Introduction
There is a high incidence of hepatocellular
carcinoma (HCC) in China, HCC accounts for more than 80% of cases
of primary liver cancer (1,2). The
majority of patients with either HCC or hepatic metastasis
carcinoma are not eligible candidates for surgical resection
(3). Transarterial
chemoembolization and the orally available targeted drug sorafenib
have been demonstrated to increase survival in selected candidates
(4). It is necessary to
investigate and develop efficient and low toxicity drugs for the
clinical treatment of HCC.
The use of thalidomide was terminated due to its
teratogenicity (5). Lenalidomide
is a new analogue of thalidomide and has been demonstrated to be
more potent than thalidomide in the stimulation of T-cells,
interleukin (IL)-2, and interferon (IFN)-γ production (6,7).
Unlike thalidomide, lenalidomide exhibits almost no sedative or
constipation-causing properties, and induces only minimal
neurotoxicity in the initial clinical application (8). Previous studies have demonstrated
that the anti-inflammatory, immunomodulatory and anti-angiogenic of
lenalidomide served important roles in its anticancer activity
(9,10). Lenalidomide induces apoptosis of
myeloma cells and exhibits an immunomodulatory effect on cytokine
secretion, enhancing T cell proliferation and IL-2 and IFN-γ
production in patients with multiple myeloma (MM), and it
additionally increases lysis of autologous MM cells through
cytotoxicity mediated by natural killer cells (11,12).
However, it remains unclear whether it may be efficacious in solid
tumors.
In the current study, SMMC-7721 hepatoma cells were
treated with lenalidomide or thalidomide at different
concentrations, and it was identified that lenalidomide
significantly inhibits proliferation of SMMC-7721 hepatoma cells
in vitro. The two drugs tested can promote cell apoptosis
and inhibit the expression of vascular endothelial growth factor
(VEGF). In addition, lenalidomide was identified to be more potent
than thalidomide, with observations of cell morphology by
microscopy confirming these results. It was suggested that
lenalidomide may induce apoptosis through the pathway of caspase-3
activation.
Materials and methods
Cells and reagents
The human HCC cell line SMMC-7721 was purchased from
the Soochow University Cell Banks (Suzhou, China). Lenalidomide
(Natco Pharma Limited, Hyderabad, India) and thalidomide
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck Millipore) to
prepare 10 and 40 mM stock solutions. Cells were stained with
Annexin V-Fluorescein Isothiocyanate (FITC) following the
manufacturer's instructions (Annexin V-FITC Apoptosis kit; Beijing
BLKW Biotechnology Co., Ltd., Beijing, China) and analyzed for
apoptosis by FACS using CellQuest software version 7.0 (BD
Bioscience, Franklin Lakes, NJ, USA). Flag-tagged caspase-3 was
purchased Bio-Box Biotech (Beijing, China). VEGF enzyme-linked
immunosorbent assay (ELISA) analysis was performed with a
commercial VEGF ELISA kit (cat. no. EH010-56; BLKW Biotechnology,
Co., Ltd.) following the manufacturer's protocol. The antibodies
for caspase-3 (cat. no. 9664P) and VEGF (cat. no. 2478S) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
GAPDH antibody (cat. no. AB22131) was obtained from Bioworld
Technology, Inc. (St. Louis Park, MN, USA) The Cell Counting Kit 8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
(Kumamoto, Japan).
Cell culture
SMMC-7721 cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (10%; Gibco; Thermo
Fisher Scientific, Inc.). The SMMC-7721 human HCC cell line was
maintained at 37°C in a humidified atmosphere with 5% CO2.
Cell proliferation assay
CCK-8 assay was used to evaluate the relative cell
viability. Briefly, cells were plated in 96-well plates at a
density of 5,000 cells/well in the media. The cells were pretreated
with compounds at 6.25, 12.5, 25, 50, 100 and 200 µg/ml in a final
concentration of 0.25% DMSO in triplicate at 37°C in a humidfied
incubator at 5% CO2 for 48 h. Subsequently, CCK-8 reagent (100
µl/ml medium) was applied and incubated with cells at 37°C, 5% CO2
for 1 h. Cells were then incubated for an additional 4 h and the
optical density (OD) was measured at 450 nm using a VersaMax
Microtiter Plate Reader (Molecular Devices, LLC, Sunnyvale, CA,
USA). Relative cell viability was calculated with the following
formula: Relative cell viability (%) = OD(treatment
group)/OD(control group) × 100%. The experiment was performed in
triplicate.
