Introduction
Hypertension is a major health problem and
predominant risk factor for the occurrence of numerous diseases,
including heart failure, myocardial infarction, stroke and
peripheral arterial disease (1,2).
Hypertension is capable of promoting arterial remodeling, which is
an adaptive process that occurs in response to long-term
alterations in the hemodynamic condition of hypertension,
predominantly including vessel wall thickening and histological
abnormalities, hemal wall/lumen ratio increases and endothelial
dysfunction (3). The process of
arterial remodeling is fundamental to numerous vascular diseases
that remain challenging to effectively treat. Thus, therapeutic
strategies directed at influencing the remodeling response may have
clinical importance.
It is evident that oxidative stress is associated
with the pathogenesis of numerous diseases, including vascular
injury (4). In addition, it has
been reported that oxidative stress is present in the arterial
remodeling of hypertension. Reactive oxygen species (ROS) at
moderate concentrations act as signaling molecules and second
messengers, which serve an important role in maintaining the
structure and function of the vascular integrity (5,6). In
contrast to these regulatory functions under physiological
conditions, excessive or sustained ROS production, when exceeding
the available antioxidant defense systems, leads to oxidative
stress. Oxidative stress damages the endothelium and impairs
endothelium-dependent vasodilatation, consequently resulting in
endothelial dysfunction, and promoting the proliferation of
vascular smooth muscle cells in addition to collagen deposition,
which cause thickening of the tunica media and narrowing of the
vascular lumen (7). This results
in the occurrence and the development of hypertension (8,9).
These observations demonstrate that oxidative stress is involved in
the occurrence and development of arterial remodeling in
hypertension, and suggest that arterial remodeling may be
alleviated by its inhibition.
Grape seed proanthocyanidin extract (GSPE) is a
combination of biologically active polyphenolic flavonoids, and
contains oligomeric proanthocyanidins, which have been demonstrated
to exhibit a spectrum of biological, pharmacological, therapeutic
and chemoprotective properties against oxygen-free radicals and
oxidative stress (10). This range
of biochemical and cellular functions suggests potential for the
prevention and treatment of a variety of human disorders caused by
oxidative stress. Due to the fact that oxidative damage has been
associated with the arterial remodeling in hypertension, the
curernt study aimed to evaluate the effect of GSPE on arterial
remodeling, which has not, to the best of our knowledge been
previously investigated.
Materials and methods
Animal preparation
A total of 20 20-week-old male spontaneously
hypertensive rats (SHRs) weighing 302.25±7.29 g and 10 20-week-old
Wistar-Kyoto rats (WKYs) weighing 298.25±6.40 g were purchased from
Vital River Laboratory Animal Co., Ltd. (Beijing, China). The rats
were kept in cages at 22±2°C and 50–55% humidity with 12-h
light/dark cycles and had access to standard rat feed and water
ad libitum. The rats were randomly assigned to three groups
(n=10 per group): WKY-C (WKY control rats treated with 1 ml 0.9%
nitric sodium orally), SHR-C (SHR control rats treated with 1 ml
0.9% nitric sodium orally) and SHR-T (SHRs treated with GSPE at a
dosage of 250 mg/kg·day). In previous studies, rats treated with
GSPE at a dosage of 250 mg/kg·day exhibited a more significant
biological effect without pharmacological toxicity (11,12).
The animals were subjected to drug administration as described
above by oral gavage for 22 weeks. All experiments were approved
and performed in accordance with the National Institute of Health
Guide for the Care and Use of Laboratory Animals (13), with approval from the Institutional
Animal Care and Use Committee of Qilu Hospital, Shandong University
(Jinan, China).
Chemicals
GSPEs (containing 56% dimeric proanthocyanidins, 12%
trimeric proanthocyanidins, 6.6% tetrameric proanthocyanidins and
small amounts of monomeric and high-molecular-weight oligomeric
proanthocyanidins and flavanols) were provided by Tianjin Jianfeng
Natural Product R&D Co., Ltd. (Tianjin, China). The components
of GSPE were analyzed using high-performance liquid chromatography
with gas chromatography-mass spectrometry detection.
Systolic blood pressure (SBP)
measurement
SBP was measured in conscious animals prior to the
start of treatment and weekly during treatment. SBP was determined
using the tail-cuff method (BP-2006A; Softron Beijing Incorporated,
Beijing, China). Prior to measurement, animals were placed in a
heated chamber at an ambient temperature of 30–34°C for 15 min and
were conditioned to numerous cuff inflation-deflation cycles by a
trained operator. Three consecutive SBP readings were collected and
averaged to obtain the exact SBP for presentation.
Tissue collection
At the end of week 22, animals in all groups were
sacrificed by decapitation under 3% sodium pentobarbital. Thoracic
aortas were removed completely and rapidly. Sections of the
thoracic aorta (4 µm) were fixed in buffered 10% formalin
for hematoxylin-eosin or sirius red-victoria blue staining, while
others were frozen in liquid nitrogen and stored at −80°C for the
biochemical assays.
Histopathological evaluation
Specimens of the middle part of the thoracic aorta
were fixed for 24 h in 10% formalin, routinely processed in
paraffin and cut into 4-µm-thick slices for staining with
hematoxylin-eosin or sirius red-victoria blue. Briefly, the
paraffin sections were deparaffinized and rehydrated in distilled
water. Following washing with 70% ethanol for 2 min, the sections
were stained in victoria blue solution for 12 h at 37°C. The
sections were then briefly washed with 95% ethanol for several
seconds and with distilled water for 2 min prior to staining with
1% picrosirius red F3BA (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) for 1 h. The sections were then washed in running tap
water for 10 min prior to dehydration, clearing and mounting.
Victoria blue- and picrosirius red-stained sections were visualized
by bright field and polarized light under an Olympus BX53
microscope (Olympus Corporation, Tokyo, Japan) and measured using
Image-Pro Plus software, version 5.0 (Media Cybernetics, Inc.,
Rockville, MD) to obtain the percentage of collagen per media area
and the collagen-elastic ratio. The wall thickness (WT), inner
diameter (ID) and aorta radius (AR) of the thoracic aorta were
determined using an Olympus DP71 camera (Olympus Corporation), and
then the outer diameter (OD), vascular cross-sectional area (VCSA),
wall cross-sectional area (WSCA), luminal cross-sectional area
(LCSA), wall-lumen ratio (wall thickness:inner diameter) and
WSCA/LCSA were calculated.
