Introduction
O-GlcNAcylation is a post-translational modification
in nuclear and cytoplasmic proteins in which O-linked
β-N-acetylglucosamine (O-GlcNAc) monosaccharide is linked to a
serine orthreonine residue (1).
O-GlcNAc cycling is catalyzed by only two enzymes: O-GlcNAc
transferase (OGT), which is responsible for the addition of
O-GlcNAc to proteins; and O-GlcNAcase (OGA), which is responsible
for the removal of O-GlcNAc from proteins (2). O-GlcNAcylation is emerging as a key
regulator of cellular biological processes, including
transcription, signaling, cell metabolism, morphogenesis, motility,
cell cycle and development (3–5).
Abnormal O-GlcNAcylation levels are associated with a variety of
human diseases including diabetes, cardiovascular disease and
neurologic disorders (6,7). An increasing number of
O-GlcNAc-modified proteins have been revealed to be closely
associated with tumorigenesis and development (8). Multiple oncogene and anti-oncogene
products, including p53, c-Myc, c-Jun, c-Fos and retinoblastoma
protein, have been demonstrated to be modified by O-GlcNAc
(9–11), suggesting that O-GlcNAcylation may
be associated with tumorigenesis and development. Aberrant
O-GlcNAcylation has been linked to several types of human cancer,
including breast (12), lung
(13), colon (14), pancreatic (15) and prostate (16) cancer. However, the effect of
O-GlcNAcylation on ovarian cancer remains poorly understood.
Ovarian cancer is the fifth most common cause of
cancer-associated mortality in females, and exhibits the highest
mortality of all gynecological malignancies (17). Aggressive ovarian cancer cells are
often able to break away from the primary tumor to invade and
spread to other parts of the body, including the lymph nodes, liver
and lungs, and the lining of the bladder, bowel and abdomen
(18,19). This results in a poor prognosis and
a high mortality rate. The malignancy of tumor cells is assessed by
measuring the migratory and invasive ability of the cells, so
investigations of the molecular mechanisms underlying these
abilities may aid the diagnosis and treatment of cancer. In the
present study, the effects of O-GlcNAcylation on ovarian cancer
cell motility were examined, including migration and invasion, and
the underlying molecular mechanism.
Ras homolog family member A (RhoA) is a member of
the Rho GTPase family associated with actin cytoskeleton
rearrangement, regulation of the cell cycle, gene transcription,
cell polarity and movement (20).
As with other GTPases, RhoA functions through the exchange between
two states: The GTP-bound active state and the GDP-bound inactive
state (21). RhoA-mediated
signaling pathways, particularly the Rho-associated protein kinase
(ROCK)/myosin light chain (MLC) pathway, are closely associated
with diverse biological activities including cytoskeleton
reorganization, gene expression, muscle contraction, cell growth
and motility (22–27). High RhoA expression levels and
activity have been observed in a variety of human types of cancer
(28–32), suggesting that it may be associated
with signaling pathways relevant to cancer. In addition, increased
RhoA and ROCK expression levels are more commonly observed in
advanced cancer compared with early stage cancer (33). Furthermore, it has been reported
(34) that RhoA silencing
significantly suppresses growth, adhesion, migration and invasion
of ovarian cancer cells. Therefore, the present study aimed to
examine whether RhoA/ROCK signaling is involved in the regulation
of O-GlcNAcylation in ovarian cancer cells, and how this affects
their capacity to migrate and invade tissues.
Materials and methods
Cell cultures
Human ovarian epithelial adenocarcinoma SKOV3 cells
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and endometrioid-type ovarian epithelial
carcinoma 59M cells were purchased from the European Collection of
Authenticated Cell Cultures; Public Health England (Salisbury, UK).
SKOV3 cells were cultured in McCoy's 5A medium (Thermo Fisher
Scientific, Inc., Waltham, MA) and 59M cells in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Lonza Group, Basel, Switzerland),
2 mM L-glutamine, 100 µg/ml streptomycin and 100 units/ml
penicillin. Cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2.
RNA interference (RNAi)
The sequence of the OGT-targeting small interfering
RNA (siRNA) used was 5′-GGATGCTTATATCAATTTAGG-3′. Negative control
siRNA oligonucleotides (F:
5′-CCGGTACGTGACACGTTCGGAGAATTCTCGAGAATTCTCCGAACGTGTCACGTTTTTTG-3′;
R:
5′-AATTCAAAAAACGTGACACGTTCGGAGAATTCTCGAGAATTCTCCGAACGTGTCACGTA-3′)
were purchased from Eurogentec (Liège, Belgium). siRNA (1.6 µg) was
diluted with Opti-MEM I Reduced Serum Medium (Invitrogen; Thermo
Fisher Scientific, Inc.) to a final volume of 100 µl. DreamFect
reagent (8 µl; OZ Biosciences, Marseille, France) was diluted with
Opti-MEM I Reduced Serum Medium to a final volume of 100 µl. The
RNAi solution and transfection reagent were then combined and
incubated for 20 min at room temperature. The 200 µl mixture was
then added to 80% confluent cells (1.2×106 cells per
well) maintained in 6-well plates, with 1.8 ml of culture medium
per well. Cells were transfected onceover 24 h for 4 days. OGT
expression and activity were detected at 96 h by western blot
analysis, and transfected cell invasion and migratory capacity were
evaluated by Transwell assay.
The sequence of the RhoA-targeting siRNA was
5′-AAGCAGATGAGAATGACGTCGGTG-3′, and negative control siRNA
(5′-ACGTGACACGTTCGGAGAATT-3′) was purchased from Invitrogen (Thermo
Fisher Scientific, Inc.). siRNAs were transfected into SKOV3 and
59M cells by electroporation with the Amaxa Nucleofector (Lonza
Group) according to the manufacturer's protocols, then lysed 24 h
later and analyzed by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blot analysis to measure RhoA
mRNA and protein expression levels, respectively.
