflammation and
oxidation, effectively attenuate plaque formation, stabilize
vulnerable plaque and prevent plaque rupture (14). However, the molecular mechanism
behind plaque stabilization and the AS-inhibitory effects of TXL
remain unclear. This may be associated with the inhibitory effect
on adventitia angiogenesis. The present study established a rabbit
model of hyperlipidemia to investigate the effects of TXL on
angiogenesis in the early stage of AS and the underlying molecular
mechanisms.
Materials and methods
Experimental animals
The present study was approved by the Animal Ethics
Committee of Shandong Wendeng Central Hospital (Weihai, China). A
total of 90 common New Zealand male white rabbits, aged 3–4 months,
with an average weight of 2.0±0.2 kg (specific pathogen-free grade,
certificate no. 2009009), were purchased from the Experimental
Animal Center of Shandong Luye Pharmaceutical Co., Ltd. (Yantai,
China). The animals were housed in individual cages at a constant
temperature (20–36°C) and humidity (40–70%).
Modeling, grouping and medication
All experimental animals were fed adaptively for 7
days and then randomly assigned to the following treatment groups
(n=15 per group): Normal group, model group, low-dose TXL group
(TXL-L), moderate-dose TXL group (TXL-M), high-dose TXL group
(TXL-H) and atorvastatin group. The normal group was fed with a
standard diet for the duration of the study. The model group and
medicine-treated groups were fed with a high cholesterol diet (1%
cholesterol, 5% lard, 7.5% yolk powder and 86.5% basal feed). The
feed was processed by the Hebei Laboratory Animal Center (Hebei,
China). Each rabbit was fed with 120 g of feed each day for 4
weeks.
The TXL-L, TXL-M and TXL-H groups were given TXL
superfine powder at 0.15, 0.30 and 0.6 g/kg/day; Yiling
Pharmaceutical Co., Inc., Shijiazhuang, China), respectively.
Considering the strong angiogenesis regulation effects of
atorvastatin, the atorvastatin group was used as the positive
control and was administered with atorvastatin by oral gavage at
2.50 mg/kg/day (Lipitor Pfizer Pharmaceuticals, Ltd., Dalian,
China). The treatments, at the aforementioned concentrations, were
prepared and administered to the rabbits by oral gavage as 3 ml/kg
body weight. Gavage administration was initiated subsequently to
grouping. The normal group and model groups were treated with 0.5%
carboxy-methyl-cellulose sodium solution according to weight
standard of 3 ml/kg. The experimental period was 4 weeks.
Specimen collection
Specimen collection for each group was performed at
the end of the 4-week experimental period. Prior to sampling,
animals were fasted for 12 h. All animals were sacrificed after an
injection with 3% pentobarbital sodium (1 ml/kg) through the ear
vein. Fasting blood samples were obtained via the abdominal aorta
of the rabbits and used to determine the lipid levels in the blood
serum. Subsequent to the removal of the right common carotid
artery, which was washed in ice-cold normal saline, the attached
connective tissues were removed with caution. One segment of the
collected sample was fixed in 10% neutral formaldehyde solution,
then embedded in paraffin for hematoxylin and eosin (HE) staining,
immunohistochemical staining and in situ hybridization
experiments. The procedure was performed under aseptic conditions.
The remaining segment was frozen in liquid nitrogen for subsequent
molecular biological assays. Additionally, the right common carotid
arteries were removed from 1 in 3 randomly selected rabbits in each
group and immediately processed into frozen sections in order to
determine O2− levels.
Determination of blood lipid levels
Enzymatic colorimetry and an automatic biochemical
analyzer were used to detect serum total cholesterol (TC),
triglyceride (TG), low density lipoprotein (LDL) and high density
lipoprotein (HDL) levels. Determination of these physiological and
biochemical parameters were measured by colorimetric enzyme kits,
according to the manufacturer's protocol (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China)
Morphological analysis and
immunohistochemical staining
Subsequent to fixing of the specimen, 5
µm-thick paraffin sections were prepared for HE staining and
CD31 immunohistochemical staining. The EnVision immunohistochemical
staining method was used to determine localization and expression
of CD31 in carotid artery tissue. Following dewaxing, the 5
µm-thick carotid specimen section was placed in 3%
H2O2 solution to block endogenous peroxidase.
The sample tissue was incubated with the CD31 primary antibody
(cat. no. ab958; Abcam, Cambridge, UK) diluted to 1:200 with 5% BSA
at 4°C overnight. Subsequently, the horseradish peroxidase
(HRP)-labeled secondary antibody (cat. no. ab6785; Abcam; dilution,
1:1,000) was added to detect the primary antibody and
3,3′-diaminobenzidine color developing was performed. Hematoxylin
was used to stain the cell nuclei. Finally, the tissue section was
dehydrated, covered and observed under an optical microscope.
The microvessel density (MVD) count method, as
described by Weidner et al (15), was used for MVD quantification
analysis of the labeled CD31. Vascular endothelial cells were
stained brownish-yellow by the secondary antibody conjugated to
CD31. The isolated endothelial cell clusters were scored as a
microvessels if they demonstrated a clear boundary with adjacent
cells and the surrounding connective tissue, irrespective of the
existence of vessel lumen. Initially, the whole section was
observed under a microscope at ×40 magnification in order to select
for a high-MVD concentration region. Subsequently, the microvessel
quantities (Q) were counted in 5 high-power fields at a
magnification of ×400 in a microscopic visual field and the
cross-sectional area (A; mm2) of the vascular wall was
determined. The MVD (n/mm2) was calculated using the
following formula: MVD=Q/A. The average of the MVDs in the five
high-power fields was used as the MVD of the sample.
Detection of superoxide anion
In accordance with a previous study (16), dihydroethidium (DHE) fluorescence
probe was used to detect O2 (•−) and evaluate the level
of reactive oxygen species (ROS) in the adventitia. Fresh tissues
(20 mm) of common right carotid artery were taken from 1 in 3
animals randomly selected from each group, quickly placed in
optimal cutting temperature embedding medium, frozen and cut into
30 µm-thick sections by a freezing micro-tome. The DHE probe
was dissolved in dimethyl sulfoxide and then diluted with
phosphate-buffered saline to a working solution of 10
µmol/l. DHE probe working solution (100 µl) was added
onto the surface of the section, which was then promptly placed in
a light-tight wet box for 30 min at 37°C, in order to oxidize DHE
and generate ethidium bromide (EB). The EB binds to DNA in the cell
nucleus to produce red fluorescence under UV light. Subsequently,
unreacted probes were washed away and the sample was observed under
a fluorescence microscope. Under excitation at a wavelength of 490
nm and an emission wavelength of 520 nm, the signal intensity of
the red fluorescence was observed. Microscopic imaging analysis was
adopted for image acquisition. Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) was used for the
quantitative analysis of the fluorescence intensity of the
image.