Apoptosis assay
Cells (5×105) were treated with 100 µg/ml or 200
µg/ml drugs (lenalidomide and thalidomide) for 48 h then washed
once with Annexin-V wash buffer (BD Biosciences). Cells were
incubated with Annexin-V binding protein (5 µl) and propidium
iodide (PI; 10 µl) (BD Biosciences) for 10 min. Cells were diluted
with 500 µl wash buffer and analyzed by a FACSCalibur flow
cytometer using CellQuest software. Furthermore, cells (5×105) were
treated with the indicated treatments for 48 h, the supernatant was
removed, 1 ml 70% cold ethanol was added along the six hole plate
edge, then cells were fixed for 15 min. The cells were then washed
with cold phosphate-buffered saline and PI (0.5 ml) was added prior
to observation under the microscope.
Caspase-3 assay
Cells (5×105) were treated with 100 µg/ml or 200
µg/ml of the drugs (lenalidomide and thalidomide) for 48 h. Lysates
(50 µl/2×106 cells) were added, then the cells were reprecipitated,
placed in an ice bath for 30 min, during which they were oscillated
3–4 times (10 sec each time), then the crude cytosol was obtained
as the supernatant as a result of centrifugation at 6,140 ×
g for 20 min at 4°C. Cell lysates (50 µl) were then
extracted and mixed with Ac-DEVD-pNA (Sigma-Aldrich; Merck
Millipore), incubated for 4 h at 37°C, and then the OD was measured
