Introduction
Aging is a component of the natural life cycle,
causing various morphological, functional and biochemical
alterations in the body, including cognitive decline, and
reductions in neuronal cytoskeleton dynamics and synaptic
plasticity (1–4). In addition, the hippocampus, a
critical region for memory, is vulnerable to damage during the
aging process, possibly due to alterations in protein levels, which
accompany the early stages of Alzheimer's disease (5,6).
Among synaptic proteins, dynamin is known to be
important in the regulation of endocytosis (7) and synaptic vesicle recycling
(8). Dynamin has three isoforms,
all of which are expressed in nerve terminals, however, dynamin 1
is detected at high levels in nerve terminals of the hippocampus
(9). Dynamin 1 is one of several
molecules involved in the pinching off of synaptic vesicles,
releasing them from the membrane during exocytosis, and then
allowing the vesicles to dock and re-enter the synaptic vesicle
pool to be refilled for further neurotransmitter release (10). Inhibition of the vesicle recycling
process or a decrease in the readily releasable pool of synaptic
vesicles affects the ability of the hippocampus to function.
Previously, it was reported that the pharmacological inhibition of
dynamin markedly impairs hippocampal-dependent associative memory
formation (11).
Several lines of evidence have demonstrated that
dynamin 1 is associated with the aging processes occurring in the
hippocampus (12–14), Alzheimer's disease (15–18)
and nicotine dependence (19,20).
However, there are conflicting reports regarding alterations to the
expression levels of dynamin 1 and associations with neurological
disorders and aging. Several studies have reported an increase in
dynamin 1 in the hippocampus associated with aging (13,14)
and Alzheimer's disease (15,18).
By contrast, other studies have found a decrease in dynamin 1 in
the hippocampus associated with Alzheimer's disease (15,21)
and nicotine dependence (19,20).
However, few studies have been performed to assess
age-related changes to the hippocampal expression of dynamin 1 in
C57BL/6 mice, a widely used experimental animal model. The present
study investigated changes to the immunoreactivity and protein
levels of dynamin 1 in the hippocampus and its correspondence with
age. In addition, the present study observed the effects of the
inhibition of dynamin 1 on the hippocampal-dependent memory in
adult mice because dynamin 1 is essential for synaptic vesicle
recycling and memory formation.
Materials and methods
Experimental animals
Young adult (4 month-old) and aged (24 month-old)
male C57BL/6 J mice were purchased from Japan SLC, Inc. (Shizuoka,
Japan). The 24 month old mice were selected as the aged group as,
in humans, this age in mice is equivalent to an age of 69 years
(22). The animals were placed in
a mouse cage (five mice/cage) in conventional conditions. They were
maintained under controlled temperature (23°C) and humidity (60%)
on a 12-h light-dark cycle. The mice were fed a commercial pelleted
diet (Purina chow diet 38057; Purina Korea, Seoul, Korea) and water
ad libitum. The procedures for the handling and caring of
animals followed the Guide for the Care and Use of Laboratory
Animals issued by the Institute of Laboratory Animal Resources, and
the experimental protocol was approved by the Institutional Animal
Care and Use Committee of Seoul National University (Seoul, Korea).
All the experiments were performed to minimize the number of
animals used and the any suffering caused by the procedures used in
the present study.
Morris water maze (MWM) task
To confirm the memory deficits in the aged group,
spatial memory was assessed using the MWM task according to a
previous study (23). The water
maze assessments were performed in order to ensure objectivity in
blind conditions. At 3 days post-training, the time required for an
individual mouse to locate the submerged platform within 2 min
(escape latency) and the swimming distance were monitored using a
digital camera and a computer system for 4 days consecutively, with
four trials per day. For each trial, the mouse (n=10 per group) was
placed in the water facing the wall at one of four starting
positions and released. The swimming speed and the time required
for the mouse to locate the hidden platform were recorded via a
visual tracking system (Noldus Information Technology, Wageningen,
The Netherlands). The probe test was performed on day 5; the
platform was removed and the time that the mouse spent swimming in
the target quadrant, and the time spent in the three non-target
quadrants (right, left and opposite quadrants), were measured in
the training and opposite quadrants in 60 sec. In addition, the
number of times the mouse crossed over the platform site was
recorded.
