Introduction
Prostate cancer primarily occurs in older males over
75 years of age (1). The American
Cancer Society predicted that 238,590 people would be diagnosed
with prostate cancer in 2013, and that 29,720 patients would
succumb to the disease. An aging population and the effects of the
environment, stress and diet have resulted in substantial
year-on-year increases in the number of prostate cancer patients.
Prostate cancer is now the second most common cause of
cancer-associated mortality in males in the USA (2) and severely affects quality of life
and life expectancy (3).
Therefore, the identification of novel strategies to diagnose and
treat prostate cancer is crucial. Currently, prostate cancer
therapies primarily involve surgical resection, radiotherapy,
chemotherapy and endocrine therapy. However, the application of
these therapies is limited by the relatively high age of onset
meaning patients may be frail, the local metastasis of the disease
and the tendency for postoperative relapse (4–10).
Previous studies have reported that traditional Chinese medicine
possesses antitumor (11–13) and anti-inflammatory (14–16)
effects, increases immunity (17),
restores the normal functions of the bone marrow, blood and
gastrointestinal tract, and improves quality of life (18,19).
Therefore, traditional Chinese medicine may potentially be useful
for the treatment of cancer.
Diosgenin is a plant steroid compound that is
isolated from the root of Dioscorea nipponica of the
Dioscoreaceae family and is an active ingredient in a
variety of traditional and patented Chinese medicines. In addition,
diosgenin (molecular formula,
C27H42O3; molecular weight, 414.61
Da) is an important raw material in the synthesis of steroid
agents. Previous studies have demonstrated that diosgenin possesses
antitumor (20,21) and antidiabetic (22,23)
activities, reduces blood lipid content (24), acts as an anti-inflammatory
(25) and vasodilator (26), and protects the myocardium
(27). With regards to the
antitumor effects of diosgenin, it has been demonstrated to inhibit
the growth of multiple tumor types, including breast, esophageal,
liver and gastric cancers (28–31),
however, few studies have investigated its effects on prostate
cancer. Furthermore, the mechanism underlying the antitumor effect
of diosgenin remains to be elucidated. It is widely accepted that
apoptosis is an important cell death pathway. In addition,
autophagy has been demonstrated to be involved in tumorigenesis.
Therefore, the present study used the DU145 human prostate cancer
cell line to investigate the effect of diosgenin on the
proliferation, apoptosis and autophagy of prostate cancer cells. In
addition, the mechanism underlying the action of diosgenin was
examined, to provide experimental evidence supporting the use of
diosgenin as a potential treatment for prostate cancer.
Materials and methods
Chemicals and reagents
Diosgenin was purchased from Nanjing Zelang Medical
Technology Co., Ltd. (Nanjing, China), with a purity of >98% as
determined by high-performance liquid chromatography. Minimum
essential medium (MEM),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
Cell Proliferation and Cytotoxicity assay kit, 3-methyladenine
(3-MA), monodansylcadaverine (MDC) Autophagy Detection kit, and
annexin V-allophycocyanin (APC)/7-aminoactinomycin D (7-AAD)
Apoptosis Detection kit were purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Fetal bovine serum (FBS), Hoechst
33,342/propidium iodide (PI) Double Staining kit,
TRIzol® reagent, First-Strand cDNA Synthesis kit and Taq
DNA Polymerase were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Bradford Protein Content assay kit (cat. no.
KGA801), SDS-PAGE Gel Preparation kit (cat. no. KGP113), Coomassie
Blue Staining kit (cat. no. KGP1001), Ponceau S staining solution
(cat. no. KGP105) and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) were obtained from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China).
Cell culture
The DU145 human prostate cancer cell line was
obtained from Nanjing KeyGen Biotech Co., Ltd. The cells were
cultured in MEM containing 10% FBS at 37°C in a 5% CO2
incubator. Cells in the logarithmic growth phase were used for
subsequent experiments.
MTT assay of cell proliferation
(IC50)
A cell suspension of 5×104 cells/ml was
prepared, and 100 µl of this was added to each well of a 96-well
culture plate, which was incubated at 37°C in a 5% CO2 incubator
for 24 h. Complete medium was used to dilute diosgenin to the
desired concentrations (100, 50, 25, 12.5, 6.25, 3.125, 1.5625,
0.78125 and 0.1953125 µg/ml), and 100 µl of the corresponding
diosgenin-containing medium was added per well. 3-MA was added to
cells at a final concentration of 5 nM. Untreated cells were the
negative control group, while cells treated with 10 µg/ml
paclitaxel were the positive control group. The 96-well plate was
incubated at 37°C in a 5% CO2 incubator for 48 h. The plate was
then subjected to MTT staining, according to the procedures
described previously (32), and
the optical density of the samples was measured at a wavelength of
490 nm. The inhibition rate and IC50 value of each group were
calculated using the following formula: Inhibition rate (%) =
[(Negative control group - Experimental group) / Negative control
group] × 100.
Annexin-V APC/7-AAD double staining to
detect apoptosis
Cells growing in the logarithmic phase were
trypsinized and seeded at a density of 5×104 cells/well
in a 6-well plate. The following day, once cells had adhered to the
plate, the corresponding diosgenin-containing medium was added
(0.6, 3 or 15 µg/ml). A negative control group, consisting of
untreated cells was included. Following incubation for 48 h at 37°C
and 5% CO2, 0.25% trypsin [without
ethylenediaminetetraacetic acid (EDTA)] was used to remove the
cells. The cells were washed twice with phosphate-buffered saline
(PBS) and centrifuged at 447.2 × g for 5 min at 20°C. A
total of 5×105 cells were resuspended in 500 µl binding
buffer, following which 5 µl of annexin V-APC was added and mixed
well, and 5 µl 7-AAD was added and mixed well. The reaction was
performed at room temperature for 5–15 min in the dark, and a flow
cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA),
together with the BD CellQuest software (BD Biosciences) was used
to detect apoptosis.
Transmission electron microscopy
DU145 cells (1×105 cells/ml) in the
logarithmic growth phase were incubated in diosgenin-containing
medium (15, 3 or 0.6 µg/ml). A negative control group consisting of
untreated cells was included. Cells were harvested 24 h later,
using trypsin (0.25%) to remove the cells from the plate. Cells
were then centrifuged at 111.8 × g for 10 min at 20°C. The
supernatants were discarded, cells were washed twice with PBS and
2.5% glutaric acid was added. The cells were fixed for 90 min at
4°C and then embedded, sectioned and stained with uranyl acetate
and lead citrate. Autophagosomes were observed under a transmission
electronic microscope (JEM-1011; JEOL, Ltd., Tokyo, Japan).
MDC staining to detect autophagy
Cells in the logarithmic growth phase were
trypsinized and seeded into a 6-well plate at a density of
5×105 cell/ml/well. The following day, once cells had
adhered, diosgenin-containing medium was added (15, 3 or 0.6
µg/ml). A negative control group consisting of untreated cells was
included. Cells were harvested using 0.25% trypsin (without EDTA)
following a 48-h incubation. Wash buffer (1X; 300 µl) was used to
wash the cells once, and cells were then resuspended in 1X wash
buffer at 1×106 cells/ml. A total of 90 µl of cell
suspension was transferred to a new microfuge tube, and 10 µl of
MDC staining solution was added and gently mixed. Following
staining at room temperature for 15–45 min in the dark, cells were
collected by centrifugation at 800 × g for 5 min. The cells
were washed three times with wash buffer and resuspended in 100 µl
of collection buffer. The cell suspension was dropped onto a slide
and covered with a coverslip. Cells were observed under a
fluorescence microscope (IX51; Olympus Corporation, Tokyo,
Japan).
Western blotting to determine protein
expression levels
Cells in the logarithmic growth phase were
trypsinized and seeded onto a 6-well plate at a density of
5×105 cell/ml/well. The following day, once cells had
adhered to the plate, diosgenin-containing medium was added (15, 3
or 0.6 µg/ml). A negative control group consisting of untreated
cells was included. Pre-chilled lysis buffer (200 µl), consisting
of 20 nM Tris (pH 7.5), 150 mM NaCl, Triton-X-100 (cat. no. KGP701;
KayGen Biotech, Co., Ltd), was added to each group and incubated on
ice for 30 min. Following vortexing, the lysate was centrifuged at
13,000 × g for 10 min at 4°C. The supernatant was retained,
and the Bradford Protein Content assay kit was used to measure the
protein concentration of the samples. Proteins (30 µg) were
resolved on a 10% SDS-PAGE gel and transferred to a polyvinylidene
difluoride membrane. Following blocking overnight with 5% non-fat
milk, the membranes were incubated overnight at 4°C in a sealed bag
with the following primary antibodies: Mouse anti-human light chain
3 (LC3)-I (cat. no. KGATG007; dilution, 1:500); mouse anti-human
LC3-II (cat. no. KGATG007; dilution, 1:500); rabbit anti-human
phosphatidylinositol 3 kinase (PI3K; cat. no. KG22639; dilution,
1:500); rabbit anti-human phosphorylated (p)-PI3K (cat. no.