Fluorescence in situ hybridization
For the localization of the carotid artery the
nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase
subunits p22phox and gp91phox mRNA were detected using in
situ hybridization with fluorescein isothiocyanate
(FITC)-conjugated DNA probes. The sequences of the probes used were
as follows: p22phox, 5′-FITC+CCAGGAGCTTCAGCACGGCGGTCAGGTAGCG-3′;
and gp91phox, 5′-FITC+ACC ATT TTATGAAAAGTGAGATTT CTG-3′. Carotid
arterial tissue was fixed in 10% neutral formaldehyde solution an
cut into 5 µm-thick paraffin sections. Subsequent to
dewaxing and air drying, the sections were soaked in proteinase K
reaction solution (100 mmol/l Tris-HCL, 50 mmol/l EDTA and 1
µg/ml proteinase K), incubated in a 37°C water bath for 20
min, rinsed 3 times with saline sodium citrate (SSC) buffer and
dehydrated through an alcohol gradient. The sections were then
placed in a staining jar containing denaturing solution (70%
methanamide, 2X SSC and 0.1 mmol/l EDTA) and incubated at 75°C for
8 min, simultaneously hybridization solution containing RNA probe
(300 µl formamide, 12 µl 50X Denhardt's solution, 120
µl 50% dextran sulfate, 10 µg/µl yeast tRNA,
10 µl RNA probe and 49 µl RNase-free deionized water)
was incubated at 75°C for 8 min. The specimen area of each slide
was covered with 50 µl hybridization mix solution, placed
into a wet box and subjected to hybridization in a 42°C oven
overnight. Subsequent to hybridization, the slide was eluted,
dehydrated through an alcohol gradient and air-dried. Signals were
observed under a fluorescence microscope.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
The total RNA was extracted using RNAiso Plus TRIzol
kit (Takara Bio, Inc., Dalian, China), according to the
manufacturer's protocol. The cDNA was synthetized using PrimeScript
RT reagent kit with gDNA Eraser (Takara Bio., Inc.) in a final
volume of 20 µl. The reverse transcription was programmed at
42°C for 2 min, followed by 37°C for 15 min and 85°C for 5 sec.
Expression of the NAD(P)H oxidase subunits p22phox and gp91phox
mRNA was determined by RT-PCR. Primers were synthesized by Generay
Biotech Co., Ltd. (Shanghai, China). Primers for amplification of
p22phox, gp91phox and GAPDH were designed using published rabbit
phagocyte sequences to amplify fragments of 77, 442 and 104 bp,
respectively. Primer sequences were as follows: p22phox
(NM_001082099), sense 5′-GTCGTGTGACTG CCACCTCTGA-3′, antisense
5′-GATGTCCACTGTGTTTACTGCAGG-3′; and gp91phox (NM_001082100.1),
sense 5′-CCAGTGCGTGCTGCTCAACAAG-3′, anti-sense 5′-GTACAA
TTCGTTCAGCTCCATGGATG-3′. GAPDH (NM_001082253.1), used as an
internal control, was amplified by the use of sense
5′-AGAGCACCAGAGGAGGACG-3′ and antisense, 5′-TGG
GATGGAAACTGTGAAGAG-3′ primers. The thermocycling conditions were as
follows: 50°C for 30 min, 94°C for 2 min, 94°C for 30 sec, 55°C for
30 sec, and 72°C for 2 min, followed by final extension at 72°C for
5 min. A total of 40 cycles were used for p22phox and gp91phox
amplification.
Western blot analysis
Three samples from each group were analyzed. Carotid
artery tissue (100 mg) was homogenized with tissue lysate solution.
Subsequent to homogenization, centrifugation was performed at
12,000 × g at 4°C to separate the supernatant. Protein
concentration was measured by the Coomassie brilliant blue method.
Equivalent protein extracts (30 µg) were obtained from each
group, separated by 10% sodium dodecyl sulfate polyacrylamide gel
and transferred onto polyvinylidene difluoride membrane via
electrotransfer, blocked with 5% skim milk powder, reacted with
VEGF antibody (cat. no. ab1316; Abcam, Cambridge, UK) and vascular
endothelial growth factor receptor-2 (VEGFR-2) antibody (cat. no.
b334966; Biorbyt, Cambridge, UK) and were incubated at 4°C
overnight and washed, and then reacted with corresponding
HRP-labeled secondary antibody (cat. nos. ab6785 and ab6881;
dilution, 1:5,000 and 1:10,000; both from Abcam) at room
temperature for 90 min. Subsequently, the membrane was washed and
enhanced chemiluminescence was used for color development. Another
identical membrane was prepared with β-actin antibody diluents
(cat. no. ab8227; dilution 1:1,000) as an internal control and
processed by the aforementioned method. The Gel Doc 2000 UVP gel
imaging system (UVP, LLC, Upland, CA, USA) was used for film
scanning. Labwork (version 4.6) Analysis software (Media
Cybernetics, Inc., Rockville, MD, USA) was used to perform gray
value analysis of the target band and β-actin internal control. The
ratio of 'gray value of targeted protein signal intensity' and
'gray value of internal control β-actin signal intensity' was
calculated and represents the relative expression level of the
protein of interest.
Statistical analysis
The statistical analysis software SPSS version 19.0
(IBM SPSS, Armonk, NY, USA) was used for data analysis. Continuous
variable data were represented by the mean ± standard deviation.
Prior to data analysis, normal distribution and homogeneity of
variance were tested using D-test and F-test methods. For data with
a normal distribution and homogenous variance, one-way analysis of
variance was used for between-group comparison and the least
significant difference was used for pairwise comparison.
