Introduction
Breast cancer is the most common cancer in women
world wide (1). Although
significant progress has been made in early diagnosis and
treatment, metastasis cannot be prevented in certain patients.
Therefore, breast cancer remains a major public health burden.
There is accumulating evidence that cancer stem cells (CSCs) are
responsible for tumor initiation, maintenance, invasion,
heterogeneity, metastasis and therapy resistance (2,3). In
breast cancer, CD44+/CD24−/low is the first
convincing marker for identifying and isolating tumorigenic CSCs
from non-tumorigenic cancer cells.
Aldehyde dehydrogenase 1 (ALDH1) is a detoxifying
enzyme that is associated with the stemness-associated markers,
octamer binding transcription factor 4 and Polycomb complex protein
BMI-1, and is proven to be a marker of stem/progenitor cells in
neural and hematopoietic systems and in the mammary gland (4). Ginestier et al (4) demonstrated that breast cancer cells
with increased ALDH activity exhibit stem/progenitor cell
properties. It was previously demonstrated that using ALDH1 as a
breast CSC marker can further divide the
CD44+/CD24−/low cell population into
fractions that are tumorigenic (4–7).
However, within breast cancer cells cultured from
fresh human specimens, few studies have analyzed the details of the
biological characteristic differences between
CD44+/CD24−/low phenotype and high ALDH1
activity cells. Based on this current knowledge, there is evidence
to support the hypothesis that the combining CD44/CD24 cell surface
expression with ALDH1 activity may be a more accurate method to
identify and isolate CSC-like cells within a population of breast
cancer cells. Furthermore, it is imperative to improve the
understanding of the biological differences among breast CSCs that
express different stem cell markers.
The CSC hypothesis has important implications for
understanding the basic biology of tumorigenesis. Cells endowed
with stem-like properties demonstrate self-renewal and high
tumorigenic potential. Current cancer treatments based on tumor
regression can kill differentiated tumor cells, while sparing the
small CSC population (8).
Therefore, the development of more effective cancer therapies may
require the targeting, identification, isolation and
characterization of CSCs.
In the present study, breast cancer cells from fresh
specimens were cultured and the percentage of three different
sub-population cells with CD44+/CD24−/low,
ALDH1+, and
ALDH1+CD44+/CD24−/low phenotypes
were analyzed. Additionally, the self-renewal, proliferative,
invasive ability of these cells was analyzed in vitro.
Finally, the capacity of these cells to generate de novo
tumors was also investigated in an in vivo mouse model.
Materials and methods
Dissociation and primary culture of
breast cancer cells
Samples of fresh breast cancer specimens were
obtained surgically from the primary tumor of one 32-year-old
female patient. The samples were dissociated mechanically and
enzymatically, based on the triple negative and basal-like
pathological type. The samples were dissociated mechanically and
enzymatically, based on pathological types that were triple
negative and basal-like. No treatment (chemotherapy or endocrine
therapy) was given to the patients before the operation and they
were treated at the Hubei Cancer Hospital (Wuhan, China) in 2014.
Breast cancer cell isolation was performed as previously described
(9). The biological specimens were
utilized according to the approved institutional review board
protocols for research in human subjects. The study was approved by
the ethical committee of Wuhan Tongji Hospital (Wuhan, China). All
patients provided written informed consent prior to participation
in the present study.
Mammosphere suspension culture
Mammosphere culture was performed as previously
described (10). Cells from one
32-year-old patient were cultured at 37°C and 5% CO2 a
density of 20,000 viable cells/ml in primary culture in serum-free
Dulbecco's modified Eagle's medium (DMEM)-F12 medium
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), which was
changed every 2 days. The daily morphological changes of
mammospheres were observed under a light microscope. Passaging was
performed after 5 days at a density of 5,000 cells/ml.
Immunofluorescence to identify cell
phenotype
The procedure was performed as previously described
(4). The main steps were as
follows: i) Cell preparations, ii) fixation, iii) permeabilization,
iv) primary antibody incubation (mouse anti-human CD44, cat. no.
BM0321; mouse anti-human CD24, cat. no. BM1723; ALDH1, cat. no.
BM3672; Wuhan Boster Biological Technology, Ltd., Wuhan, China), v)
secondary antibody incubation [goat anti-mouse IgG phycoerythrin
(PE), cat. no. BA1031; rabbit anti-mouse IgG-fluorescein
isothiocyanate, cat. no. BA1101; Wuhan Boster Biological
Technology, Ltd.], vi) mounting and vii) imaging.
Flow cytometry
The procedure was performed according to the method
of Al-Hajj et al (11). The
cells were collected by centrifugation, trypsin was added for
digestion, serum-free medium was added to terminate the digestion,
and a single-cell suspension was obtained. Test tube and control
tube were set to adjust the cell concentration, and the number of
cells was ≥1×105. Anti-human CD44-phycoerythrin (PE) CY5
(15-0441-81) and anti-human CD24-PE (12-0241-81) antibodies
(eBioscience, Inc., San Diego, CA, USA) were added to the test
tube, while isotype control antibody was added to the control tube,
mixed and incubated at room temperature in the dark for 30 min.
Samples were washed twice with PBS, the supernatant was discarded
after centrifugation at 200 × g for 5 min at room
temperature, and the cells were resuspended in PBS containing 1%
paraformaldehyde to fix the cells. Finally, 300 µl PBS was added.
The analysis was performed using a FACStarPLUS (BD Biosciences,
Franklin Lakes, NJ, USA) flow cytometer.
The ALDEFLUOR kit (Stemcell Technologies, Inc.,
Vancouver, BC, Canada) was used to isolate the cell population with
a high ALDH enzymatic activity. Cells were suspended in serum-free
DMEM-F12 medium. ALDEFLUOR assay buffer containing activated
ALDEFLUOR substrate (BAAA; 1 µmol/l per 1×106 cells) was
added to the cell suspension, mixed and incubated at 37°C for 30
min. The cell suspension was centrifuged, washed with PBS and
re-suspended in DMEM-F12 serum-free medium. Then cells were diluted
using ALDEFLUOR buffer, adjusting the cell concentration to
1×106 cells/ml. The flow cytometry detection was
immediately performed or performed within 24 h at 4°C.