Ultrastructural examination
A portion of the thoracic aorta was fixed in 3%
glutaraldehyde (Nanjing Chemical Reagent Co., Ltd., Nanjing,
China). Ultrathin sections cut from the embedded blocks were
stained with uranyl acetate and lead citrate and then observed with
an H-800 transmission electron microscope (Hitachi, Ltd., Tokyo,
Japan).
Pulse wave velocity (PWV)
Two polyethylene cannulas were inserted into the
aorta via the left carotid and the left femoral arteries for
central and peripheral blood pressure measurements, respectively.
The aorta cannulas were connected to a pressure recording system
(MP100CE) and with AcqKnowledge software, version 3.7.3 (BioPAC
Systems, Inc., Goleta, CA, USA). PWV (cm/s) was calculated as the
distance between the central and peripheral cannula tips divided by
the transit time. The distance between the two central and
peripheral cannula tips was measured in situ subsequent to
postmortem fixation by sticking a damp cotton thread onto the
aorta. Transit times between the two central and peripheral
pressure signals were measured online for each 5 sec period by peak
detectors of the AcqKnowledge software, which systematically
shifted the peripheral pressure waveform in time with respect to
the central pressure waveform and determined the value of the
time.
Enzyme-linked immunosorbent assay
The aorta tissue was sectioned as small as possible
that were homogenized 1:9 (w:v) in 0.9% saline. The homogenates
were then centrifuged at 1,500 × g for 5 min at 4°C, and the
supernatant was used to determine the content of nitric oxide (NO)
and endothelin-1 (ET-1) and the levels of superoxide dismutase
(SOD), catalase (CAT) and malondialdehyde (MDA). The quantity of NO
and ET-1 in the aorta tissues was measured according to
manufacturer's instructions using the NO Assay kit and the ET-1
Assay kit, respectively (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). The levels of SOD, CAT and MDA in aorta
tissue were performed according to manufacturer's instructions
using the SOD Assay kit, the CAT Assay kit and the Microscale MDA
Assay kit, respectively (Nanjing Jiancheng Bioengineering
Institute).
Statistical analysis
Statistical analysis was performed by one-way
analysis of variance using SPSS software, version 19.0 (SPSS, Inc.,
Chicago, IL, USA). The results are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
GSPE ameliorates alterations in arterial
remodeling
As presented in Table
I, wall thickness, OD, AR, VCSA, WCSA, wall-lumen ratio,
WCSA/LCSA and PWV were significantly increased in the SHR-C group
compared with the WKY-C group. Following administration of GSPE (at
a dose of 250 mg/kg·day), the above parameters were reversed,
indicating that GSPE reversed arterial remodeling. To further
investigate the effect of GSPE on arterial remodeling, thoracic
aortas were stained with hematoxylin-eosin or sirius red-victoria
blue.
 | Table IEffect of grape seed proanthocyanidin
extract on the parameters of arterial remodeling. |
Table I
Effect of grape seed proanthocyanidin
extract on the parameters of arterial remodeling.
| Parameter | Groups
|
|---|
| WKY-C | SHR-C | SHR-T |
|---|
| WT (µm) | 109.99±17.14 | 234.69±51.76b | 123.44±21.52d |
| ID (µm) | 1939.72±188.25 | 2286.03±37.18a | 2146.63±121.73 |
| Wall-lumen ratio
(%) | 5.76±1.40 | 10.26±2.27a | 5.74±0.77c |
| OD (µm) | 2551.94±168.60 | 2863.10±94.76b |
2457.40±160.16c |
| AR (µm) | 1079.85±77.17 |
1377.71±56.68b |
1196.75±78.02c |
| VCSA
(µm2) |
3995782.31±600797.63 |
6439627.69±423165.96b |
4753893.19±624694.74c |
| WCSA
(µm2) |
1023674.09±31811.67 |
2336546.56±374561.53b |
1128830.56±241649.31d |
| LCSA
(µm2) |
2972108.22±580826.92 |
4103081.13±133875.61a |
3625062.64±410015.65 |
| WCSA/LCSA (%) | 35.21±5.86 | 56.94±9.05b | 31.01±4.04d |
| PWV (cm/s) | 2492.10±789.70 |
6681.09±2154.93 | 4283.10±946.05 |
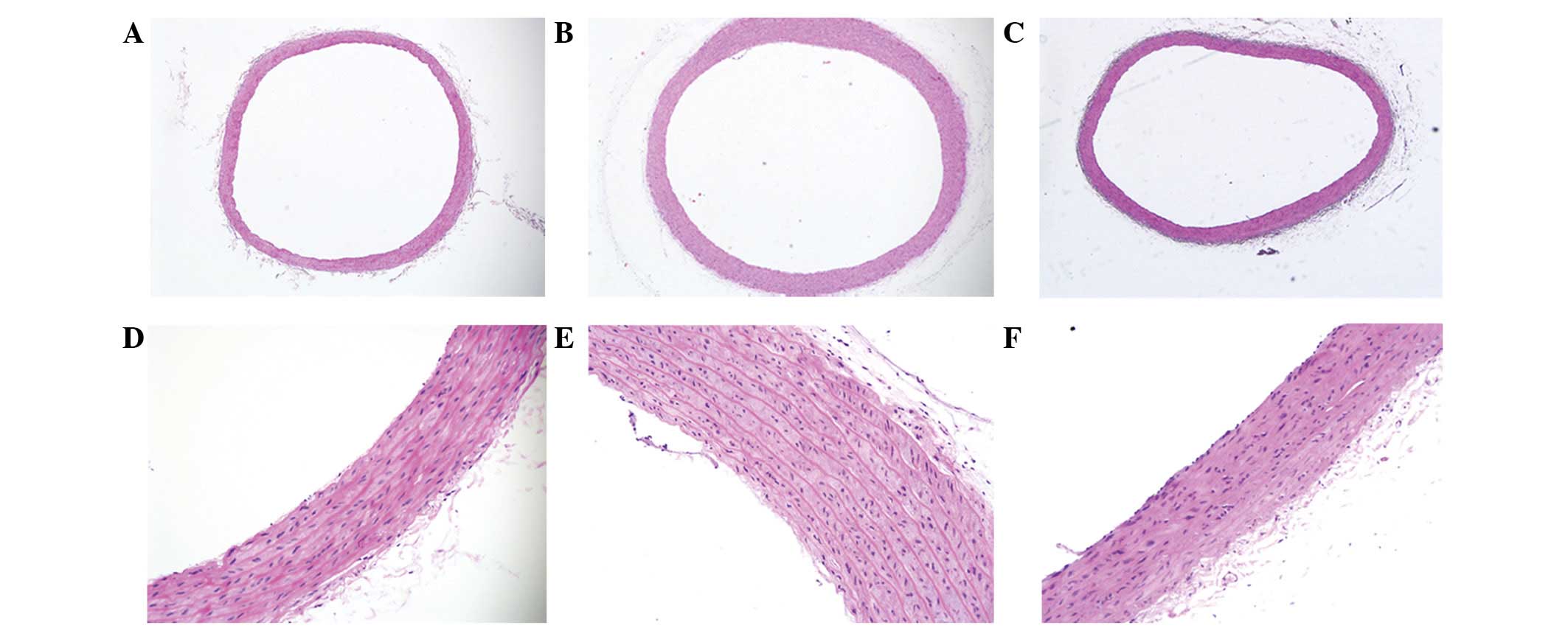
Hematoxylin and eosin staining indicated that the
arrangement of the elastic fibers in the aortas in the WKY-C group
rats was normal and that there was no collagen hyperplasia in the
vessel wall, while the aortic wall in the SHR-C group rats was
thickened, with hyperplastic collagen fibers in the media and with
reduced, disordered and even ruptured elastic fibers. However, the
aortic elastic fibers in the SHR-T group remained ordered (Fig. 1).