KG22638-2; dilution, 1:500); rabbit anti-human protein kinase B
(Akt; cat. no. KG21502; dilution, 1:200); rabbit anti-human p-Akt
(cat. no. KG11054-2; dilution, 1:200); rabbit anti-human mammalian
target of rapamycin (mTOR; Ser 2448; cat. no. KGYT2914-7; dilution,
1:200); rabbit anti-human p-mTOR (cat. no. KGYP0176-6; dilution,
1:200); and anti-GAPDH (cat. no. KGAA002-2; dilution, 1:200). All
primary antibodies were obtained from KayGen Biotech Co., Ltd.
Tris-buffered saline and Tween 20 was used to wash the membrane
three times for 10 min before it was incubated with horseradish
peroxidase-conjugated goat anti-mouse IgG (cat. no. KGAA37;
dilution, 1:4,000) or goat anti-rabbit IgG (cat. no. KGAA35;
dilution, 1:4,000; both from KayGen Biotech, Co., Ltd.) secondary
antibodies for 1 h at 37°C. Finally, the membrane was visualized
using an enhanced chemiluminescence method (cat. no. KGP1201;
KayGen Biotech Co., Ltd.) and exposed to film. Protein band
densities were quantified using Quantity One analysis software
(version, V4.4.0.36; Bio-Rad, Inc., Hercules, CA, USA).
Hoechst 33,342/PI double staining to
detect apoptosis
Cells in the logarithmic growth phase were
trypsinized and seeded into a 6-well plate at a density of
1×105 cell/ml/well. The following day, once cells had
adhered, diosgenin-containing medium was added. A negative control
group consisting of untreated cells was included. Cells were
harvested with 0.25% trypsin (without EDTA) following incubation
for 48 h. Cells (105-106) were resuspended in
1 ml of medium and 10 µl Hoechst 33,342 staining solution was added
to the cells and incubated at 37°C for 5–15 min. The cells were
centrifuged at 111.8 × g for 5 min at 4°C, and the
supernatant was discarded. Buffer A (1 ml) was used to resuspend
the cells, and 5 µl PI staining solution was added and incubated at
room temperature for 5–15 min in the dark. The suspension was mixed
well and observed under a fluorescence microscope. The number of
apoptotic cells was determined by counting the number of
PI-positive cells in 3 random fields of view for each sample.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) to detect gene expression
Pre-cooled TRIzol (1 ml) was added to DU145 cells
(1×106) growing in logarithmic phase, and the solution
was repeatedly retro-pipetted until it was transparent. The
solution was then transferred to a 1.5 ml centrifuge tube, and
incubated at room temperature for 5 min. Chloroform (0.2 ml) was
subsequently added and the cover was fastened. The tube was shaken
for 15s until the solution was white in color, before it was
incubated at room temperature for 3 min. The solution was then
centrifuged at 16099.2 × g for 15 min at 4°C, before 0.6 ml
of the supernatant was transferred to a fresh centrifuge tube.
Ispropyl alcohol (0.6 ml) was subsequently added and, after gentle
vortexing, the sample was incubated at room temperature for 10 min
and centrifuged at 16099.2 × g for 10 min at 4°C. The
supernatant was removed and 1 ml 70% ethanol was added along the
tube wall, before it was vortexed gently and centrifuged at 16099.2
× g for 10 min at 4°C. The supernatant was removed,
air-dried and precipitated at room temperature for approximately 5
min, before 30–50 µl RNase-free water was added to dissolve the RNA
precipitate. Following complete dissolution, the solution was
preserved at −70°C until required. The RNA was reverse transcribed
into cDNA using the First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), and RT-qPCR was performed using SYBR Green
Realtime PCR Master Mix (cat. no. QPK-201; Toyobo, Co., Ltd.,
Osaka, Japan) and the StepOnePlus software program (version 2.0;
Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers
were synthesized by Gen Script (Nanjing) Co., Ltd. (Nanjing, China)
and had the following sequences: Sense,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and antisense,
5′-GCCATCACGCCACAGTTTC-3′ for GAPDH (101 bp); sense,
5′-ATGTCCACAGAAAGTGCCAA-3′ and antisense,
5′-GGGTGATCCACATCTGTCTG-3′ for Beclin 1 (140 bp); and sense,
5′-AAATCCGACCACTAATTGCC-3′ and antisense,
5′-TGCTCTTCAGATGGTGATCC-3′ for B-cell lymphoma 2 (Bcl2; 114 bp).
The amplification conditions were 95°C pre-denaturation for 5 min
followed by 40 cycles of 95°C denaturation for 15 sec, 60°C
annealing for 20 sec, and 72°C extension for 40 sec. The
specificity of the amplified products was monitored by melting
curves. ABI StepOne software (version, 2.3; Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to calculate the relative
expression (using 2-ΔΔCq) of the target genes in each group, and
GAPDH served as an internal reference to assess the expression of
target genes (33).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analyses were performed in SPSS software
version 16.0 (SPSS, Inc., Chicago, IL, USA). Analysis of variance
was used to compare differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
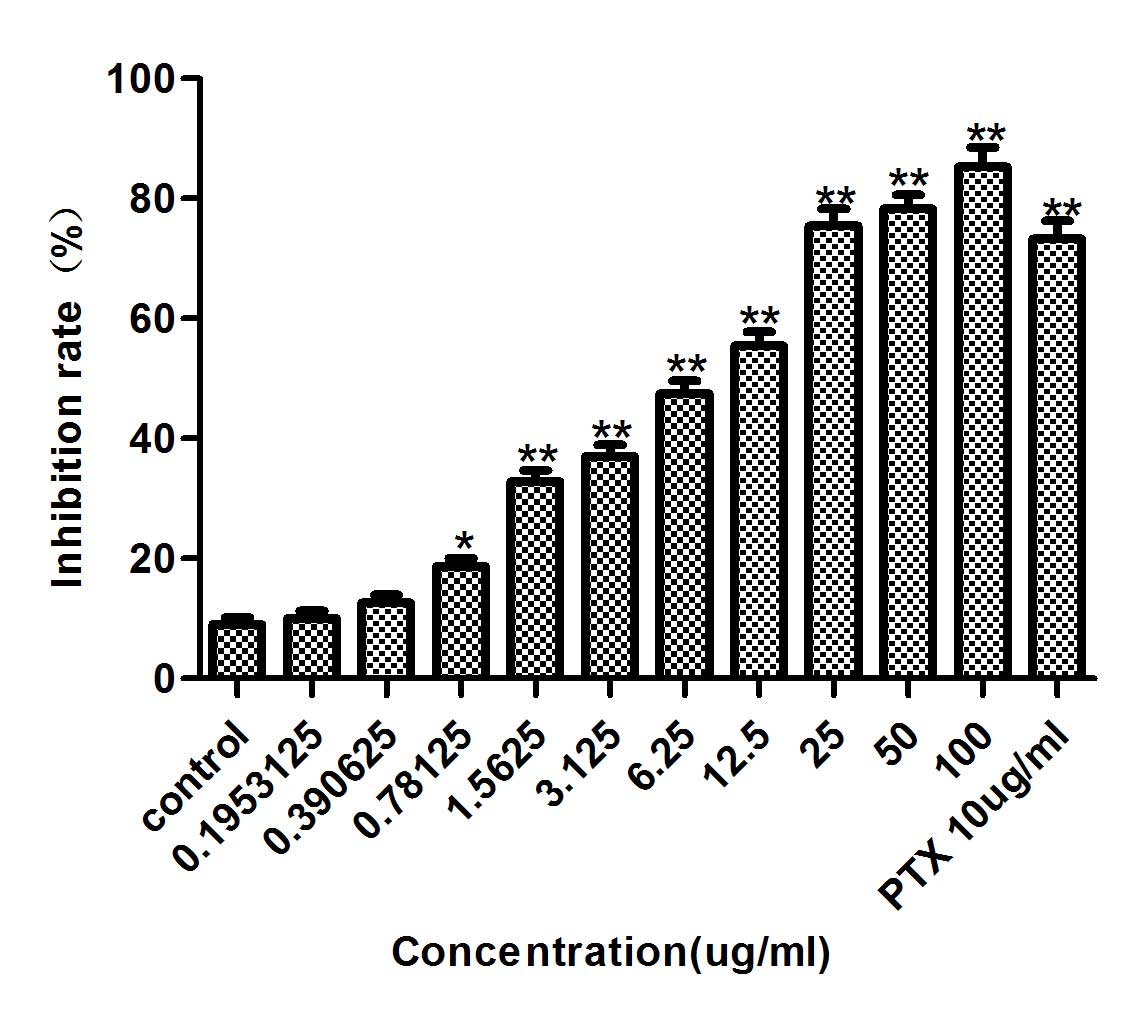
Inhibitory effect of diosgenin on the
proliferation of DU145 cells
DU145 cells were treated with diosgenin (100, 50,
25, 12.5, 6.25, 3.125, 1.5625, 0.78125 or 0.1953125 µg/ml) for 48
h. Diosgenin inhibited DU145 cell proliferation in a dose-dependent
manner (Fig. 1). At 48 h, the IC50
was 6.757 µg/ml, therefore 0.6, 3 and 15 µg/ml diosgenin
concentrations were used for subsequent experiments.
