Introduction
Sepsis, a major medical problem often leading to
multiple organ failure, is one of the major causes of mortality in
critical care medicine (1).
Respiratory failure is a devastating consequence of septic shock,
it contributes to reduced systemic oxygen delivery and leads to
multiple organ failure and mortality. Contractile dysfunction of
the diaphragm muscles has a central role in respiratory failure
during sepsis (2–4). Previous studies indicate several
mechanisms are responsible for sepsis-induced contractile
dysfunction of diaphragm muscle, including the implication of
oxygen-deprived free radicals, mitochondrial injury,
excitation-contraction coupling damage and reduced expression of
dihydropyridine receptor α1 subunit (DHPRα1s) and ryanodine
receptor 1 (RyR1) (5–7). However, little is known about the
status of the endoplasmic reticulum (ER) within the diaphragm, a
crucial regulator of muscle contraction, during sepsis.
ER is an extensive intracellular membranous network
that is essential for the maintenance of cellular processes,
including calcium signaling, protein assembly, calcium homeostasis
and lipid biosynthesis (8,9). Efficient functioning of the ER is
crucial for cell function and survival. Perturbations of ER
homeostasis by energy deprivation, infection, increased protein
trafficking, expression of mutant proteins with folding defects and
chemical triggers interfere with the proper functioning of the ER
to create a condition called ER stress (10). To cope with ER stress conditions,
the cell initiates several signaling cascades known as the unfolded
protein response (UPR). UPR sensors are in an inactive state when
coupled to glucose-regulated protein 78 kDa (GRP78). During ER
stress and accumulation of unfolded proteins, GRP78 is released,
which allows upregulation of ER chaperones and activation of the
ER-associated protein degradation pathway (8).
ER stress is considered an important contributing
factor in a wide variety of pathologies, including diabetes,
neurodegenerative disorders, obesity and ischemia-reperfusion heart
diseases (11–13). It has been previously reported that
ER stress may result in diminished cardiomyocyte contractile
function, indicating a role for ER stress in compromised muscle
function under pathological conditions including sepsis (14). To the best of our knowledge, the
status of ER stress in the diaphragm during sepsis has not been
previously reported.
The aim of the present study was to assess the
effects of sepsis on the ER stress status of the diaphragm in a rat
sepsis model. ER damage of the diaphragm during sepsis was
evaluated by electron microscopy (EM). The present study also
examined the expression of a series of ER stress markers using
western blot analysis and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR).
Materials and methods
Animal preparation
The protocol of the present study was approved by
the ethics committee of Shengjing Hospital of China Medical
University (Shenyang, China) and conducted in accordance with the
Animal Care Center Guidelines of the institution. Animals used in
the present study were adult male Wistar rats (age, 3 weeks;
weight, 250–350 g) obtained from the National Research Center
(Giza, Egypt). The animals were housed in polyacrylic cages (five
animals per cage) and provided access to food/water ad
libitum. Rats were maintained at room temperature (22–25°C)
with a 12-h light/dark cycle. Lipopolysaccharides (LPS), from
Escherichia coli 055:B5 (cat no. L2880; lot no. 110M4086V;
containing 3,000,000 endotoxin units per mg LPS) were obtained from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany) and were
administered intraperitoneally to rats (8 per group) in three
experimental groups (24, 48 h and 7 days) at a dose of 8 mg/kg. For
the 24 h group, rats were euthanized 24 h after the first dose. For
the 48 h and 7 day groups, an additional dose of 8 mg/kg was given
24 h after the first dose and rats were euthanized 48 h or 7 days
after the original dose. An additional 8 rats were enrolled in the
control group and were injected intraperitoneally with 1 ml of
normal saline as a placebo. All rats received normal saline
subcutaneously to prevent dehydration. Animals were euthanized with
an overdose of pentobarbital sodium (100 mg/kg), delivered
intravenously. Diaphragm muscles were immediately excised from the
midcostal region, with the insertion of fibers at the ribs and
central tendon kept intact. The excised diaphragm muscles were
prepared for investigation as previously described (15,16).
Measurement of lung wet-to-dry ratio
(W/D) and lung histopathology
For W/D, left lungs were weighed and subsequently
dried for 3 days in an oven at 65°C. The ratio of wet weight to dry
weight was calculated to estimate lung edema (17). For hematoxylin and eosin (H&E)
staining, both lungs were cannulated and inflated with 4%
paraformaldehyde. After overnight fixation at 4°C, tissue was
embedded in paraffin, sectioned, and stained. H&E staining was
performed and the scale of lung injury was determined by a
pathologist who was blind to the experimental groups. Six sections
(4-µm thick) from left and right lower lobes in each animal were
examined to determine the scale of lung injury on the basis of
alveolar congestion, hemorrhage, neutrophil infiltration into the
airspace or vessel wall and thickness of alveolar wall/hyaline
membrane formation. The scale of lung injury was measured on a 0–4
scale as follows: 0, no injury; 1, injury in up to 25% of the
field; 2, injury in up to 50% of the field; 3, injury in up to 75%
of the field; and 4, diffuse injury (18–20).
Measurements of the contractile
function of the diaphragm
The in vitro techniques used to measure
isometric contractile force and fatigue index (FI) have been
previously described (16,21). A strip of diaphragm was stimulated
in vitro using monophasic rectangular pulses through a Grass
S88 stimulator provided by AstroNova (West Warwick, RI, USA).
Current intensity was adjusted until muscle produced maximal
isometric tension (P0; 500 msec train and 50 Hz)
responses were obtained. The stimulus intensity was set at 125% of
this current intensity value. The length at which the muscle
produced maximal isometric tension (L0) was determined.
Then, L0 was measured using digital calipers. At
L0, the peak twitch force (Pt) was determined
from a series of contractions induced by single-pulse stimuli. At
L0, diaphragm force-frequency curves (measured at 10,
20, 40, 50, 75 and 100 Hz) were determined using 1 sec duration
trains of stimuli with a minimum of 2-min intervals between each
stimulus train. Forces were normalized for the muscle
cross-sectional area (CSA), which was determined by the following
formula: [Muscle mass (g)]/L0(cm) × muscle density
(g/cm3). The fatigability of diaphragm muscles was
evaluated through use of a test described in a previous report,
which involved repetitive stimulation at 40 Hz in trains of 330
msec duration, with one train repeated each sec (22). The FI was calculated as the ratio
of magnitude of force generated after 2 min of stimulation to the
magnitude of the initial force.
Assessment of endoplasmic reticulum
damage
EM was performed, as previously described (21), on diaphragm muscle segments from
the control and sepsis groups. After diaphragm muscle segments were
fixed, dehydrated, polymerized and sliced, the muscle fibril
orientation was determined using light microscopy. The blocks were
then reoriented and ultra-thin sections (50–70 nm) were cut
transversely, or parallel, to the muscle fiber axis. These sections
were contrasted with uranyl acetate and bismuth subnitrate for
transmission EM.