RT-qPCR
Total RNA in cells was extracted using a total RNA
isolation kit (A&A Biotechnology, Gdynia, Poland). cDNA was
obtained by reverse transcription of 1 µg of total RNA in a 20 µl
reaction using a RevertAid™ First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) and was amplified using
a TaqMan® Gene Expression Assay (Applied Biosystems;
Thermo Fisher Scientific, Inc.), using primers specific for the
target proteins. The sequences of the primers were as follows:
5′-CGGGAGCTAGCCAAGATGAAG-3′ (F) and 5′-GCTTGCAGAGCAGCTCTCGTA-3′ (R)
for RhoA. 5′-GGCCGTGAAGTCGTCAGAAC-3′ (F) and
5′-GCCACGATGCCCAGGAA-3′ (R) for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). The two genes were amplified by a first step
of 120 sec at 95°C, followed by 45 cycles of 30 sec at 95°C, 30 sec
at 60°C, and 30 sec at 72°C. mRNA expression of RhoA was calculated
using the formula 2−ΔΔCq (35) and was normalized to GAPDH
expression levels. mRNA expression levels in cells transfected with
RhoA siRNA are presented relative to mRNA expression levels in
cells with negative control siRNA.
Migration and invasion assays
Cell migration was evaluated by Transwell assay
using Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA)
(36). A total of 600 µl cell
culture medium, with or without 5 µM Thiamet-G (an OGN inhibitor)
or 50 µM Y-27632 (a ROCK inhibitor) was added in the lower chamber.
Culture medium (100 µl) containing 1×105 SKOV3 or 59M
cells and 1% FBS, was plated into the upper chamber, with or
without Thiamet-G or Y-27632. The cells on the undersurface of the
upper chamber were stained with crystal violet 20 h later and were
observed using a light microscope. A total of six fields at ×100
magnification were selected at random to measure the average cell
coverage using ImageJ software version 1.45s (National Institutes
of Health, Bethesda, MD, USA). Invasion assays were performed using
the same protocol as the migration assay, but the upper face of the
polycarbonate membrane in the upper chamber was covered with 1
mg/ml Matrigel (BD Biosciences) and the invasive cells were
detected following 24 h incubation.
Western blot analysis
Cells were lysed for 15 min at ice bath using a
lysis buffer (1% Triton X-100, 20 mM Tris pH 7.5, 1 mM
MgCl2, 150 mM NaCl, 1 mM Na3VO4,
50 mM NaF, 1.5 mM EDTA, 10% glycerol, 20 mM β-glycerophosphate, 10
µg/ml aprotonin, 1 µM pepstatin A) containing 5 µM PUGNAc (an OGA
inhibitor; Toronto Research Chemicals, Inc., North York, Canada).
Protein samples (50 µg) were separated by 10% SDS-PAGE and
transferred to polyvinylidene fluoride membranes (Merck KGaA,
Darmstadt, Germany). The membrane was blocked with 5% non-fat dried
milk in TBST for 1 h at room temperature and incubated overnight at
4°C with primary antibodies. Antibodies specific to O-GlcNAcylation
(RL2; 1:1,000; Affinity BioReagents, Golden, CO, USA) and OGT
(F-12; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were
used. RhoA (67B9; 1:1,000), MLC (3672; 1:1,000) and phosphorylated
(p-)MLC (3671; 1:1,000) antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). GAPDH antibody
(sc-25778; 1:2,000) and horseradish peroxidase-linked goat
anti-mouse (sc-2005; 1:2,000) and goat anti-rabbit (sc-2004;
1:2,000) IgG secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc. Development was carried out using an enhanced
chemiluminescence western blotting detection reagent (GE Healthcare
Life Sciences, Chalfont, UK).
RhoA activity detection
RhoA activity was analyzed using Rhotekin Rho
binding domain (Upstate Biotechnology; Thermo Fisher Scientific,
Inc.) bound to glutathione agarose beads to pulldown the active
GTP-bound RhoA form from ovarian cell lysates in lysis buffer (20
mM HEPES, pH 7.5, 0.5% NP-40, 100 mM NaCl, 0.2% deoxycholic acid,
10% glycerol, and 10 mM MgCl2) supplemented with
protease and phosphatase inhibitors (37). GTP-bound RhoA and total RhoA were
evaluated by western blot analysis as above, using RhoA antibody
(67B9; 1:1,000; Cell Signaling Technology, Inc.).
MLC phosphorylation detection
Cells were starved in serum-free medium for 24 h,
then incubated at 37°C for 60 min with or without Thiamet-G at 5 µM
concentration. The cells were subsequently lysed for 15 min at
4°Cin cell lysis buffer [100 mM NaCl, 1 mM
Na3VO4, 40 mM
Na4P2O7, 20 mM NaF, 30 mM
HEPES/NaOH (pH 7.4), 1% Triton X-100, 1 mM EGTA, 1 mM PMSF, 10
µg/ml pepstatin, 10 µg/ml leupeptin and 10 µg/ml aprotinin], and
cell lysates were centrifuged for 10 min at 4°C. The cell extracts
were then used for western blot analysis using MLC and p-MLC
antibodies, as above.
Statistical analysis
All experiments were repeated at least three times.