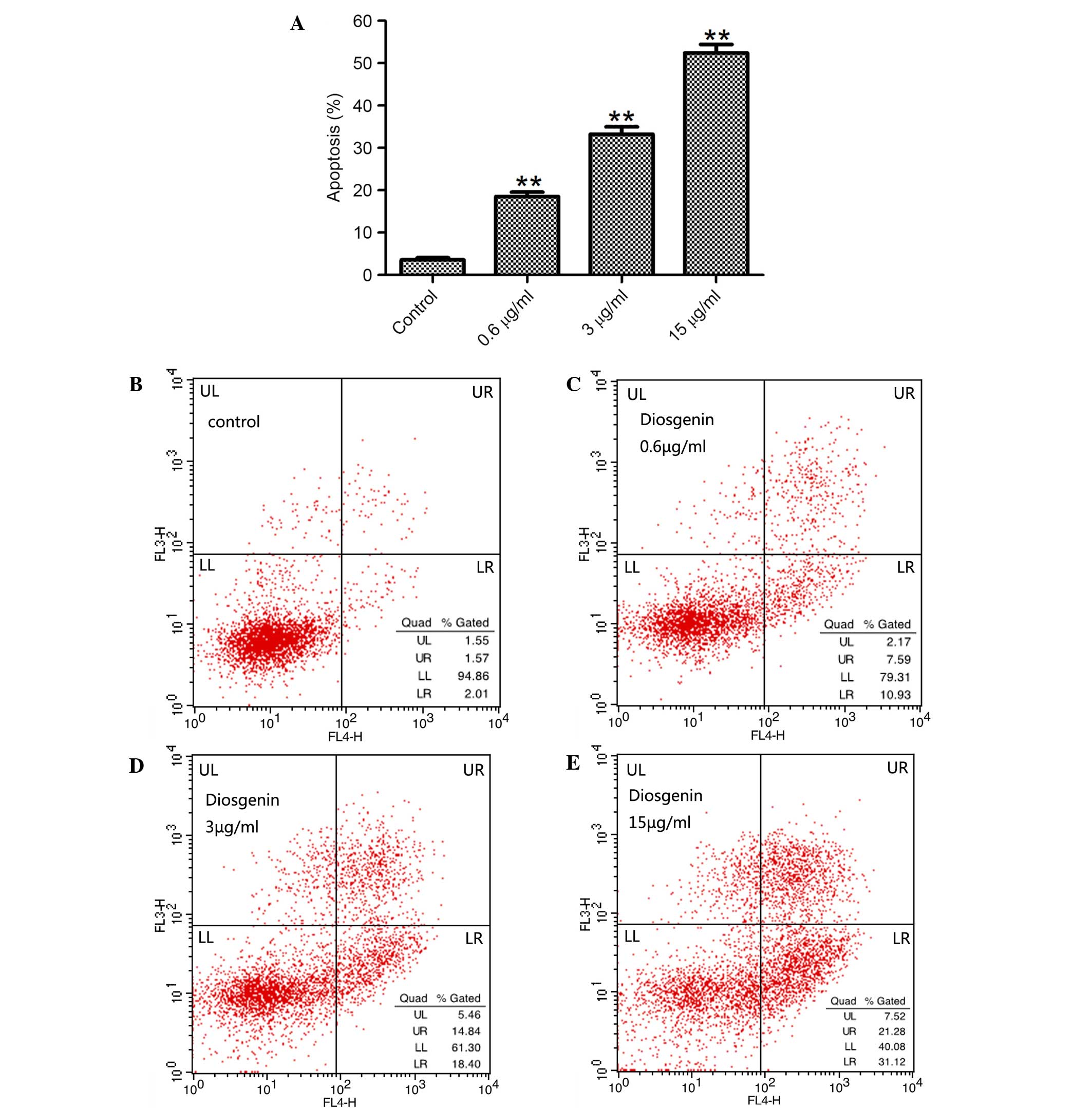
Effect of diosgenin on the apoptosis
of DU145 cells
To assess whether the inhibition of proliferation by
diosgenin was associated with apoptosis, DU145 cells were treated
with diosgenin for 48 h, and the percentage of apoptotic cells was
analyzed by flow cytometry. Diosgenin induced apoptosis in DU145
cells in a dose-dependent manner (P<0.01 for 0.6, 3 and 15 µg/ml
diosgenin; Fig. 2).
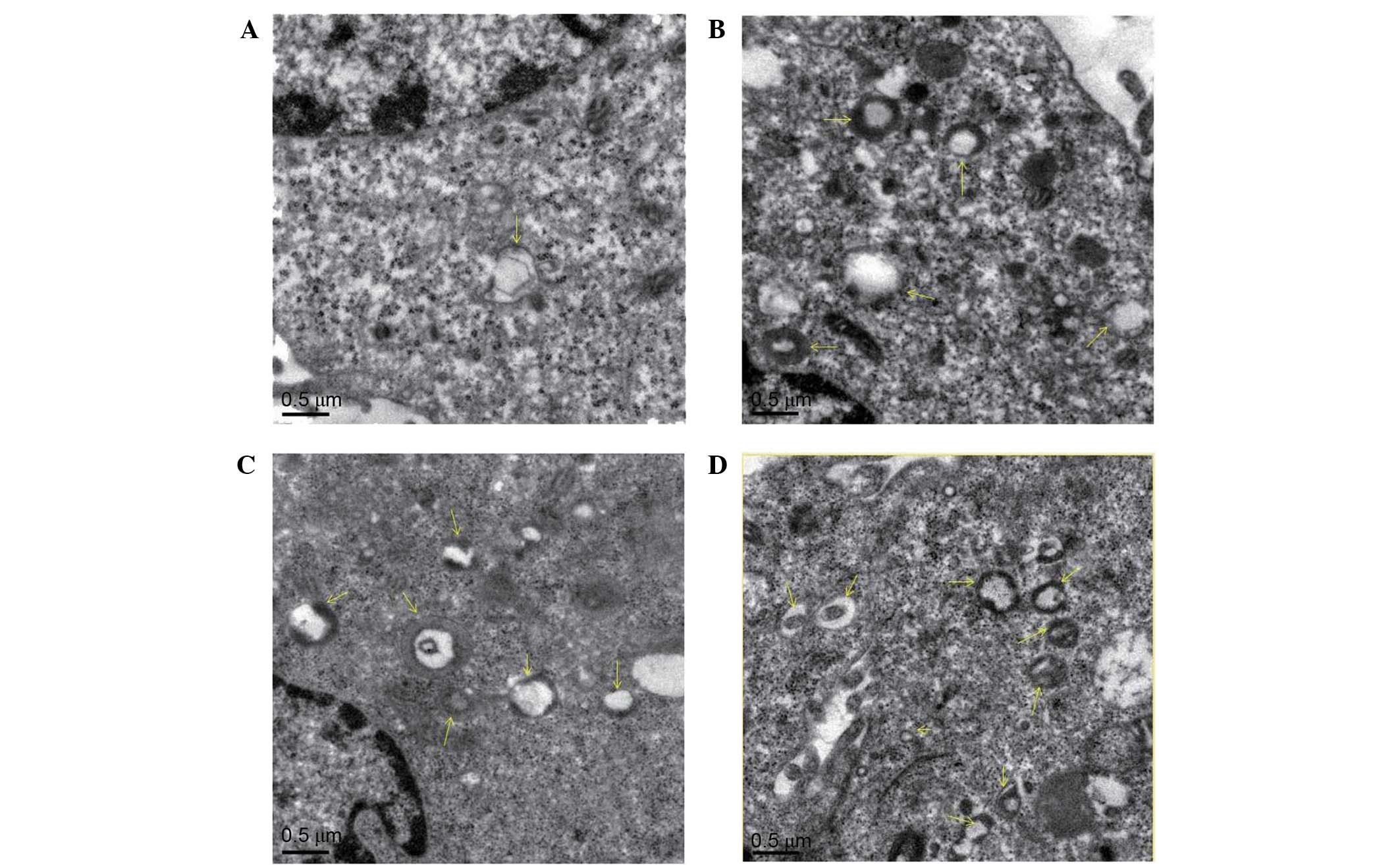
Effect of diosgenin on the
microstructural morphology of DU145 cells
To verify whether the cytoplasmic vacuoles observed
by inverted microscopy were associated with autophagy, transmission
electron microscopy was used to observe autophagosomes in DU145
cells treated with diosgenin. As presented in Fig. 3, untreated cells contained normal
nuclei, cytoplasm, and organelles, whereas diosgenin-treated cells
exhibited a large number of autophagosomes of various sizes. In
addition, autophagosomes containing mitochondria were observed.
This suggests that autophagy occurred in the cells following
diosgenin treatment.