To isolate the
ALDH1+CD44+/CD24−/low cells,
CD44+/CD24−/low cells were suspended
(1×106 cells/ml) in in ALDEFLUOR assay buffer and 5
µl/ml ALDEFLUOR substrate was added to the cell suspension. Then
cells were separated as described above.
Analysis of proliferation using
MTT
Each cell sub-population and cells in the control
group (primary cells without sorting) were re-suspended in DMEM-F12
[1:1; 2% fetal bovine serum (FBS; Gibco Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA); 20 ng/ml basic fibroblast
growth factor; 20 ng/ml epidermal growth factor, 2% B27 and 1%
penicillin-streptomycin], and cultured in 96-well plate
(approximately 103 cells/well, five wells/group), and
then cultured at 37°C. Culture medium (25 µl) was added to each
well every 2 days, the MTT assay was performed and the absorbance
at 490 nm was measured using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) every 24 h for consecutive 8
days to obtain a curve of the measured values.
Mammosphere formation ability
assay
Mammosphere formation rate or cloning efficiency is
an important indicator of tumor cell self-renewal ability. The
experimental procedure used to compare the cloning efficiency of
cells in each sub-population was as follows: A single cell
suspension of each sub-population (after sorting) and a non-sorting
cell suspension as a control group were re-suspended in serum-free
medium containing growth factors (20 ng/ml basic fibroblast growth
factor and 20 ng/ml epidermal growth factor; BD Biosciences) to
adjust the concentration to 103 cells/ml, and were
seeded in 96-well plates. Subsequently, 100 cells were seeded, 25
µl medium containing growth factors was added to each well every 2
days, and breast cancer mammosphere numbers were counted in each
well. Breast mammosphere formation rate is calculated as follows:
Mammosphere (MS) % = microsphere number/inoculated cells ×100%.
Invasion ability of cells in each
sub-population by Transwell assay
The 24-well Transwell chambers (8.0 µm) were placed
into the culture plates, pre-warmed serum-free DMEM-F12 medium (300
µl) was added to the chamber and incubated at room temperature for
30 min, and then the medium was removed. The cell suspension was
prepared to for experiments, digestion, and the supernatant was
discarded following centrifugation and re-suspended with serum-free
medium containing 0.2% bovine serum albumin (Gibco Invitrogen;
Thermo Fisher Scientific, Inc.). A 200 µl cell suspension
(1×105 cells/ml) was added to the Transwell chamber.
Another 500 µl DMEM-F12 medium containing 10% FBS was added to the
24-well plate lower chamber, avoiding the formation of air bubbles.
The suspension was incubated in a sterile incubator for 24 h.
Furthermore, Matrigel was added to coat the upper chamber, while
500 µl complete medium (containing 0.5 mg/ml MTT) was added to the
lower house of a 24-well plate, and incubated at 37°C for 4 h
before chamber. Next, the chamber was immersed in 500 µl dimethyl
sulfoxide and incubated for 10 min. The absorbance was measured on
a microplate reader at a wavelength of 490 nm.
In vivo tumorigenicity experiment
The animal experiments were approved by the ethics
committee of Hubei Cancer Hospital (Wuhan, China). Female
BALB/C-nude mice (n=60; specific-pathogen free; age, 4–6 weeks old;
weight, 14–22 g) were purchased from Hunan Slack King of Laboratory
Animal Co., Ltd. (Changsha, China). The mice were kept at a
temperature of 20–26°C with a relative humidity of 40–70%, with an
average of 5 g/100 g weight food and 6–7 ml/100 g weight water per
day, with a light/dark cycle of 12 h/12 h. The mice were divided
into three batches, and each batch contained four groups with five
nude mice in each group. The four groups were represented by the
control group, CD44+CD24−/low group,
ALDH1+ group and ALDH1+
CD44+CD24−/low group. The tumorigenicity
experiments were performed within a laminar flow cabinet. Unsorted
primary cells were inoculated in the mice of the control group,
while CD44+CD24−/low cells, ALDH1+
cells, and ALDH1+ CD44+CD24−/low
cells were inoculated in the mice of the other three groups. The
first batch of nude mice were injected with 500 cells per mouse in
each group; the second batch was injected with 5,000 cells per
mouse, and the third batch was injected with 50,000 cells per
mouse. The method of Al-Hajj et al (11), was followed, including the
following stages: i) Cell collection; ii) cell mixing; and iii)
cell inoculation. A volume of 0.1 ml of cells, was subcutaneously
inoculated on one side of the chest of each mouse. Subsequently,
the mice were fed under standard conditions for 8 weeks. Mice were
monitored once a week, and the tumor dimension was measured and
recorded. The mice were sacrificed by cervical dislocation after 8
weeks of monitoring.