using a microplate reader. Caspase-3 activation was determined by
the rate of OD induced and OD control. All experiments were
performed in triplicates.
ELISA assay
Cells (5×105) were treated with lenalidomide and
thalidomide at the indicated doses for 48 h. ELISA analysis was
performed as previously described (13). VEGF ELISA was performed using 200
µl culture supernatant in duplicates using the Quantikine VEGF
ELISA kit (BLKW Biotechnology, Co., Ltd.) according to the
manufacturer's instructions. Briefly, 200 µl culture supernatants
was added to the wells and they were incubated for 2 h at 37°C. The
plate was washed with 400 µl wash buffer twice and 200 µl VEGF
conjugate was added followed by incubation for 2 h. Subsequent to
the addition of substrate and stop solution, the optical density
was determined using a microplate reader (RT-21000; Rayto Life and
Analytical Sciences Co., Ltd., Shenzhen, China) at 450 nm with
wavelength correction of 570 nm.
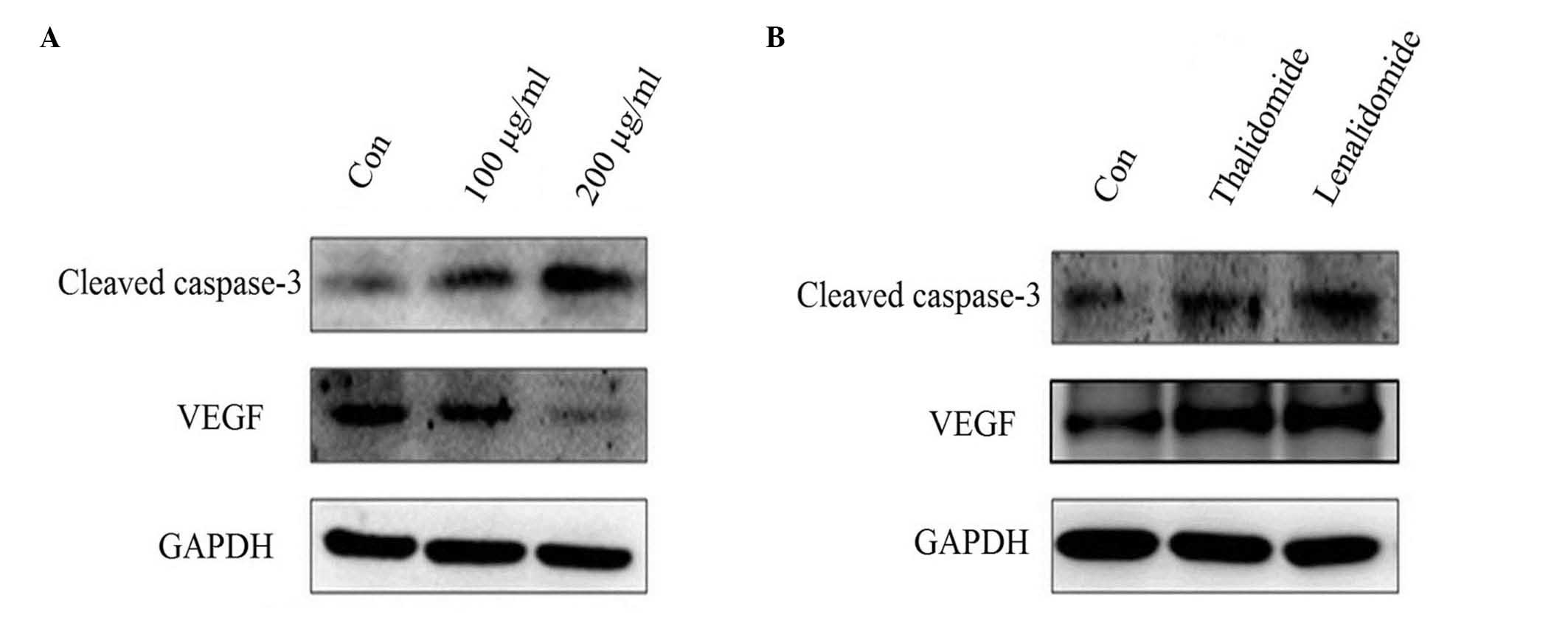
Western blotting
To determine the level of VEGF proteins,
lenalidomide and thalidomide-treated cell lysates were prepared as
described. A total of 20 µg proteins was analyzed by western blot
analysis. The PVDF membranes with the transferred proteins were