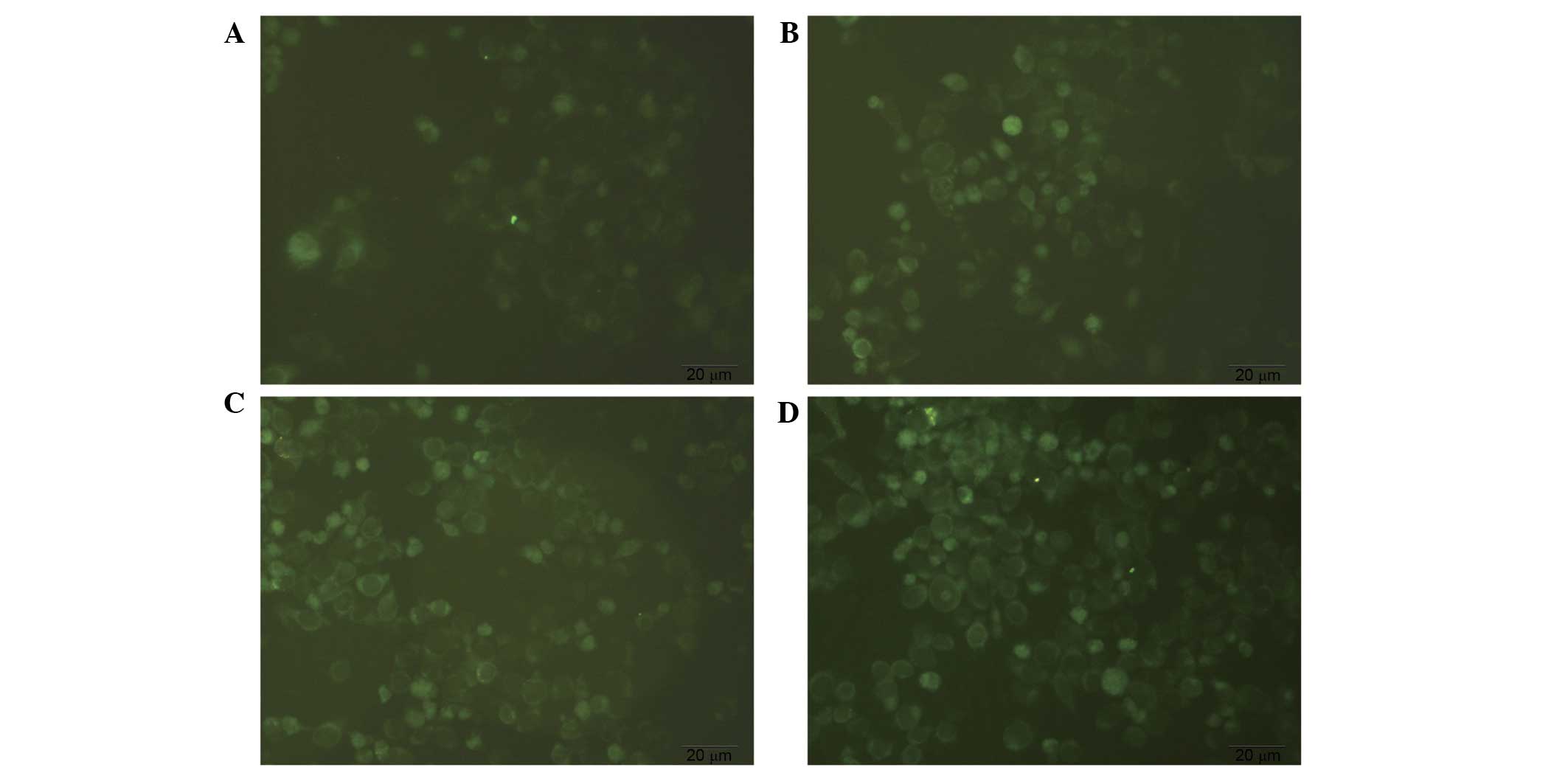
MDC staining of autophagosomes
MDC-labeled autophagic vacuoles were observed under
an inverted fluorescence microscope and exhibited clear vesicles in
the cytoplasm and perinuclear region, and the changes of the
particles inside the cell were used to determine the level of
autophagy. As presented in Fig. 4,
diosgenin-treated cells exhibited increased fluorescence intensity
and numbers of autophagic vacuoles compared with the control group.
The number and staining intensity of the vacuoles increased in a
dose-dependent manner. This suggests that diosgenin induces
autophagy.
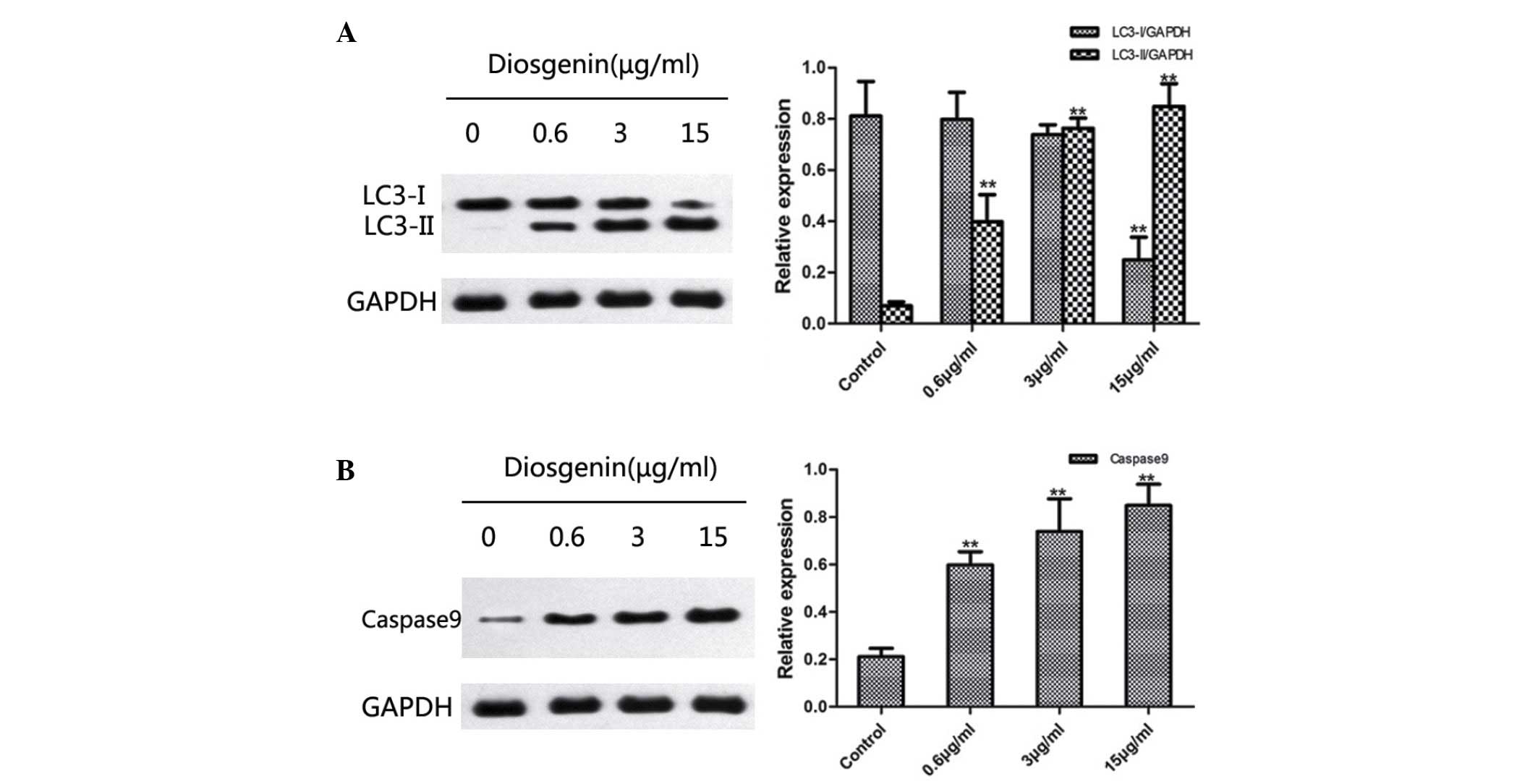
Diosgenin induces
microtubule-associated protein 1A/1B-LC3 and caspase 9 protein
expression in DU145 cells
Western blotting was used to detect changes in the
protein expression levels of LC3 and caspase 9 following treatment
of DU145 cells with diosgenin. On SDS-PAGE gels, LC3-II ran faster
than LC3-I, producing two bands by western blot. Fig. 5A indicates that untreated cells
exhibited only a faint LC3-I band, whereas LC3-II was not detected.
By contrast, following treatment with diosgenin, the protein
expression levels of LC3-II increased significantly in a
dose-dependent manner (0.6 µg, P=0.006; 3 µg, P<0.001; 15 µg,
P<0.001; Fig. 5A). In addition,
diosgenin treatment significantly increased the protein expression
levels of caspase 9 compared with the control group, in a
dose-dependent manner (0.6 µg/ml, P<0.001; 3 µg/ml, P=0.002; 15
µg/ml, P<0.001; Fig. 5B).