Statistical analysis
The results were statistically analyzed using SPSS
13.0 (SPSS Inc., Chicago, IL, USA). The continuous variables are
expressed as the mean ± standard deviation. Categorical variables
are expressed as frequencies and/or percentages. The data were
compared by analysis of variance analysis and followed by least
significant difference post hoc analysis. For two independent
samples, a t-test was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
CD44+CD24−/low
phenotype and ALDH1 activity in breast cancer cells
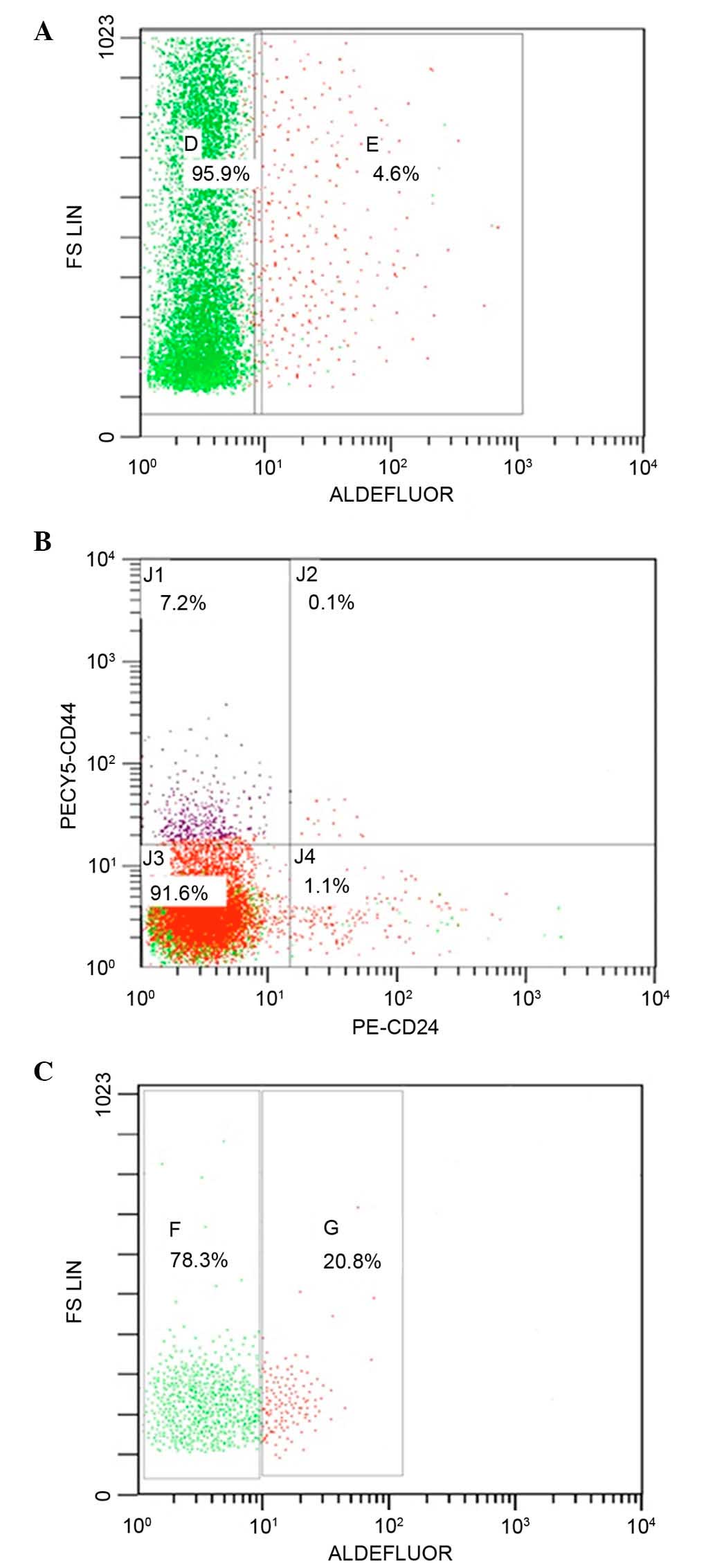
Flow cytometry analysis enables the separation of
different cell populations. As demonstrated in the current study,
the proportion of ALDH1+ cells in the breast cancer
specimens was 4.6% (Fig. 1A),
whereas 7.2% of the population were
CD44+CD24−/low phenotype tumor cells
(Fig. 1B). Further sorting of the
cells by ALDEFLUOR was performed to isolate the population with
high ALDH enzymatic activity; 20.8% of these cells were
CD44+CD24−/low (Fig. 1C). A small overlap of the two
groups, ALDH1+CD44+CD24−/low
sub-population, was represented by 1.5% of the total cells.
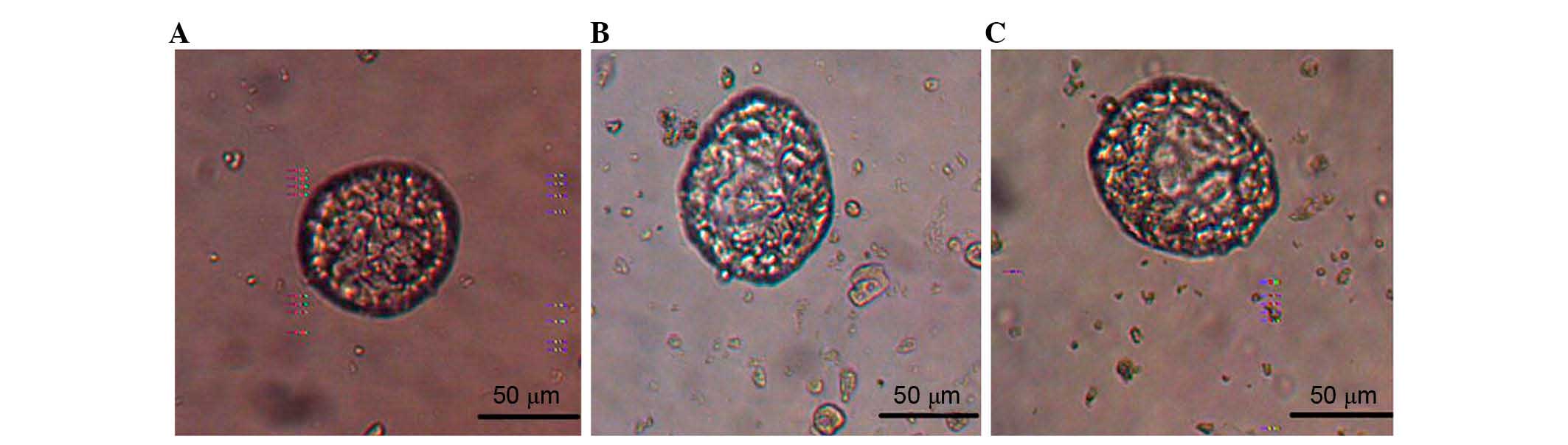
Difference in the mammosphere
formation of different cells population
The sorted CD44+CD24−/low,
ALDH1+ and
ALDH1+CD44+CD24−/low cells in each
sub-population were suspended and cultured in serum-free medium. A
few mammospheres was observed at 4–5 days, with a diameter of 20–30
µm. Typical mature mammospheres of
CD44+CD24−/low cells formed after ~1 week in
culture, with a diameter of 80–100 µm (Fig. 2A). For the ALDH1+ and
ALDH1+CD44+CD24−/low cells,
mammospheres were formed with an increased number and size in the
following days (Fig. 2B and C),
and remained stable and in shape until 10–12 days. Mammospheres
occurred earliest in the
ALDH1+CD44+CD24−/low cells after 2
days in serum-free medium and exhibited the largest diameter; up to
110–120 µm (Fig. 2C).