EM images were evaluated independently by two
pathologists from Shengjing Hospital of China Medical University. A
total of 100 ER per muscle sample (n=8) were evaluated. Each ER was
assessed for membrane integrity and normal morphological size.
Membrane integrity was scored as 0 (normal) or 1 (damaged). The
morphological size of the ER was scored as 0 (normal) or 1
(dilated). The scores of each ER were added to produce a final
score. An ER with an intact membrane and normal morphological size
was given a final score of 0, while one with a damaged membrane and
dilated ER was given a final score of 2. The percentage of ER in
each final score group (0, 1 and 2) within each sample was
calculated.
Determination of ER stress markers
using RT-qPCR
Rat diaphragms were homogenized with 1 ml of RNAiso
reagent (Takara Biotechnology Co., Ltd., Dalian, China), and total
RNA was isolated according to the manufacturer's protocol.
Recombinant DNase I (RNase-free; 2 µl; Takara Biotechnology Co.,
Ltd.) was added to each tube for 10 min. The total RNA (1 µg) was
converted to cDNA by using PrimeScript RT Master Mix kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
qPCR was performed using SYBR Premix Ex Taq II (Takara
Biotechnology Co., Ltd.) on the 7900HT Fast Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc. Waltham, MA,
USA) as follows: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C
for 15 sec, and 60°C for 60 sec. A dissociation procedure was
performed to generate a melting curve for confirmation of
amplification specificity. GAPDH served as the reference gene. The
relative levels of gene expression were determined by ΔCq = (Cq
gene - Cq reference gene), and the fold change of gene expression
was calculated by the 2−ΔΔCq method (23). Experiments were repeated in
triplicate. The sequences of the primer pairs are as follows: GRP78
forward, 5′-AACATGGACCTGTTCCGCTCT-3′ and reverse,
5′-CGAGTAGATCCGCCAACCAG-3′; C/EBP homologous protein (CHOP)
forward, 5′-CCCTCGCTCTCCAGATTCC-3′ and reverse,
5′-GACCACTCTGTTTCCGTTTCCT-3′; glucose-regulated protein 94 kDa
(GRP94) forward, 5′-AAACGGCAACTCTTCGGTCA-3′ and reverse,
5′-CCTCTGGCTCTTCCTCTACCTG-3′; endoplasmic reticulum protein 44
(ERP44) forward, 5′-AAGTCACCAATCTTGATCGCAGT-3′ and reverse,
5′-GCAGAAAGGAAGGCACAGTCAT-3′; protein disulfide-isomerase like
protein (ERP57) forward, 5′-GAAGGTGGCCGTGAATTAAATG-3′ and reverse,
5′-CATTTGGCTGTTGCTTTAGAGGT-3′; protein disulfide isomerase family A
member 4 (ERP72) forward, 5′-CAAATTTCACCACACTTTCAGCAC−3′ and
reverse, 5′-CATCCTGGGCTCATACTTGGAC-3′; and GAPDH forward,
5′-GCTGGTCATCAACGGGAAA-3′ and reverse,
5′-CGCCAGTAGACTCCACGACAT-3′.
Western blot analysis
Total protein from rat diaphragm tissue was
extracted using cell lysis buffer (Pierce; Thermo Fisher
Scientific, Inc.) and quantified using the Bradford assay. Protein
(50 µg) was loaded per lane and separated on a 12% SDS-PAGE gel.
After transferring to a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA), the membrane was blocked with 5%
bovine serum albumin (BSA) buffer (5 g BSA/100 ml Tris-buffered
saline with Tween-20) and then incubated overnight at 4°C with
antibodies against GRP78 (rabbit monoclonal; 1:1,000; cat. no.
3177), CHOP (rabbit monoclonal; 1:1,000; cat. no. 5554), GRP94
(rabbit polyclonal; 1:1,000; cat. no. 2104), ERP44 (rabbit
polyclonal; 1:1,000; cat. no. 2886), ERP57 (rabbit polyclonal;
1:1,000; cat. no. 2887), ERP72 (rabbit polyclonal; 1:1,000; cat.
no. 2798), caspase-12 (rabbit polyclonal; 1:1,000; cat. no. 2202)
(all from Cell Signaling Technology, Inc., Danvers, MA, USA) and
GAPDH (rabbit polyclonal; 1:2,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; cat. no. sc-25778). Following incubation with
peroxidase-conjugated anti-mouse/rabbit IgG (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 5127) at 37°C for 2 h, bound
proteins were visualized using Pierce ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc.) and detected using DNR
imaging systems (DNR Bio-Imaging Systems, Ltd., Jerusalem, Israel).
The relative protein levels were calculated by normalizing to GAPDH
protein as a loading reference from three blots using ImageJ
software (imagej.nih.gov/).
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance, followed by Tukey's post hoc test,
was applied to identify any significant differences between the
control and sepsis groups using the SPSS software (version 17.0;
SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Sepsis causes lung injury and
increases lung W/D
As presented in Table
I, the lung W/D, which indicates the level of lung edema, was
significantly increased in the 3 sepsis groups compared with the
control (P<0.05). The ratio reached its highest level at 48 h
and decreased at day 7 (P=0.013).
 | Table I.Lung injury scores and W/D in control
and sepsis groups. |
Table I.
Lung injury scores and W/D in control
and sepsis groups.