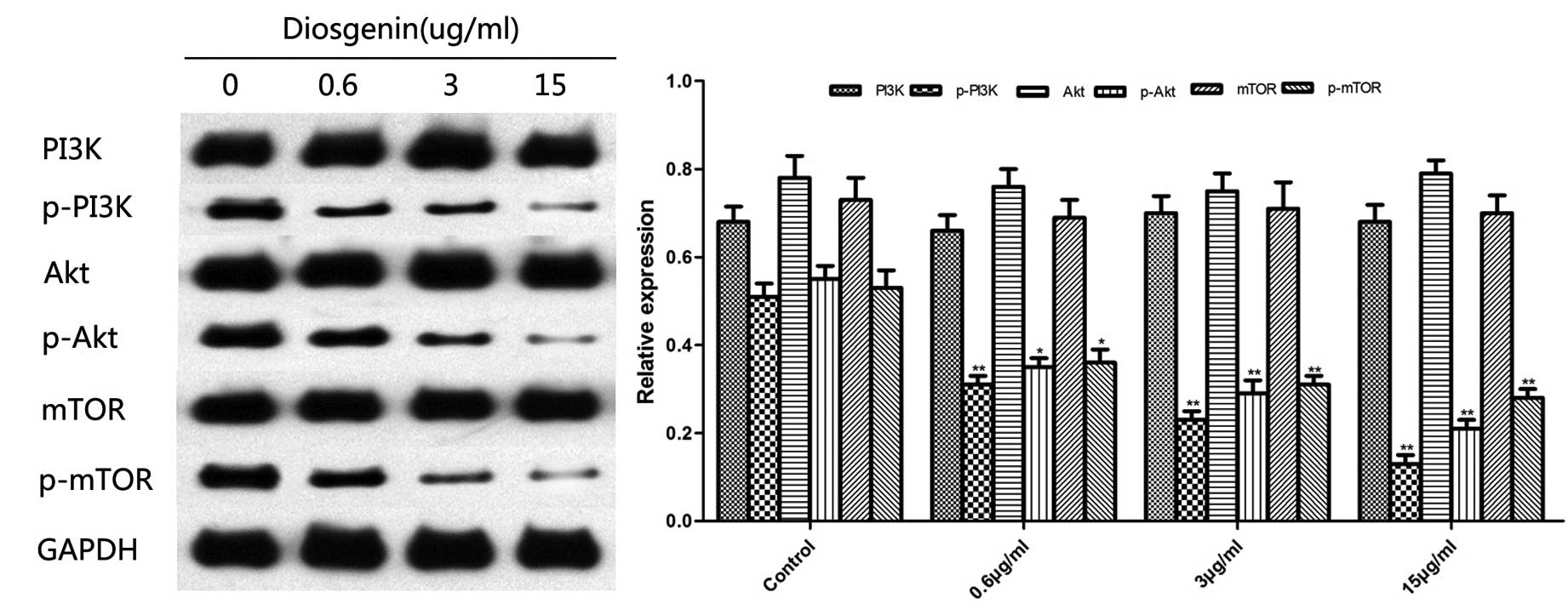
Effect of diosgenin on the
PI3K/Akt/mTOR signaling pathway
The PI3K/Akt/mTOR signaling pathway is the canonical
pathway that negatively regulates the initiation of autophagy. It
has been reported that inhibition of this signaling pathway induces
cell autophagy. As presented in Fig.
6, western blotting demonstrated that diosgenin inhibited the
phosphorylation of PI3K, Akt, and mTOR in the PI3K/Akt/mTOR
signaling pathway.
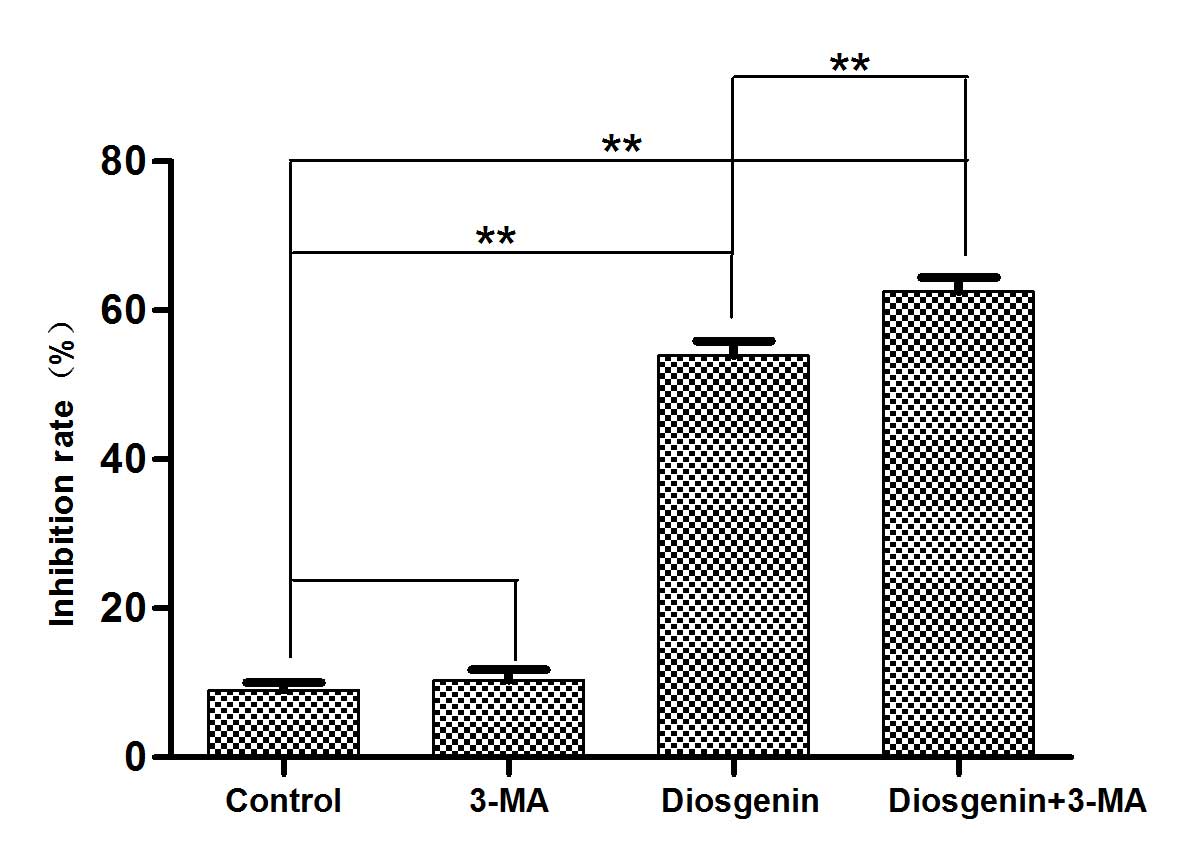
Inhibition of autophagy increases the
cytotoxicity of diosgenin
Preliminary experiments determined that diosgenin
treatment induced autophagy and apoptosis in DU145 cells and that
the inhibition of DU145 proliferation by diosgenin occurred in a
dose-dependent manner. To determine whether the cytotoxicity
exhibited by diosgenin was mediated by autophagy, the autophagy
inhibitor 3-MA was used, and MTT assays were performed to assess
cytotoxicity. Compared with cells treated with diosgenin alone, the
addition of 3-MA increased the percentage of non-viable DU145 cells
(P<0.01; Fig. 7).
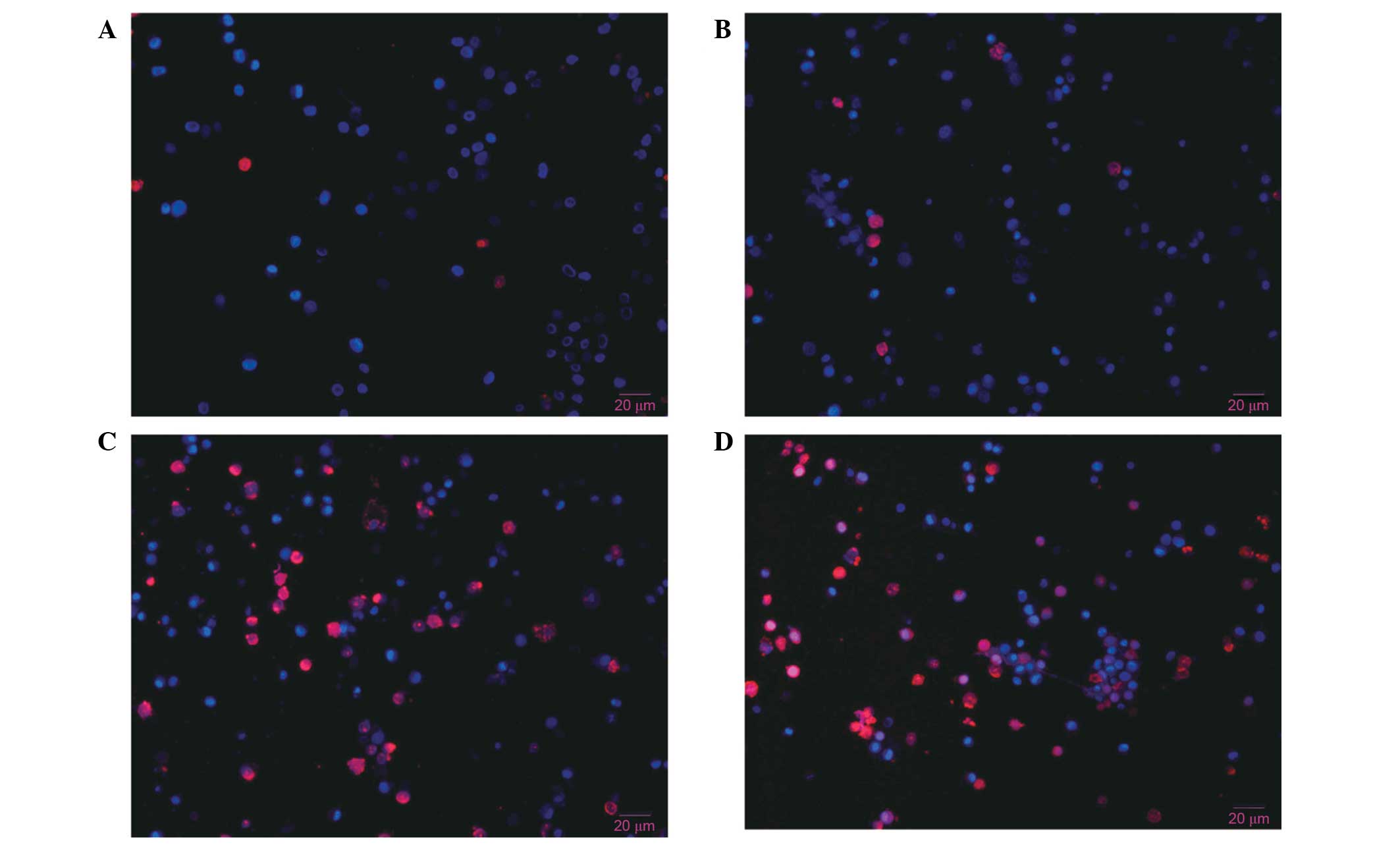
Hoechst 33342 and PI double
fluorescence staining of live cells
Hoechst 33342 and PI double staining distinguish
live and dead cells. The nuclei of cells in the late apoptotic or
early necrotic stages stain red, whereas the nuclei of live cells
stain blue. No significant differences in the percentages of
apoptotic cells were observed between control cells (Fig. 8A) and cells treated with 3-MA alone
(Fig. 8B). However, cells treated
with diosgenin (Fig. 8C) for 48 h
exhibited nuclei with a bead-like shape, forming apoptotic bodies.