Additionally, ALDH1+CD44+CD24−/low
cells exhibited the longest stability, starting to disintegrate
later than the other sub-groups.
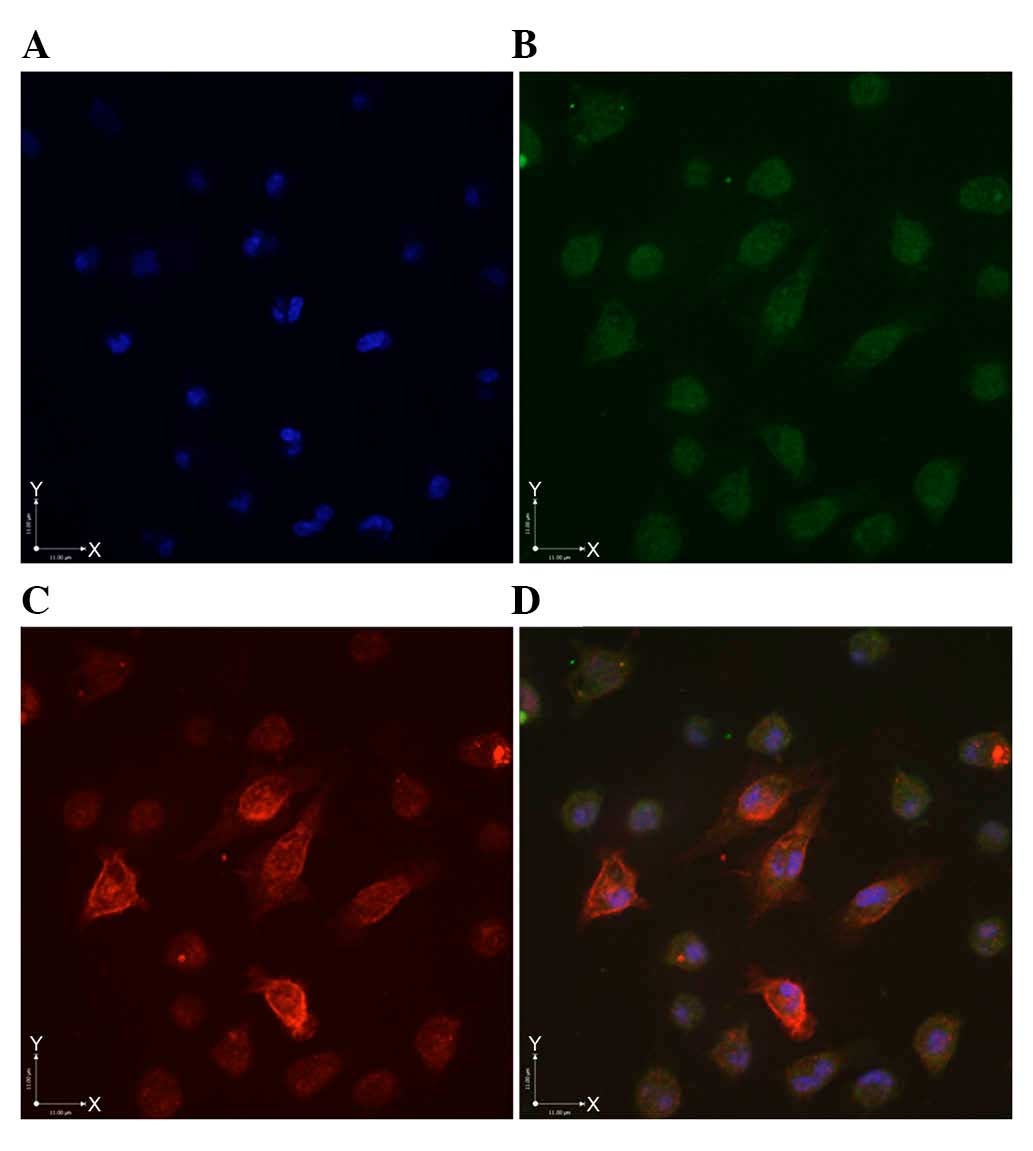
Immunofluorescence findings
Following the suspension of the unsorted primary
cells in serum-free medium with growth factors for 7 days,
mammospheres were collected, and corresponding fluorescent
antibodies were added. Green fluorescence was detected in the
cytoplasm of ALDH1+ cell, with no staining in the
membrane and the nucleus (Fig.
3B). CD44+CD24−/low cells exhibited
brownish/red fluorescence, predominantly in the membrane, although
cytoplasmic staining was observed in certain cells (Fig. 3C). Cells exhibiting green
fluorescence in the cytoplasm and the brownish/red fluorescence in
the membrane (Fig. 3D) indicated
the presence of the
ALDH1+CD44+CD24−/low
sub-population.
