Introduction
Cardiac fibrosis is a common pathological alteration
that occurs as a result of hypertension and myocardial infarction,
and during valvular heart disease, which induces notable left
ventricular hypertrophy (1).
Cardiac fibroblasts (CFs) are the most abundant cell type in the
myocardium, which serve an important role in the maintenance of
myocardial structure and function (2). The abnormal proliferation of CFs, and
deposition of extracellular matrix (ECM) proteins and collagens,
results in the development of cardiac fibrosis, which then
adversely affects cardiac performance (3). Previous studies have demonstrated
that angiotensin II (Ang II), which is one of the most important
renin-angiotensin system components, has a crucial role in the
progression of cardiac fibrosis (4–6). Ang
II has been reported to be aberrantly activated in patients with
myocardial fibrosis (7). In
addition, Ang II acts as a potent profibrotic molecule that
promotes myofibroblast differentiation, and aberrant ECM production
and degradation (8). Therefore,
suppressing Ang II-induced ECM synthesis in CFs may provide a
potential target for the prevention of cardiac fibrosis.
Diosgenin is a steroidal saponin present in various
plants, including the Solanum and Dioscorea species.
Numerous studies have indicated that diosgenin possesses important
pharmacological activities, including anti-inflammatory,
antiatherosclerotic, antitumor and antioxidant activities (9–12).
Badalzadeh et al reported that diosgenin may exert
cardioprotective effects against myocardial reperfusion injury via
activation of mitochondrial KATP channels (13). A recent study reported that
diosgenin inhibited high glucose-induced renal tubular fibrosis
(14); however, to the best of our
knowledge, no studies have focused on the effects of diosgenin
against cardiac fibrosis. Therefore, the present study aimed to
explore the effects of diosgenin on Ang II-induced ECM remodeling
and its possible mechanism in CFs.
Materials and methods
Cell culture and treatment
The experimental protocol and associated animal
handling procedures complied with the Guidelines for Animal
Experiments from the Ethical Committee for Animal Research of
Tianjin Medical University Metabolic Diseases Hospital (Tianjin,
China). Primary cultures of neonatal rat CFs were prepared as
previously described (15).
Briefly, female Sprague-Dawley rats (n=6, 180±200 g) were obtained
from the Animal Experimental Center, Tianjin Medical University
Metabolic Diseases Hospital. They were housed at 20–24°C under 12 h
light-dark cycles and were allowed free access to water and
commercial pellets. Rats were anesthetized with 10% chloral hydrate
(350 mg/kg, intraperitoneal; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). The right ventricles from the rats were then
minced and digested in collagenase for 24 h at 37°C (450 U/ml;
Sigma-Aldrich; Merck Millipore). Cells were pelleted via
centrifugation at 8,000 × g at 4°C for 24 h, and were suspended in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.). CFs
were cultured for 3–4 days and CFs in passages 9 to 20 were
used.
Once the cells reached 80–90% confluence, the medium
was replaced with serum-free DMEM 1 day prior to pretreatment with
diosgenin (1, 5 and 10 µM; Sigma-Aldrich; Merck Millipore) for 24 h
at 37°C. The cells were then exposed to Ang II (100 nM;
Sigma-Aldrich; Merck Millipore) at 37°C for 30 min or 24 h.
Cell proliferation assay
Cell proliferation was detected with a colorimetric
assay using the CellTiter 96 AQueous One Solution (MTS) reagent
(Promega Corporation, Madison, WI, USA). CFs were added to 96-well
plates at a density of 1×104 cells/well. Following
treatment for 24 h, 20 µl MTS reagent was added to each well and
the CFs were incubated at 37°C for a further 4 h. The optical
density was measured at a wavelength of 490 nm. FBS (10%) was used
as a positive control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from CFs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and cDNA was generated from total RNA using the iScript cDNA
synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturer's protocol. The mRNA expression
levels were analyzed by RT-qPCR using the SYBR-Green PCR Mix
(Invitrogen; Thermo Fisher Scientific, Inc.) and run on the Bio-Rad
iCycler system (Bio-Rad Laboratories, Inc.). The primers used were
as follows: α-smooth muscle actin (α-SMA), forward
5′-GCTATTCAGGCTGTGCTGTC-3′, reverse 5′-GGTAGTCGGTGAGATCTCGG-3′;
transforming growth factor (TGF)-β1, forward
5′-CCAACTATTGCTTCAGCTCCA-3′, reverse 5′-GTGTCCAGGCTCCAAATGT-3′; and
GAPDH, forward 5′-ACTCCCATTCTTCCACCTTTG-3′ and reverse
5′-CCCTGTTGCTGTAGCCATATT-3′. The PCR cycling conditions were as
follows: Initial denaturation at 94°C for 4 min; 40 cycles of 94°C
for 20 sec, 55°C for 30 sec and 72°C for 20 sec; 2 sec for plate
reading for 40 cycles; melting curve, 65–95°C. GAPDH was used as a
control for normalizing gene expression levels. The data obtained
were analyzed using the 2−ΔΔCq method (16).