Tissue processing for histology
For histological analysis, the mice in the adult and
aged groups (n=6 per group) were terminally anesthetized the day
following the MWM task with 1 g/kg urethane (Sigma-Aldrich; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The animals were
perfused transcardially with 0.1 M phosphate-buffered saline (PBS;
pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer
(pH 7.4). The brains were removed and post-fixed in the same
fixative for 12 h. The brain tissues were cryoprotected by
infiltration with 30% sucrose overnight. Subsequently, 30-µm-thick
brain sections were serially cut in the coronal plane using a
cryostat (Leica Microsystems GmbH, Wetzler, Germany). The sections
were collected in six-well plates containing PBS and stored in
storage solution at −20°C until further processing.
Immunohistochemistry
In order to obtain accurate data for
immunohistochemistry, free-floating sections were carefully
processed under the same conditions. Sections were selected located
between −1.46 and −2.46 mm posterior to the Bregma in reference to
a mouse atlas (24). The sections
were sequentially treated with 0.3% hydrogen peroxide in 0.1 M PBS
at 25°C for 30 min and 10% normal goat serum in 0.1 M PBS. They
were then incubated with diluted polyclonal rabbit anti-dynamin 1
(1:200; cat. no. ab55397; Abcam, Cambridge, UK) overnight at 25°C,
and subsequently exposed to biotinylated goat anti-rabbit IgG
(diluted 1:200; cat. no. BA-1000; Vector Laboratories, Inc.,
Burlingame, CA, USA) and streptavidin peroxidase complex (diluted
1:200, Vector Laboratories, Inc.) for 2 h at 25°C. Subsequently,
the sections were visualized by reaction with 3,3′-diaminobenzidine
tetrahydrochloride for 1 min (Sigma-Aldrich; Thermo Fisher
Scientific, Inc.). Digital images were captured with a BX51 light
microscope (Olympus Corporation, Tokyo, Japan) equipped with a
digital camera (DP72; Olympus Corporation) connected to a computer
monitor.
Western blot analysis
To confirm the alterations in dynamin 1 with age,
six mice from each group were sacrificed and for western blot
analysis. Following sacrifice of the mice (n=4 per group) and
removal of their brains, the hippocampi were dissected out with a
surgical blade. The hippocampal tissues were pooled from tissues of
three animals to increase the efficiency of normalization, and were
homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol
bis (2-aminoethyl Ether)-N, N, N',
N'-tetraacetic acid (pH 8.0), 0.2% Nonidet P-40, 10 mM
ethylendiamine-tetraacetic acid (pH 8.0), 15 mM sodium
pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2
mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 1
mM dithiothreitol (DTT). Following centrifugation at 16,000 ×
g for 20 min in a pre-cooled centrifuge, the protein level
was determined in the supernatants using a Micro BCA protein assay
kit with bovine serum albumin as the standard (Pierce; Thermo
Fisher Scientific, Inc,). Aliquots containing 50 µg of total
protein were boiled at 95°C in a loading buffer containing 150 mM
Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30%
glycerol for 5 min. The aliquots were then loaded onto a 7.5%
polyacrylamide gel. Following electrophoresis, the proteins were
transferred from the gel onto nitrocellulose transfer membranes
(Pall Corp., East Hills, NY, USA). To reduce background staining,
the membranes were incubated with 5% non-fat dry milk in PBS
containing 0.1% Tween 20 for 45 min at 25°C, followed by incubation
with rabbit anti-dynamin 1 (1:1,000; cat. no. ab55397; Abcam) at
4°C for 12 h, peroxidase-conjugated anti-rabbit IgG (cat. no.
PI-1000; Vector Laboratories, Inc.) and an use of an enhanced
luminol-based chemiluminescence kit (Pierce; Thermo Fisher
Scientific, Inc.). The blot was densitometrically scanned for
quantification of the relative optical density of each band using
ImageJ 1.59 software (NIH, Bethesda, MD, USA).