Differences in data with normal distribution and heterogeneous
variance were tested using Dunnett's method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Serum lipid levels
Blood serum TC, TG, LDL and HDL levels of the
rabbits in the model group were significantly higher compared with
those in the normal group (P<0.002; Table I). Serum TC, TG and LDL levels in
the TXL-H (P<0.004), TXL-M (P<0.047) and atorvastatin
(P<0.007) groups were significantly lower compared with the
model group (Table I). HDL levels
in the TXL-H group and atorvastatin group were significantly higher
compared with the model group (P<0.002; Table I). No significant difference was
identified between TC, TG, LDL and HDL levels in the TXL-H, TXL-M
and atorvastatin groups (Table
I).
 | Table IChanges in the blood lipid levels of
the various treatment groups. |
Table I
Changes in the blood lipid levels of
the various treatment groups.
| Group | TC | TG | LDL | HDL |
|---|
| Normal | 4.66±1.09 | 1.89±0.55 | 0.68±0.23 | 1.05±0.40 |
| Model | 58.75±15.22a | 4.23±1.26a | 17.28±4.27a | 2.98±0.75a |
| TXL-L | 58.67±13.72 | 3.87±0.99 | 15.90±4.43 | 3.03±0.78 |
| TXL-M | 27.93±8.02c | 2.83±0.96c | 9.48±2.11b | 3.32±0.61 |
| TXL-H | 25.71±10.24c | 2.84±0.52c | 7.80±3.45c | 4.21±0.80c |
| Atorvastatin | 17.43±5.49c | 2.69±0.75c | 7.26±1.41c | 4.31±0.99c |
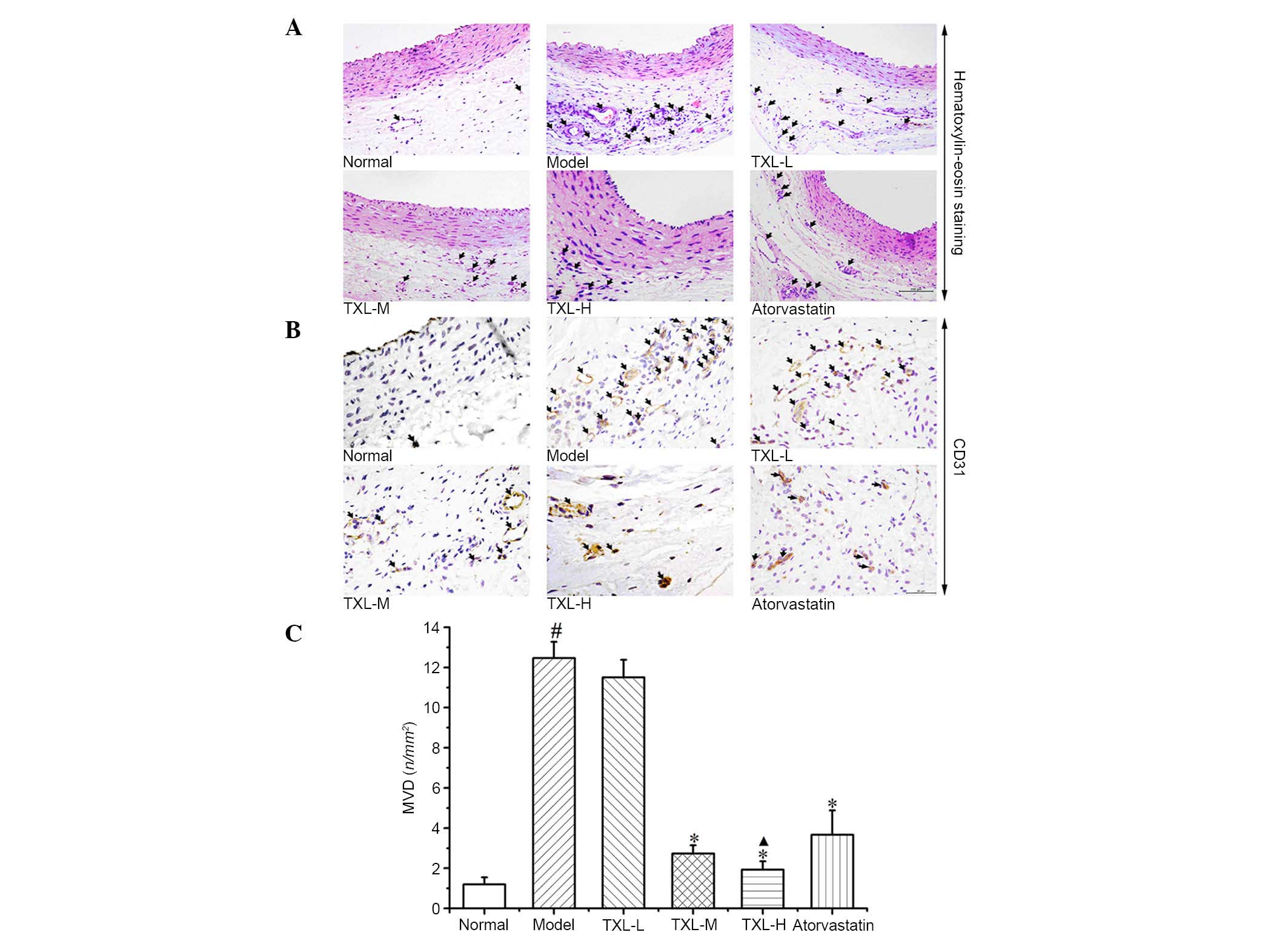
Density of vasa vasorum in carotid
arterial wall
As illustrated in Fig.
1, outward from the lumen, there were HE-positively stained
cells in the intima, tunica media and adventitia with internal and
external elastic membranes of carotid artery as boundaries. The
tissues from the normal group exhibited smooth intima, continuous
and complete monolayer of endothelial cells and a few vasa vasorum
in the adventitia. In the model group, carotid neointima was formed
and the quantity of vasa vasorum in the adventitia was increased.
In the TXL-H, TXL-M and atorvastatin groups, intimal hyperplasia
was reduced and the quantity of vasa vasorum in the adventitia was
reduced to varying degrees.
The CD31 immunohistochemical staining demonstrated
several CD31-positive microvessels in the carotid arteries of the
normal group. The quantity of vasa vasorum was greater in the model
group compared with the normal group. The TXL-L, TXL-M, TXL-H and
atorvastatin groups demonstrated different degrees of decline in
the quantity of vasa vasorum in the adventitia. Quantitative
analysis demonstrated that the MVD-positive labeling index in the
model group tissues was significantly greater compared with the
normal group tissues (P<0.006; Fig.