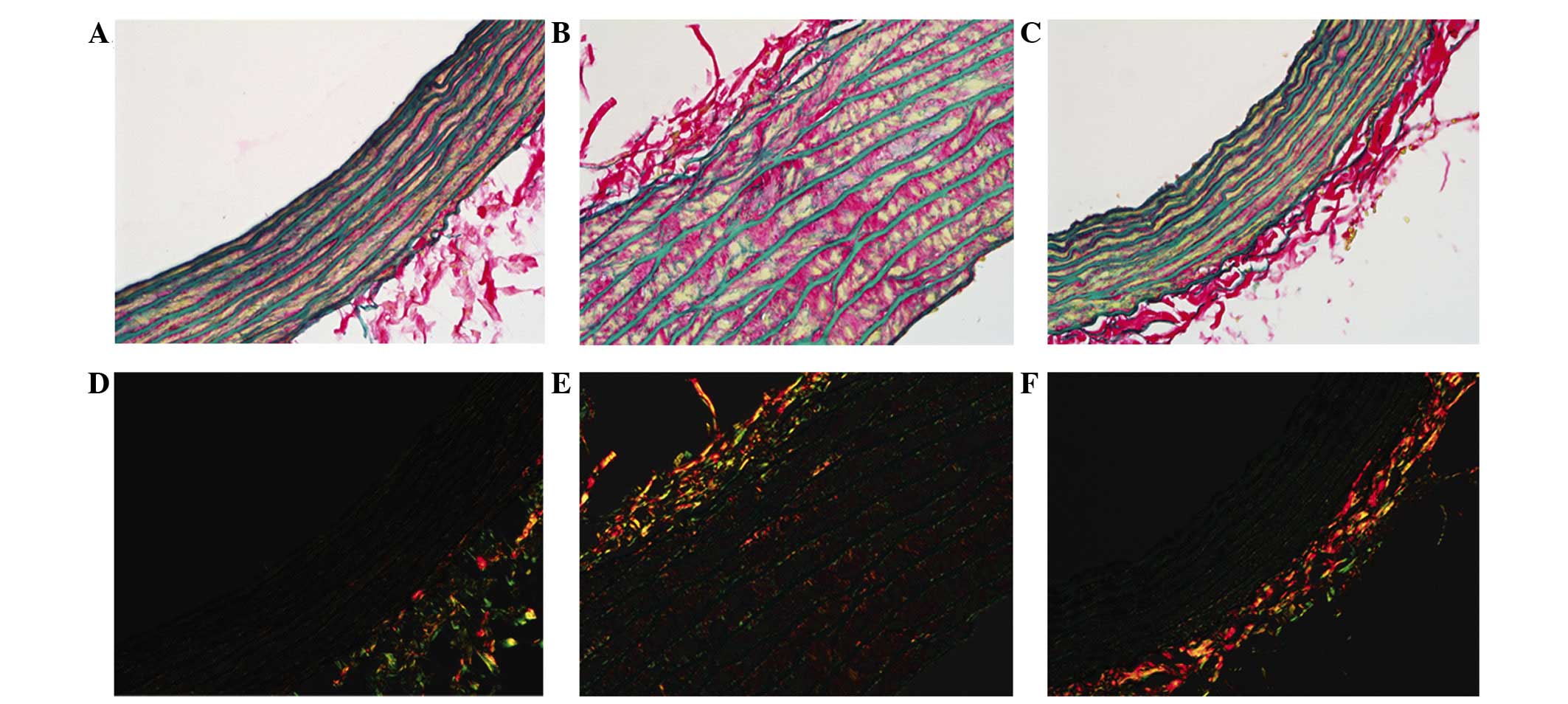
Sirius red-victoria blue staining was observed in
the histological images (Fig. 2),
where red fibers represent collagen and blue fibers show elastin in
the bright fields, and under polarized light, the collagen I fibers
presented orange-red, whereas the thinner collagen III fibers
appeared yellow-green (14).
Previous studies demonstrated that certain conditions, such as
hypertension and atherosclerosis, stimulate vascular smooth muscle
cells (VSMCs) to produce extra collagen in the vessel wall
(15,16) and that the arterial collagen
content increases progressively when blood pressure rises (17,18).