Effects of dynamin 1 inhibition on
hippocampal-dependent memory
Dynasore (Sigma-Aldrich; Thermo Fisher Scientific,
Inc.), an inhibitor of dynamin, was prepared as described in a
previous study (11). Briefly, the
dynasore was dissolved in DMSO to obtain a 200 mM stock
concentration and then stored at −80°C. Working solutions (80 µM
dynasore) were diluted in artificial cerebrospinal fluid (CSF)
containing 124 mM NaCl, 4.4 mM KCl, 1 mM Na2HPO4, 25 mM NaHCO3, 10
mM glucose, 2 mM CaCl2 and 2 mM MgCl2, supplemented with
0.3% DMSO, in a low light environment. The animals (n=7 in each
group) were anesthetized with isoflurane and a 26-gauge guide
cannula was placed above the dorsal hippocampi under stereotaxic
coordination (anteroposterior, +2.4; mediolateral, ± 1.5;
dorsoventral, −1.3 mm) (24). At 1
week post-surgery, the same volume (1.5 µl) of artificial CSF or 80
µM dynasore was bilaterally injected through the intracerebral
cannulas connected to a microsyringe with polyethylene tubing. At
20 min post-dynasore treatment, the mice were placed in a novel
environment of a fear conditioning box and exposed to a mild foot
electric shock (2 sec; 0.45 mA) together with an auditory tone (30
sec; 85 dB sound at 2,800 Hz). The electric shock was delivered
during the last 2 sec of the auditory tone. Freezing, whereby mice
do not move other than to breathe, was scored using FreezeView
(version 2.04; Coulbourn Instruments, Holliston, MA, USA). Learning
was assessed 24 h later by measuring freezing behavior for 5 min,
in the chamber in which the mice were trained, in response to
representation of the context without the auditory cue.
Statistical analysis
The data shown represent the mean of experiments
performed for each experimental area. Differences among the means
were statistically analyzed using a two-tailed Mann-Whitney
t-test in order to elucidate differences between adult and
aged groups. Analysis was performed using GraphPad Prism 5.01
software (GraphPad Software, Inc., La Jolla, CA, USA). All data are
presented as the mean ± standard error of the mean. P<0.05 was
considered to indicate a significantly significant difference.
Results
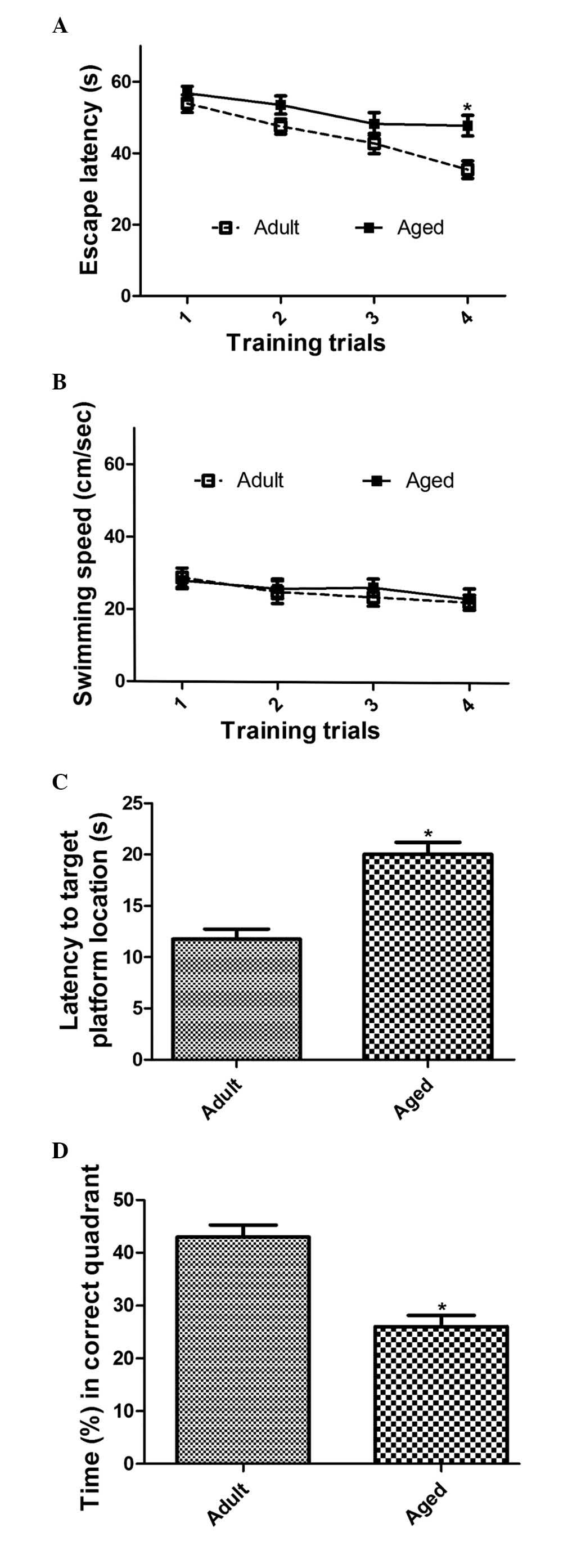
Spatial memory in aged mice
Spatial memory the in adult and aged mice was
assessed using the MWM task. In the training trial of the escape
latency task, the mean escape latency in the aged group was
marginally longer, compared with the adult group on days 2 and 3.