1C). The MVD-positive labeling indexes in the TXL-H, TXL-M and
atorvastatin group tissues were significantly lower compared with
the model group tissues (P<0.003; Fig. 1C). The TXL-H group exhibited a
significant decrease in the MVD-positive labeling index compared
with the atorvastatin group (P<0.038; Fig. 1C).
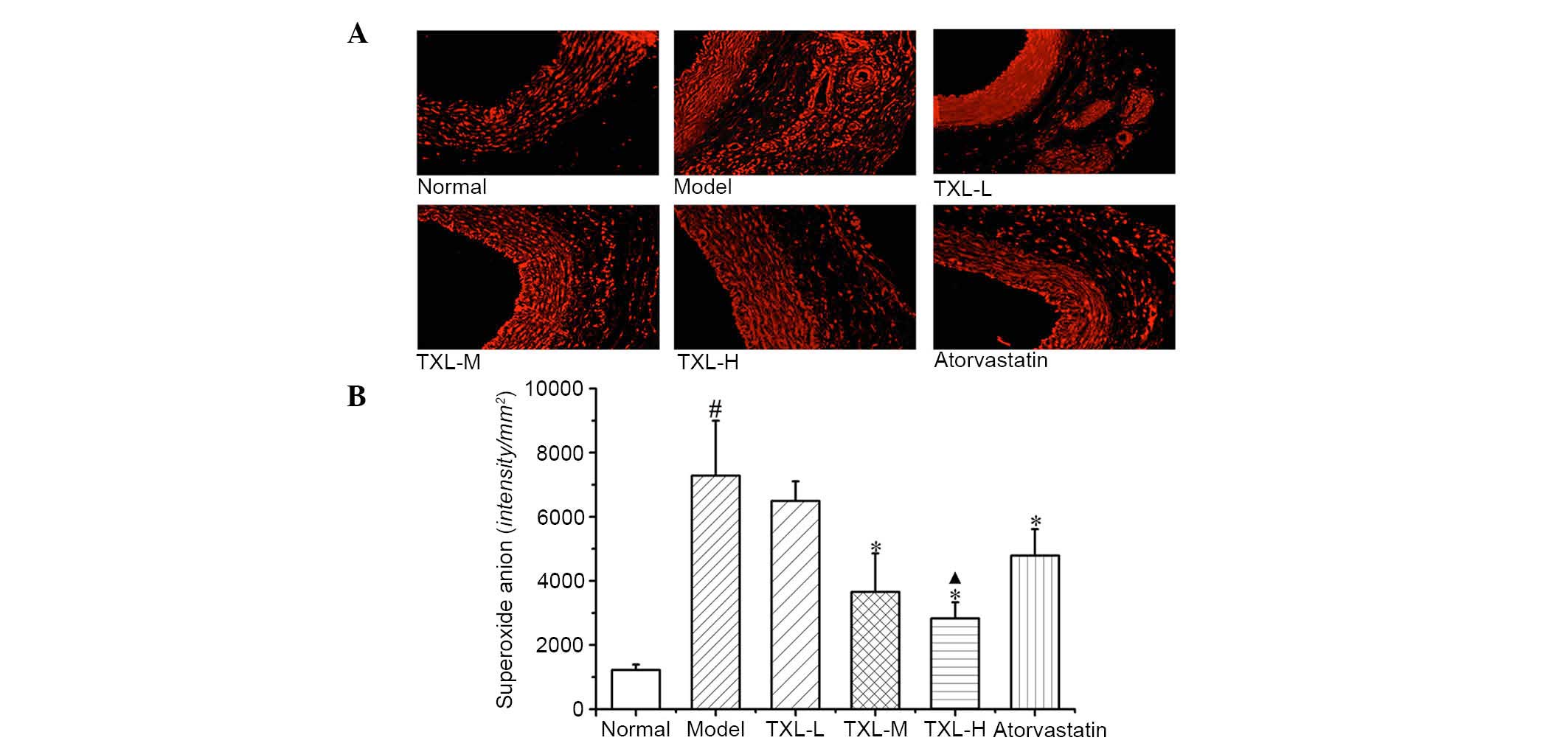
Superoxide production
As demonstrated in Fig.
2A, low O2− levels were detected in the
carotid intima, tunica media and adventitia of the normal group. In
the model group, all layers of vessels exhibited increased
O2− generation, primarily in the adventitia,
the endothelium of vasa vasorum and the surrounding areas.
Adventitia O2− levels in the TXL-H, TXL-M,
TXL-L and atorvastatin groups decreased to different extents.
According to quantitative analysis of fluorescence
intensity, the model group exhibited significantly higher
O2− fluorescence intensity in the adventitia
compared with the normal group (P<0.007; Fig. 2B). The TXL-H, TXL-M and
atorvastatin groups exhibited significantly lower levels of
O2− fluorescence intensity in the adventitia
compared with the model group tissues (P<0.002; Fig. 2B). The O2−
fluorescence intensity in the adventitia of the TXL-H group was
significantly lower compared with the atorvastatin group
(P<0.041; Fig. 2B).
Localization and expression of NADPH
oxidase subunits p22phox and gp91phox mRNA in carotid artery
According to the results of the in situ
hybridization localization experiments presented in Fig. 3A and B, the weak p22phox gene
hybridization signal associated with its expression in carotid
artery was confined to the adventitia in the normal group. The
distribution of the gp91phox gene hybridization signal indicated
that it was primarily expressed in the adventitia and to a lesser
extent in the intima. However, the gp91phox gene hybridization
signal detected in the adventitia was greater compared with
p22phox. In all layers of the vessels in the model group, the
p22phox and gp91phox gene hybridization signal associated with
their expression was strongly positive and primarily distributed in
the intima and adventitia. No difference was identified between the
expression of the p22phox and gp91phox genes in the TXL-L group and
the model group. In the TXL-H, TXL-M and atorvastatin groups, the
expression of p22phox and gp91phox were reduced in the
adventitia.