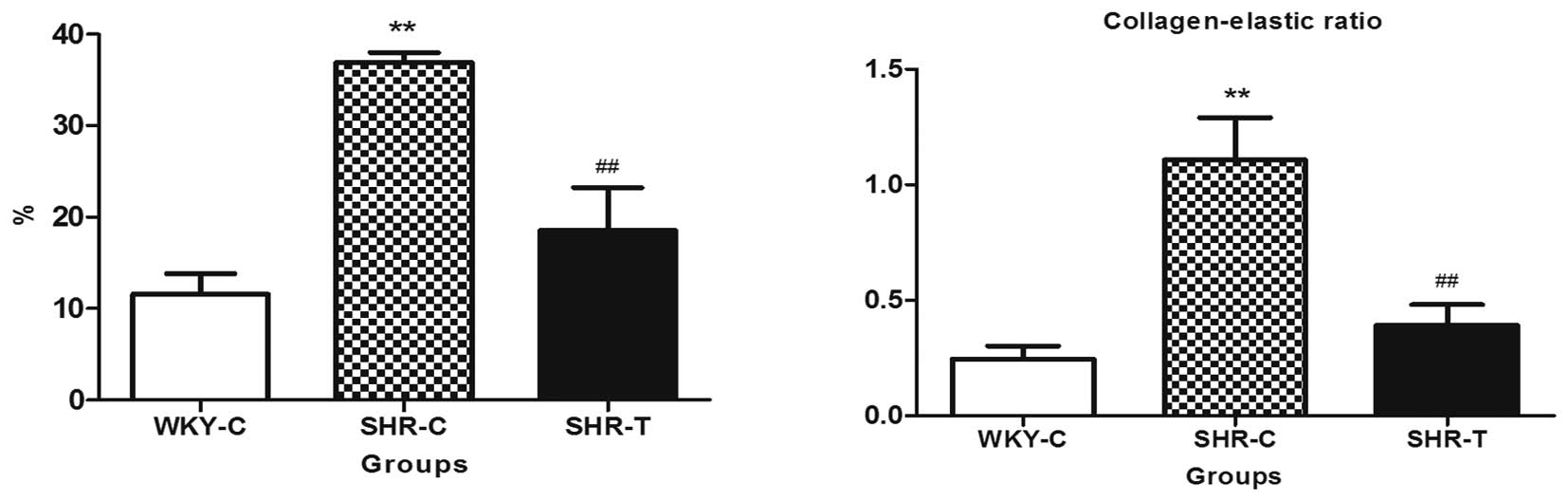
The present study supports this theory by
demonstrating a marked increase in total collagen content per media
area of the aortic segment for the SHRs compared with the WKY rats.
In the SHR-C group, the aortic fibration was promoted by increasing
collagen deposition in the wall, particularly collagen type I,
while it was lower in the SHR-T group when compared with the SHR-C
group. In addition, the ratio of collagen fibers and elastic fibers
was reduced more in the SHR-T group than in the SHR-C group.
Collagen content and the collagen-elastic ratio were lower in the
SHR-T group than in the SHR-C tissues, indicating that GSPE reduced
collagen deposition (Fig. 3).
GSPE preserves ultrastructural
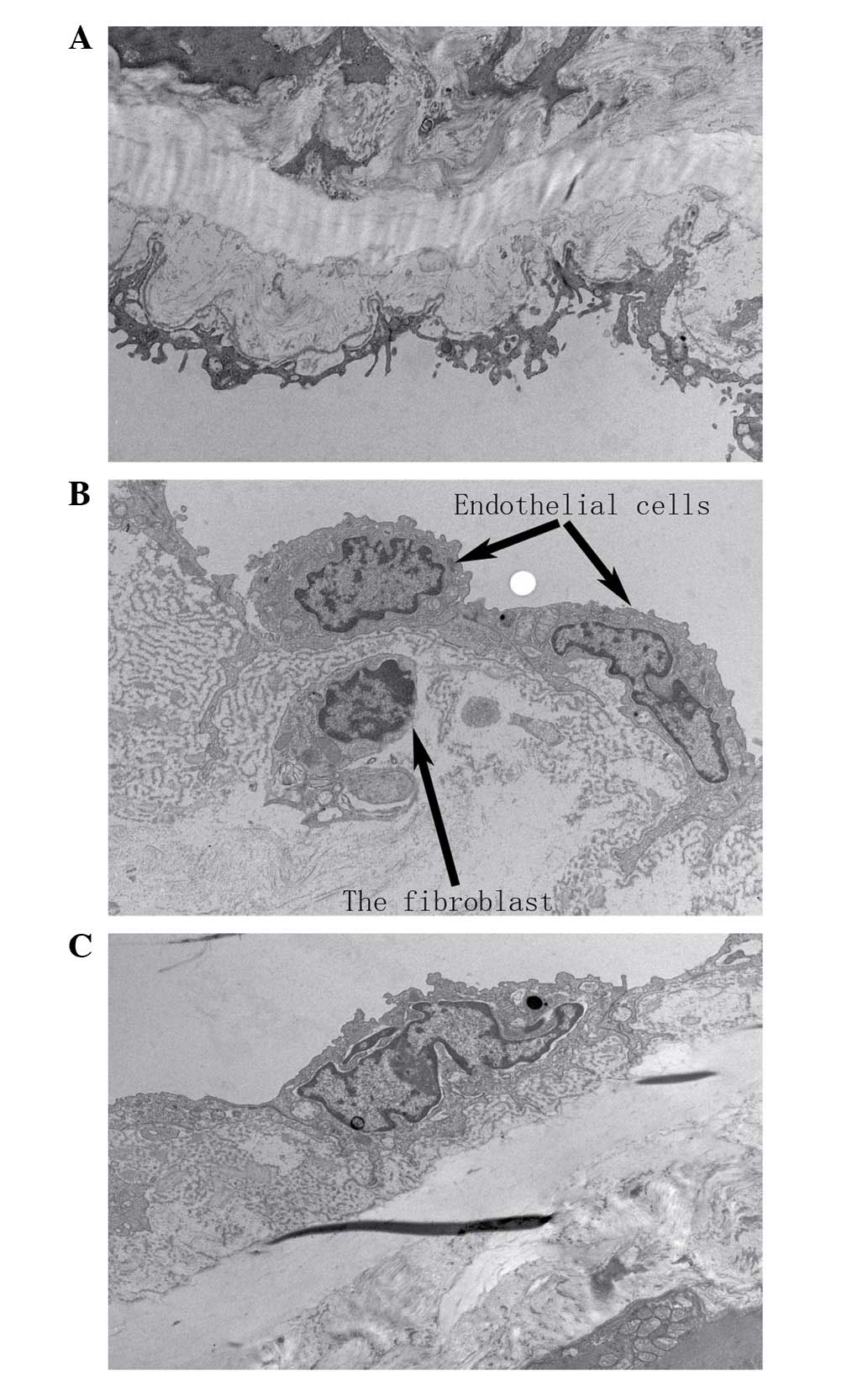
alterations of the thoracic aorta
In the SHR-C group, the repeatedly repaired basement
membrane had numerous holes, and the internal elastic lamina was
split, with abundant extracellular matrix content intertwined with
the basement membrane. In addition, fibroblast-like cells were
present in the subendothelium, and the apoptosis of endothelial
cells was observed in the SHR-C group, whereas normal
ultrastructure was observed in the aortic tissues of the WKY-C
group. The administration of GSPE resulted in improvements to the
preservation of the fine structure of the basement membrane and
internal elastic lamina and a reduction in the number of inserted
fibroblast-like cells in the subendothelium (Fig. 4).