Western blot analysis
Proteins were extracted from CFs using
radioimmunoprecipitation assay lysis buffer (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 1 mmol/l
phenylmethylsulfonyl fluoride and phosphatase inhibitor (Pierce;
Thermo Fisher Scientific, Inc.), and the protein concentration was
quantified using the Bradford assay. A total of 20–30 µg protein
was fractionated by 12% SDS-PAGE and was transferred to
nitrocellulose membranes (Amersham; GE Healthcare Life Science,
Little Chalfont, UK). Subsequently, the membranes were incubated in
Tris-buffered saline (TBS) containing 5% non-fat dry milk to block
non-specific binding at room temperature for 1 h. The membrane was
then washed with TBS and incubated with primary antibodies against
α-SMA (1:1,500; sc-53142), TGF-β1 (1:2,500; sc-130348), Smad3
(1:3,000; sc-101154), phosphorylated-Smad3 (1:3,000; sc-130218),
fibronectin (1:2,000; sc-9068), type I collagen (1:1,500;
sc-25,974) and GAPDH (1:3000; sc-367,714) (all Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Subsequently, the membranes were washed extensively with TBS
containing 0.1% Tween and were incubated with a horseradish
peroxidase-conjugated secondary antibody (1:3,000; sc-516087; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. The signal
was detected using Enhanced Chemiluminescence Plus (Thermo Fisher
Scientific, Inc.). The optical densities of the bands were
semi-quantified using Gel-Pro Analyzer v4.0 (Media Cybernetics,
Inc., Rockville, MD, USA). GAPDH was used as the endogenous
control.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Statistical significance was assessed using one-way
analysis of variance followed by Tukey's post hoc test for
multiple-group comparisons. Differences between groups were
compared using a paired-sample Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Diosgenin inhibits Ang II-induced
proliferation of rat CFs
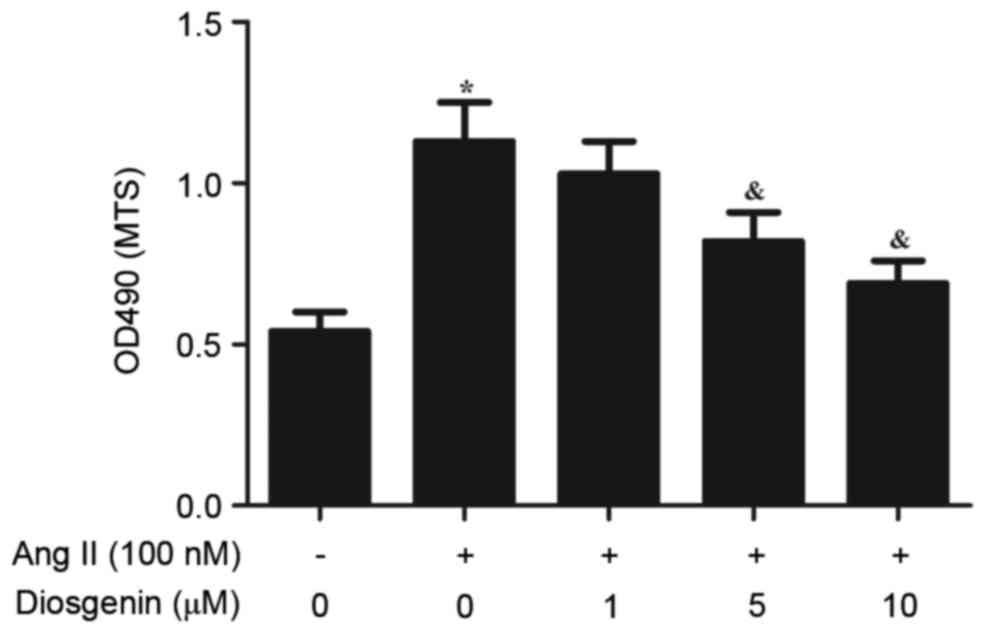
Ang II was revealed to induce the proliferation of
CFs. To determine whether diosgenin may reverse the effects of Ang
II on CF proliferation, rat CFs were treated with various
concentrations of diosgenin prior to Ang II. As presented in
Fig. 1, diosgenin significantly
inhibited cell proliferation in Ang II-stimulated CFs in a
dose-dependent manner.