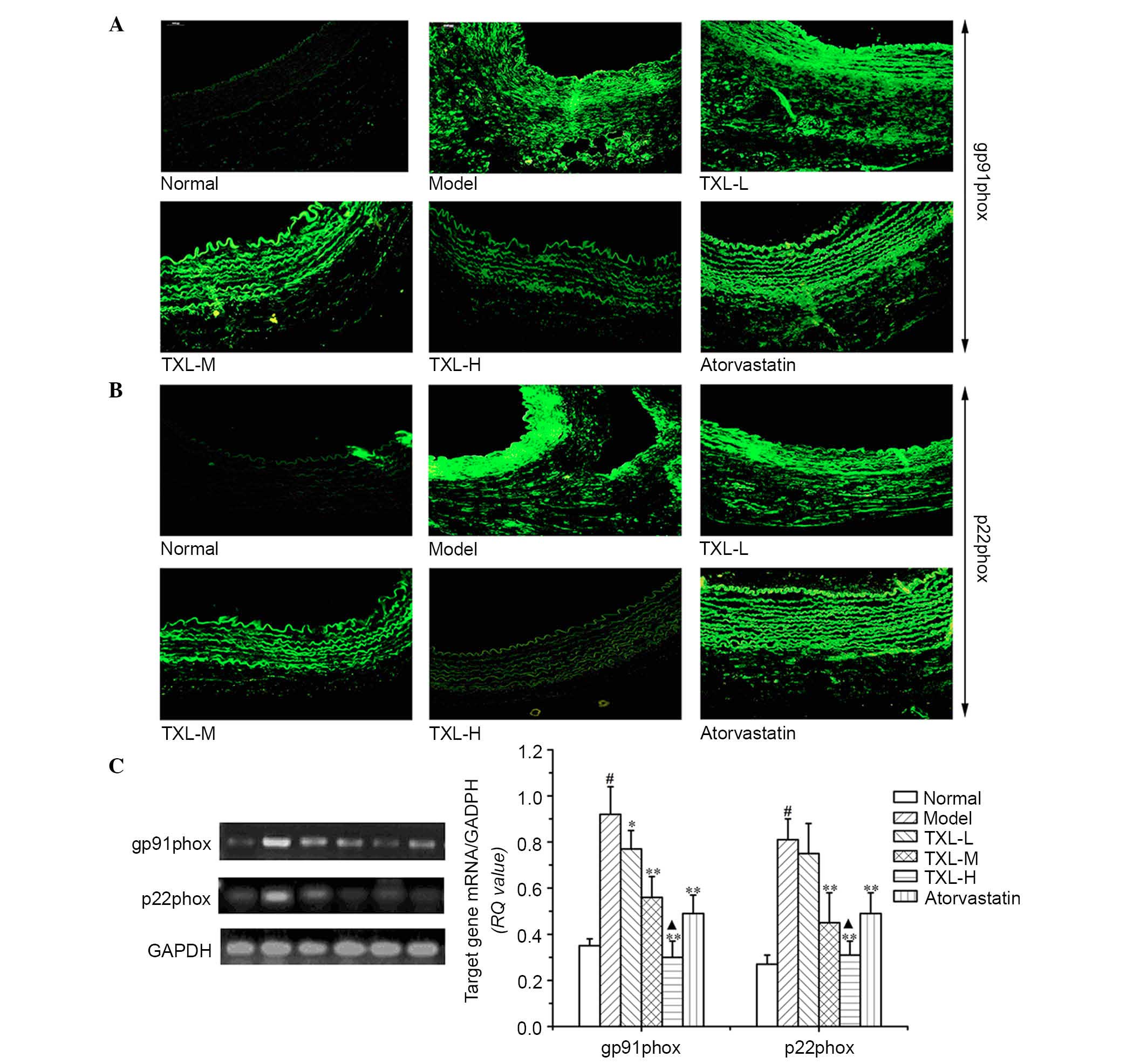 | Figure 3Localization and expression levels of
the NAD(P)H oxidase subunits p22phox and gp91phox mRNA in carotid
arteries. Each image is representative of results from 3 animals.
(A) p22phox mRNA were localized primarily in the adventitia of
normal arteries and in the adventitia, media and neointima of
injured arteries. (B) gp91phox mRNA were localized to the
adventitia or the intima in normal arteries and to the adventitia,
media or neointima in injured arteries, detected by in situ
hybridization. Magnification, ×200. (C) Expression levels of
p22phox and gp91phox mRNA of NAD(P)H oxidase subunits analyzed by
reverse transcription-polymerase chain reaction in carotid
arteries. Representative findings showing 77 and 442 bp products of
p22phox and gp91phox mRNA. The histograms represent the ratios of
the optical density of quantitative analyses. Densitometric
analysis was performed, data are presented as the mean ± standard
deviation of 3 experiments. #P<0.05 vs. normal group;
*P<0.05, **P<0.01 vs. model group;
▲P<0.05 vs. atorvastatin group. NAD(P)H, nicotinamide
adenine dinucleotide phosphate; TXL, tongxinluo; L, low; M, medium;
H, high. |
For evaluating the effects of ROS induced NADPH
oxidase were evaluated by analyzing, p22phox and gp91phox mRNA
expression using RT-PCR analysis. It was determined that the
p22phox and gp91phox mRNA expression levels in the model group were
significantly higher compared with the normal group (P<0.001;
Fig. 3C). No significant
difference was identified between the expression levels of p22phox
and gp91phox mRNAs between the TXL-L group and the model group
(Fig. 3C). The p22phox and
gp91phox mRNA expression levels in the TXL-H, TXL-M and
atorvastatin groups were significantly reduced compared with the
model group (P<0.005; Fig. 3C).
There was a significant reduction in the p22phox and gp91phox mRNA
expression levels in the TXL-H group compared with the atorvastatin
group (P<0.019; Fig. 3C).