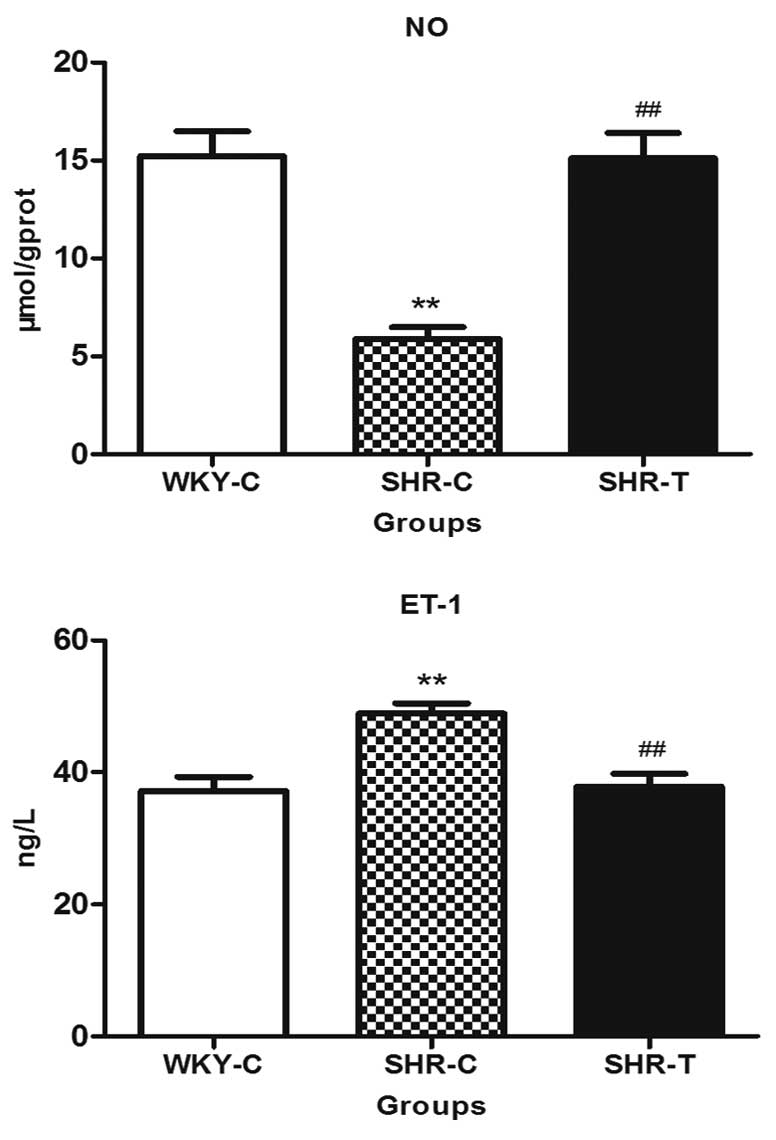
GSPE significantly improves endothelial
function
The content of NO in the thoracic aorta in the SHR-C
group reduced significantly, while GSPE treatment increased NO
production when comparing the SHR-T group with the SHR-C group.
However, ET-1 expression was significantly increased in the SHR-C
group when compared with that of the WKY-C group, which was capable
of being reversed by GSPE treatment, indicating that GSPE
significantly improved endothelial function (Fig. 5).