|
| Groups |
|---|
|
|
|
|---|
| Parameter | Control | 24 h | 48 h | 7 days |
|---|
| Lung injury
score | 0.08±0.05 |
3.16±0.24a |
3.73±0.22a,b |
2.26±0.26a–c |
| W/D | 4.68±0.58 |
6.49±0.74a |
6.68±0.61a |
5.92±0.62a,c |
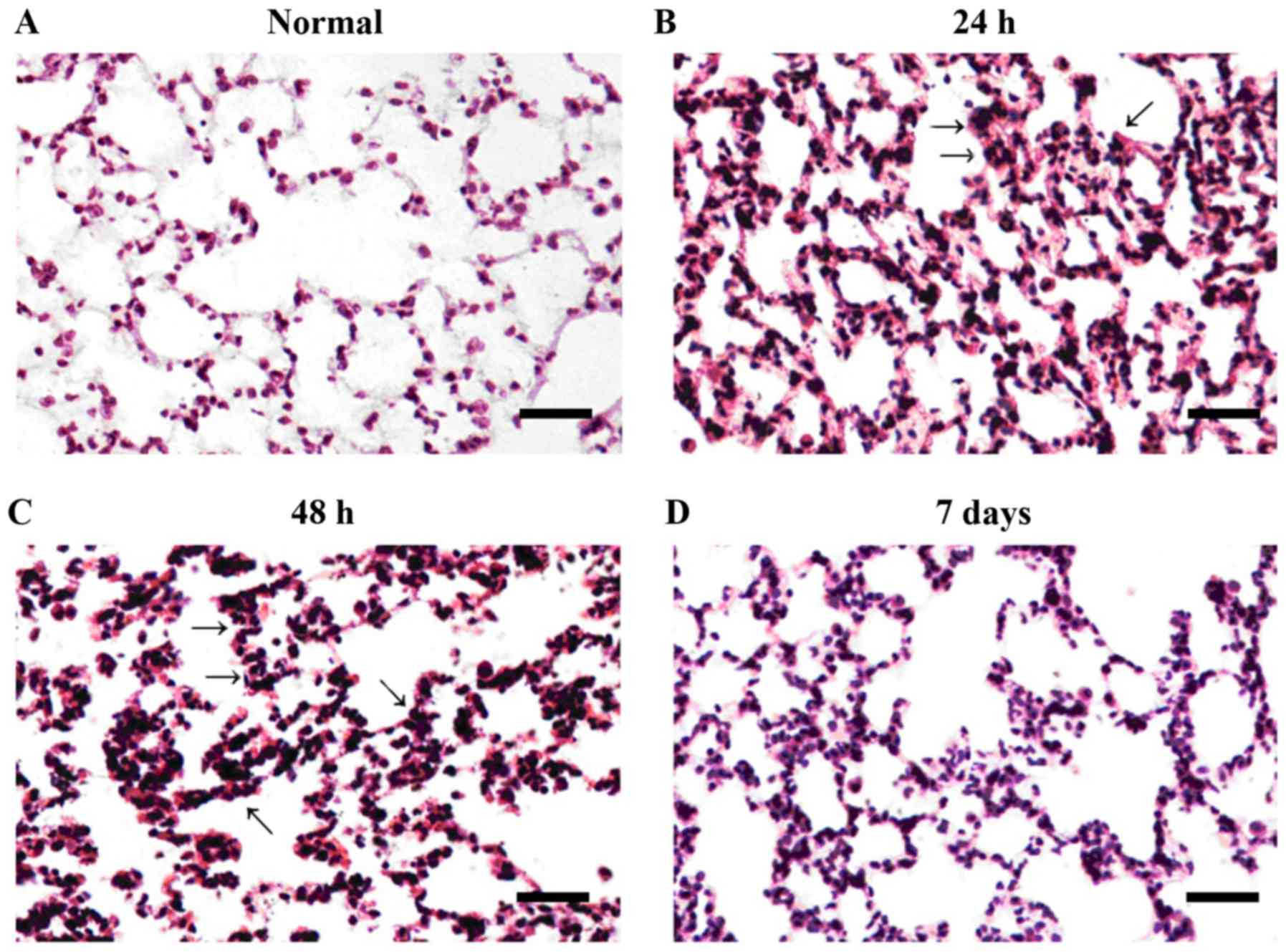
As demonstrated in Fig.
1, microscopic analysis of the control group revealed marked
alveoli, a thin alveolar septum and absence of congestion or edema.
At 24 and 48 h following the first LPS dose, significant pulmonary
alveolar congestion, hemorrhage and pulmonary interstitial edema
associated with inflammatory infiltration were visible. The level
of alveolar congestion and hemorrhage was reduced at day 7 compared
with at 24 and 48 h. The boundaries of the alveolar septum became
thick and inflammatory infiltration was reduced compared with 24
and 48 h following the initial LPS dose. Lung injury scores are
summarized in Table I. The level
of lung injury was most severe at 48 h and reduced at day 7
compared with at 24 and 48 h (P<0.05).
Sepsis reduces contractile force and
FI of diaphragms
Pt/CSA and FI were significantly lower in
the sepsis groups compared with the control group (P<0.05), with
the exception of Pt/CSA in the 7 day group.
Pt/CSA and FI were lowest at 48 h following the original
dose of LPS (P<0.05 vs. control; Table II). At day 7, Pt
(P=0.007) and FI (P=0.016) increased significantly compared with
the 48 h group. Compared with the control group, P0/CSA
in the sepsis groups declined across the entire stimulation
frequency range (10–100 Hz), which was most significant at 48 h
following the initial LPS dose (P<0.05; Table III). At day 7, P0/CSA
was increased compared with at 48 h across the entire stimulation
frequency range (P<0.05).
 | Table II.FI and Pt/CSA in control
and sepsis groups. |
Table II.
FI and Pt/CSA in control
and sepsis groups.
|
| Groups |
|---|
|
|
|
|---|
| Parameter | Control | 24 h | 48 h | 7 days |
|---|
| FI (%) | 31.88±3.59 |
23.12±3.14a |
21.63±2.37a |
25.63±3.38a,c |
| Pt/CSA
(N/cm2) | 2.53±0.30 |
1.98±0.25a |
1.87±0.29a |
2.47±0.35b,c |
 | Table III.Force/CSA at different stimulation
frequencies in control and sepsis groups. |
Table III.
Force/CSA at different stimulation
frequencies in control and sepsis groups.