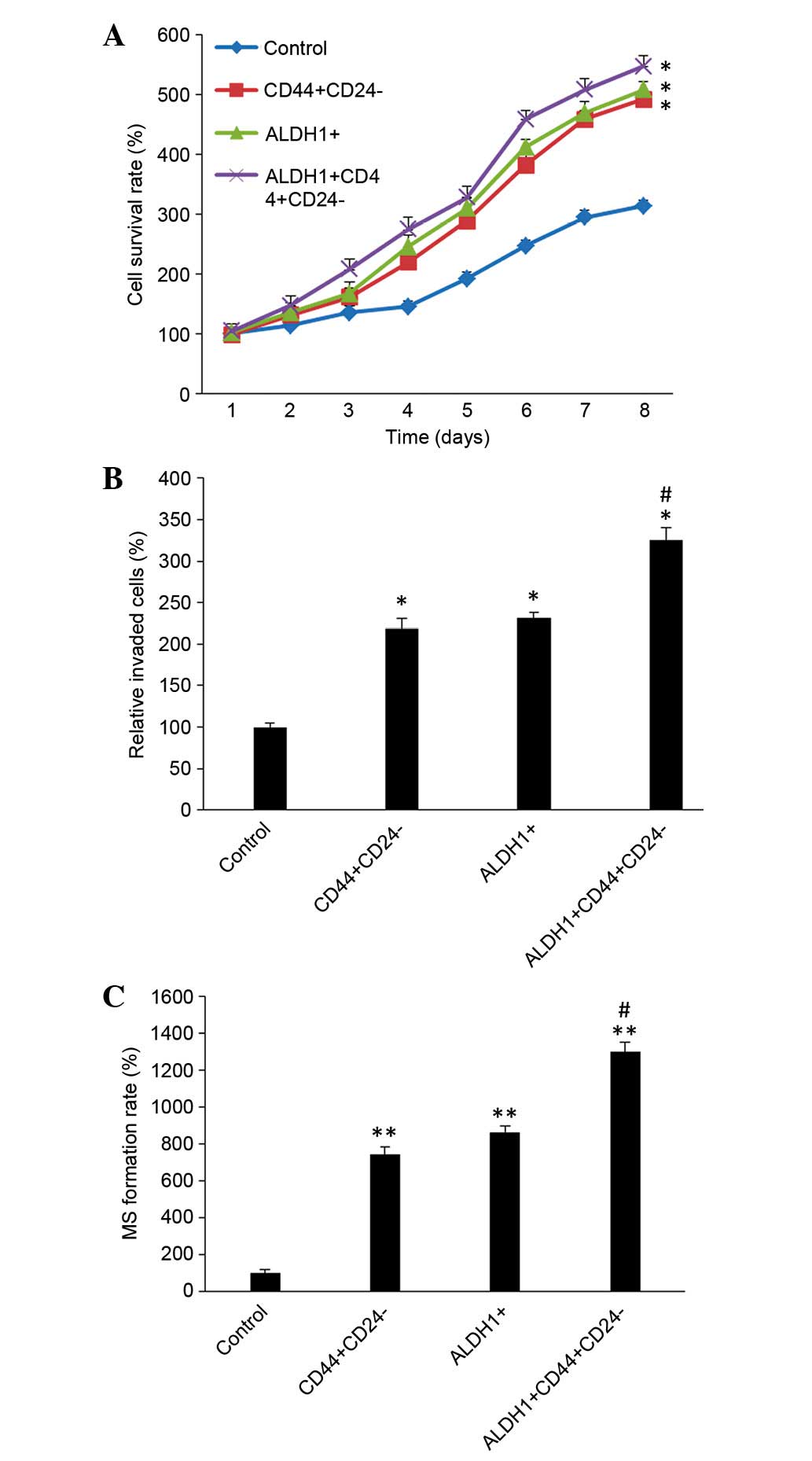
Differences in proliferation among the
sub-populations
The cells were divided into four groups: Control
cells (unsorted primary cells),
CD44+CD24−/low, ALDH1+ and
ALDH1+CD44+CD24−/low cells, using
flow cytometry. The proliferation ability of cells in each
sub-population was then compared. The number of living cells was
subsequently measured by MTT assay (Fig. 4A). After 8 days of culture, the
results demonstrated that CD44+CD24−/low,
ALDH1+ and
ALDH1+CD44+CD24−/low cells were
continuously proliferating, with no observable of quiescence. On
day 8, the values of control, CD44+CD24−/low,
ALDH1+ and
ALDH1+CD44+CD24−/low cells were
0.160±0.005, 0.251±0.005, 0.259±0.007 and 0.279±0.009,
respectively. The cell proliferation of the three sub-populations
was significantly increased compared with the control group cells
(P=0.0113; Fig. 4A), however,
there were no significant differences among the three
sub-population groups (P=0.151).
Differences in invasion of each cell
sub-population
To compare the invasion and migration ability of
each cell group, Transwell experiments were conducted. After 48 h
in culture, a large number of CD44+CD24−/low,
ALDH1+ and
ALDH1+CD44+CD24−/low cells passed
through the Transwell membrane. The MTT absorbance of the control
group was 0.48±0.021, and the CD44+CD24−/low,
ALDH1+ and
ALDH1+CD44+CD24−/low groups were
1.05±0.058, 1.11±0.036 and 1.56±0.075, respectively. The invasion
and migration abilities of CD44+CD24−/low,
ALDH1+, and
ALDH1+CD44+CD24−/low cells were
significantly increased compared with the control group (P=0.0129).
Additionally, the invasion and migration abilities of
ALDH1+CD44+CD24−/low cells were
increased compared with the CD44+CD24−/low
and ALDH1+ sub-populations (P=0.0287; Fig. 4B).
Differences in the mammosphere
formation rate in each sub-population
At day 8 after suspension, the mammosphere formation
rates were 4.80±1.10, 35.70±1.92, 41.50±1.71 and 62.45±2.50% in the
control group, CD44+CD24−/low,
ALDH1+ and
ALDH1+CD44+CD24−/low groups,
respectively. The mammosphere formation rate was significantly
increased in the three sub-populations groups compared with the
control group (P<0.001). Additionally, the mammosphere formation
rate of ALDH1+CD44+CD24−/low cells
was significantly increased compared with the other two
sub-populations (P=0.0185; Fig.
4C).