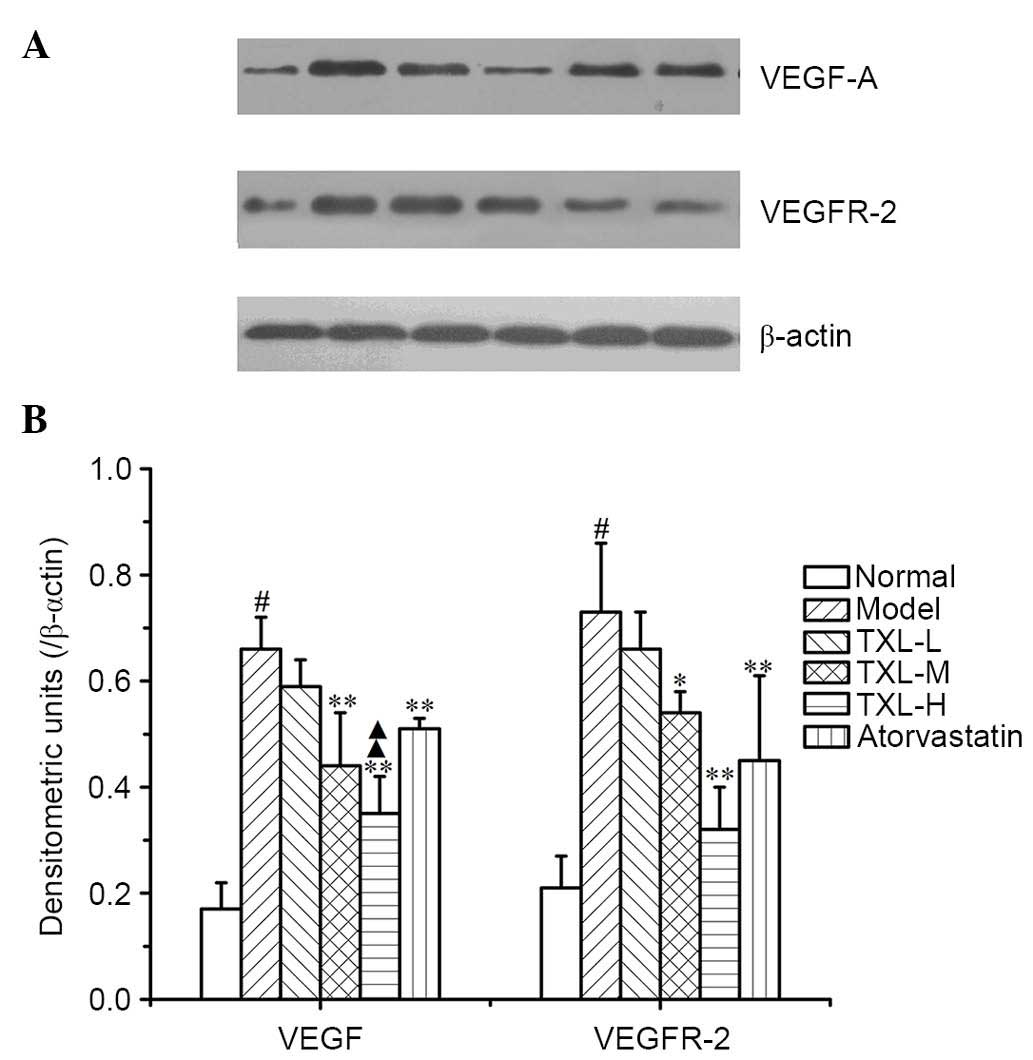
Relative protein expression levels of
VEGF and VEGFR-2
The model group exhibited significantly greater
protein expression levels of VEGF and VEGFR-2 in the carotid artery
compared with the normal group (P<0.003; Fig. 4). The TXL-H, TXL-M and atorvastatin
groups demonstrated significantly lower VEGF protein expression
levels compared with the model group (P<0.004; Fig. 4). The relative VEGF protein
expression level was significantly lower in the TXL-H group
compared with the atorvastatin group (P<0.004; Fig. 4). Additionally, the relative
expression level of the VEGFR-2 protein in the TXL-M group was
significantly lower compared with the model group (P<0.031;
Fig. 4). The VEGFR-2 expression
levels in the TXL-H and atorvastatin groups were also significantly
lower compared with the model group (P<0.008; Fig. 4). The expression levels of the
VEGFR-2 protein in the TXL-H group were reduced compared with the
atorvastatin group, however, no significant difference was
identified (Fig. 4).
Discussion
AS is a common arterial disease that seriously
threatens human health. Additionally, it is the pathological basis
underlying the development of cardiovascular and cerebrovascular
diseases. Previous studies have demonstrated that pathological
angiogenesis in the arterial wall is frequently observed during the
atherosclerotic process (17–20).
Angiogenesis is an important factor in the development of unstable
atherosclerotic lesions (21). The
results of the present study indicate that TXL inhibits adventitia
angiogenesis. The moderate dose of TXL (TXL-M group) exerted an
anti-angiogenic effect comparable with atorvastatin and the
high-dose of TXL (TXL-H group) exhibited a greater anti-angiogenic
effect when compared with atorvastatin, which was used as a
positive control medicine in the present study, as it regulates
angiogenesis. Previous studies have reported that atorvastatin
treatment may decrease angiogenesis and suppress formation of
plaques (22–24). It is possible that the anti-AS
mechanism of TXL is associated with its inhibitory effect on
angiogenesis. This may be due to its ability to reduce blood
perfusion and flow velocity in the vasa vasorum, decreasing the
exchanged endothelial area in vascular walls, thus preventing
infiltration of pro-inflammatory and pro-arteriosclerotic blood
components into the arterial wall; therefore, weakening the
inflammatory reaction in the vascular wall (25–27).
Anti-angiogenic treatment with TXL may be an effective AS treatment
strategy.
Previous studies have identified ROS as regulators
of cellular signaling and survival, with cell signaling and disease
being investigated in in vivo and in vitro studies.
Additionally, ROS involvement has been identified in pathological
neovascularization (28–30). ROS may trigger various redox
signaling pathways and lead to altered expression of
angiogenesis-associated genes, migration and proliferation of
endothelial cells, re-arrangement of the cytoskeleton (31) and the occurrence of tubular shape
(32), which may further
facilitate angiogenesis. The results of the present study indicated
that compared with the model group, TXL treatment decreased ROS
levels in adventitia in a dose-dependent manner and downregulated
expression of the pro-angiogenic factors involved in the
VEGF/VEGFR-2 signaling pathway. This indicated that the possible
mechanism of inhibition of angiogenesis by TXL treatment in
hyperlipidemic rabbits is associated with reduced adventitia ROS
generation and the resulting activation of the downstream
VEGF/VEGFR-2 signaling pathway. In angiogenesis, ROS may directly
stimulate VEGF, which induces proliferation and migration of
endothelial cells. Additionally, VEGF may activate NADPH oxidase,
increase ROS generation (33),
initiate VEGFR-2 autophosphorylation, provoke endothelial cell
proliferation and migration and lumen formation; therefore, TXL may
inhibit a positive feedback pathway involved in oxidative stress
during angiogenesis.