SPSS software version 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for analysis, and data were expressed as the mean ± standard error
of the mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
Differential regulation of
O-GlcNAcylation in ovarian cancer cells
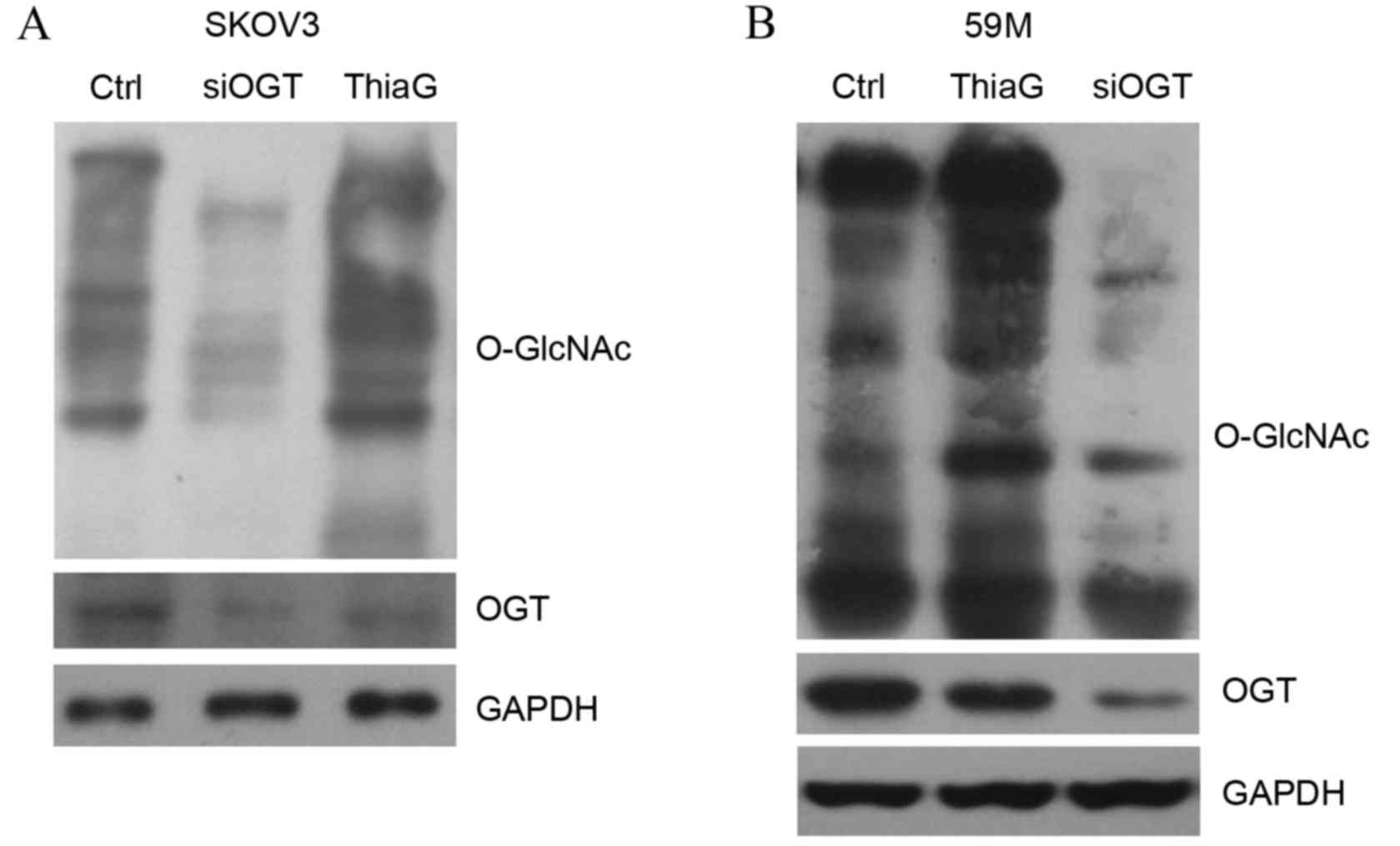
Two human ovarian cancer cell lines, SKOV3 and 59M,
were used to determine the involvement of O-GlcNAcylation in
ovarian cancer. To alter the O-GlcNAcylation level, cells were
transfected with OGT-targeting siRNA or treated with the OGA
inhibitor Thiamet-G (5 µM) for 24 h. Western blot analysis revealed
that OGT silencing decreased the level of global O-GlcNAc in SKOV3
(Fig. 1A) and 59M cells (Fig. 1B) compared with cells transfected
with control siRNA. OGT protein expression levels were also visibly
reduced in SKOV3 (Fig. 1A) and 59M
cells (Fig. 1B) transfected with
OGT siRNA compared with cells transfected with control siRNA.
Thiamet-G treatment visibly increased global O-GlcNAc levels in
SKOV3 (Fig. 1A) and 59M cells
(Fig. 1B) compared with untreated
control cells, indicating that it effectively inhibited OGA
activity.
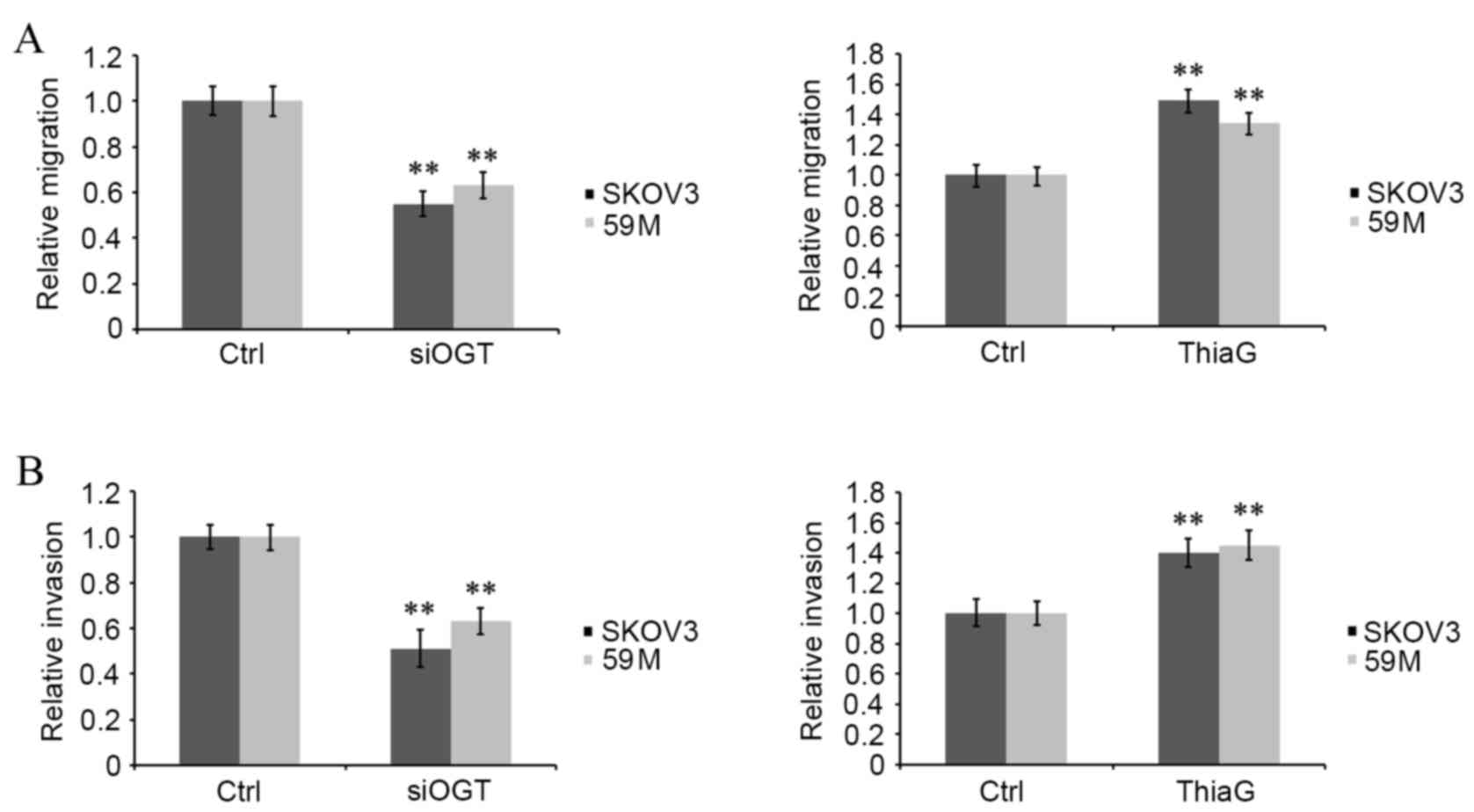
O-GlcNAcylation affects ovarian cancer
cell migration and invasion
The effect of O-GlcNAcylation on ovarian cancer
malignancy was investigated via in vitro analysis of cell
migration and invasion using a Transwell assay. Transfection with
OGT siRNA significantly decreased migration (SKOV3 cells, P=0.007;
59M cells, P=0.009; Fig. 2A) and
invasion (SKOV3 cells, P=0.006; 59M cells, P=0.008; Fig. 2B) in OGT siRNA transfected cells
compared with control siRNA transfected cells. However, Thiamet-G
treatment significantly increased migration (SKOV3 cells, P=0.007;
59M cells, P=0.009; Fig. 2A) and
invasion (SKOV3 cells, P=0.007; 59M cells, P=0.006; Fig. 2B) in treated cells compared with
untreated controls. This indicates that a positive correlation
exists between the intracellular global O-GlcNAcylation level and
the motility of ovarian cancer cells.
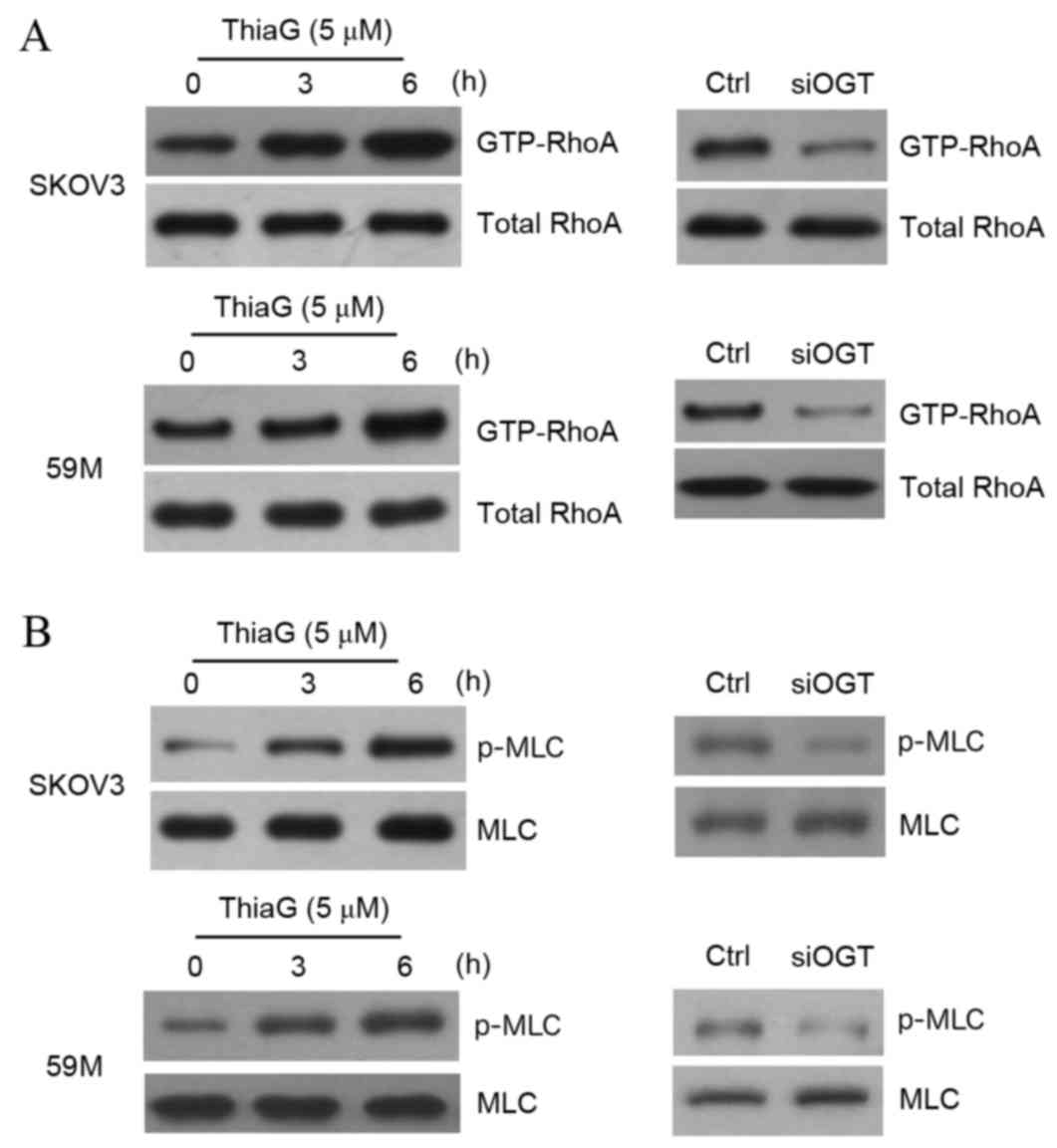
O-GlcNAcylation affects the
RhoA/ROCK/MLC signal pathway
It has previously been reported (22–27)