|
| Groups |
|---|
|
|
|
|---|
| P0/CSA)
(N/cm2) | Control | 24 h | 48 h | 7 days |
|---|
| 10
Hz | 2.78±0.35 |
2.15±0.29a |
2.03±0.22a |
2.6±0.39b,c |
| 20
Hz | 6.53±0.69 |
4.51±0.56a |
4.22±0.65a |
5.02±0.51a,c |
| 40
Hz | 11.29±0.89 |
7.85±0.82a |
7.34±0.65a |
8.64±0.74a,c |
| 50
Hz | 14.32±1.57 |
10.45±1.06a |
8.75±1.05a,b |
12.83±1.10a–c |
| 75
Hz | 16.29±1.43 |
11.06±1.04a |
10.98±1.56a |
14.23±2.01a–c |
| 100 Hz | 13.19±1.49 |
10.32±0.96a |
8.96±0.91a,b |
11.49±1.09a–c |
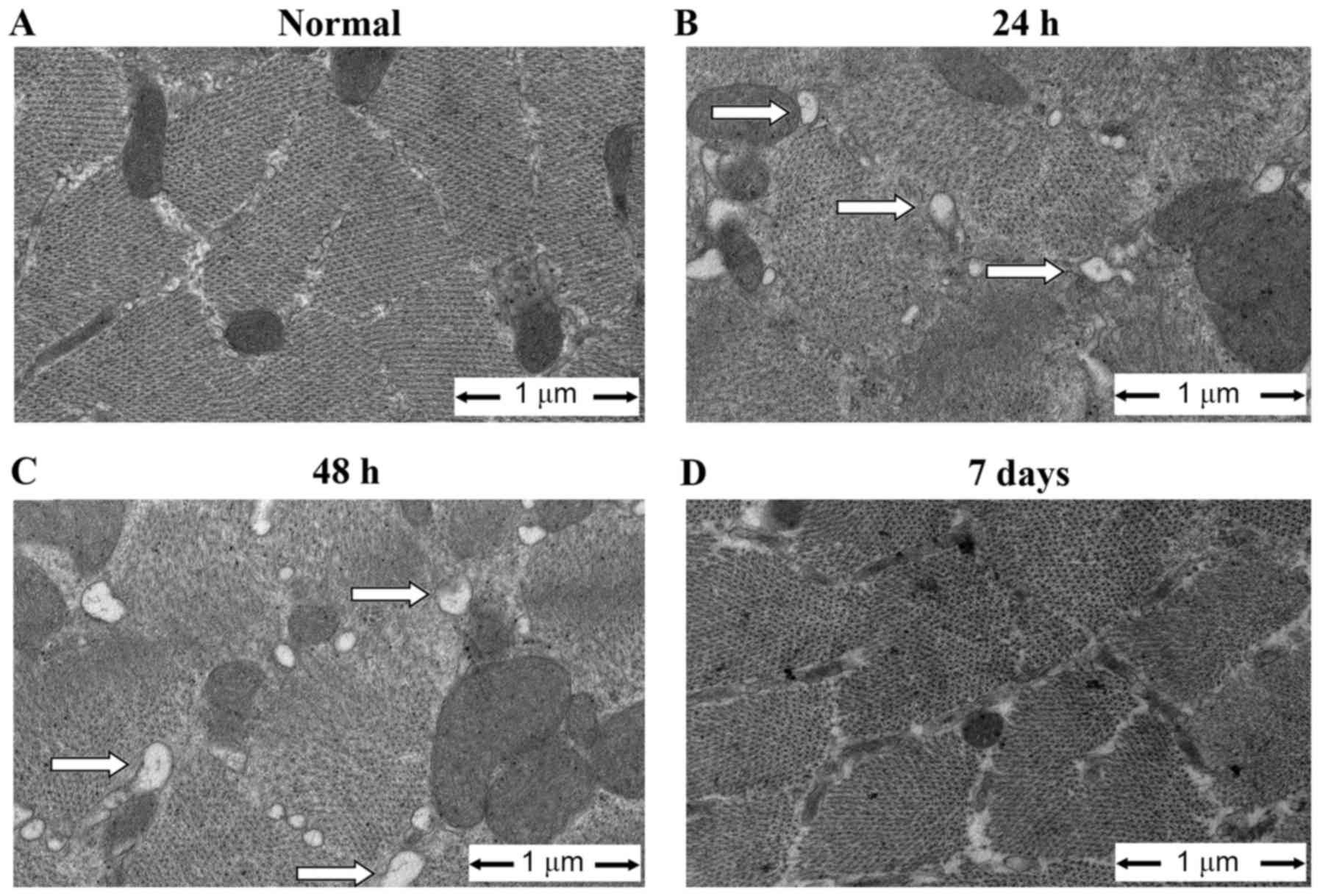
Sepsis causes ultrastructural
alteration of the ER
Ultrastructural changes of the ER were investigated
using EM. As demonstrated in Fig.
2, the diaphragm in sepsis groups exhibited swollen and
distended ER with an irregular shape. Other ultrastructural
abnormalities included swollen, degenerated mitochondria with a
loss of cristae. ER damage was most obvious in the 48 h group and
alleviated at 7 days. Additional ER ultrastructural changes were
quantified by assessing membrane integrity and morphological size.
As demonstrated in Table IV, the
percentage of intact ER in each muscle sample (score 0) was
decreased, and the percentage of damaged ER in each muscle sample
(scores 1 and 2) was increased, in sepsis groups compared with the
control (P<0.05).
 | Table IV.ER injury scores in control and
sepsis groups. |
Table IV.
ER injury scores in control and
sepsis groups.
|
| Groups |
|---|
|
|
|
|---|
| Score | Control | 24 h | 48 h | 7 days |
|---|
| 0 | 93.38±0.92 |
73.00±4.14a |
71.13±3.13a |
82.63±2.72a–c |
| 1 | 5.87±1.13 |
21.75±4.06a |
22.50±2.73a |
13.63±1.41a–c |
| 2 | 0.75±0.71 |
5.25±1.04a |
6.38±1.30a,b |
2.00±0.93a–c |
Sepsis regulates protein and mRNA
expression of ER stress markers in diaphragm
The role of ER stress in the pathophysiology of
sepsis and its complications has been demonstrated recently
(24,25). However, whether ER stress is
involved in contractile dysfunction of the diaphragm in septic rats
has not been investigated. GRP78, CHOP and GRP94 are key markers of
ER stress. ERP44, ERP57 and ERP72 are ER chaperones that can be
upregulated by the UPR at transcriptional level. In the present
study, western blot analysis and RT-qPCR were performed to detect
the expression of these proteins in the diaphragm. As demonstrated
in Fig. 3, in the 24 h group, the
expression levels of CHOP and ERP57 were significantly higher than
in the control group (P<0.05). In the 48 h group, levels of
GRP78, CHOP, GRP94, ERP44, ERP57 and ERP72 were significantly
higher than in the control and 24 h groups (P<0.05). Expression
of these proteins returned to normal levels at day 7. In addition,
a caspase-12 antibody, which indicated cleaved caspase-12 at 35
kDa, was used to indicate the level of ER stress. The level of
cleaved caspase-12 increased in sepsis groups and reached its
highest level in the 48 h group, where the increase was
statistically significant compared with the control (P=0.001).