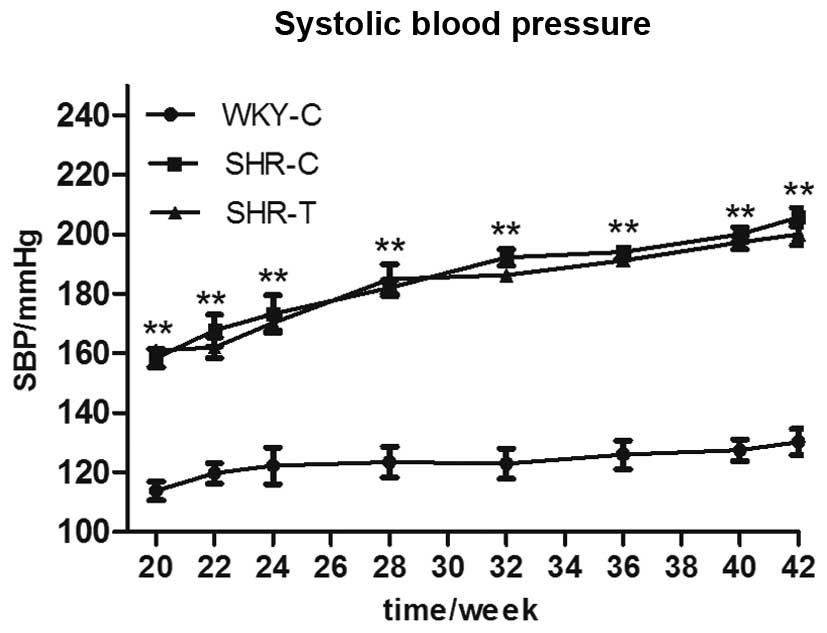
GSPE acts via the reduction of oxidative
stress
During the 22 weeks of treatment, SBP was similarly
increased in both the SHR-C and the SHR-T groups, indicating that
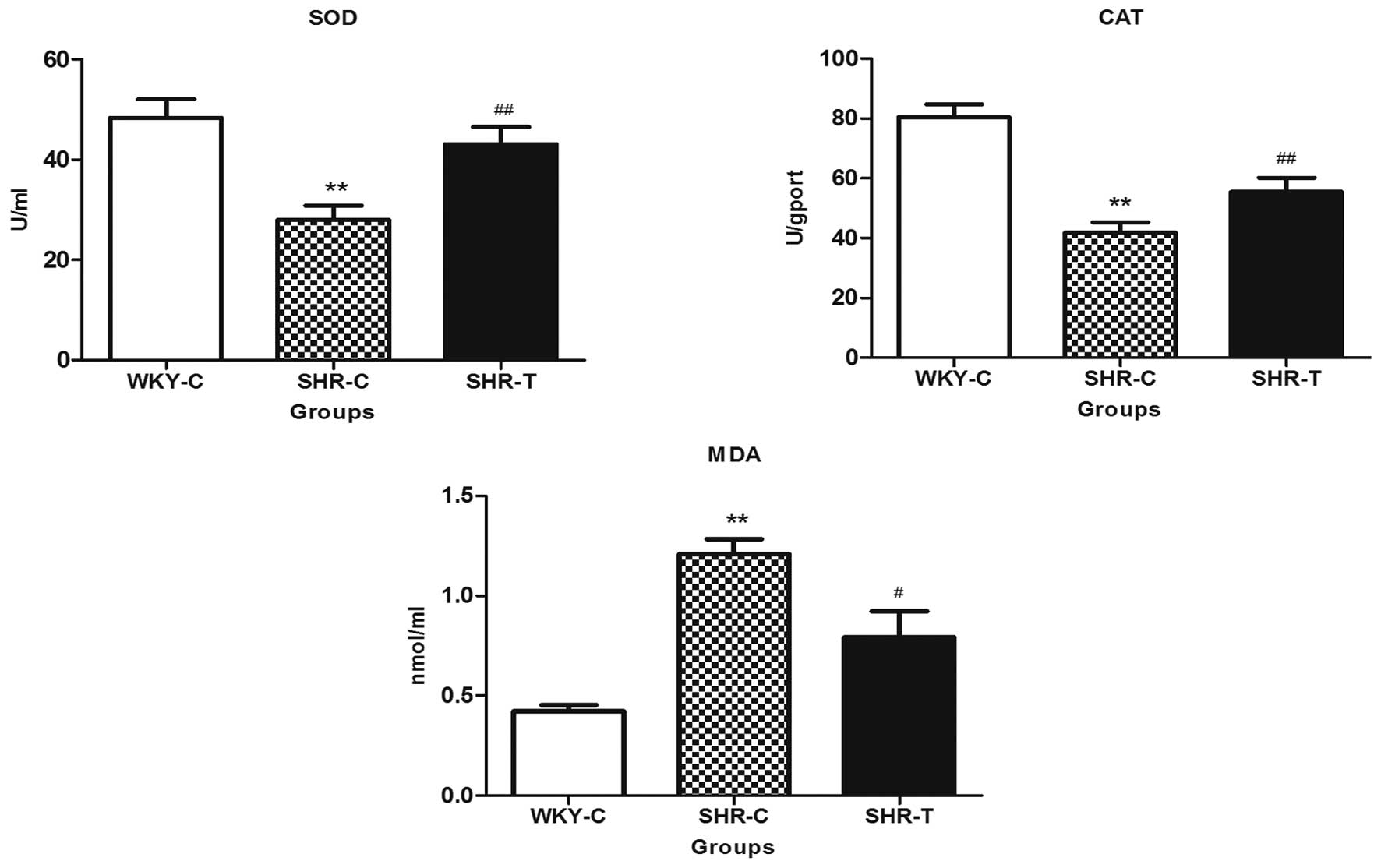
GSPE had no effect on blood pressure (Fig. 6). As presented in Fig. 7, compared with the WKY-C group, MDA
expression was greater in the aorta tissue of the SHRs, while SOD
and CAT activities were reduced, which was accompanied by arterial
remodeling in SHRs, as demonstrated by a significant increase in
wall thickness, wall-lumen ratio (Table I) and increased collagen fibers
(Fig. 3). All of these parameters
were reversed in SHRs chronically supplemented with GSPE.
Discussion
Arterial remodeling is an active process of
structural alteration that involves the vessel wall thickening and
histological abnormalities, hemal wall/lumen ratio increases and
finally endothelial dysfunction. Morphological observations from
the current study indicated that a marked increase in wall
thickness, OD, AR, VCSA, WCSA, wall-lumen ratio and WCSA/LCSA was
observed in the SHR-C groups. Previous studies suggested that
arterial remodeling leads to increased wall thickness and
wall-lumen ratios, which appears to be due to degradation and
reorganization of the extracellular matrix scaffold, in addition to
hypertrophy and/or hyperplasia of the VSMCs (11,16).
Consistent with these results, collagen deposition was observed in
the aortas of the SHR group. Pulse wave velocity (PWV), which is
defined as the propagation speed of the pressure or flow wave front
traveling along the aorta, has been regarded as a predictor for
cardiovascular events and mortality (19,20).
The increased PWV reflected arterial stiffness as a result of
structural alterations in the arterial wall (21). The structure of vessels is altered
when an increase in blood pressure causes an augmentation of
vascular tension in hypertension. These effects, in turn, lead to
an increase in aortic wall stiffness and a quickening of PWV in
hypertension. In the current study, SHRs exhibited a significantly
increased PWV.
A previous study demonstrated that the imbalance of
endothelium-derived factors may elevate vasomotor tone, promote
VSMC proliferation and induce arterial remodeling (11). NO is the key endothelium-derived
relaxing factor that serves an important role in the regulation of
vascular function, and it appears that the abnormalities in the
production or actions of NO lead to endothelial dysfunction and
abnormal arterial remodeling (22). ET-1 is the dominant
vasoconstrictive factor. A previous study identified that aortic
ET-1 content is significantly increased in deoxycorticosterone
acetate-salt hypertensive rats compared with that of age-matched
control rats (23). Studies
investigating clinical and experimental hypertension observed an
imbalance between NO and ET-1 (24,25).