incubated with primary antibodies (cleaved caspase-3, 1:500; VEGF,
1:1,000; GAPDH, 1:10,000) at 4°C overnight and horseradish
peroxidase-conjugated secondary antibodies (1:10,000; cat. no.
7074P2; Cell Signaling Technology, Inc.) at room temperature for 2
h. The signal was developed by the enhanced chemiluminescence
reagent (EMD Millipore, Billerica, MA, USA) and visualized by
FluorChem FC2 Imaging System (Alpha Innotech, San Leandro, CA,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data analysis was performed using the SPSS software, version 18.0
(SPSS, Inc., Chicago, IL, USA). Difference between groups were
assessed with Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Lenalidomide inhibits SMMC-7721 cell
proliferation
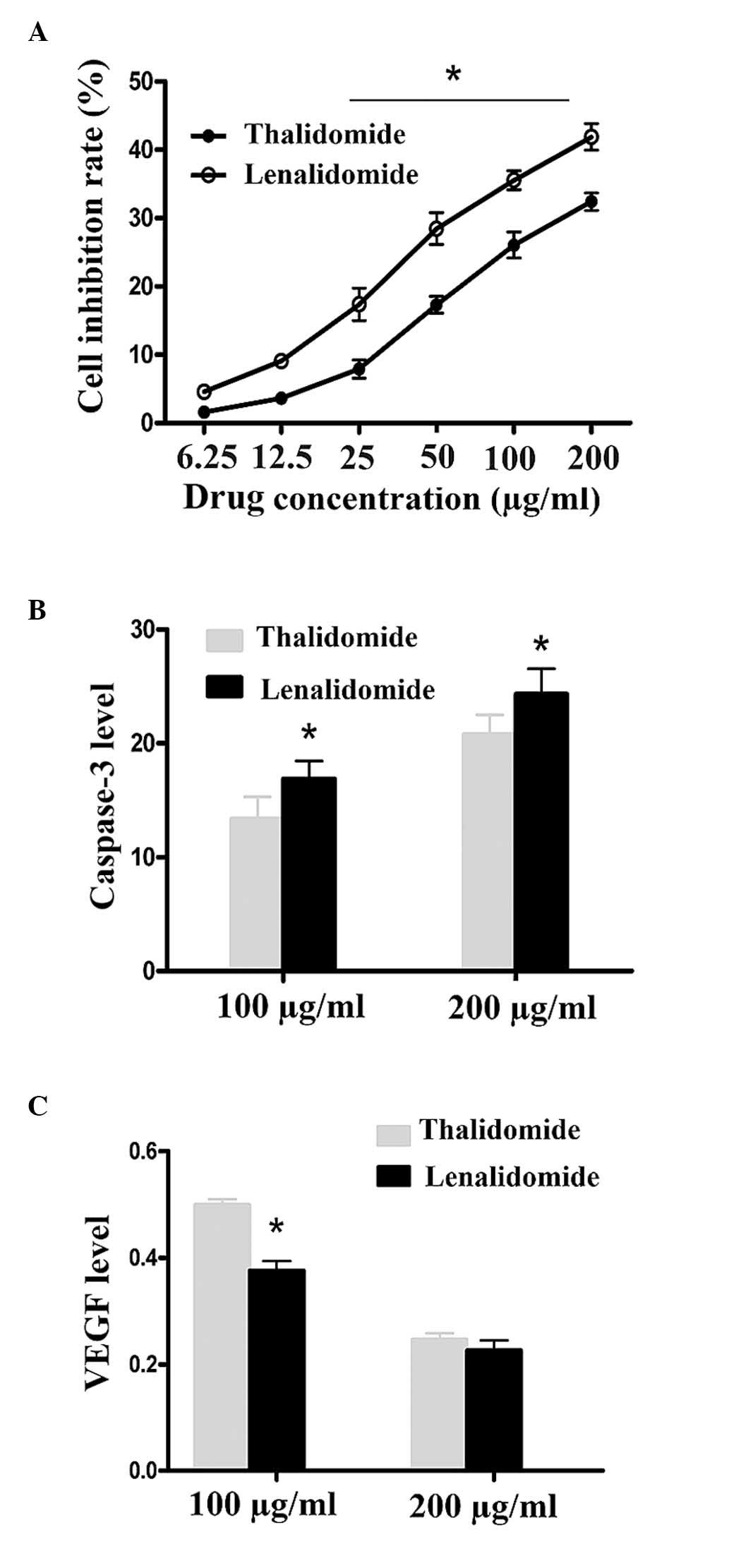
The anti-proliferative rate was detected by CCK-8,
results are expressed as the mean ± standard deviation. Treatment
of cells with lenalidomide and thalidomide in different
concentrations for 48 h led to a dose-dependent induction of the
inhibition of cell proliferation. The anti-proliferative effects of
lenalidomide were identified to be more potent than that of
thalidomide in the 25, 50, 100 and 200 µg/ml groups (P<0.01;
Fig. 1A and Table I).
 | Table I.Cell growth inhibition rates in each
drug concentration group. |
Table I.