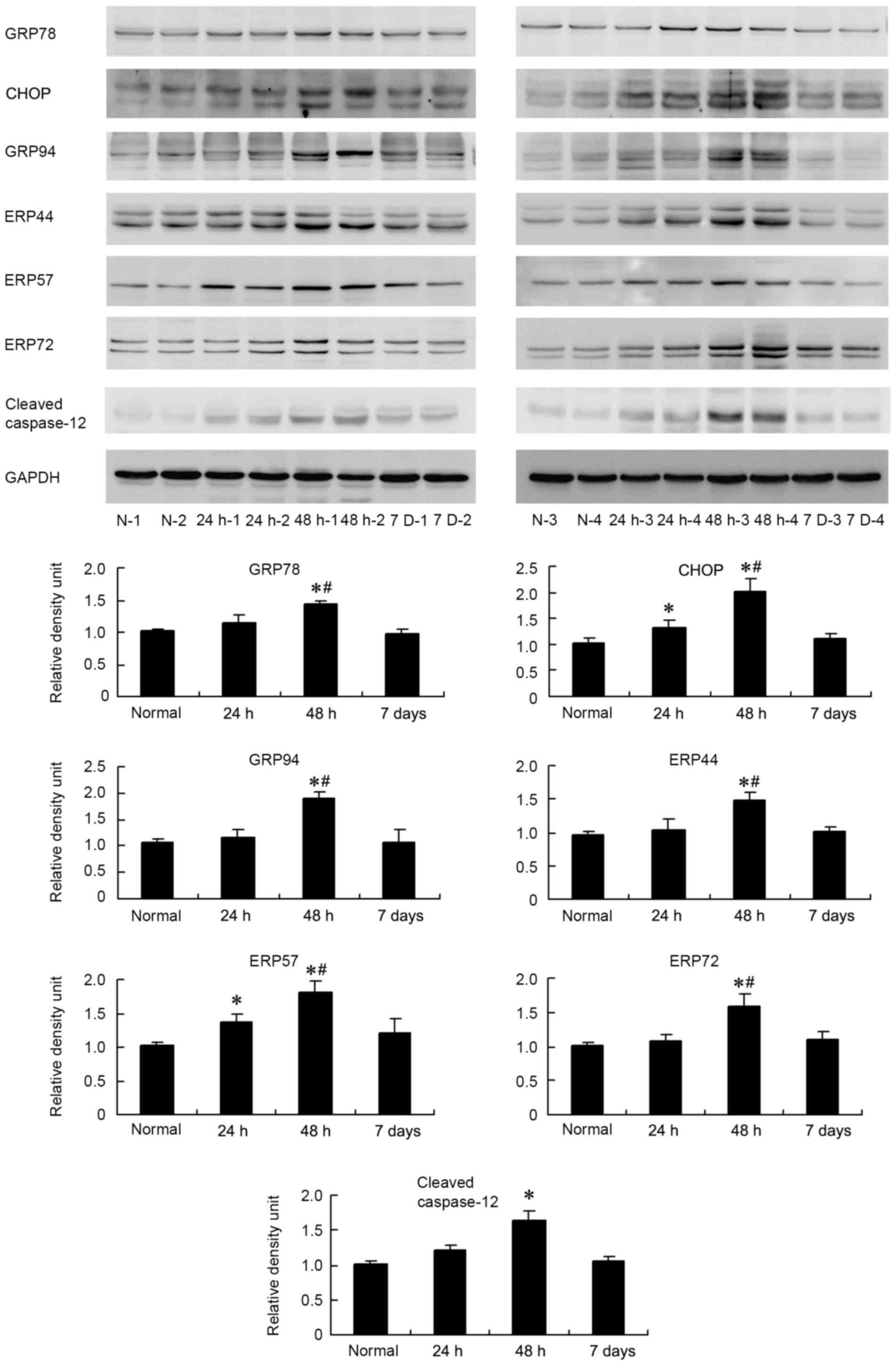 | Figure 3.Western blot analysis of protein
expression levels of ER stress markers in the diaphragm of the
sepsis and control groups. Western blot and grey value analysis
results indicated that expression of CHOP and ERP57 in the 24 h
group was significantly higher than that in control group. In the
48 h group, levels of GRP78, CHOP, GRP94, ERP44, ERP57 and ERP72
were significantly higher than that in control and 24 h groups.
Expression of cleaved caspase-12 in 48 h group was significantly
higher than that in control group. Samples from 4 subjects in each
group are presented. Values in the bar graph are presented as the
mean ± standard deviation. *P<0.05 vs. the control,
#P<0.05 vs. the 24 h group. ER, endoplasmic
reticulum; GRP78, glucose-regulated protein 78 kDa; CHOP, C/EBP
homologous protein; GRP94, glucose-regulated protein 94 kDa; ERP44,
endoplasmic reticulum protein 44; ERP57, protein
disulfide-isomerase like protein; ERP72, protein disulfide
isomerase family A member 4. |
Changes in the mRNA levels of these ER
stress markers were also examined by RT-qPCR
As demonstrated in Fig.
4, significant increases in GRP78, CHOP, GRP94, ERP44, ERP57
and ERP72 mRNA were observed at 24 and 48 h compared with the
control (P<0.05). Upregulated mRNA expression of ER stress
markers declined at day 7, to a level that was similar to the
control group.
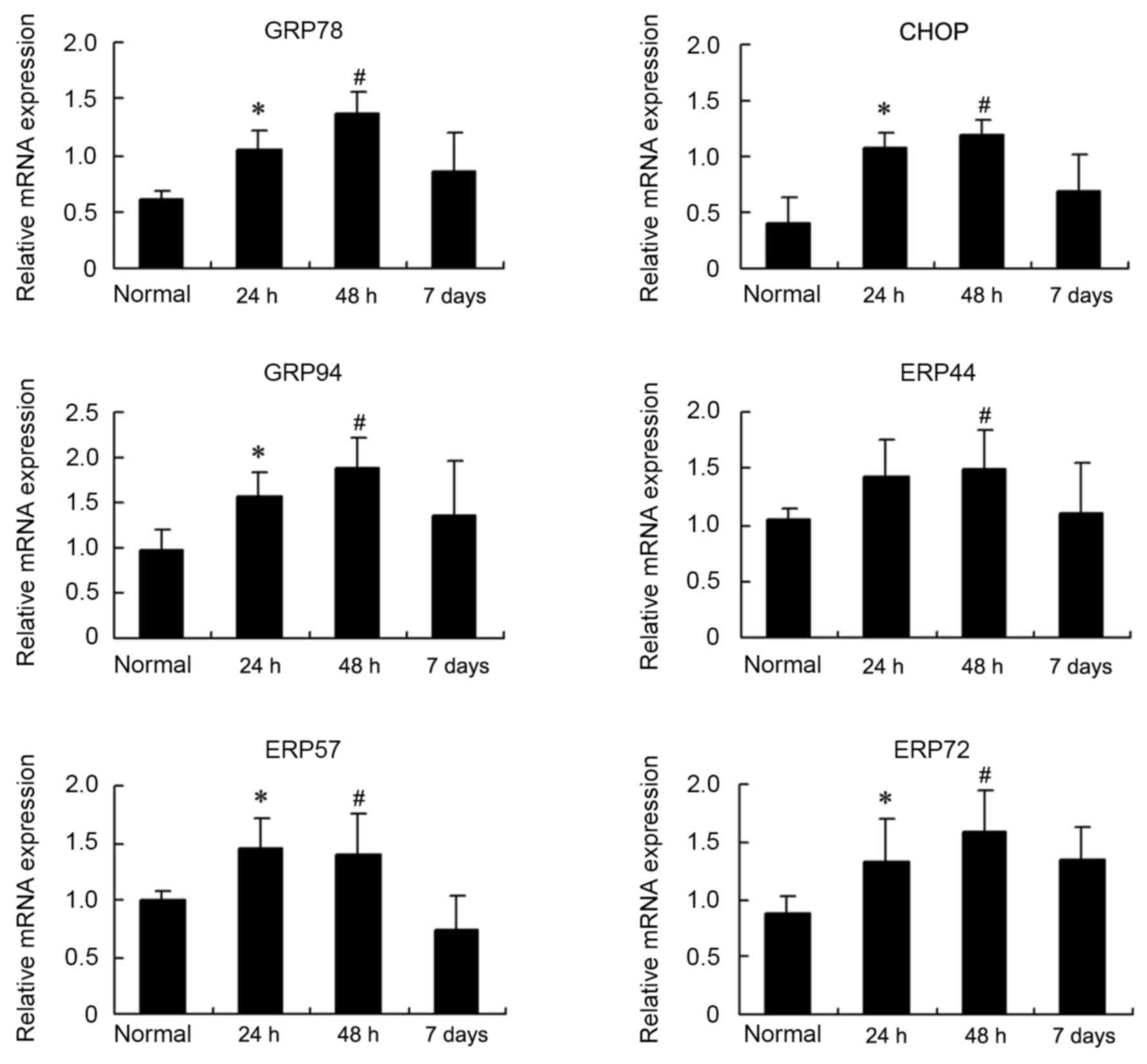 | Figure 4.Reverse transcription-quantitative
polymerase chain reaction analysis of mRNA expression levels of ER
stress markers in the diaphragm from sepsis and control groups.