Comparison of the tumorigenic ability
of each sub-population in vivo
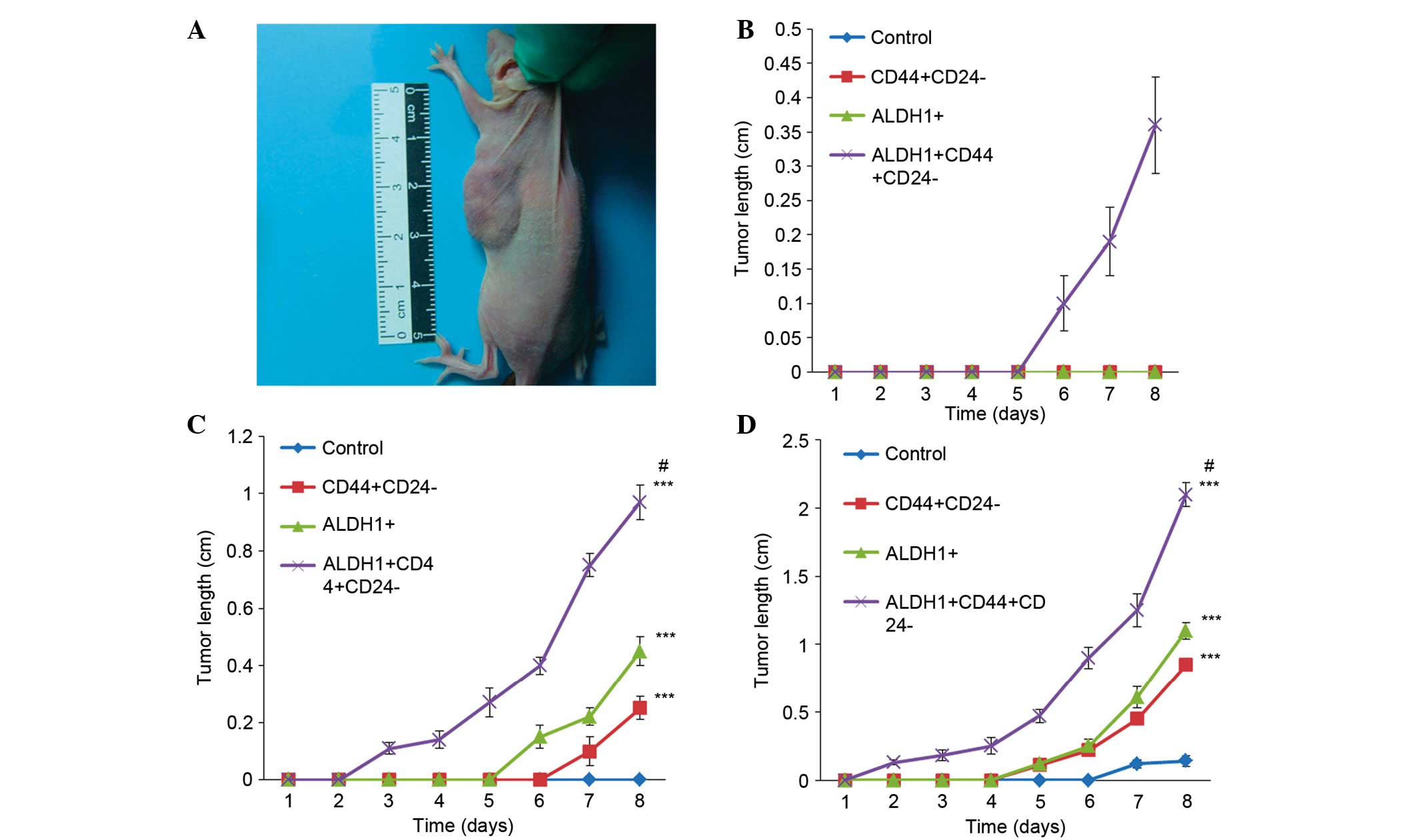
A nude mice tumorigenic experiment was performed to
discover the tumorigenicity of the tumor cell sub-populations.
Fig. 5A demonstrates a nude mouse
bearing a tumor developed following injection of
ALDH+CD44+CD24−/low sub-population
cells. The batch of nude mice that received an injection of 500
cells of the unsorted control cells,
CD44+CD24−/low and ALDH+
sub-populations exhibited no tumor formation after 8 weeks.
However, mice that received an injection of 500
ALDH+CD44+CD24−/low sub-population
cells demonstrated clear and palpable subcutaneous nodules by week
6, reaching a size of up to 0.36±0.07 cm at week 8, (Fig. 5B). The second batch of nude mice
that received an injection of 5,000 cells of the
CD44+CD24−/low sub-population exhibited tumor
development from the week 6 onward. Nude mice that received an
injection of 5,000 cells of the ALDH1+ sub-population
exhibited tumor development from week 5 onward, and finally, nude
mice that received an injection of 5,000 cells of the
ALDH+CD44+CD24−/low sub-population exhibited
tumor development from week 2 onward (Fig. 5C). The tumor lengths at week 8 were
0.25±0.04, 0.45±0.05 and 0.97±0.06 cm in the
CD44+CD24−/low, ALDH1+ and
ALDH+CD44+CD24−/low
sub-populations, respectively. Statistical analysis demonstrated
that the tumorigenic abilities of the cells in the three
sub-populations were significantly increased compared with the
control group. The
ALDH+CD44+CD24−/low sub-population
exhibited the strongest ability of tumor formation and the tumor
length was significantly increased compared with the other
sub-populations (P=0.0162; Fig.
5C). The third batch of nude mice that received an injection of
50,000 cells of the
ALDH+CD44+CD24−/low
sub-populations exhibited tumor formation from the week 1 onward,
with a fast growth and a large tumors developing (Fig. 5D). Nude mice that received an
injection of 50,000 cells of the ALDH+ and of the
CD44+CD24−/low sub-populations exhibited
tumor formation from week 4 onward, and at week 8, the tumor
lengths were 2.10±0.09, 1.10±0.06, 0.85±0.05 and 0.15±0.04 cm in
the ALDH+CD44+CD24−/low,
ALDH+ and CD44+CD24−/low
sub-populations, and the control group, respectively. Statistical
analysis demonstrated that the tumorigenic abilities of the cells
in the three sub-populations were significantly increased compared
with the control group (P<0.05). The
ALDH+CD44+CD24−/low sub-population
exhibited the strongest tumor formation ability, and the tumor
length was significantly increased compared with the other two
sub-populations
(ALDH+CD44+CD24−/low vs.