However, there was no significant difference in the escape latency
between the adult and aged groups. By day 4, the escape latency was
significantly longer in the aged group, compared with that in the
adult group (Fig. 1A). However, no
significant differences were found between the adult and aged
groups in the average swimming speed or the total distance traveled
during the probe trial (Fig.
1B).
In the probe trial for the escape latency task, the
animals in the aged groups took significantly longer to locate the
target platform location, compared with those in the adult group
(P=0.0007; Fig. 1C). In addition,
the aged group spent less time in the correct quadrant, compared
with the adult group (P=0.0001; Fig.
1D).
Expression of dynamin 1 in the
hippocampus
Changes in the expression of dynamin 1 were
examined. In the hippocampal CA1 region of the adult group, the
immunoreactivity of dynamin 1 was widely detected in the stratum
radiatum and the stratum pyramidale (Fig. 2A). However, in the aged group,
immunoreactivity of dynamin 1 was only marginal in the stratum
radiatum region of CA1 (Fig.
2B).
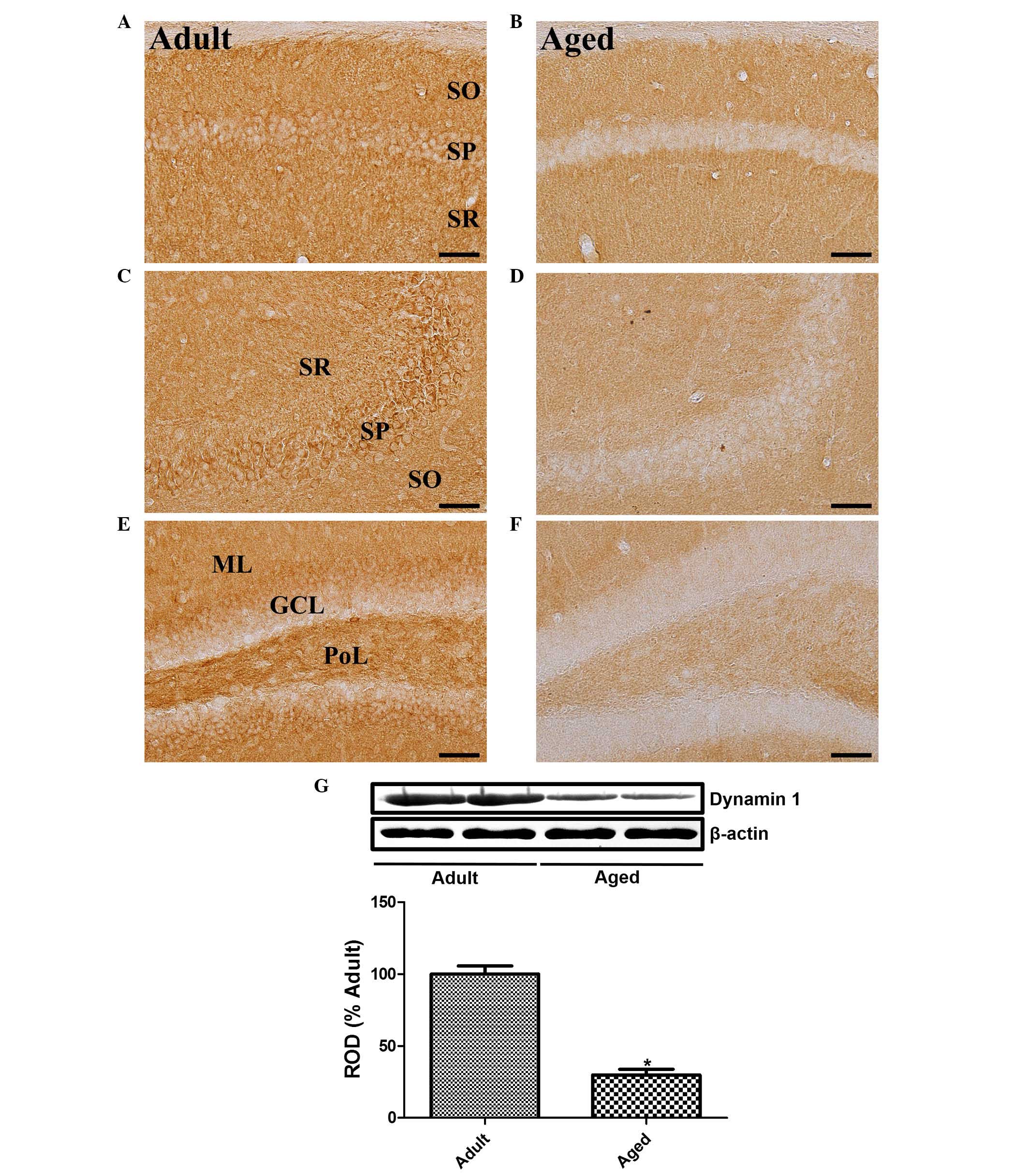 | Figure 2.Immunohistochemical assessment of
dynamin 1. Immunohistochemistry was used to detect dynamin 1 in the
(A) adult and (B) aged hippocampal CA1 region, (C) adult and (D)
aged CA3 region, and (E) adult and (F) aged dentate gyrus. In the
adult group, dynamin 1 immunoreactivity was found in the SR of the
CA1 and CA3 regions, and in the PoL of the dentate gyrus. Dynamin 1
immunoreactivity was also found in the SP of the hippocampal CA1-3
region and GCL of dentate gyrus. In the aged group, dynamin 1
immunoreactivity was detected in these regions at low levels. Scale
bar=50 µm. (G) Western blot analysis of the protein expression of
dynamin 1 in the hippocampi of adult and aged groups. The RODs of
the immunoblot bands are shown as percentages (n=6 per group).
Vales are presented as the mean + standard error of the mean.