Cell growth inhibition rates in each
drug concentration group.
|
| Inhibition rate
(%) |
|---|
|
|
|
|---|
| Drug concentration
(µg/ml) | Thalidomide | Lenalidomide |
|---|
| 6.25 |
1.57±0.291 |
4.55±0.852 |
| 12.5 |
3.01±0.447 |
11.05±0.859 |
| 25 |
7.89±0.349 |
17.35±2.366a |
| 50 |
17.28±1.223 |
28.44±2.331a |
| 100 |
26.03±1.897 |
35.51±0.383a |
| 200 |
32.40±1.296 |
41.87±0.949a |
Lenalidomide promotes apoptosis in
SMMC-7721 cells
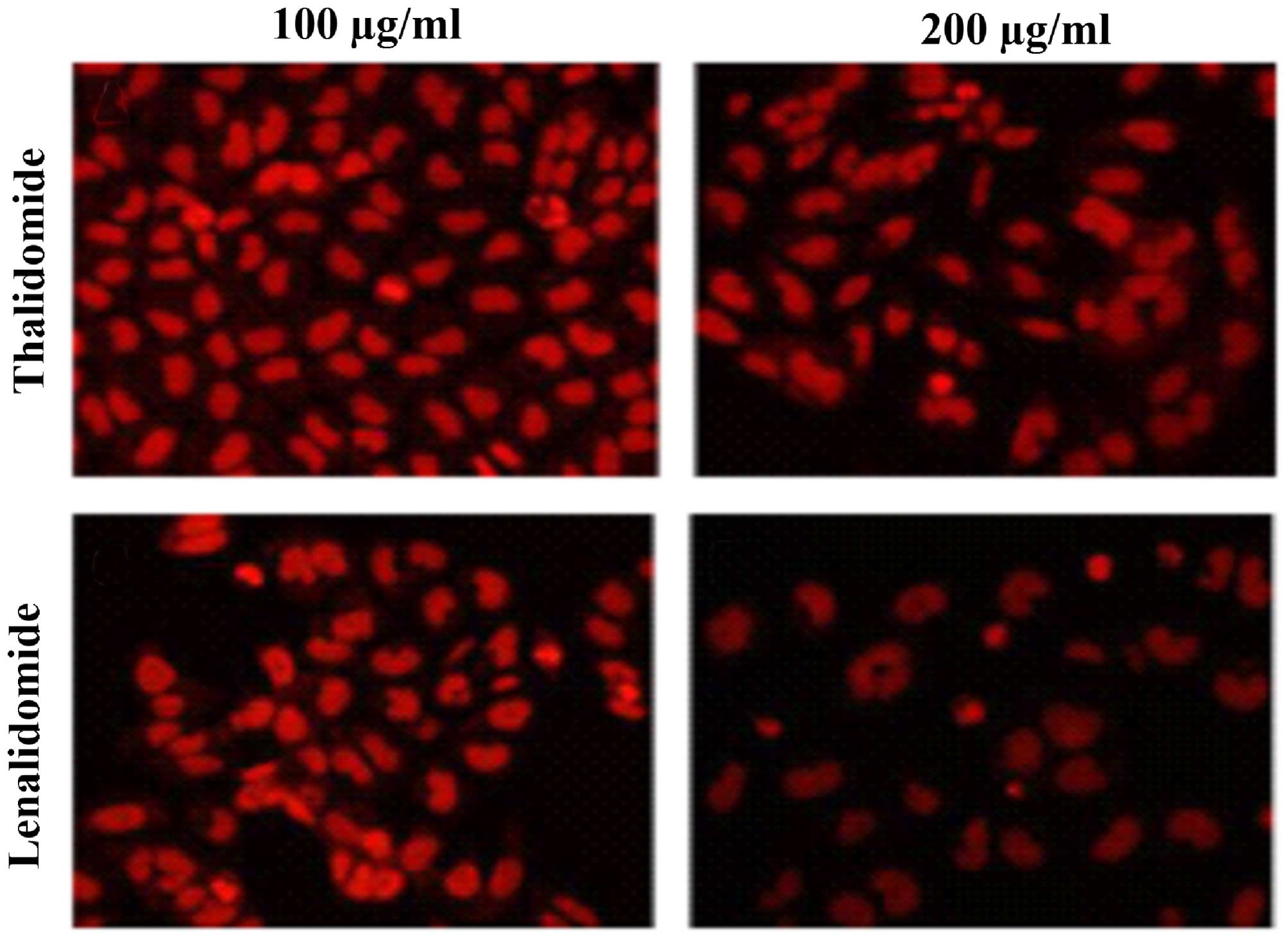
Cells (5×105) were treated with lenalidomide and
thalidomide at the indicated doses for 48 h. Typical apoptotic
morphological alterations were observed using fluorescence
microscopy, and included cell nucleus shrinkage, chromatin
condensation and the appearance of apoptotic bodies when treated
with different doses of the drugs tested (Fig. 2). Lenalidomide was observed to
exhibit an increased effect of inducing cell apoptosis than
thalidomide at the same concentration (Fig. 2). Caspase-3 activity of samples was
detected using a microplate reader, and the OD was analyzed at 405
nm. Activity of caspase-3 was upregulated with increased
lenalidomide and thalidomide concentrations. Caspase-3 activity in
the lenalidomide groups was greater than in the thalidomide groups
at the same concentrations, and this difference was significant