that Rho GTPases are associated with cell motility, with RhoA
stimulating ROCK and MLC to regulate these cellular events. To
determine how O-GlcNAcylation modulates ovarian cancer cell
motility, RhoA activity was detected by pull-down assay. The
results revealed that Thiamet-G treatment-induced O-GlcNAcylation
upregulation visibly enhanced RhoA activity at 3 and 6 h in SKOV3
and 59M cells compared with untreated control cells (Fig. 3A), while downregulation of
O-GlcNAcylation induced by OGT silencing visibly reduced RhoA
activity in SKOV3 and 59M cells compared with control cells
(Fig. 3A). MLC phosphorylation is
stimulated by RhoA through ROCK activation (25), so MLC phosphorylation was analyzed
by western blotting. The results indicated that O-GlcNAcylation
upregulation increased MLC phosphorylation in SKOV3 and 59M cells
compared with untreated controls (Fig.
3B), and O-GlcNAcylation downregulation attenuated this
phosphorylation in SKOV3 and 59M cells compared with control cells
(Fig. 3B). This suggests that the
RhoA/ROCK/MLC signal pathway may be closely associated with
O-GlcNAcylation and the regulation of motility in ovarian cancer
cells.
RhoA silencing reverses
O-GlcNAcylation-induced cell motility
To determine whether O-GlcNAcylation affected
ovarian cancer cell motility by targeting RhoA/ROCK signaling, RhoA
was knocked down by RNAi and interference efficiency was assessed
using RT-qPCR and western blot analysis to measure mRNA and protein
expression levels, respectively. RhoA mRNA and protein expression
levels were effectively decreased in SKOV3 cells transfected with
RhoA siRNA compared with control cells (Fig. 4A and B, respectively). RhoA
silenced and non-silenced cells were subsequently treated with or
without Thiamet-G, and cell migration and invasion were evaluated
by Transwell assay. Thiamet-G treatment resulted in a significant
increase in migration and invasion compared with control cells in
SKOV3 (P=0.005 and P=0.006, respectively; Fig. 4C and D, respectively) and 59M cells
(P=0.009 and P=0.005, respectively; Fig. 4C and D, respectively). RhoA
silencing significantly attenuated cell migration and invasion in