Changes in the relative expression of mRNA, in control and the
three different sepsis groups, for the following ER stress markers
are presented as the mean ± standard deviation: GRP78, CHOP, GRP94.
ERP44, ERP57 and ERP72. *P<0.05 vs. the control,
#P<0.05 vs. the control. ER, endoplasmic reticulum;
GRP78, glucose-regulated protein 78 kDa; CHOP, C/EBP homologous
protein; GRP94, glucose-regulated protein 94 kDa; ERP44,
endoplasmic reticulum protein 44; ERP57, protein
disulfide-isomerase like protein; ERP72, protein disulfide
isomerase family A member 4. |
Discussion
The present study demonstrated that weakened
diaphragm muscle contractile force is associated with lung injury,
ER damage and increased protein and mRNA expression of ER
stress-associated factors, GRP78, CHOP, GRP94, ERP44, ERP57 and
ERP72.
Sepsis is the most common clinical condition in
which acute lung injury and respiratory failure develops. Various
studies have examined the effects of sepsis models on diaphragm
contractility in spontaneously breathing animals (4,26),
however, few studies provide a comprehensive characterization of
the physiological, structural and ultrastructural effects on the
lungs and diaphragm. The present study employed a rat model of
sepsis by injecting 8 mg/kg LPS intraperitoneally, a model which
has been used in other studies (7,27,28).
Responses to LPS were also characterized, including the acute and
recovery phases of the response, and the integration of such data
may provide additional insights into the pathogenesis of the
endotoxin response. In a previous study, which used the same model,
8 mg/kg LPS was administered, and decreased contractile force and
FI of diaphragms was observed after 24 h (21). In the current study, this dose was
administered to rats and diaphragm function was examined at 24, 48
h and 7 days. It was observed that sepsis reduced Pt, FI
and contractile forces of the diaphragm, P0, to their
lowest levels at 48 h following the initial LPS treatment.
Suppression of diaphragm function was alleviated by day 7. Lung W/D
was examined to evaluate the level of edema. Microscopic
examination of lung tissues by H&E staining was employed to
evaluate lung tissue injury, which was manifested by alveolar
congestion, hemorrhage and inflammatory infiltration. Similar to
diaphragm function, the degree of lung edema and injury peaked at
48 h and declined at day 7. These results demonstrated that the
level of lung injury was positively associated with diaphragm
dysfunction. Regarding the association between lung injury and
muscle dysfunction, it was assumed that lung injury led to
decreased oxygen intake and the release of inflammatory mediators
and free radical species including superoxide, nitric oxide and,
the free radical-derived product, hydrogen peroxide (29–31).
These factors may be involved in LPS- or infection-induced
alterations in skeletal muscle function.
Sepsis is known to induce diaphragm muscle weakness,
which in turn contributes to the development of respiratory
failure. A number of studies have explored the mechanism of
sepsis-induced diaphragm dysfunction and it has been reported that
caspase-12 activation, RNA-dependent protein kinase activation and
dysfunction of the calcium release mechanism are responsible for
this effect (32,33). In our previous study it was
demonstrated that, in a septic rat model, expression of DHPRα1s and
RyR1, which are essential for calcium release during
excitation-contraction coupling in striated muscles, were reduced
in the diaphragm (21).
Accumulating evidence suggests that ER stress serves a role in the
pathogenesis of muscle dysfunction (34–36).
The involvement of ER stress has not been investigated in
sepsis-induced diaphragm contractile weakness. The ultrastructural
changes of ER in the diaphragm were investigated by EM. The results
demonstrated swollen, distended ER and mitochondrial lesions of
diaphragm muscle cells in septic rats, which indicated ER injury.
This structural injury may be the result of changes at the
molecular level (37,38). In muscle cells, the sarcoplasmic
reticulum, smooth ER found in myocytes, stores calcium ions and
pumps them out into the cytoplasm following stimulation of the
muscle fiber. After release from the ER, calcium ions interact with
contractile proteins that utilize ATP to shorten the muscle fiber
(39). It was assumed that, during
the ER stress process, ER function is jeopardized and calcium ion
flow is dysregulated, which leads to muscle dysfunction.
The ER is extremely sensitive to a variety of
different stimuli, and signals are transduced from the ER to the
cytoplasm and nucleus, eventually resulting in adaptation for
survival or induction of apoptosis. In response, UPR genes are
induced, increasing the capacity to fold proteins. The UPR is
regulated by three ER transmembrane receptors, which mediate signal
transduction: Inositol requiring ER-to-nucleus signal kinase 1,
activating transcription factor 6 and double-stranded RNA-activated
kinase (protein kinase R)-like ER kinase (40). On accumulation of unfolded
proteins, ER resident chaperones, including GRP78 and GRP94, are
upregulated, which triggers the UPR (41). Subsequently, GRP78 and GRP94 bind
to misfolded proteins to prevent forming aggregates and assists
with correct refolding. The present study demonstrated that sepsis
stimulated ER stress, as reflected by increased expression of GRP78
and GRP94, and by increased expression of CHOP, which are
well-established markers of ER stress. The role of CHOP in
ER-stressed cells is associated with its role in promoting protein
synthesis and oxidative stress inside the ER. This study also
observed that several other ER chaperones, including ERP44, ERP57
and ERP72 were upregulated in the diaphragm of septic rats
(42–44). ERP57, ERP44 and ERP72 are suggested
to be involved in the isomerization of disulfide bonds on certain
glycoproteins during ER stress. During ER stress, these
ER-associated proteins are activated and help to return proteins to
their native conformation and to reconstitute cellular balance.