Diosgenin treatment increased the percentage of apoptotic cells
compared with control cells. Cells treated with diosgenin and 3-MA
(Fig. 8D) exhibited an increased
percentage of apoptotic cells compared with cells treated with
diosgenin alone. These results demonstrated that apoptosis
increased significantly following the inhibition of
diosgenin-induced autophagy.
Effect of diosgenin on apoptosis
following the inhibition of autophagy
As cytotoxicity of diosgenin was increased following
the inhibition of autophagy, it was investigated whether autophagy
inhibited the effect of diosgenin on apoptosis. RT-qPCR was
performed to analyze mRNA expression levels in DU145 cells treated
with diosgenin. Using Beclin 1 and Bcl2 as markers, the effect of
diosgenin on autophagy and apoptosis was examined following the
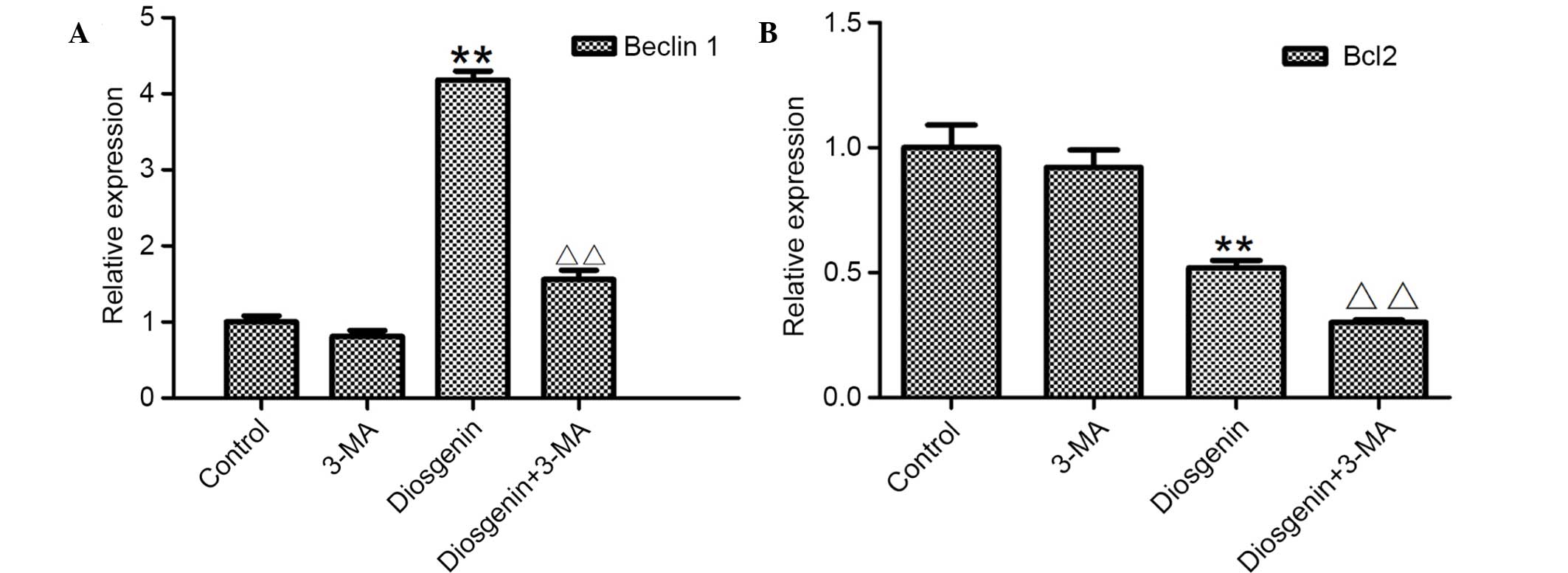
inhibition of autophagy. As presented in Fig. 9, following treatment with 5 mM 3-MA
alone, the mRNA expression levels of Beclin 1 decreased compared
with the control cells. Following treatment with 6.8 µg/ml
diosgenin, the mRNA expression levels of Beclin1 increased
significantly compared with control cells (P<0.01). However,
when diosgenin was added following simultaneous treatment with
3-MA, Beclin 1 mRNA expression levels significantly decreased
compared with cells treated with diosgenin alone (P<0.01).
Following treatment with diosgenin alone, Bcl2 mRNA expression
levels decreased significantly compared with control cells
(P<0.01). Simultaneous treatment with 3-MA significantly
decreased Bcl2 mRNA expression levels compared with cells treated
with diosgenin alone (P<0.01). These results suggest that when
autophagy was inhibited, the apoptotic effect of diosgenin was
increased.
Discussion
The results of the MTT assay indicated that
diosgenin significantly inhibits the proliferation of DU145 cells
in a dose-dependent manner, suggesting that diosgenin induces DU145
cell death.
Apoptosis is an important pathway of cell death.
Therefore, flow cytometry was performed in the present study to
detect the effect of diosgenin on apoptosis. The results
demonstrated that the percentage of apoptotic diosgenin-treated
cells increased significantly in a dose-dependent manner,
suggesting that diosgenin inhibits proliferation by promoting
apoptosis in tumor cells.
Apoptosis is a complex physiological process that
involves numerous genes, in which the caspase family is important.
Caspase 9 is a cysteine protease that uses aspartic acid as a
substrate and is a core component of the apoptotic pathway
(34). Caspase 9 is the most
important initiator of the endogenous apoptotic pathway and is an
upstream initiating caspase that activates downstream factors of
apoptosis, initiates cascade activation reactions, and inactivates
proteins that regulate cell structure, cell cycle and DNA repair,
resulting in the initiation of apoptosis (35–37).
The present study determined the protein expression levels of
caspase 9 in DU145 cells by western blotting. The results indicated
that the expression of caspase 9 significantly increased in cells
treated with diosgenin, which further supports the induction of
apoptosis by diosgenin.
Autophagy is a cell death process that is distinct
from apoptosis. As a mechanism underlying cell defense and stress
regulation, autophagy has been extensively investigated in cancer
research. Autophagy refers to the process in eukaryotic cells in
which a double membrane wraps around a portion of cytoplasm and the
intracellular organelles and proteins to be degraded form an
autophagosome. This fuses with endosomes to form an amphisome,
which eventually fuses with lysosomes to form an autophagolysosome
that degrades the packaged contents (38–41).
Under physiological conditions, autophagy occurs at low levels.
When cells are under various stress conditions, including
insufficient nutrition, a lack of growth factors and hypoxia, cells
initiate autophagy (42,43), which is crucial for the maintenance
of the stability of the intracellular environment and the normal
physiological functions of the cell. Previous studies have
demonstrated that autophagy is closely involved in the pathogenesis
and progression of tumors. Autophagy inhibits tumor growth,
particularly during the early stages of tumor formation; however,
autophagy may lead to tumor cell adaptation to adverse metabolic
stress and, thus, allow survival of tumor cells (44–46).
In addition, numerous antitumor therapeutic agents induce
autophagy; however, whether this results the death of tumor cells
or instead promotes tumor cell survival remains controversial
(47). Therefore, the present
study performed transmission electronic microscopy to observe DU145
cells and determined that the cytoplasm of cells treated with
diosgenin contained numerous autophagic vacuoles of various sizes.