In the current study, an increase in ET-1 and a reduction in NO
were observed. The present study confirms that SHRs have
endothelial dysfunction, which may contribute to arterial
remodeling.
As identified in the current study, the process of
arterial remodeling leads to increased wall thickness, VCSA, WCSA,
AR, wall-lumen ratio, WCSA/LCSA and PWV, which appear to be due to
arterial wall hyperplasia and hypertrophy, and an imbalance in NO
and ET-1 leading to endothelial dysfunction. However, these
alterations were reversed by GSPE. A previous study reported that
phenolic compounds prevent target organ damage in hypertensive rats
(26). GSPE, a phenolic compound,
has been reported to protect against oxidative injury during
doxorubicin-induced/cyclosporine-induced cardiac injury and
ischemia/reperfusion in the rat heart (27–29).
In a controlled registry study involving 119 healthy, pre- and
mildly hypertensive subjects (30), GSPE significantly reduced blood
pressure, however, in the present study, its effect could not be
observed in the SHRs. In the current study, the starting time of
treatment, age and weight of the SHRs, which may serve an important
role in the treatment of high blood pressure, were different from
previous studies (11,12). It is suggested that this explains
the discrepancies between the results obtained in the current study
and those of previous studies. These results indicated that the
vascular remodeling was improved independent of reducing blood
pressure, however was associated with the direct effects of GSPE.
Therefore, it is hypothesized that significantly reduced wall
thickness, OD, AR, VCSA, WCSA, wall-lumen ratio, WCSA/LCSA, PWV and
ET-1, and increased NO in the SHR-T group may be due to the
antioxidant activity of GSPE.
Oxidative stress occurs when there is an excessive
or sustained ROS production that exceeds the available antioxidant
defense systems. ROS are highly reactive and unstable by nature;
hence, they can damage various cellular components, including lipid
membranes. Lipid peroxides are derived from polyunsaturated fatty
acid (PUFA) oxidation and are capable of initiating lipid
peroxidation via a free radical chain reaction. MDA is a major
end-product of PUFA peroxidation and is often used as an indicator
of cell injury. An increase in the production of MDA may be due to
the formation of reactive oxidants. GSPE significantly inhibited
oxidative stress, as indicated by the reduced level of MDA in SHRs
treated with GSPE. The increase in MDA in the SHR-C group may be a
reflection of the reductions in the enzymatic and nonenzymatic
antioxidants defense system (31).
Thus, the observations of the present study confirm those of a
previous study that indicated that oxidative stress is present in
hypertension (32). The results of
the current study additionally illustrate that the oral
administration of GSPE reduces the production of MDA.
SOD and CAT balance together to eliminate ROS, and
small deviations in physiological concentrations may have marked
effects on the resistance of cellular lipids, proteins and DNA to
oxidative damage (33). Consistent
with previous reports, SHRs indicated significantly depleted
activities of the antioxidant enzymes SOD and CAT (27,34).
Upon GSPE treatment, antioxidant enzyme activities were
significantly increased in the SHR-T group, and the high free
radical scavenging activity of GSPE could be a possible reason for
this reversal effect of the lipid peroxidation levels and
antioxidative enzyme activities (35).
All of these results suggested that oxidative stress
was closely involved in the development of arterial remodeling, and
GSPE exerted a significantly beneficial effect on preventing the
development of arterial remodeling by improving the antioxidant
system, thus reducing oxidative stress.
Acknowledgments
The current study was supported by grants from the
National Natural Science Foundation of China (grant no. 30700884),
the Shandong Science and Technology Research Plan (grant no.
2010GGC10294), the Shandong Science and Technology Project Plan
(grant no. 2012GB021817) and the National Science and Technology
Major Project: Technology Platform Construction for Clinical
Evaluation of Cardiovascular New Drug (grant no.
2012ZX09303016-003).
References
|
1
|
Chobanian AV, Bakris GL, Black HR, Cushman
WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright
JT Jr, et al: Seventh report of the joint national committee on
prevention, detection, evaluation, and treatment of high blood
pressure. Hypertension. 42:1206–1252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Behradmanesh S and Nasri P: Serum
cholesterol and LDL-C in association with level of diastolic blood
pressure in type 2 diabetic patients. J Renal Inj Prev. 1:23–26.
2012.PubMed/NCBI
|
|
3
|
Intengan HD and Schiffrin EL: Vascular
remodeling in hypertension: Roles of apoptosis, inflammation, and
fibrosis. Hypertension. 38:581–587. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tousoulis D, Briasoulis A, Papageorgiou N,
Tsioufis C, Tsiamis E, Toutouzas K and Stefanadis C: Oxidative
stress and endothelial function: Therapeutic interventions. Recent
Pat Cardiovasc Drug Discov. 6:103–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cakir Y and Ballinger SW: Reactive
species-mediated regulation of cell signaling and the cell cycle:
The role of MAPK. Antioxid Redox Signal. 7:726–740. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lyle AN and Griendling KK: Modulation of
vascular smooth muscle signaling by reactive oxygen species.
Physiology (Bethesda). 21:269–280. 2006. View Article : Google Scholar
|
|
7
|
McIntyre M, Bohr DF and Dominiczak AF:
Endothelial function in hypertension: The role of superoxide anion.
Hypertension. 34:539–545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berk BC: Redox signals that regulate the
vascular response to injury. Thromb Haemost. 82:810–817.
1999.PubMed/NCBI
|
|
9
|
Cave AC, Brewer AC, Narayanapanicker A,
Ray R, Grieve DJ, Walker S and Shah AM: NADPH oxidases in
cardiovascular health and disease. Antioxid Redox Signal.
8:691–728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bagchi D, Garg A, Krohn RL, Bagchi M,
Bagchi DJ, Balmoori J and Stohs SJ: Protective effects of grape
seed proanthocyanidins and selected antioxidants against
TPA-induced hepatic and brain lipid peroxidation and DNA
fragmentation, and peritoneal macrophage activation in mice. Gen
Pharmacol. 30:771–776. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Qiu J, Zhao S, You B, Ji X, Wang Y,
Cui X, Wang Q and Gao H: Grape seed proanthocyanidin extract
alleviates ouabain-induced vascular remodeling through regulation
of endothelial function. Mol Med Rep. 6:949–954. 2012.PubMed/NCBI
|
|
12
|
Li XL, Li BY, Gao HQ, Cheng M, Xu L, Li XH
and Ma YB: Effects of grape seed proanthocyanidin extracts on
aortic pulse wave velocity in streptozocin induced diabetic rats.