*P<0.05, compared with the adult group. SR, stratum radiatum;
SO, stratum oriens; SP, stratum pyramidale; PoL, polymorphic layer;
GCL, granule cell layer; ML, molecular layer; ROD relative optical
density. |
In the hippocampal CA3 region of the adult mice, a
high level of dynamin 1 immunoreactivity was detected in the
pyramidal cell layer. In addition, dynamin 1 was detected in the
stratum radiatum (Fig. 2C).
However, in the aged group, the immunoreactivity of dynamin 1 was
significantly decreased in the pyramidal cell layer and almost
absent in the hippocampal CA3 region (Fig. 2D).
In the dentate gyrus of the adult mice, dynamin 1
was found in the outer half of the granule cell layer and the
polymorphic layer (Fig. 2E).
However, in the aged group, the immunoreactivity of dynamin 1 was
significantly decreased in the granule cell layer and the
polymorphic layer of the dentate gyrus (Fig. 2F).
The protein levels of dynamin 1 in the aged group
were significantly lower, compared with those in the adult group.
In the aged group, the protein expression of dynamin 1 was 34.8% of
the expression of dynamin 1 in the adult group (Fig. 2G).
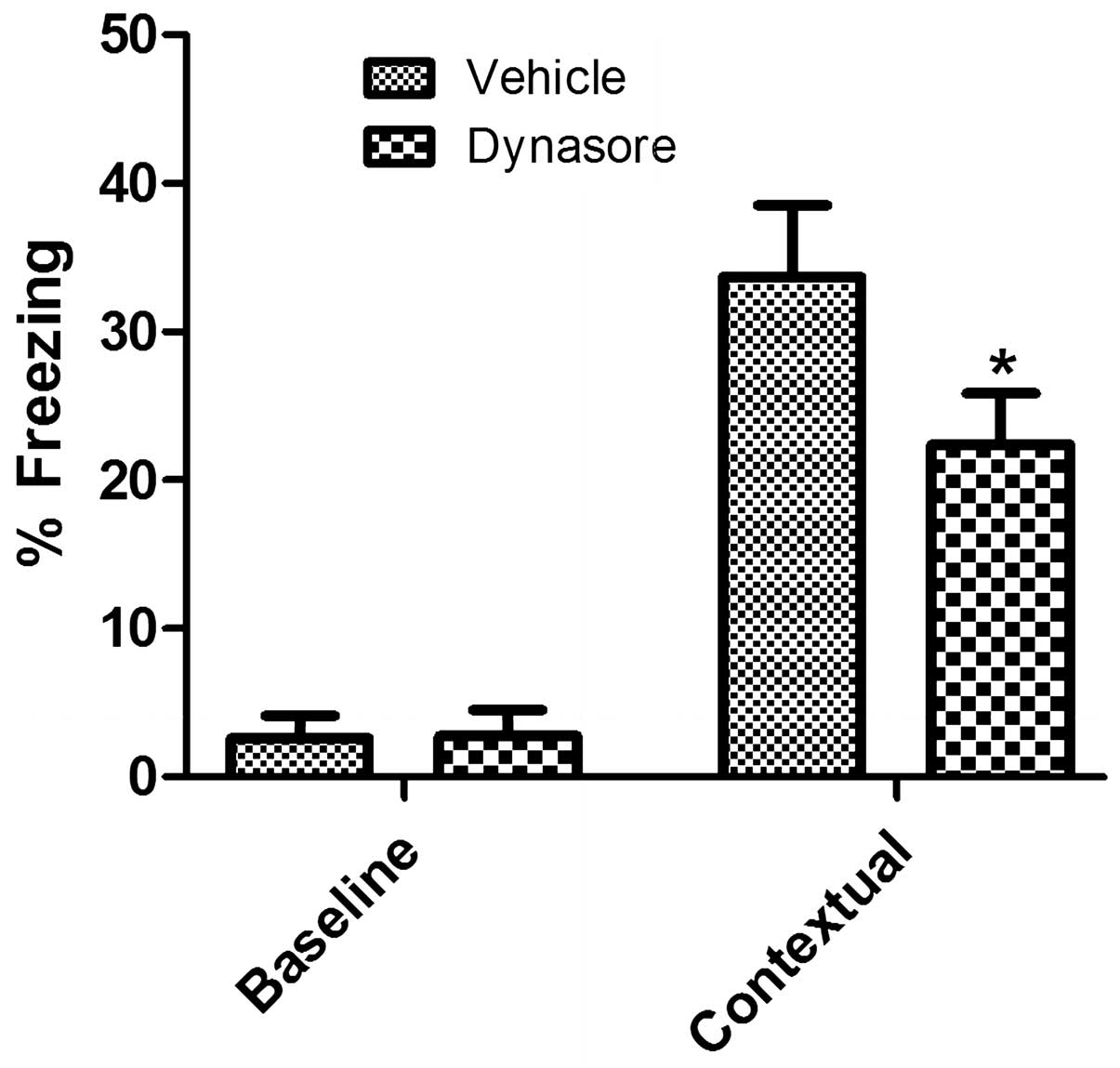
Contextual memory in adult mice
following inhibition of dynamin 1
Under basal conditions, the administration of
vehicle or dynasore did not lead to any significant differences in
freezing between groups. However, following electric and auditory
shock, freezing was observed in the vehicle-treated group. In the
dynasore-treated group, the level of freezing was significantly
decreased, compared with that in the vehicle-treated group
(Fig. 3).
Discussion
There is increasing evidence suggesting that several
presynaptic proteins are involved in altering synaptic activity in
patients with Alzheimer's disease and in animal models of
Alzheimer's disease (21,25,26).
In addition, synaptic proteins are essential for the regulation of
memory. In the present study, a significant reduction in the