(16.69±1.54 vs. 13.37±1.59; 24.31±2.24 vs. 20.75±1.75; P<0.01,
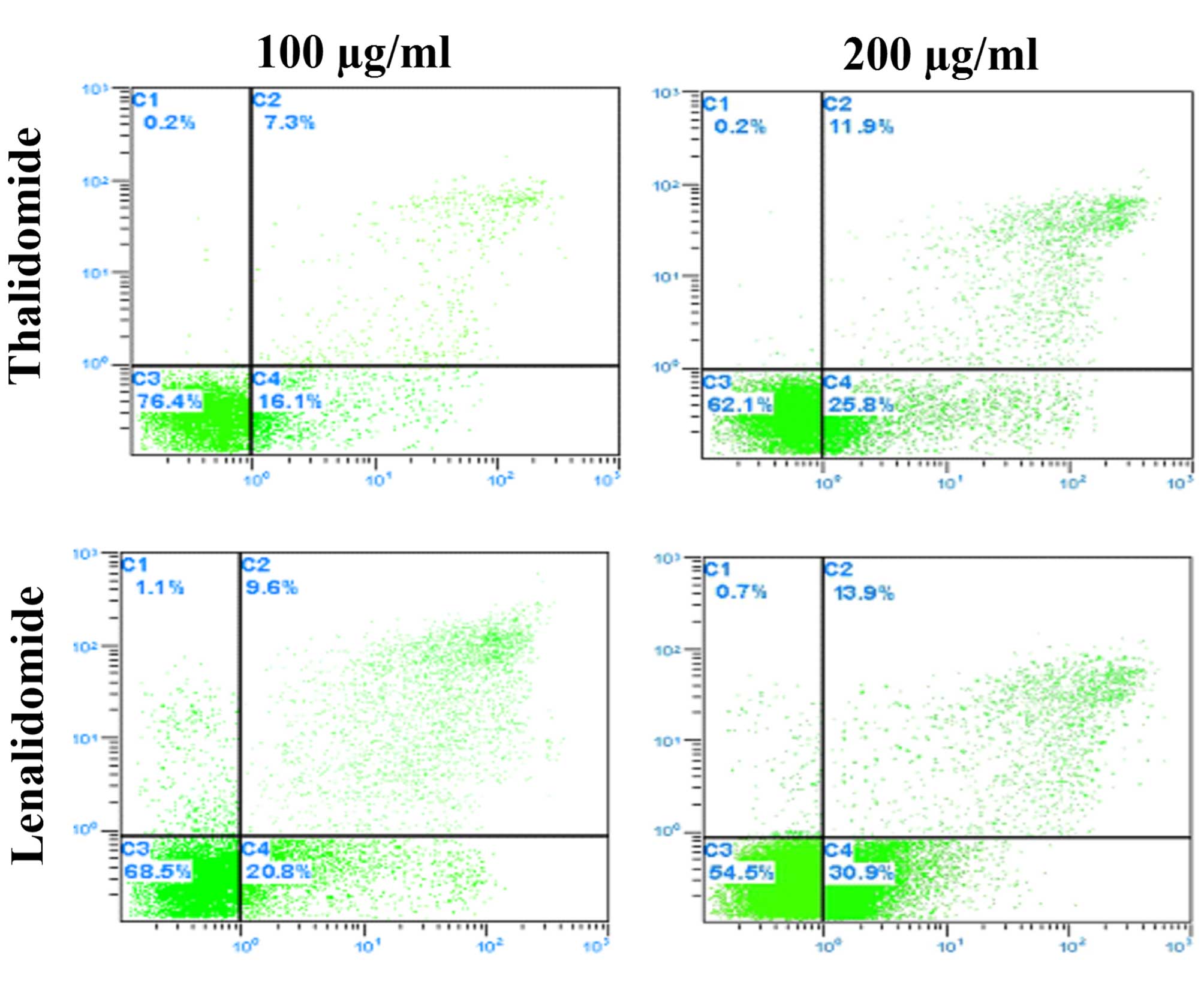
P<0.05; Fig. 1B, Table II). Hepatoma SMMC-7721 cell
apoptosis was examined using the Annexin-V staining-based FACS
assay following lenalidomide and thalidomide treatment for 48 h.
The rate of apoptosis is presented in Fig. 3. Lenalidomide has a higher rate of
induced cell apoptosis than thalidomide of the same concentration
(P<0.01; Fig. 3), and the
effect was observed to be greater with the increase of the
lenalidomide concentration (Fig.
4).
 | Table II.Expression of activated caspase-3 in
each drug concentration group. |
Table II.
Expression of activated caspase-3 in
each drug concentration group.
| Drug | 100 µg/ml | 200 µg/ml |
|---|
| Thalidomide |
13.37±1.95 |
20.75±1.75 |
| Lenalidomide |
16.90±1.54a |
24.31±2.24a |
Lenalidomide inhibits VEGF expression
in SMMC-7721 cells
The data were presented as the mean ± standard
deviation. In the current study, it was observed that lenalidomide
can significantly inhibit VEGF expression of SMMC-7721 cells in
vitro (Fig. 4) and is more
potent than thalidomide in the 100 µg/ml groups (0.3760±0.01813;
0.4985±0.01097; P<0.05). No significant differences were
observed between the 200 µg/ml groups (0.2255±0.01921;
0.2460±0.01192; P>0.05; Fig.