Biosci Biotechnol Biochem. 73:1348–1354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guide for the Care and Use of Laboratory
Animals. National Research Council (US) Institute for Laboratory
Animal Research, National Academies Press (US); Washington (DC):
1996
|
|
14
|
Rizzoni D, Paiardi S, Rodella L, Porteri
E, De Ciuceis C, Rezzani R, Boari GE, Zani F, Miclini M, Tiberio
GA, et al: Changes in extracellular matrix in subcutaneous small
resistance arteries of patients with primary aldosteronism. J Clin
Endocrinol Metab. 91:2638–2642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lopez-Andres N, Fortuno MA, Diez J, Zannad
F, Lacolley P and Rossignol P: Vascular effects of cardiotrophin-1:
A role in hypertension? J Hypertens. 28:1261–1272. 2010.PubMed/NCBI
|
|
16
|
Wolinsky H: Long-term effects of
hypertension on the rat aortic wall and their relation to
concurrent aging changes. Morphological and chemical studies. Circ
Res. 30:301–309. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zhang J, Gao H, Zhao S, Ji X, Liu
X, You B, Li X and Qiu J: Profilin-1 promotes the development of
hypertension-induced artery remodeling. J Histochem Cytochem.
62:298–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Safar M, Chamiot-Clerc P, Dagher G and
Renaud JF: Pulse pressure, endothelium function, and arterial
stiffness in spontaneously hypertensive rats. Hypertension.
38:1416–1421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan YH, Yiu KH, Lau KK, Yiu YF, Li SW,
Lam TH, Lau CP, Siu CW and Tse HF: The CHADS2 and CHA2DS2-VASc
scores predict adverse vascular function, ischemic stroke and
cardiovascular death in high-risk patients without atrial
fibrillation: Role of incorporating PR prolongation.
Atherosclerosis. 237:504–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka M, Ishii H, Aoyama T, Takahashi H,
Toriyama T, Kasuga H, Takeshita K, Yoshikawa D, Amano T and
Murohara T: Ankle brachial pressure index but not brachial-ankle
pulse wave velocity is a strong predictor of systemic
atherosclerotic morbidity and mortality in patients on maintenance
hemodialysis. Atherosclerosis. 219:643–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ng K, Butlin M and Avolio AP: Persistent
effect of early, brief angiotensin-converting enzyme inhibition on
segmental pressure dependency of aortic stiffness in spontaneously
hypertensive rats. J Hypertens. 30:1782–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rudic RD and Sessa WC: Nitric oxide in
endothelial dysfunction and vascular remodeling: Clinical
correlates and experimental links. Am J Hum Genet. 64:673–677.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujita K, Matsumura Y, Kita S, Miyazaki Y,
Hisaki K, Takaoka M and Morimoto S: Role of endothelin-1 and the
ETA receptor in the maintenance of deoxycorticosterone
acetate-salt-induced hypertension. Br J Pharmacol. 114:925–930.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Statsenko ME and Derevianchenko MV:
Correction of endothelial dysfunction in hypertensive patients with
type II diabetes mellitus during combined antihypertensive therapy.
Ter Arkh. 86:90–93. 2014.In Russian.
|
|
25
|
Zhu WW, Liu XP, Wu N, Zhao TT, Zhao Y,
Zhang J and Shao JH: Beneficial effects of losartan on vascular
injury induced by advanced glycosylation end products and their
receptors in spontaneous hypertension rats. Mol Cell Biochem.
304:35–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jalili T, Carlstrom J, Kim S, Freeman D,
Jin H, Wu TC, Litwin SE and David Symons J: Quercetin-supplemented
diets lower blood pressure and attenuate cardiac hypertrophy in
rats with aortic constriction. J Cardiovasc Pharmacol. 47:531–541.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boghdady NA: Antioxidant and antiapoptotic
effects of proanthocyanidin and ginkgo biloba extract against
doxorubicin-induced cardiac injury in rats. Cell Biochem Funct.
31:344–351. 2013. View
Article : Google Scholar
|
|
28
|
Ozkan G, Ulusoy S, Alkanat M, Orem A,
Akcan B, Ersöz S, Yuluğ E, Kaynar K and Al S: Antiapoptotic and
antioxidant effects of GSPE in preventing cyclosporine A-induced
cardiotoxicity. Ren Fail. 34:460–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shao ZH, Wojcik KR, Dossumbekova A, Hsu C,
Mehendale SR, Li CQ, Qin Y, Sharp WW, Chang WT, Hamann KJ, et al:
Grape seed proanthocyanidins protect cardiomyocytes from ischemia
and reperfusion injury via Akt-NOS signaling. J Cell Biochem.
107:697–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belcaro G, Ledda A, Hu S, Cesarone MR,
Feragalli B and Dugall M: Grape seed procyanidins in pre- and mild
hypertension: A registry study. Evid Based Complement Alternat Med.
2013:3131422013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu BP: Cellular defenses against damage
from reactive oxygen species. Physiol Rev. 74:139–162.
1994.PubMed/NCBI
|
|
32
|
González J, Valls N, Brito R and Rodrigo
R: Essential hypertension and oxidative stress: New insights. World
J Cardiol. 6:353–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matés JM and Sánchez-Jiménez F:
Antioxidant enzymes and their implications in pathophysiologic
processes. Front Biosci. 4:D339–D345. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng H and Yu YS: Chronic hydrogen-rich
saline treatment attenuates vascular dysfunction in spontaneous
hypertensive rats. Biochem Pharmacol. 83:1269–1277. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ulker S, McMaster D, McKeown PP and
Bayraktutan U: Impaired activities of antioxidant enzymes elicit
endothelial dysfunction in spontaneous hypertensive rats despite
enhanced vascular nitric oxide generation. Cardiovasc Res.
59:488–500. 2003. View Article : Google Scholar : PubMed/NCBI
|