CD44+CD24−/low, P=0.0074;
ALDH+CD44+CD24−/low vs.
ALDH1+, P=0.0104), and the comparison among the three
sub-populations were significantly different (P=0.010; Fig. 5D).
Discussion
The CSC hypothesis has important implications for
understanding the basic biology of tumorigenesis. Cells endowed
with stem-like properties demonstrate self-renewal and high
tumorigenic potential. Current cancer treatments based on tumor
regression can kill differentiated tumor cells, while sparing the
small CSC population (8).
Therefore, the development of more effective cancer therapies may
require the identification, isolation and characterization of
CSCs.
In recent years, advances have been made in the
research of stem cell markers, including the marker set of
CD44/CD24 and ALDH1 (11–22) Based on the cell surface markers,
Al-Hajj et al (11)
isolated the carcinogenic sub-population in breast cancer cells.
CD44+/CD24− cells possessed the ability to
develop into tumors, whereas the alternate phenotypes failed to
form tumors in mice. Ginestier et al (4) observed that breast cancer cells with
high ALDH1 activity were able to generate tumors in nude mice with
low cell numbers. The previously reported percentages of
CD44+/CD24− cells and ALDH1+ vary
widely (11–22). In the current study,
CD44+CD24−/low breast cancer cells were
isolated from fresh tissue at a proportion of 7.2% of the total
cell population, and ALDH1+ cells at 4.6%. By further
sorting, an overlap in the two-sub-population cells was detected,
with 1.5% of the total breast cancer cells exhibiting the
CD44+CD24−/low phenotype and ALDH1 activity.
Immunofluorescence experiments also confirmed the presence of these
three sub-populations cells in human breast cancer. The
immunohistochemical expression of ALDH1 and its used for clinical
prognosis have also been widely explored. High AlDH1 expression is
correlated with poor prognosis in various types of cancer (23–27).
Additionally, by analyzing the
CD44+CD24−/low, epithelial specific
antigen+, CD133+ and other multiple stem cell
markers, Hwang-Verslues et al (15) observed significant differences in
the biological characteristics among breast cancer cells with
different markers, including CD44+/CD24−,
ESA+ or CD133+, and even in different
pathological types.
By MTT assay and Transwell experiments, the current
study demonstrated that there were evident increases in
self-renewal, proliferation and invasion ability among the
CD44+CD24−/low, ALDH1+ and
ALDH1+CD44+CD24−/low cells
compared with the unsorted control cells, and
ALDH1+CD44+CD24−/low cells were
the strongest. Additionally, mammospheres were formed when the
cancer cells were cultured in serum-free medium, and after
continuous passage culture, they can produce new mammospheres. The
difference of self-renewal capacity among these cell populations
was also clearly demonstrated. The mammosphere formation rate of
ALDH1+CD44+CD24−/low cells was
significantly increased compared with the other groups. Increased
number and size of mammospheres, as demonstrated in the current
study, reflects the typical self-renewal of breast CSCs (16).
Dey et al (17) reported that, after long period in
serum-free culture, breast CSCs exhibit difficulties in maintaining
their undifferentiated state. With increasing passages, a
high-oxygen environment led to telomerase loss, resulting in the
aging of stem cells and interfering with the stem cells phenotype,
which caused decreased self-renewal ability of the stem cells. In
the present study, the cells were, therefore, passaged only 1–2
times to avoid stem cell aging.
In the present study, experiments using a nude
mouse tumor model demonstrated that
CD44+CD24−/low, ALDH1+, and
ALDH1+CD44+CD24−/low breast cancer
cells all exhibited tumorigenic ability, however, significant
differences between the sub-populations was also observed.
Inoculation with 500
ALDH1+CD44+CD24−/low cells formed
tumors, whereas, 500 of the other sub-population or control cells
did not generate tumors.
ALDH1+CD44+CD24−/low cells formed
tumors earliest after injection, indicating that this cell
population possessed the strongest tumorigenicity. Thus, in-depth
study of the biological characteristics of different subsets of
breast CSCs may provide a reference for clinical research and tumor
treatment.
Previous studies have demonstrated that tumorigenic
ALDH1+ cells are biologically aggressive, and their
presence tends to be associated with poor patient prognosis.
CD44+/CD24−/low cells and ALDH1+
cells are more frequently detected in basal-like tumors (4–7). In
the current study, the primary breast cancer cells were obtained
from basal-like tumors. According to the preliminary experiments,
ALDH1+ cells were easily detected and isolated from
basal-like cancers, however, it was difficult to obtain these cells
from other types of tumor. Thus, the primary cells used in the
present study were from patients with basal-like breast cancer.
Therefore, effort should be made to investigate the expression of
stem cell markers in other types of breast cancer.
In conclusion,
CD44+/CD24−/low, ALDH1+, and
ALDH1+ CD44+/CD24−/low cells have
stem/progenitor properties, and are capable of self-renewal and
generating tumors. There are distinct biological properties among
the three cell sub-population;
ALDH1+CD44+/CD24−/low cells
exhibit the strongest self-renewal, proliferation, invasion and
tumorigenic capacity, indicating that these sub-populations with
different markers may potentially not originate from the same stem
cells, which is helpful to understand the biological
characteristics and heterogeneity of breast CSCs. Diverse
phenotypes of CD44+/CD24−/low,
ALDH1+ and
ALDH1+CD44+/CD24−/low may be used
to isolate and identify breast CSCs with distinct levels of
heterogeneity, which display distinct biological characteristics.