SKOV3 (P=0.004 and P=0.006, respectively; Fig. 4C and D, respectively) and 59M cells
(P=0.007 and P=0.004, respectively; Fig. 4C and D, respectively) compared with
control cells. No significant difference was observed in migration
or invasion between RhoA silenced cells and RhoA silenced cells
treated with Thiamet-G (Fig. 4C and
D, respectively). These findings suggest that RhoA is involved
in the regulation of O-GlcNAcylation in ovarian cancer cell
motility.
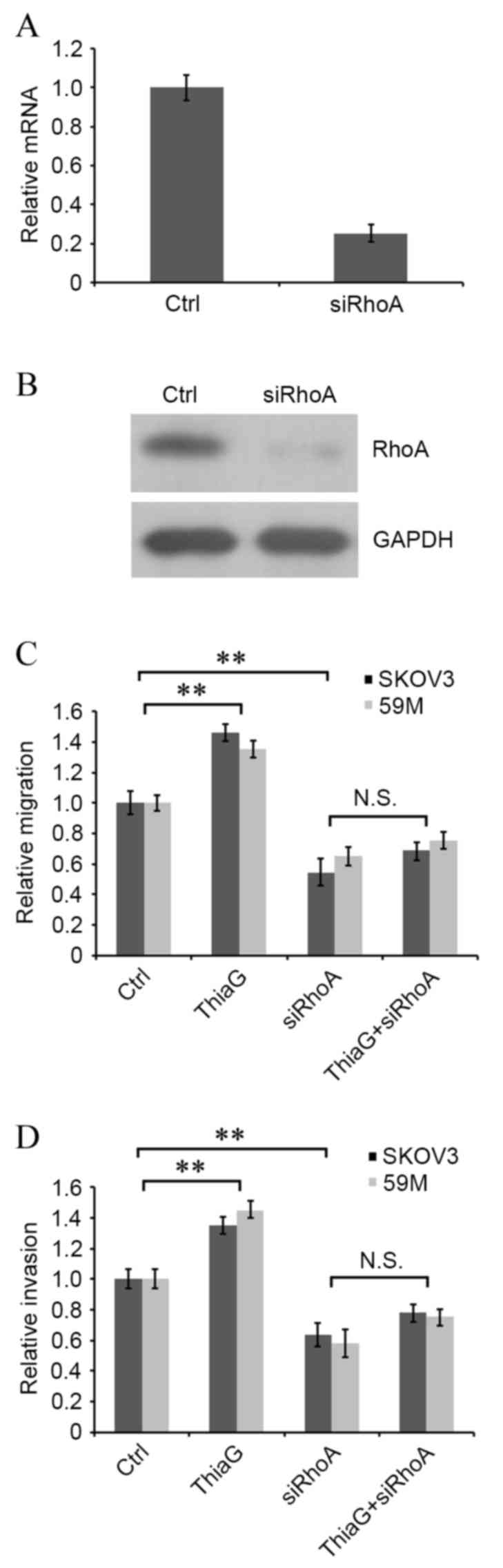 | Figure 4.siRhoA attenuates
O-GlcNAcylation-induced cell migration and invasion in SKOV3 and
59M human ovarian cancer cells. The effect of siRhoA transfection
on RhoA (A) mRNA and (B) protein expression levels, assessed by
reverse transcription-quantitative polymerase chain reaction and
western blotting, respectively. (C) Migration and (D) invasion in
RhoA silenced and non-silenced cells following ThiaG treatment,
assessed by Transwell assay. **P<0.01, with comparisons
indicated by lines. O-GlcNAc, O-linked β-N-acetylglucosamine;
siRhoA, Ras homolog family member A small interfering RNA; RhoA,
Ras homolog family member A; Ctrl, control; GADPH, glyceraldehyde
3-phosphate dehydrogenase; ThiaG, Thiamet-G; N.S., not
significant. |
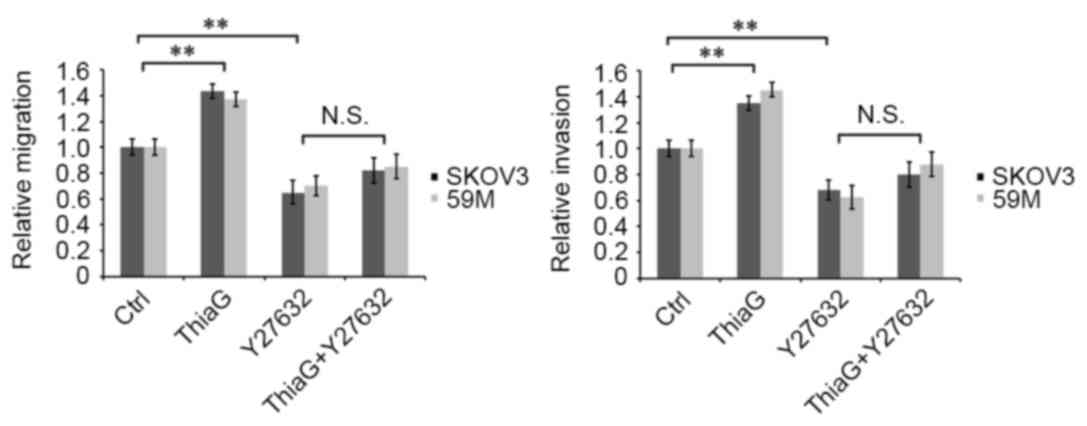
Y-27632 inhibited
O-GlcNAcylation-induced cell migration and invasion
Y-27632 is able to effectively inhibit ROCK activity
(38) and is often used in the
investigation of the ROCK signal pathways (39). Therefore, Thiamet-G treated and
untreated SKOV3 and 59M cells were treated with or without 50 µM
Y-27632, and cell motility was analyzed. Thiamet-G treatment
resulted in a significant increase in migration and invasion
compared with control cells in SKOV3 (P=0.005 and P=0.008,
respectively; Fig. 5) and 59M
cells (P=0.007 and P=0.003, respectively; Fig. 5). Y-27632 treatment significantly
inhibited cell migration and invasion in SKOV3 (P=0.006 and
P=0.004, respectively; Fig. 5) and
59M cells (P=0.007 and P=0.003, respectively; Fig. 5) compared with control cells. No
significant difference was observed in invasion or migration
between Y-27632 treated and Thiamiet-G+Y-27632 treated cells