In addition, the formation of autophagosomes was observed,
confirming that diosgenin induced autophagy. To verify this
finding, MDC was used to stain for autophagic vacuoles. MDC is a
fluorescent dye that is absorbed by cells and stains autophagic
vacuoles. Fluorescence microscopy of MDC-labeled autophagic
vacuoles identified vesicles in the cytoplasm and perinuclear
region. The change in intracellular particles was used to determine
the autophagy level. Compared with control cells, diosgenin-treated
cells exhibited a greater fluorescence intensity and an increased
number of MDC-labeled autophagic particles, suggesting that
diosgenin induces autophagy.
LC3 is the homolog of the yeast protein Atg8 and
serves as a marker of autophagy in mammalian cells (48). In tumor cells, LC3 may be processed
to generate cytoplasmic LC3-I, which undergoes a
ubiquitination-like modification of a covalent linkage to
phosphatidylethanolamine on autophagosome membranes to form LC3-II
(49,50). Based on the observation that the
level of LC3-II corresponds to the level of autophagy (51), the initiation of autophagy may be
determined by the measurement of LC3-II protein expression levels.
The present study performed western blotting to determine the
protein expression levels of LC3-II, and the results revealed that
the ratio of LC3-II/LC3-I in DU145 cells treated with diosgenin
increased, further confirming that diosgenin induces autophagy in
tumor cells.
The PI3K/Akt/mTOR signaling pathway is widely
recognized to regulate cell proliferation, autophagy, apoptosis and
motility, and is critical for the pathogenesis, progression and
prognosis of cancer (52–54). Following PI3K activation, the
second messengers phosphatidylinositol (3,4,5)-biphosphate and phosphatidylinositol
(3,4,5)-triphosphate are generated at the cell
membrane and in turn activate the downstream kinase Akt. Activated
Akt is anti-apoptotic, promotes cell survival and performs other
biological functions via the activation of downstream substrates
(55). The downstream protein mTOR
activates protein translation and promotes cell growth (56) and is a critical inhibitory factor
of autophagy. Under normal growth conditions, mTOR is in an active
state, and intracellular autophagy is inhibited. Under stress
conditions, including insufficient nutrition, the activity of mTOR
is inhibited to promote autophagy (57,58).
In addition, caspase 9 is a downstream target of the PI3K/Akt/mTOR
signaling pathway. Akt reduces caspase 9 activity by
phosphorylating S196 on the caspase 9 precursor protein, thus
inhibiting apoptosis and promoting tumorigenesis (59). To further investigate the mechanism
underlying diosgenin activity, western blotting was performed to
determine the protein expression levels of members of the
PI3K/Akt/mTOR signaling pathway. The results indicated that
diosgenin significantly inhibited the phosphorylation of PI3K, Akt,
and mTOR, suggesting that diosgenin activates tumor cell autophagy
and apoptosis potentially by inhibiting the PI3K/Akt/mTOR signaling
pathway.
These results demonstrated that diosgenin inhibits
the proliferation of DU145 cells and activates autophagy and
apoptosis. Inhibition of the phosphorylation of members of the
PI3K/Akt/mTOR signaling pathway is an underlying antitumor
mechanisms of diosgenin.
To further verify the effect of autophagy on the
induction of apoptosis by diosgenin, the autophagy inhibitor 3-MA
was used in combination with diosgenin to treat DU145 cells. The
results of the MTT assays demonstrated that the percentage of dead
cells increased significantly following combined treatment. Hoechst
33342/PI live-cell staining demonstrated a significant increase in
apoptosis of cells administered the combined treatment, suggesting
that the inhibition of diosgenin-activated autophagy increases
apoptosis to achieve increased antitumor effects.
RT-qPCR was performed to determine Beclin 1 and Bcl2
mRNA expression levels. Beclin 1 is the mammalian homolog of the
yeast ATG6-Vps30 protein, which has a key regulatory role in
tumorigenesis and autophagosome formation (60,61).
Bcl2 inhibits apoptosis (62) and
interacts with Beclin 1. Beclin 1 is considered to induce
autophagy, whereas Bcl2 inhibits Beclin 1-dependent autophagy
(63,64). Therefore, Bcl2/Beclin 1 signaling
is an important regulator of autophagy. The results of the present
study demonstrated that the mRNA expression levels of Bcl2 in cells
treated with diosgenin decreased significantly, whereas the Beclin
1 mRNA expression levels increased significantly, confirming that
diosgenin induces apoptosis and autophagy. Following the inhibition
of autophagy, apoptosis increased significantly, consistent with
the Hoechst 33342/PI staining result.
In conclusion, the results of the present study
demonstrate that diosgenin has marked antitumor activity in the
DU145 prostate cancer cell line. Diosgenin inhibits the
proliferation of DU145 cells by activating apoptosis and autophagy,
and the mechanism underlying this activation may be associated with
the inhibition of the PI3K/Akt/mTOR signaling pathway. In addition,
the inhibition of autophagy mediated by diosgenin increases
apoptosis and, thus, increases the therapeutic effect. The
combination of diosgenin with an autophagy inhibitor may
potentially be an effective strategy to further increase the
antitumor effect of diosgenin.
Acknowledgements
The present study was supported by the Health
Department of Jiangsu Province (grant no. J201406) and Qing Lan
Project.
References
|
1
|
Zhou CK, Check DP, Lortet-Tieulent J,
Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB and Devesa SS:
Prostate cancer incidence in 43 populations worldwide: An analysis
of time trends overall and by age group. Int J Cancer.
138:1388–1400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peskoe SB, Joshu CE, Rohrmann S, McGlynn
KA, Nyante SJ, Bradwin G, Dobs AS, Kanarek N, Nelson WG and Platz
EA: Circulating total testosterone and PSA concentrations in a
nationally representative sample of men without a diagnosis of
prostate cancer. Prostate. 75:1167–1176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glybochko PV, Aliaev IuG, Krupinov GE,
Rapoport LM, Amosov AV, Bezrukov EA, Novichkov ND, Lachinov ÉL,
Ganzha TM, Obukhov AA and Lerner IuV: Diagnosis and treatment of
local recurrence of prostate cancer using hystoscanning and
high-intensity focused ultrasound in patients after radical
prostatectomy. Urologiia. 72–76. 2014.(In Russian).
|
|
5
|
Kelly BD, Miller N, Sweeney KJ, Durkan GC,
Rogers E, Walsh K and Kerin MJ: A circulating MicroRNA signature as
a biomarker for prostate cancer in a high risk group. J Clin Med.
4:1369–1379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahl A, Hoefeler H, Duran I, Hechmati G,
Garzon-Rodriguez C, Ashcroft J, Lorusso V, Ghelani P, Wei R, Thomas
E and Lüftner D: Health resource utilization associated with
skeletal-related events in patients with advanced prostate cancer:
A European subgroup analysis from an observational, multinational
study. J Clin Med. 3:883–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh P, Pal SK, Alex A and Agarwal N:
Development of PROSTVAC immunotherapy in prostate cancer. Future
Oncol. 11:2137–2148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyamae K, Kitani K, Hara K, Nakakuma K,
Hamada Y, Yamasaki Y, Horio M and Miyamura S: Clinical study of
long-term docetaxel based chemotherapy treatment for patients with
castration-resistant prostate cancer. Nihon Hinyokika Gakkai
Zasshi. 105:172–177. 2014.PubMed/NCBI
|
|
9
|
Luo HC, Cheng HH, Lin GS, Fu ZC and Li DS:
Intensity- modulated radiotherapy combined with endocrine therapy
for intermediate and advanced prostate cancer: Long-term outcome of
Chinese patients. Asian Pac J Cancer Prev. 14:4711–4715. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishizaki F, Hara N, Takizawa I, Nishiyama
T, Isahaya E, Kawasaki T and Takahashi K: Deficiency in androgens
and upregulation of insulin-like growth factor-1 are involved in
high bone turnover in men receiving androgen deprivation therapy
for prostate cancer. Growth Horm IGF Res. 22:122–128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HQ, Jin JJ and Wang J: Matrine
induces mitochondrial apoptosis in cisplatin-resistant non-small
cell lung cancer cells via suppression of β-catenin/survivin
signaling. Oncol Rep. 33:2561–2566. 2015.PubMed/NCBI
|
|
12
|
Tuorkey MJ: Curcumin a potent cancer
preventive agent: Mechanisms of cancer cell killing. Interv Med
Appl Sci. 6:139–146. 2014.PubMed/NCBI
|
|
13
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu X, Liu S, Zhou J, Wang H, Fu R, Wu X,
Wang J and Lu F: Effect of Astragalus polysaccharides on chronic
atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine
in rats. Drug Res (Stuttg). 63:597–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ku SK, Kwak S and Bae JS: Orientin
inhibits high glucose-induced vascular inflammation in vitro and in
vivo. Inflammation. 37:2164–2173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lucas CD, Dorward DA, Sharma S, Rennie J,
Felton JM, Alessandri AL, Duffin R, Schwarze J, Haslett C and Rossi
AG: Wogonin induces eosinophil apoptosis and attenuates allergic
airway inflammation. Am J Respir Crit Care Med. 191:626–636. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma HD, Deng YR, Tian Z and Lian ZX:
Traditional Chinese medicine and immune regulation. Clin Rev
Allergy Immunol. 44:229–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SG, Lee AJ, Bae SH, Kim SM, Lee JH,
Kim MJ and Jang HB: Total extract of Korean red ginseng facilitates
human bone marrow hematopoietic colony formation in vitro. Blood
Res. 49:177–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao L, Wang Y, Shen HL, Shen XD, Nie Y,
Wang Y, Han T, Yin M and Zhang QY: Structural characterization and
radioprotection of bone marrow hematopoiesis of two novel
polysaccharides from the root of Angelica sinensis (Oliv.) Diels.