spatial memory abilities of the aged group were observed, compared
with the adult group, on assessment using the MWM task. The present
study then investigated the correlation between the memory
impairment observed in the aged group and the expression of dynamin
1. These results suggested that, compared with the adult group, the
aged mice used in the present study showed a decline in
hippocampal-dependent memory formation. Dynamin 1 is a major
dynamin isoform found in neurons (5) and is detected at high levels in
presynaptic terminals. In the present study, age-related changes in
the expression of dynamin 1 in the hippocampus were observed. The
immunoreactivity and protein levels of dynamin 1 were significantly
decreased in the hippocampi of the aged group, compared with the
levels in the adult group. A reduction in dynamin 1 impairs the
axonal trafficking of vesicles through interactions with other
endocytotic accessory proteins present in hippocampal neurons
(17,27,28).
It has also been reported that dynamin 1-depleted neurons
accumulate synaptic vesicles at the plasma membrane and decreases
the readily releasable pool of synaptic vesicles (29).
However, there have been contradictory reports
regarding the changes in the expression levels of dynamin 1 in the
brain and its association with age or Alzheimer's disease. A
previous study found that the expression level of dynamin 1 was
significantly increased overall in the brains of aged (80-week-old)
C57BL/6 mice, compared to that in the brains of young (6-week-old)
mice (13). However, in the
olfactory bulb, the protein expression of dynamin 1 was found to be
significantly decreased in the olfactory bulbs of aged
(80-week-old) mice, compared with young (6-week-old) mice (12). In a mouse model of Alzheimer's
disease, the protein levels of dynamin 1 have been reported to be
increased in the brains of Tg2576 mice with plaque deposition
(15) and
APPE693Δ-transgenic mice in the hippocampus (18) based on a proteomic approach.