(Fig. 5). These results suggest
that O-GlcNAcylation regulates ovarian cancer cell motility through
the RhoA/ROCK signal pathway.
Discussion
Ovarian cancer exhibits the highest mortality of all
gynecological malignancies due to a high rate of metastasis
(17). Metastasis is the final
step in the progression of numerous solid tumors, and previous
studies (40–42) have demonstrated that ovarian cancer
cells often spread to distant sites including the lung, spleen,
liver and bone aspirates, leading to increased complications and
higher mortality rates. Therefore, the investigation of mechanisms
associated with ovarian cancer is necessary to improve the
diagnosis and treatment of ovarian cancer.
A growing body of evidence (12–16)
has demonstrated that O-GlcNAcylation is a critical regulator of
several human tumors and is associated with anchorage independent
growth, proliferation, adhesion, migration and invasion in cancer
cells, which are closely associated with tumor cell malignancy
(3–5,8).
Cellular migration and invasion particularly represent the
metastatic ability of tumor cells. In the present study,
O-GlcNAcylation upregulation was demonstrated to promote migration
and invasion of ovarian cancer cells, whereas O-GlcNAcylation
downregulation inhibited migration and invasion. This finding is
supported by previous studies (12,13)
regarding the involvement of O-GlcNAcylation in breast, lung and
colon cancer progression.
High RhoA mRNA and protein expression levels have
been reported in several human types of cancer, including bladder
(28), gastric (29), breast (30), testicular (31) and ovarian (32) cancer. RhoA expression is also
significantly increased in prostate cancer cells compared with
normal prostate cells, contributing to aberrant cell growth, and
knockdown of RhoA decreases prostate cancer cell viability and
motility (43). ROCK affects the
growth, formation, migration, invasion and metastasis of tumor
cells by modulating cell stress-fiber and intercellular connection
formation (28,44–51)
and RhoA-mediated signaling pathways, particularly the
RhoA/ROCK/MLC pathway, are involved in regulating cell motility
(23,24,28).