Their expression reflects the level of ER stress. In addition, this
study demonstrated that the level of ER stress-associated proteins
peaked at 48 h and then declined at 7 days, which corresponded with
the changes in diaphragm function, lung injury and ER damage.
Several previous studies have indicated that ER stress is involved
in muscle dysfunction. ER stress compromises cardiac contractile
dysfunction in LPS-induced animal models and drugs that inhibit ER
stress rescued ER stress-induced contractile dysfunction (14,45,46).
ER stress also causes muscle injury in rats with diabetic
gastroparesis (47). To the best
of our knowledge, the present study is the first to investigate
protein and mRNA changes of ER stress markers in diaphragm muscles
during sepsis and the results implicate ER stress in diaphragm
weakness.
Extended ER stress may lead to caspase activation
and cell apoptosis, which links sepsis-induced ER stress and
diaphragm dysfunction. Caspase-12 activation is believed to have an
important role in ER stress-induced muscle apoptosis (48). In a previous study, caspase-12
mediated ER-specific apoptosis and mice with caspase-12 deficiency
became resistant to apoptosis induced by ER stress (49). Upregulation of cleaved caspase-12
was also observed in myocardial apoptosis induced by ER stress
(50,51). The current study demonstrated that
cleaved caspase-12 expression is increased in the diaphragm of
septic rats, suggesting that ER stress-induced apoptosis occurs in
diaphragm muscle cells, which may eventually lead to muscle
weakness.
In conclusion, the present study demonstrated that
the weakened diaphragm contraction observed in septic rats is
associated with lung tissue injury, ER damage and elevated mRNA and
protein expression of ER stress markers. These results further
support the hypothesis that ER stress was enhanced in the diaphragm
and that diaphragm weakness was, at least partially, induced by ER
stress in septic rats. The present study provides a novel insight
into the mechanisms required for the development of sepsis-induced
diaphragm dysfunction. Further studies are required to investigate
signal transduction in ER stress, which may improve the
characterization of the development of respiratory failure.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170068, 31071004
and 81100108). This abstract has been previously published as part
of the proceedings of the American Thoracic Society 2016 in San
Francisco (CA, USA) on May 13-18, 2016.
References
|
1
|
Sprung CL, Annane D, Keh D, Moreno R,
Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H,
et al: Hydrocortisone therapy for patients with septic shock. N
Engl J Med. 358:111–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson TA, Legrand A, Gevenois PA and De
Troyer A: Respiratory effects of the external and internal
intercostal muscles in humans. J Physiol. 530:319–330. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Supinski G, Nethery D, Stofan D and
DiMarco A: Comparison of the effects of endotoxin on limb,
respiratory, and cardiac muscles. J Appl Physiol (1985).
81:1370–1378. 1996.PubMed/NCBI
|
|
4
|
Shindoh C, Murakami Y, Shishido R, Sasaki
K, Nishio T and Miura M: Tulobuterol patch maintains diaphragm
muscle contractility for over twenty-four h in a mouse model of
sepsis. Tohoku J Exp Med. 218:271–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lanone S, Taillé C, Boczkowski J and
Aubier M: Diaphragmatic fatigue during sepsis and septic shock.
Intensive Care Med. 31:1611–1617. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Supinski GS and Callahan LA: Calpain
activation contributes to endotoxin-induced diaphragmatic
dysfunction. Am J Respir Cell Mol Biol. 42:80–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Callahan LA and Supinski GS: Sepsis
induces diaphragm electron transport chain dysfunction and protein
depletion. Am J Respir Crit Care Med. 172:861–868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deniaud A, el dein O Sharaf, Maillier E,
Poncet D, Kroemer G, Lemaire C and Brenner C: Endoplasmic reticulum
stress induces calcium-dependent permeability transition,
mitochondrial outer membrane permeabilization and apoptosis.
Oncogene. 27:285–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao L and Ackerman SL: Endoplasmic
reticulum stress in health and disease. Curr Opin Cell Biol.
18:444–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li SY, Gilbert SA, Li Q and Ren J:
Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol
ingestion-induced myocardial insulin resistance and endoplasmic
reticulum stress. J Mol Cell Cardiol. 47:247–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miki T, Miura T, Hotta H, Tanno M, Yano T,
Sato T, Terashima Y, Takada A, Ishikawa S and Shimamoto K:
Endoplasmic reticulum stress in diabetic hearts abolishes
erythropoietin-induced myocardial protection by impairment of
phospho-glycogen synthase kinase-3beta-mediated suppression of
mitochondrial permeability transition. Diabetes. 58:2863–2872.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minamino T, Komuro I and Kitakaze M:
Endoplasmic reticulum stress as a therapeutic target in
cardiovascular disease. Circ Res. 107:1071–1082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Xia Z, La Cour KH and Ren J:
Activation of Akt rescues endoplasmic reticulum stress-impaired
murine cardiac contractile function via glycogen synthase
kinase-3β-mediated suppression of mitochondrial permeation pore
opening. Antioxid Redox Signal. 15:2407–2424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sassoon CS, Caiozzo VJ, Manka A and Sieck
GC: Altered diaphragm contractile properties with controlled
mechanical ventilation. J Appl Physiol (1985). 92:2585–2595. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sassoon CS, Zhu E, Fang L, Ramar K, Jiao
GY and Caiozzo VJ: Interactive effects of corticosteroid and
mechanical ventilation on diaphragm muscle function. Muscle Nerve.
43:103–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolthuis EK, Vlaar AP, Hofstra JJ, Roelofs
JJ, de Waard V, Juffermans NP and Schultz MJ: Plasminogen activator
inhibitor-type I gene deficient mice show reduced influx of
neutrophils in ventilator-induced lung injury. Crit Care Res Pract.
2011:2178962011.PubMed/NCBI
|
|
18
|
Rojas M, Woods CR, Mora AL, Xu J and
Brigham KL: Endotoxin-induced lung injury in mice: Structural,
functional, and biochemical responses. Am J Physiol Lung Cell Mol
Physiol. 288:L333–L341. 2005.PubMed/NCBI
|
|
19
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Li D, Liu X, Tang S and Wei F: Human
umbilical cord mesenchymal stem cells reduce systemic inflammation
and attenuate LPS-induced acute lung injury in rats. J Inflamm
(Lond). 9:332012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiao GY, Hao LY, Gao CE, Chen L, Sun XF,
Yang HL, Li Y and Dai YN: Reduced DHPRα1S and RyR1 expression
levels are associated with diaphragm contractile dysfunction during
sepsis. Muscle Nerve. 48:745–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burke RE, Levine DN, Tsairis P and Zajac
FE III: Physiological types and histochemical profiles in motor
units of the cat gastrocnemius. J Physiol. 234:723–748. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khan MM, Yang WL and Wang P: Endoplasmic
reticulum stress in sepsis. Shock. 44:294–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma T, Han L and Hu WQ: Study of the role
of endoplasmic reticulum stress mediated apoptosis signal pathway
in sepsis-induced splenic lymphocyte apoptosis. Zhongguo Wei Zhong
Bing Ji Jiu Yi Xue. 21:48–50. 2009.(In Chinese). PubMed/NCBI
|
|
26
|
Narimatsu E, Nakayama Y, Sumita S, Iwasaki
H, Fujimura N, Satoh K and Namiki A: Sepsis attenuates the
intensity of the neuromuscular blocking effect of d-tubocurarine
and the antagonistic actions of neostigmine and edrophonium
accompanying depression of muscle contractility of the diaphragm.