1C, Table III).
 | Table III.Expression of vascular endothelial
growth factor in each drug concentration group. |
Table III.
Expression of vascular endothelial
growth factor in each drug concentration group.
| Drug | 100 µg/ml | 200 µg/ml |
|---|
| Thalidomide |
0.4985±0.01097 |
0.2460±0.01192 |
| Lenalidomide |
0.3760±0.01813a |
0.2255±0.01921 |
Discussion
Lenalidomide is a novel analogue of thalidomide and
previous studies have demonstrated its anticancer effects (8,9,14).
The results of the current study demonstrated that lenalidomide and
thalidomide can significantly inhibit the proliferation of the
human SMMC-7721 HCC cell line. These suggested that lenalidomide
has anti-proliferative activity for HCC cell lines in vitro,
and the same effect of lenalidomide inhibiting cell proliferation
has been observed in multiple myeloma cell lines (15). In addition, in the present study
typical apoptotic morphological alterations were identified by
fluorescence microscopy, including cell nucleus shrinkage,
chromatin condensation and the presence of apoptotic bodies with
treatment with different doses of the drugs.
Caspase-3 is an intracellular protease activated
early during apoptosis of cells and serves an important role in
cell apoptosis. This protease activity can be measured
spectrophotometrically by detection of the chromophore
(p-nitroanilide) subsequent to cleavage from the labeled
substrate (DEVD-pNA). In previous studies, Dmoszynska et al
(11) reported that the mixture of
lovastatin and thalidomidemay increases the rate of multiple
myeloma cell apoptosis. Ezell et al (16) observed that low dose thalidomide
treatment of human T leukemic cells exhibited rapid increases in
caspase-3 activity, in addition, thalidomide and its
immunomodulatory analogs trigger activation of caspase-8, enhancing
MM cell sensitivity to Fas-induced apoptosis (17). The current study identified that
activity of caspase-3 is upregulated with increases in lenalidomide
and thalidomide concentration, and caspase-3 activity in
lenalidomide groups is significantly higher than that of the
thalidomide groups with the same concentrations (P<0.05;
Figs. 1B and 4 and Table
II). The present study indicated that lenalidomide inhibits
proliferation of SMMC-7721 cells via the induction of cell
apoptosis and suggested that caspase-3 may serve an important role
in this process.
Angiogenesis serves an important role during tumor
growth, invasion and metastasis (18), and VEGF is an endothelial-specific
growth factor that stimulates endothelial function and angiogenesis
(15). A previous study identified
that lenalidomide possesses anti-angiogenic activity, and enhances
T cell proliferation in MM patients (8). Tan et al (7) demonstrated that thalidomide can
suppress VEGF and hypoxia-inducible factor 1α in a dose-dependent
manner (P<0.05). In the present study, it was demonstrated that
lenalidomide can significantly inhibit VEGF expression of SMMC-7721
cells in vitro, and is more potent than that of thalidomide
in the 100 µg/ml groups (P<0.05; Figs. 1C and 4 and Table
III). No significant difference was observed between the 200
µg/ml groups (P>0.05), These results indicated that the
anti-angiogenesis activity of lenalidomide was induced through
suppression of VEGF, and this may be a critical factor in the
inhibition of cell proliferation.
Taken together, the results suggest that the
inhibition of SMMC-7721 cell proliferation by lenalidomide in
vitro is more potent than that of thalidomide and in addition,
induction of apoptosis and inhibition of angiogenesis may be two
potential mechanisms for its anti-HCC activity.
Acknowledgements
The present study was supported by the Science and
Technology Projects (grant no. CY20119002) from the Changzhou
Science and Technology Bureau of China. The authors would like to
thank Dr Peng Jiang for the technical assistance and Professor Yong
Jiang for the helpful discussion.
References
|
1
|
El-Serag HB and Mason AC: Rising incidence
of hepatocellular carcinoma in the United States. N Engl J Med.