In order to explore the underlying molecule mechanisms behind
O-GlcNAcylation modulation of motility in ovarian cancer cells, the
present study investigated the RhoA/ROCK/MLC signal pathway. The
data demonstrated that O-GlcNAcylation activated the RhoA/ROCK/MLC
pathway by stimulating the formation of activated GTP-bound RhoA
and MLC phosphorylation. Deficiencies in this pathway, mediated by
either RhoA silencing or the inhibition of ROCK by Y-27632, blocked
O-GlcNAcylation and induced increased migration and invasion. These
results suggest that O-GlcNAcylation modulates motility in ovarian
cancer cells by stimulating RhoA/ROCK/MLC signaling. However, RhoA
activity is regulated by a variety of proteins. p27 regulates the
activation of the RhoA/ROCK/MLC signaling pathway by binding with
RhoA, which affects biological functions of the cell (52). p27-Rho is able to activate RhoA and
induce invadopodia, thus regulating tumor cell invasion (53). RhoA/ROCK/MLC signaling is also
activated by guanine-nucleotide exchange factor-H1 to regulate cell
contractility (54). However,
whether altered RhoA activity is the result of direct modification
or an indirect effect of O-GlcNAc remains to be elucidated, with
more study required.
In conclusion, O-GlcNAcylation enhanced
RhoA/ROCK/MLC signaling, which promoted the migration and invasion
of ovarian cancer cells. This finding suggests valuable novel
targets to control metastasis, and lays a theoretical foundation
for the diagnosis and treatment of ovarian cancer.
References
|
1
|
Torres CR and Hart GW: Topography and
polypeptide distribution of terminal N-acetylglucosamine residues
on the surfaces of intact lymphocytes. Evidence for O-linked
GlcNAc. J Biol Chem. 259:3308–3317. 1984.PubMed/NCBI
|
|
2
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and phosphorylation:
Roles in signaling, transcription, and chronic disease. Annu Rev
Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins.
Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wells L, Vosseller K and Hart GW:
Glycosylation of nucleocytoplasmic proteins: Signal transduction
and O-GlcNAc. Science. 291:2376–2378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanover JA: Glycan-dependent signaling:
O-linked N-acetylglucosamine. FASEB J. 15:1865–1876. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lazarus BD, Love DC and Hanover JA:
O-GlcNAc cycling: Implications for neurodegenerative disorders. Int
J Biochem Cell Biol. 41:2134–2146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Ongusaha PP, Miles PD, Havstad JC,
Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ and
Evans RM: Phosphoinositide signalling links O-GlcNAc transferase to
insulin resistance. Nature. 451:964–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slawson C and Hart GW:
O-GlcNAc-signalling: Implications for cancer cell biology. Nat Rev
Cancer. 11:678–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zachara NE and Hart GW: Cell signaling,
the essential role of O-GlcNAc. Biochim Biophys Acta. 1761:599–617.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chou TY, Hart GW and Dang CV: C-Myc is
glycosylated at threonine 58, a known phosphorylation site and a
mutational hot spot in lymphomas. J Biol Chem. 270:18961–18965.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamemura K, Hayes BK, Comer FI and Hart
GW: Dynamic interplay between O-glycosylation and O-phosphorylation
of nucleocytoplasmic proteins. Alternative
glycosylation/phosphorylation of Thr-58, a known mutational hot
spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem.
277:19229–19235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C,
Yang J, Han F, Lu X and Yu W: GlcNAcylation plays an essential role
in breast cancer metastasis. Cancer Res. 70:6344–6351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylationis a novel regulator of lung and
colon cancer malignancy. Biochim Biophys Acta. 1812:514–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yehezkel G, Cohen L, Kliger A, Manor E and
Khalaila I: O-linked β-N-acetylglucosaminylation (O-GlcNAcylation)
in primary and metastatic colorectal cancer clones and effect of
N-acetyl-beta-D-glucosaminidase silencing on cell phenotype and
transcriptome. J Biol Chem. 287:28755–28769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Z, Vocadlo DJ and Vosseller K:
Hyper-OglcNAcylation is anti-apoptotic and maintains constitutive
NF-κB activity in pancreatic cancer cells. J Biol Chem.
288:15121–15130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lynch TP, Ferrer CM, Jackson SR, Shahriari
KS, Vosseller K and Reginato MJ: Critical role of O-Linked
β-N-acetylglucosamine transferase in prostate cancer invasion,
angiogenesis, and metastasis. J Biol Chem. 287:11070–11081. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008v1.2. Cancer incidence,
mortality and prevalence worldwideIARC Cancer Base No 10
[Internet]. Lyon, France: IARC Press; 2010
|
|
18
|
Treating advanced ovarian cancer.
https://www.cancerresearchuk.orgMay
16–2015.
|
|
19
|
Ruddon RW: Cancer biology. 4th edition.
Oxford University Press; Oxford: pp. 2232007
|
|
20
|
Basile JR, Gavard J and Gutkind JS:
Plexin-B1 utilizes RhoA and Rho kinase to promote the
integrin-dependent activation of Akt and ERK and endothelial cell
motility. J Biol Chem. 282:34888–34895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nikonova E, Tsyganov MA, Kolch W, Fey D
and Kholodenko BN: Control of the G-protein cascade dynamics by GDP
dissociation inhibitors. Mol Biosyst. 9:2454–2462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samuel MS, Lopez JI, McGhee EJ, Daniel R,
Croft DR, Strachan D, Timpson P, Munro J, Schröder E, Zhou J, et
al: Actomyosin-mediated cellular tension drives increased tissue
stiffness and β-catenin activation to induce epidermal hyper-plasia
and tumor growth. Cancer Cell. 19:776–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rösel D, Brábek J, Tolde O, Mierke CT,
Zitterbart DP, Raupach C, Bicanová K, Kollmannsberger P, Panková D,
Vesely P, et al: Up-regulation of Rho/ROCK signaling in sarcoma
cells drives invasion and increased generation of protrusive
forces. Mol Cancer Res. 6:1410–1420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gadea G, De Toledo M, Anguille C and Roux
P: Loss of p53 promotes RhoA-ROCK-dependent cell migration and
invasion in 3D matrices. J Cell Biol. 178:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amano M, Ito M, Kimura K, Fukata Y,
Chihara K, Nakano T, Matsuura Y and Kaibuchi K: Phosphorylation and
activation of myosin by Rho-associated kinase (Rho-kinase). J Biol
Chem. 271:20246–20249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riento K and Ridley AJ: Rocks:
Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolodney MS and Elson EL: Contraction due
to microtubule disruption is associated with increased
phosphorylation of myosin regulatory light chain. Proc Natl Acad
Sci USA. 92:10252–10256. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
29
|
Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y
and Fan D: Expression of seven main Rho family members in gastric
carcinoma. Biochem Biophys Res Commun. 315:686–691. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang WG, Watkins G, Lane J, Cunnick GH,
Douglas-Jones A, Mokbel K and Mansel RE: Prognostic value of rho
GTPases and rho guanine nucleotide dissociation inhibitors in human
breast cancers. Clin Cancer Res. 9:6432–6440. 2003.PubMed/NCBI
|
|
31
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
CDC42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horiuchi A, Imai T, Wang C, Ohira S, Feng
Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA
and RhoC, is associated with tumor progression in ovarian
carcinoma. Lab Invest. 83:861–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao XQ and Lu X: Expression of RhoA and
ROCK in breast carcinomas and their significance. J Radioimmunol.