As ALDH1+CD44+/CD24−/low cells
exhibited the strongest stem-like properties, it may be useful as a
more specific stem cell marker. The utilization of reliable
biomarkers to distinguish the breast CSC pool will be important in
the development of specific target therapies for breast cancer.
Acknowledgements
This work was supported by Hubei Provincial Health
Department (grant no. JX4A07).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shackleton M, Quintana E, Fearon ER and
Morrison SJ: Heterogeneity in cancer: Cancer stem cells versus
clonal evolution. Cell. 138:822–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sottoriva A, Verhoeff JJ, Borovski T,
McWeeney SK, Naumov L, Medema JP, Sloot PM and Vermeulen L: Cancer
stem cell tumor model reveals invasive morphology and increased
phenotypical heterogeneity. Cancer Res. 70:46–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morimoto K, Kim SJ, Tanei T, Shimazu K,
Tanji Y, Taguchi T, Tamaki Y, Terada N and Noguchi S: Stem cell
marker aldehyde dehydrogenase 1-positive breast cancers are
characterized by negative estrogen receptor, positive human
epidermal growth factor receptor type 2, and high Ki67 expression.
Cancer Sci. 100:1062–1068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng S, Yang X, Lassus H, Liang S, Kaur S,
Ye Q, Li C, Wang LP, Roby KF, Orsulic S, et al: Distinct expression
levels and patterns of stem cell marker, aldehyde dehydrogenase
isoform 1 (ALDH1), in human epithelial cancers. PLoS One.
5:e102772010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Croker AK, Goodale D, Chu J, Postenka C,
Hedley BD, Hess DA and Allan AL: High aldehyde dehydrogenase and
expression of cancer stem cell markers selects for breast cancer
cells with enhanced malignant and metastatic ability. J Cell Mol
Med. 13:2236–2252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: An old idea-a paradigm shift. Cancer Res. 66:1883–1890;
discussion 1895–1896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stingl J, Eirew P, Ricketson I, Shackleton
M, Vaillant F, Choi D, Li HI and Eaves CJ: Purification and unique
properties of mammary epithelial stem cells. Nature. 439:993–997.
2006.PubMed/NCBI
|
|
10
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bane A, Viloria-Petit A, Pinnaduwage D,
Mulligan AM, O'Malley FP and Andrulis IL: Clinical-pathologic
significance of cancer stem cell marker expression in familial
breast cancers. Breast Cancer Res Treat. 140:195–205. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang-Verslues WW, Kuo WH, Chang PH, Pan
CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, et al:
Multiple lineages of human breast cancer stem/progenitor cells
identified by profiling with stem cell markers. PLoS One.
4:e83772009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dey D, Saxena M, Paranjape AN, Krishnan V,
Giraddi R, Kumar MV, Mukherjee G and Rangarajan A: Phenotypic and
functional characterization of human mammary stem/progenitor cells
in long term culture. PLoS One. 4:e53292009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueda K, Ogasawara S, Akiba J, Nakayama M,
Todoroki K, Ueda K, Sanada S, Suekane S, Noguchi M, Matsuoka K and
Yano H: Aldehyde dehydrogenase 1 identifies cells with cancer stem
cell-like properties in a human renal cell carcinoma cell line.
PLoS One. 8:e754632013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasper M, Schäfer A, Piontek G, Teufel J,
Brockhoff G, Ringel F, Heindl S, Zimmer C and Schlegel J: Aldehyde
dehydrogenase 1 positive glioblastoma cells show brain tumor stem
cell capacity. Neuro Oncol. 12:1024–1033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mieog JS, de Kruijf EM, Bastiaannet E,
Kuppen PJ, Sajet A, de Craen AJ, Smit VT, van de Velde CJ and
Liefers GJ: Age determines the prognostic role of the cancer stem
cell marker aldehyde dehydrogenase-1 in breast cancer. BMC Cancer.
12:422012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuroda T, Hirohashi Y, Torigoe T, Yasuda
K, Takahashi A, Asanuma H, Morita R, Mariya T, Asano T, Mizuuchi M,
et al: ALDH1-high ovarian cancer stem-like cells can be isolated
from serous and clear cell adenocarcinoma cells and ALDH1 high
expression is associated with poor prognosis. PLoS One.
8:e651582013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lohberger B, Rinner B, Stuendl N, Absenger
M, Liegl-Atzwanger B, Walzer SM, Windhager R and Leithner A:
Aldehyde dehydrogenase 1, a potential marker for cancer stem cells
in human sarcoma. PLoS One. 7:e436642012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krause U, Ryan DM, Clough BH and Gregory
CA: An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a
novel cancer survival mechanism through modulation of
aldehyde-dehydrogenase-1 activity. Cell Death Dis. 5:e10932014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lohberger B, Stuendl N, Wolf E,
Liegl-Atzwanger B, Leithner A and Rinner B: The novel
myxofibrosarcoma cell line MUG-Myx1 expresses a tumourigenic
stem-like cell population with high aldehyde dehydrogenase 1
activity. BMC Cancer. 13:5632013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wegman-Points LJ, Teoh-Fitzgerald ML, Mao
G, Zhu Y, Fath MA, Spitz DR and Domann FE: Retroviral-infection
increases tumorigenic potential of MDA-MB-231 breast carcinoma
cells by expanding an aldehyde dehydrogenase (ALDH1) positive
stem-cell like population. Redox Biol. 2:847–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim YS, Jung MJ, Ryu DW and Lee CH:
Clinicopathologic characteristics of breast cancer stem cells
identified on the basis of aldehyde dehydrogenase 1 expression. J
Breast Cancer. 17:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou W, Yang Y, Gu Z, Wang H, Xia J, Wu X,
Zhan X, Levasseur D, Zhou Y, Janz S, et al: ALDH1 activity
identifies tumor-initiating cells and links to chromosomal
instability signatures in multiple myeloma. Leukemia. 28:1155–1158.
2014. View Article : Google Scholar : PubMed/NCBI
|