However, these changes to the levels of dynamin 1 in the whole
brain or hippocampus were not confirmed by immunohistochemistry or
western blot analysis, respectively. Other studies have shown a
significant decrease in the mRNA and protein levels of dynamin 1 in
the frontal cortex of patients with Alzheimer's disease (21). In addition, the presence of
ameyloid β induces a significant decrease in the expression of
dynamin 1 through the calpain-mediated cleavage of dynamin 1
(16), which is induced by a
sustained calcium influx mediated by N-methyl-D-aspartate
receptors in hippocampal neurons (17). The increase or decrease of dynamin
1 may be associated with the severity of aging or Alzheimer's
disease, or the brain regions used for analysis. However, dynamin 1
is likely to be involved in hippocampal-dependent memory formation.
In the present study, the involvement of dynamin 1 was demonstrated
by directly infusing dynasore, an inhibitor of dynamin 1, into the
hippocampus. This infusion reduced the ability of the mice to
perform on hippocampal-dependent memory tasks, including the
fear-conditioning task. However, no impairment is observed on
hippocampal-independent tasks, including cued conditioning
(11). The present study also
confirmed the effects of dynamin 1 on hippocampal functions using
dynasore. The administration of dynasore significantly decreased
the contextual memory by electric and auditory shock, compared with
that in the vehicle-treated group. This result suggested that
dynamin 1 is one of the key factors affecting hippocampal-dependent
function.
In conclusion, the immunoreactivity and protein
levels of dynamin 1 were found to be significantly reduced in the
hippocampus of aged animals, compared with adult mice, and this
reduction may be associated with the reduction in
hippocampal-dependent memory.
Acknowledgements
This study was supported by the Priority Research
Centers Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
no. NRF-2009-0094071) and the Research Institute for Veterinary
Science, Seoul National University (Seoul, Korea).
References
|
1
|
Rosenzweig ES and Barnes CA: Impact of
aging on hippocampal function: Plasticity, network dynamics, and
cognition. Prog Neurobiol. 69:143–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Himeda T, Mizuno K, Kato H and Araki T:
Effects of age on immunohistochemical changes in the mouse
hippocampus. Mech Ageing Dev. 126:673–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Stefano G, Casoli T, Fattoretti P,
Balietti M, Grossi Y, Giorgetti B and Bertoni-Freddari C: Level and
distribution of microtubule-associated protein-2 (MAP2) as an index
of dendritic structural dynamics. Rejuvenation Res. 9:94–98. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Filipek A, Schneider G, Mietelska A,
Figiel I and Niewiadomska G: Age-dependent changes in neuronal
distribution of CacyBP/SIP: Comparison to tubulin and the tau
protein. J Neural Transm (Vienna). 115:1257–1264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferguson SM, Brasnjo G, Hayashi M, Wölfel
M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S,
et al: A selective activity-dependent requirement for dynamin 1 in
synaptic vesicle endocytosis. Science. 316:570–574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fjell AM, McEvoy L, Holland D, Dale AM and
Walhovd KB: Alzheimer's Disease Neuroimaging Initiative: What is
normal in normal aging? Effects of aging, amyloid and Alzheimer's
disease on the cerebral cortex and the hippocampus. Prog Neurobiol.
117:20–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daulatzai MA: Early stages of pathogenesis
in memory impairment during normal senescence and Alzheimer's
disease. J Alzheimers Dis. 20:355–367. 2010.PubMed/NCBI
|
|
8
|
Watanabe T, Iwasaki K, Takasaki K,
Yamagata N, Fujino M, Nogami A, Ii M, Katsurabayashi S, Mishima K
and Fujiwara M: Dynamin 1 depletion and memory deficits in rats
treated with Abeta and cerebral ischemia. J Neurosci Res.