Fitoterapia. 83:1712–1720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Z, Chen H, Li G, Zhu H, Gao Y, Zhang L
and Sun J: Diosgenin inhibits the migration of human breast cancer
MDA-MB-231 cells by suppressing Vav2 activity. Phytomedicine.
21:871–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang HP, Yue L, Jiang WW, Liu Q, Kou JP
and Yu BY: Diosgenin inhibits tumor necrosis factor-induced tissue
factor activity and expression in THP-1 cells via down-regulation
of the NF-κB, Akt, and MAPK signaling pathways. Chin J Nat Med.
11:608–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalailingam P, Kannaian B, Tamilmani E and
Kaliaperumal R: Efficacy of natural diosgenin on cardiovascular
risk, insulin secretion, and beta cells in streptozotocin
(STZ)-induced diabetic rats. Phytomedicine. 21:1154–1161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato K, Fujita S and Iemitsu M: Acute
administration of diosgenin or dioscorea improves hyperglycemia
with increases muscular steroidogenesis in STZ-induced type 1
diabetic rats. J Steroid Biochem Mol Biol. 143:152–159. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McKoy ML, Thomas PG, Asemota H, Omoruyi F
and Simon O: Effects of Jamaican bitter yam (Dioscorea
polygonoides) and diosgenin on blood and fecal cholesterol in rats.
J Med Food. 17:1183–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ebrahimi H, Badalzadeh R, Mohammadi M and
Yousefi B: Diosgenin attenuates inflammatory response induced by
myocardial reperfusion injury: Role of mitochondrial ATP-sensitive
potassium channels. J Physiol Biochem. 70:425–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manivannan J, Shanthakumar J, Arunagiri P,
Raja B and Balamurugan E: Diosgenin interferes coronary
vasoconstriction and inhibits osteochondrogenic
transdifferentiation of aortic VSMC in CRF rats. Biochimie.
102:183–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Badalzadeh R, Yousefi B, Majidinia M and
Ebrahimi H: Anti-arrhythmic effect of diosgenin in
reperfusion-induced myocardial injury in a rat model: Activation of
nitric oxide system and mitochondrial KATP channel. J Physiol Sci.
64:393–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghosh S, More P, Derle A, Kitture R, Kale
T, Gorain M, Avasthi A, Markad P, Kundu GC, Kale S, et al:
Diosgenin functionalized iron oxide nanoparticles as novel
nanomaterial against breast cancer. J Nanosci Nanotechnol.
15:9464–9472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding W, Jiang Y, Jiang Y, Zhu T, Xu Y,
Jiang W, Zhu W, Tang Z, Ge Z, Ma T and Tan Y: Role of SB203580 in
the regulation of human esophageal cancer cells under the effection
of Diosgenin. Int J Clin Exp Med. 8:2476–2479. 2015.PubMed/NCBI
|
|
30
|
Li Y, Wang X, Cheng S, Du J, Deng Z, Zhang
Y, Liu Q, Gao J, Cheng B and Ling C: Diosgenin induces G2/M cell
cycle arrest and apoptosis in human hepatocellular carcinoma cells.
Oncol Rep. 33:693–698. 2015.PubMed/NCBI
|
|
31
|
Mao ZJ, Tang QJ, Zhang CA, Qin ZF, Pang B,
Wei PK, Liu B and Chou YN: Anti-proliferation and anti-invasion
effects of diosgenin on gastric cancer BGC-823 cells with HIF-1α
shRNAs. Int J Mol Sci. 13:6521–6533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ganot N, Meker S, Reytman L, Tzubery A and
Tshuva EY: Anticancer metal complexes: Synthesis and cytotoxicity
evaluation by the MTT assay. J Vis Exp. e507672013.PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Würstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saleem M, Qadir MI, Perveen N and Ahmad B,
Saleem U, Irshad T and Ahmad B: Inhibitors of apoptotic proteins:
New targets for anticancer therapy. Chem Biol Drug Des. 82:243–251.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Floyd DH, Zhang Y, Dey BK, Kefas B, Breit
H, Marks K, Dutta A, Herold-Mende C, Synowitz M, Glass R, et al:
Novel anti-apoptotic microRNAs 582-5p and 363 promote human
glioblastoma stem cell survival via direct inhibition of caspase 3,
caspase 9, and Bim. PLoS One. 9:e962392014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ando M, Hoyos V, Yagyu S, Tao W, Ramos CA,
Dotti G, Brenner MK and Bouchier-Hayes L: Bortezomib sensitizes
non-small cell lung cancer to mesenchymal stromal cell-delivered
inducible caspase-9-mediated cytotoxicity. Cancer Gene Ther.
21:472–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng Y, He D, Yao Z and Klionsky DJ: The
machinery of macroautophagy. Cell Res. 24:24–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Belaid A, Ndiaye PD, Klionsky DJ, Hofman P
and Mograbi B: Signalphagy: Scheduled signal termination by
macroautophagy. Autophagy. 9:1629–1630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klionsky DJ and Codogno P: The mechanism
and physiological function of macroautophagy. J Innate Immun.
5:427–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Münz C: Regulation of innate immunity by
the molecular machinery of macroautophagy. Cell Microbiol.
16:1627–1636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hönscheid P, Datta K and Muders MH:
Autophagy: Detection, regulation and its role in cancer and therapy
response. Int J Radiat Biol. 90:628–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Patel AS, Morse D and Choi AM: Regulation
and functional significance of autophagy in respiratory cell
biology and disease. Am J Respir Cell Mol Biol. 48:1–9. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lorin S, Hamaï A, Mehrpour M and Codogno
P: Autophagy regulation and its role in cancer. Semin Cancer Biol.
23:361–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thorburn A, Thamm DH and Gustafson DL:
Autophagy and cancer therapy. Mol Pharmacol. 85:830–838. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Polewska J: Autophagy-molecular mechanism,
apoptosis and cancer. Postepy Hig Med Dosw (Online). 66:921–936.
2012.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen HY and White E: Role of autophagy in
cancer prevention. Cancer Prev Res (Phila). 4:973–983. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Slobodkin MR and Elazar Z: The Atg8
family: Multifunctional ubiquitin-like key regulators of autophagy.
Essays Biochem. 55:51–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Romao S and Münz C: LC3-associated
phagocytosis. Autophagy. 10:526–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rogov V, Dötsch V, Johansen T and Kirkin
V: Interactions between autophagy receptors and ubiquitin-like
proteins form the molecular basis for selective autophagy. Mol
Cell. 53:167–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gong J, Muñoz AR, Chan D, Ghosh R and
Kumar AP: STAT3 down regulates LC3 to inhibit autophagy and
pancreatic cancer cell growth. Oncotarget. 5:2529–2541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xia P and Xu XY: PI3K/Akt/mTOR signaling
pathway in cancer stem cells: From basic research to clinical
application. Am J Cancer Res. 5:1602–1609. 2015.PubMed/NCBI
|
|
53
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang YQ, Chang GL, Wang Y, Zhang DY, Cao
L and Liu J: Geniposide prevents hypoxia/reoxygenation-induced
apoptosis in H9c2 Cells: Improvement of mitochondrial dysfunction
and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell
Physiol Biochem. 39:407–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Inoki K: mTOR signaling in autophagy
regulation in the kidney. Semin Nephrol. 34:2–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sangawa A, Shintani M, Yamao N and
Kamoshida S: Phosphorylation status of Akt and caspase-9 in gastric
and colorectal carcinomas. Int J Clin Exp Pathol. 7:3312–3317.
2014.PubMed/NCBI
|
|
60
|
Won KY, Kim GY, Lim SJ, Sung JY, Kim YW,
Park YK, Lee J and Choi HS: Autophagy is related to the hedgehog
signaling pathway in human gastric adenocarcinoma: Prognostic
significance of Beclin-1 and Gli2 expression in human gastric
adenocarcinoma. Pathol Res Pract. 211:308–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhong Y, Morris DH, Jin L, Patel MS,
Karunakaran SK, Fu YJ, Matuszak EA, Weiss HL, Chait BT and Wang QJ:
Nrbf2 protein suppresses autophagy by modulating Atg14L
protein-containing Beclin 1-Vps34 complex architecture and reducing
intracellular phosphatidylinositol-3 phosphate levels. J Biol Chem.
289:26021–26037. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Salminen A, Kaarniranta K and Kauppinen A:
Beclin 1 interactome controls the crosstalk between apoptosis,
autophagy and inflammasome activation: Impact on the aging process.
Ageing Res Rev. 12:520–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fukui M, Yamabe N, Choi HJ, Polireddy K,
Chen Q and Zhu BT: Mechanism of ascorbate-induced cell death in
human pancreatic cancer cells: Role of Bcl-2, Beclin 1 and
autophagy. Planta Med. 81:838–846. 2015. View Article : Google Scholar : PubMed/NCBI
|