In order to determine the effect of ROS on
angiogenesis in the arterial wall, the mRNA levels of the NADPH
oxidase subunits were detected. NADPH oxidase is a crucial enzyme
involved in endovascular ROS generation in cardiovascular diseases
(34). As a combined enzyme agent
with multiple subunits, it is composed of heterodimers that include
the membrane subunits p22phox and gp91phox, and several cytoplasmic
protein components, including p47phoX, p67phox, p40phox and a small
G protein, Rac1. gp91phox and p22phox are the core of NADPH oxidase
(35). A previous study determined
that NADPH oxidase is an important regulator of angiogenesis
(36). To the best of our
knowledge, the effects of p22phox and gp91phox on pathological
angiogenesis in the arterial wall at the early stage of AS
development have not been widely reported. The present study
determined that during neovascularization, the levels of p22phox
and gp91phox mRNA adjacent to the vasa vasorum and the carotid
artery were increased. TXL reduced the p22phox and gp91phox
expression levels in the carotid artery and adventitia. These
results indicated that p22phox and gp91phox may be important
regulators of adventitia angiogenesis, and that TXL may reduce ROS
generation by downregulating p22phox and gp91phox expression levels
in adventitia. As demonstrated by a previous study, p22phox gene
knock-out mice exhibited reduced tumor neovascularization (37). Additionally, during in vitro
culture of endothelial cells, gene silencing of p22phox partially
limited ROS generation, reduced peroxisome proliferator-activated
receptor expression and inhibited angiogenesis (37). In vitro experiments
performed in a previous study determined that gp91phox was involved
in endothelial cell proliferation, survival and that gp91phox gene
silencing may influence endothelial cell morphology (38). Additionally, the hind limb ischemia
model determined that gp91phox gene knock-out mice exhibited a
delayed increase in capillary density. This mechanism may be
associated with the inhibited combination of gp91phox with actin,
IQ motif containing GTPase activating protein 1 and Rac1 binding
protein, and the promotion of endothelial cell migration (39). The current study has determined
that TXL may suppress neovascularization through the downregulation
of p22phox and gp91phox gene expression in the adventitia.
In conclusion, the present study identified that TXL
may inhibit adventitia angiogenesis in hyperlipidemic rabbits. This
is potentially associated with the downregulation of adventitia ROS
generation and the VEGF/VEGFR-2 signaling pathway. Therefore, TXL
may be a potential therapeutic agent for treating hyperlipidemia
and inhibiting angiogenesis. However, no direct causal association
was observed in the present study and whether the observed effect
is the cause or consequence of the anti-AS function of TXL has not
been fully determined. The current study was performed using
rabbits; however, human clinical trials should be conducted in the
future in order to elucidate the effect of TXL on AS.
References
|
1
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palinski W, Ord VA, Plump AS, Breslow JL,
Steinberg D and Witztum JL: ApoE-deficient mice are a model of
lipoprotein oxidation in atherogenesis. Demonstration of
oxidation-specific epitopes in lesions and high titers of
autoantibodies to malondialdehyde-lysine in serum. Arterioscler
Thromb. 14:605–616. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitka M: Cholesterol drug lowers LDL-C
levels but again fails to show clinical benefit. JAMA. 303:211–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herrmann J, Lerman LO, Rodriguez-Porcel M,
Holmes DR Jr, Richardson DM, Ritman EL and Lerman A: Coronary vasa
vasorum neovascularization precedes epicardial endothelial
dysfunction in experimental hypercholesterolemia. Cardiovasc Res.
51:762–766. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Staub D, Patel MB, Tibrewala A, Ludden D,
Johnson M, Espinosa P, Coll B, Jaeger KA and Feinstein SB: Vasa
vasorum and plaque neovascularization on contrast-enhanced carotid
ultrasound imaging correlates with cardiovascular disease and past
cardiovascular events. Stroke. 41:41–47. 2010. View Article : Google Scholar
|
|
7
|
Langheinrich AC, Michniewicz A, Sedding
DG, Walker G, Beighley PE, Rau WS, Bohle RM and Ritman EL:
Correlation of vasa vasorum neovascularization and plaque
progression in aortas of apolipoprotein E(−/−)/low-density
lipoprotein(−/−) double knockout mice. Arterioscler Thromb Vasc
Biol. 26:347–352. 2006. View Article : Google Scholar
|
|
8
|
Tanaka K, Nagata D, Hirata Y, Tabata Y,
Nagai R and Sata M: Augmented angiogenesis in adventitia promotes
growth of atherosclerotic plaque in apolipoprotein E-deficient
mice. Atherosclerosis. 215:366–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gössl M, Herrmann J, Tang H, Versari D,
Galili O, Mannheim D, Rajkumar SV, Lerman LO and Lerman A:
Prevention of vasa vasorum neovascularization attenuates early
neointima formation in experimental hypercholesterolemia. Basic Res
Cardiol. 104:695–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson SH, Herrmann J, Lerman LO, Holmes
DR Jr, Napoli C, Ritman EL and Lerman A: Simvastatin preserves the
structure of coronary adventitial vasa vasorum in experimental
hypercholesterolemia independent of lipid lowering. Circulation.