4:439–442. 2012.
|
|
34
|
Wang X, Jiang W, Kang J, Liu Q and Nie M:
Knockdown of RhoA expression alters ovarian cancer biological
behavior in vitro and in nude mice. Oncol Rep. 34:891–899.
2015.PubMed/NCBI
|
|
35
|
Slack JL, Bi W, Livak KJ, Beaubier N, Yu
M, Clark M, Kim SH, Gallagher RE and Willman CL: Pre-clinical
validation of a novel, highly sensitive assay to detect
PML-RARalpha mRNA using real-time reverse-transcription polymerase
chain reaction. J Mol Diagn. 3:141–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu Y, Zhang J, Mi W, Yang J, Han F, Lu X
and Yu W: Silencing of GM3 synthase suppresses lung metastasis of
murine breast cancer cells. Breast Cancer Res. 10:R12008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yanagisawa M and Anastasiadis PZ: p120
catenin is essential for mesenchymal cadherin-mediated regulation
of cell motility and invasiveness. J Cell Biol. 174:1087–1096.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uehata M: Y-27632. Selective probe of
ROCK/Rho-kinase. JikkenIgaku. 17:850–855. 1999.
|
|
40
|
Magtibay PM, Adams PB, Silverman MB, Cha
SS and Podratz KC: Splenectomy as part of cytoreductive surgery in
ovarian cancer. Gynecol Oncol. 102:369–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim MC, Kang S, Lee KS, Han SS, Park SJ,
Seo SS and Park SY: The clinical significance of hepatic
parenchymal metastasis in patients with primary epithelial ovarian
cancer. Gynecol Oncol. 112:28–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Braun S, Schindlbeck C, Hepp F, Janni W,
Kentenich C, Riethmüller G and Pantel K: Occult tumor cells in bone
marrow of patients with locoregionally restricted ovarian cancer
predict early distant metastatic relapse. J ClinOncol. 19:368–375.
2001. View Article : Google Scholar
|
|
43
|
Schmidt LJ, Duncan K, Yadav N, Regan KM,
Verone AR, Lohse CM, Pop EA, Attwood K, Wilding G, Mohler JL, et
al: RhoA as a mediator of clinically relevant androgen action in
prostate cancer cells. Mol Endocrinol. 26:716–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
45
|
Somlyo AV, Bradshaw D, Ramos S, Murphy C,
Myers CE and Somlyo AP: Rho-kinase inhibitor retards migration and
in vivo dissemination of human prostate cancer cells. Biochem
Biophys Res Commun. 269:652–659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ying H, Biroc SL, Li WW, Alicke B, Xuan
JA, Pagila R, Ohashi Y, Okada T, Kamata Y and Dinter H: The Rho
kinase inhibitor fasudil inhibits tumor progression in human and
rat tumor models. Mol Cancer Ther. 5:2158–2164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakajima M, Katayama K, Tamechika I,
Hayashi K, Amano Y, Uehata M and Kondo T: WF-536 inhibits
metastatic invasion by enhancing the host cell barrier and
inhibiting tumour cell motility. Clin Exp Pharmacol Physiol.
30:457–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wong CC, Wong CM, Tung EK, Man K and Ng
IO: Rho-kinase 2 is frequently overexpressed in hepatocellular
carcinoma and involved in tumor invasion. Hepatology. 49:1583–1594.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakajima M, Hayashi K, Egi Y, Katayama K,
Amano Y, Uehata M, Ohtsuki M, Fujii A, Oshita K and Kataoka H:
Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16
melanoma. Cancer Chemother Pharmacol. 52:319–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sahai E, Ishizaki T, Narumiya S and
Treisman R: Transformation mediated by RhoA requires activity of
ROCK kinases. Curr Biol. 9:136–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xue F, Takahara T, Yata Y, Xia Q, Nonome
K, Shinno E, Kanayama M, Takahara S and Sugiyama T: Blockade of
Rho/Rho-associated coiled coil-forming kinase signaling can prevent
progression of hepatocellular carcinoma in matrix
metalloproteinase-dependent manner. Hepatol Res. 38:810–817. 2006.
View Article : Google Scholar
|
|
52
|
Larrea MD, Wander SA and Slingerland JM:
p27 as Jekyll and Hyde: Regulation of cell cycle and cell motility.
Cell Cycle. 8:3455–3461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hoshino D, Tomari T, Nagano M, Koshikawa N
and Seiki M: A novel protein associated with membrane-type 1 matrix
metalloproteinase binds p27(kip1) and regulates RhoA activation,
actin remodeling and matrigel invasion. J Biol Chem.
284:27315–27326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chang YC, Nalbant P, Birkenfeld J, Chang
ZF and Bokoch GM: GEF-H1 couples nocodazole-induced microtubule
disassembly to cell contractility via RhoA. Mol Biol Cell.
19:2147–2153. 2008. View Article : Google Scholar : PubMed/NCBI
|