88:1908–1917. 2010.PubMed/NCBI
|
|
9
|
Sontag JM, Fykse EM, Ushkaryov Y, Liu JP,
Robinson PJ and Südhof TC: Differential expression and regulation
of multiple dynamins. J Biol Chem. 269:4547–4554. 1994.PubMed/NCBI
|
|
10
|
Clark SG, Shurland DL, Meyerowitz EM,
Bargmann CI and van der Bliek AM: A dynamin GTPase mutation causes
a rapid and reversible temperature-inducible locomotion defect in
C. elegans. Proc Natl Acad Sci USA. 94:10438–10443. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fà M, Staniszewski A, Saeed F, Francis YI
and Arancio O: Dynamin 1 is required for memory formation. PLoS
One. 9:e919542014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poon HF, Vaishnav RA, Butterfield DA,
Getchell ML and Getchell TV: Proteomic identification of
differentially expressed proteins in the aging murine olfactory
system and transcriptional analysis of the associated genes. J
Neurochem. 94:380–392. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poon HF, Vaishnav RA, Getchell TV,
Getchell ML and Butterfield DA: Quantitative proteomics analysis of
differential protein expression and oxidative modification of
specific proteins in the brains of old mice. Neurobiol Aging.
27:1010–1019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CH and Won MH: Increased dynamin-1 and
−2 protein expression in the aged gerbil hippocampus. Cell Mol
Neurobiol. 34:791–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shin SJ, Lee SE, Boo JH, Kim M, Yoon YD,
Kim SI and Mook-Jung I: Profiling proteins related to amyloid
deposited brain of Tg2576 mice. Proteomics. 4:3359–3368. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly BL, Vassar R and Ferreira A:
Beta-Amyloid-induced dynamin 1 depletion in hippocampal neurons. A
potential mechanism for early cognitive decline in Alzheimer
disease. J Biol Chem. 280:31746–31753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kelly BL and Ferreira A:
beta-Amyloid-induced dynamin 1 degradation is mediated by
N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem.
281:28079–28089. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takano M, Yamashita T, Nagano K, Otani M,
Maekura K, Kamada H, Tsunoda S, Tsutsumi Y, Tomiyama T, Mori H, et
al: Proteomic analysis of the hippocampus in Alzheimer's disease
model mice by using two-dimensional fluorescence difference in gel
electrophoresis. Neurosci Lett. 534:85–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang YY and Li MD: Proteins
differentially expressed in response to nicotine in five rat brain
regions: Identification using a 2-DE/MS-based proteomics approach.
Proteomics. 6:3138–3153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Q and Li MD: Nicotine modulates
expression of dynamin 1 in rat brain and SH-SY5Y cells. Neurosci
Lett. 489:168–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao PJ, Zhu M, Pyun EI, Brooks AI,
Therianos S, Meyers VE and Coleman PD: Defects in expression of
genes related to synaptic vesicle trafficking in frontal cortex of
Alzheimer's disease. Neurobiol Dis. 12:97–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fox JG, Barthold SW, Davisson MT, Newcomer
CE, Quimby FW and Smith AL: The Mouse in Biomedical Research:
Normative Biology, Husbandry and Models (American College of
Laboratory Animal Medicine). 3. 2nd. Elsevier; Burlington: 2007
|
|
23
|
Yoo DY, Kim W, Lee CH, Shin BN, Nam SM,
Choi JH, Won MH, Yoon YS and Hwang IK: Melatonin improves
D-galactose-induced aging effects on behavior, neurogenesis, and
lipid peroxidation in the mouse dentate gyrus via increasing pCREB
expression. J Pineal Res. 52:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franklin KBJ and Paxinos G: The Mouse
Brain in Stereotaxic Coordinates. 4th. Academic Press; San Diego:
1997
|
|
25
|
Leal SL and Yassa MA: Perturbations of
neural circuitry in aging, mild cognitive impairment, and
Alzheimer's disease. Ageing Res Rev. 12:823–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lithfous S, Dufour A and Després O:
Spatial navigation in normal aging and the prodromal stage of
Alzheimer's disease: Insights from imaging and behavioral studies.
Ageing Res Rev. 12:201–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kitzmueller E, Krapfenbauer K, Hoeger H,
Weitzdoerfer R, Lubec G and Lubec B: Life-long effects of perinatal
asphyxia on stress-induced proteins and dynamin 1 in rat brain.
Neurochem Res. 29:1767–1777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang S, Avraham HK, Kim TA, Rogers RA and
Avraham S: Receptor-type PTP-NP inhibition of Dynamin-1 GTPase
activity is associated with neuronal depolarization. Cell Signal.
18:1439–1446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferguson SM and De Camilli P: Dynamin, a
membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 13:75–88.
2012.PubMed/NCBI
|