105:415–418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moulton KS, Vakili K, Zurakowski D,
Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K
and Folkman J: Inhibition of plaque neovascularization reduces
macrophage accumulation and progression of advanced
atherosclerosis. Proc Natl Acad Sci USA. 100:4736–4741. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moulton KS, Heller E, Konerding MA, Flynn
E, Palinski W and Folkman J: Angiogenesis inhibitors endostatin or
TNP-470 reduce intimal neovascularization and plaque growth in
apolipoprotein E-deficient mice. Circulation. 99:1726–1732. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen WQ, Zhong L, Zhang L, Ji XP, Zhao YX,
Zhang C, Jiang H, Wu YL and Zhang Y: Chinese medicine tongxinluo
significantly lowers serum lipid levels and stabilizes vulnerable
plaques in a rabbit model. J Ethnopharmacol. 124:103–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Liu Y, Lu XT, Wu YL, Zhang C, Ji
XP, Wang R, Liu CX, Feng JB, Jiang H, et al: Traditional Chinese
medication Tongxinluo dose-dependently enhances stability of
vulnerable plaques: A comparison with a high-dose simvastatin
therapy. Am J Physiol Heart Circ Physiol. 297:H2004–H2014. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weidner N, Carroll P, Flax J, Blumenfeld W
and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
16
|
Purushothaman KR, Sanz J, Zias E, Fuster V
and Moreno PR: Atherosclerosis neovascularization and imaging. Curr
Mol Med. 6:549–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy H, Bhardwaj S, Babu M, Kokina I,
Uotila S, Ahtialansaari T, Laitinen T, Hakumaki J, Laakso M, Herzig
KH and Ylä-Herttuala S: VEGF-A, VEGF-D, VEGF receptor-1, VEGF
receptor-2, NF-kappaB, and RAGE in atherosclerotic lesions of
diabetic Watanabe heritable hyperlipidemic rabbits. FASEB J.
20:2159–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moulton KS: Angiogenesis in
atherosclerosis: gathering evidence beyond speculation. Curr Opin
Lipidol. 17:548–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura R, Sene A, Santeford A, Gdoura A,
Kubota S, Zapata N and Apte RS: IL10-driven STAT3 signalling in
senescent macrophages promotes pathological eye angiogenesis. Nat
Commun. 6:78472015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szöcs K, Lassègue B, Sorescu D, Hilenski
LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD and
Griendling KK: Upregulation of Nox-based NAD(P)H oxidases in
restenosis after carotid injury. Arterioscler Thromb Vasc Biol.
22:21–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moreno PR, Purushothaman KR, Fuster V,
Echeverri D, Truszczynska H, Sharma SK, Badimon JJ and O'Connor WN:
Plaque neovascularization is increased in ruptured atherosclerotic
lesions of human aorta: Implications for plaque vulnerability.
Circulation. 110:2032–2038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weis M, Heeschen C, Glassford AJ and Cooke
JP: Statins have biphasic effects on angiogenesis. Circulation.
105:739–745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian J, Hu S, Jia H, Hou J, Zhang S, Yu B
and Jang IK: Correlation of vasa vasorum and plaque progression and
response to atorvastatin therapy an a rabbit model of
atherosclerosis: In vivo intravascular ultrasound and
contrast-enhanced ultrasound imaging study. J Am Coll Card.
59:E10722012. View Article : Google Scholar
|
|
24
|
Bot I, Jukema JW, Lankhuizen IM, van
Berkel TJ and Biessen EA: Atorvastatin inhibits plaque development
and adventitial neovascularization in ApoE deficient mice
independent of plasma cholesterol levels. Atherosclerosis.
214:295–300. 2011. View Article : Google Scholar
|
|
25
|
Fleiner M, Kummer M, Mirlacher M, Sauter
G, Cathomas G, Krapf R and Biedermann BC: Arterial
neovascularization and inflammation in vulnerable patients: Early
and late signs of symptomatic atherosclerosis. Circulation.
110:2843–2850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moreno PR, Purushothaman KR, Sirol M, Levy
AP and Fuster V: Neovascularization in human atherosclerosis.
Circulation. 113:2245–2252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rademakers T, Douma K, Hackeng TM, Post
MJ, Sluimer JC, Daemen MJ, Biessen EA, Heeneman S and van Zandvoort
MA: Plaque-associated vasa vasorum in aged apolipoprotein
E-deficient mice exhibit proatherogenic functional features in
vivo. Arterioscler Thromb Vasc Biol. 33:249–256. 2013. View Article : Google Scholar
|
|
28
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar :
|
|
29
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Liu B, Yuan J, Yang J, Zhang J, An
Y, Tie L, Pan Y and Li X: Atorvastatin reduces vascular endothelial
growth factor (VEGF) expression in human non-small cell lung
carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS)
production. Mol Oncol. 6:62–72. 2012. View Article : Google Scholar
|
|
31
|
Vepa S, Scribner WM, Parinandi NL, English
D, Garcia JG and Natarajan V: Hydrogen peroxide stimulates tyrosine
phosphorylation of focal adhesion kinase in vascular endothelial
cells. Am J Physiol. 277:L150–L158. 1999.PubMed/NCBI
|
|
32
|
Shono T, Ono M, Izumi H, Jimi SI,
Matsushima K, Okamoto T, Kohno K and Kuwano M: Involvement of the
transcription factor NF-kappaB in tubular morphogenesis of human
microvascular endothelial cells by oxidative stress. Mol Cell Biol.
16:4231–4239. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giacca M and Zacchigna S: VEGF gene
therapy: Therapeutic angiogenesis in the clinic and beyond. Gene
Ther. 19:622–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Griendling KK, Sorescu D and Ushio-Fukai
M: NAD(P)H oxidase: Role in cardiovascular biology and disease.
Circ Res. 86:494–501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vignais PV: The superoxide-generating
NADPH oxidase: Structural aspects and activation mechanism. Cell
Mol Life Sci. 59:1428–1459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Konior A, Schramm A, Czesnikiewicz-Guzik M
and Guzik TJ: NADPH oxidases in vascular pathology. Antioxid Redox
Signal. 20:2794–2814. 2014. View Article : Google Scholar :
|
|
37
|
Garrido-Urbani S, Jemelin S, Deffert C,
Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet
X, Michalik L, et al: Targeting vascular NADPH oxidase 1 blocks
tumor angiogenesis through a PPARα mediated mechanism. PLoS One.
6:e146652011. View Article : Google Scholar
|
|
38
|
Ikeda S, Yamaoka-Tojo M, Hilenski L,
Patrushev NA, Anwar GM, Quinn MT and Ushio-Fukai M: IQGAP1
regulates reactive oxygen species-dependent endothelial cell
migration through interacting with Nox2. Arterioscler Thromb Vasc
Biol. 25:2295–2300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coso S, Harrison I, Harrison CB, Vinh A,
Sobey CG, Drummond GR, Williams ED and Selemidis S: NADPH oxidases
as regulators of tumor angiogenesis: Current and emerging concepts.
Antioxid Redox Signal. 16:1229–1247. 2012. View Article : Google Scholar : PubMed/NCBI
|