Acta Anaesthesiol Scand. 43:196–201. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Supinski GS, Wang W and Callahan LA:
Caspase and calpain activation both contribute to sepsis-induced
diaphragmatic weakness. J Appl Physiol (1985). 107:1389–1396. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doi K, Leelahavanichkul A, Yuen PS and
Star RA: Animal models of sepsis and sepsis-induced kidney injury.
J Clin Invest. 119:2868–2878. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng H, Chen Q and Tan Y: Frequent
ejaculation associated free radical and lactic acid accumulation
cause noninfectious inflammation and muscle dysfunction: A
potential mechanism for symptoms in Chronic Prostatitis/Chronic
Pelvic Pain Syndrome. Med Hypotheses. 73:372–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Supinski GS and Callahan LA: Free
radical-mediated skeletal muscle dysfunction in inflammatory
conditions. J Appl Physiol (1985). 102:2056–2063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Supinski G: Free radical induced
respiratory muscle dysfunction. Mol Cell Biochem. 179:99–110. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu SH, Lai JL, Yang RS and Lin-Shiau SY:
Nitric oxide is not involved in the endotoxemia-induced alterations
in Ca2+ and ryanodine responses in mouse diaphragms.
Naunyn Schmiedebergs Arch Pharmacol. 366:327–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Supinski GS and Callahan LA:
Double-stranded RNA-dependent protein kinase activation modulates
endotoxin-induced diaphragm weakness. J Appl Physiol (1985).
110:199–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bohnert KR, Gallot YS, Sato S, Xiong G,
Hindi SM and Kumar A: Inhibition of ER stress and unfolding protein
response pathways causes skeletal muscle wasting during cancer
cachexia. FASEB J. 30:3053–3068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deldicque L, Bertrand L, Patton A,
Francaux M and Baar K: ER stress induces anabolic resistance in
muscle cells through PKB-induced blockade of mTORC1. PLoS One.
6:e209932011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chalil S, Pierre N, Bakker AD, Manders RJ,
Pletsers A, Francaux M, Klein-Nulend J, Jaspers RT and Deldicque L:
Aging related ER stress is not responsible for anabolic resistance
in mouse skeletal muscle. Biochem Biophys Res Commun. 468:702–707.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hrincius ER, Liedmann S, Finkelstein D,
Vogel P, Gansebom S, Samarasinghe AE, You D, Cormier SA and
McCullers JA: Acute lung injury results from innate sensing of
viruses by an ER stress pathway. Cell Rep. 11:1591–1603. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Inagi R, Nangaku M, Onogi H, Ueyama H,
Kitao Y, Nakazato K, Ogawa S, Kurokawa K, Couser WG and Miyata T:
Involvement of endoplasmic reticulum (ER) stress in podocyte injury
induced by excessive protein accumulation. Kidney Int.
68:2639–2650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao X, Li Y, Cheng X and Li H: ER stress
contributes to alpha-naphthyl isothiocyanate-induced liver injury
with cholestasis in mice. Pathol Res Pract. 212:560–567. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mori K: Tripartite management of unfolded
proteins in the endoplasmic reticulum. Cell. 101:451–454. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Travers KJ, Patil CK, Wodicka L, Lockhart
DJ, Weissman JS and Walter P: Functional and genomic analyses
reveal an essential coordination between the unfolded protein
response and ER-associated degradation. Cell. 101:249–258. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vattemi G, Engel WK, McFerrin J and
Askanas V: Endoplasmic reticulum stress and unfolded protein
response in inclusion body myositis muscle. Am J Pathol. 164:1–7.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dihazi H, Dihazi GH, Bibi A, Eltoweissy M,
Mueller CA, Asif AR, Rubel D, Vasko R and Mueller GA: Secretion of
ERP57 is important for extracellular matrix accumulation and
progression of renal fibrosis, and is an early sign of disease
onset. J Cell Sci. 126:3649–3663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simmen T, Lynes EM, Gesson K and Thomas G:
Oxidative protein folding in the endoplasmic reticulum: Tight links
to the mitochondria-associated membrane (MAM). Biochim Biophys
Acta. 1798:1465–1473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ceylan-Isik AF, Zhao P, Zhang B, Xiao X,
Su G and Ren J: Cardiac overexpression of metallothionein rescues
cardiac contractile dysfunction and endoplasmic reticulum stress
but not autophagy in sepsis. J Mol Cell Cardiol. 48:367–378. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang B, Zhang Y, La Cour KH, Richmond KL,
Wang XM and Ren J: Mitochondrial aldehyde dehydrogenase obliterates
endoplasmic reticulum stress-induced cardiac contractile
dysfunction via correction of autophagy. Biochim Biophys Acta.
1832:574–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X, Fu XS, Li CP and Zhao HX: ER
stress and ER stress-induced apoptosis are activated in gastric
SMCs in diabetic rats. World J Gastroenterol. 20:8260–8267. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Di Sano F, Ferraro E, Tufi R, Achsel T,
Piacentini M and Cecconi F: Endoplasmic reticulum stress induces
apoptosis by an apoptosome-dependent but caspase 12-independent
mechanism. J Biol Chem. 281:2693–2700. 2006.PubMed/NCBI
|
|
49
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ding W and Zhang X, Huang H, Ding N, Zhang
S, Hutchinson SZ and Zhang X: Adiponectin protects rat myocardium
against chronic intermittent hypoxia-induced injury via inhibition
of endoplasmic reticulum stress. PLoS One. 9:e945452014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yao W, Gu H, Zhu J, Barding G, Cheng H,
Bao B, Zhang L, Ding A and Li W: Integrated plasma and urine
metabolomics coupled with HPLC/QTOF-MS and chemometric analysis on
potential biomarkers in liver injury and hepatoprotective effects
of Er-Zhi-Wan. Anal Bioanal Chem. 406:7367–7378. 2014. View Article : Google Scholar : PubMed/NCBI
|