340:745–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sangiovanni A, Del Ninno E, Fasani P, De
Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R and Colombo
M: Increased survival of cirrhotic patients with a hepatocellular
carcinoma detected during surveillance. Gastroenterology.
126:1005–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Merchant N, David CS and Cunningham SC:
Early hepatocellular carcinoma: Transplantation versus resection:
The case for liver resection. Int J Hepatol. 2011:1420852011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burrel M, Reig M, Forner A, Barrufet M, de
Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI and Bruix J:
Survival of patients with hepatocellular carcinoma treated by
transarterial chemoembolisation (TACE) using drug eluting beads.
Implications for clinical practice and trial design. J Hepatol.
56:1330–1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Settles B, Stevenson A, Wilson K, Mack C,
Ezell T, Davis MF and Taylor LD: Down-regulation of cell adhesion
molecules LFA-1 and ICAM-1 after in vitro treatment with the
anti-TNF-alpha agent thalidomide. Cell Mol Biol (Noisy-le-grand).
47:1105–1114. 2001.PubMed/NCBI
|
|
6
|
Sun P, Zhang LM, Sun DJ and Dong LL:
Inhibitory effect of thalidomide on growth of human hepatoma cell
line SMMC 7721 cells. Zhonghua Zhong Liu Za Zhi. 31:582–586.
2009.(In Chinese). PubMed/NCBI
|
|
7
|
Tan H, Chen H, Xu C, Ge Z, Gao Y, Fang J,
Liu W and Xiao S: Role of vascular endothelial growth factor in
angiodysplasia: An interventional study with thalidomide. J
Gastroenterol Hepatol. 27:1094–1101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galustian C, Meyer B, Labarthe MC, Dredge
K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, et
al: The anti-cancer agents lenalidomide and pomalidomide inhibit
the proliferation and function of T regulatory cells. Cancer
Immunol Immunother. 58:1033–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agliano A, Martin-Padura I, Marighetti P,
Gregato G, Calleri A, Prior C, Redrado M, Calvo A and Bertolini F:
Therapeutic effect of lenalidomide in a novel xenograft mouse model
of human blastic NK cell lymphoma/blastic plasmacytoid dendritic
cell neoplasm. Clin Cancer Res. 17:6163–6173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park E, Levis WR, Greig N, Jung E and
Schuller-Levis G: Effect of thalidomide on nitric oxide production
in lipopolysaccharide-activated RAW 264.7 cells. J Drugs Dermatol.
9:330–333. 2010.PubMed/NCBI
|
|
11
|
Dmoszynska A, Podhorecka M, Klimek P and
Grzasko N: Lovastatin and thalidomide have a combined effect on the
rate of multiple myeloma cell apoptosis in short term cell
cultures. Eur J Clin Pharmacol. 62:325–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dmoszynska A, Podhorecka M, Manko J,
Bojarska-Junak A, Rolinski J and Skomra D: The influence of
thalidomide therapy on cytokine secretion, immunophenotype, BCL-2
expression and microvessel density in patients with resistant or
relapsed multiple myeloma. Neoplasma. 52:175–181. 2005.PubMed/NCBI
|
|
13
|
Vaithilingam V, Oberholzer J, Guillemin GJ
and Tuch BE: Beneficial effects of desferrioxamine on encapsulated
human islets-in vitro and in vivo study. Am J Transplant.
10:1961–1969. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van de Donk NW, Wittebol S, Minnema MC and
Lokhorst HM: Lenalidomide (Revlimid) combined with continuous oral
cyclophosphamide (endoxan) and prednisone (REP) is effective in
lenalidomide/dexamethasone-refractory myeloma. Br J Haematol.
148:335–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao KV: Lenalidomide in the treatment of
multiple myeloma. Am J Health Syst Pharm. 64:1799–1807. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ezell TN, Maloney N, Githua JW and Taylor
LD: Exposure to the anti-TNF-alpha drug thalidomide induces
apoptotic cell death in human T leukemic cells. Cell Mol Biol
(Noisy-le-grand). 49:1117–1124. 2003.PubMed/NCBI
|
|
17
|
Mitsiades N, Mitsiades CS, Poulaki V,
Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP and
Anderson KC: Apoptotic signaling induced by immunomodulatory
thalidomide analogs in human multiple myeloma cells: Therapeutic
implications. Blood. 99:4525–4530. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|