Diosgenin reduces the differentiation
of CFs to myofibroblasts
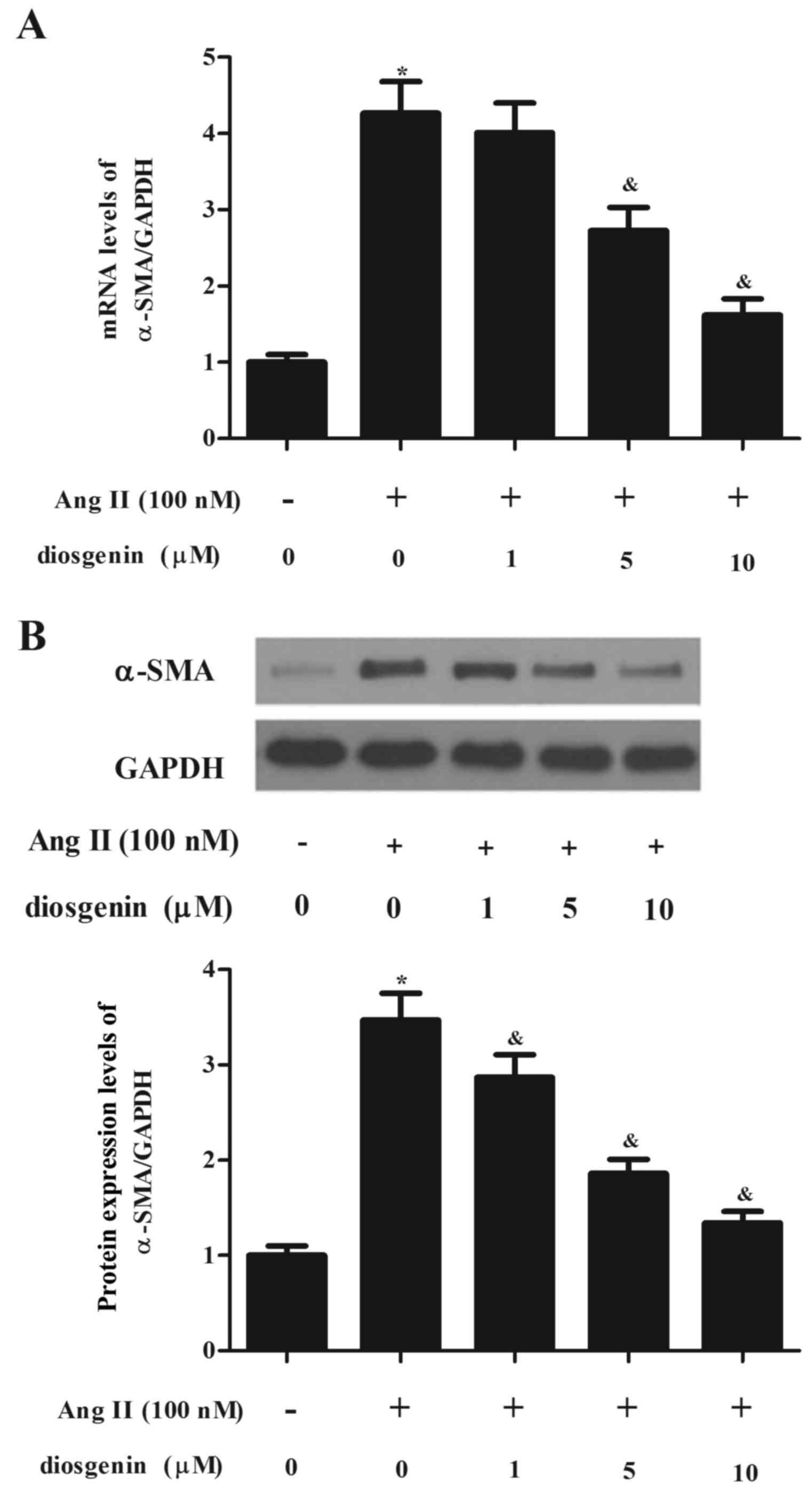
Treatment with Ang II was able to stimulate the
differentiation of CFs to myofibroblasts, and α-SMA acted as a
marker of myofibroblast differentiation. Therefore, the present
study investigated the effects of diosgenin on the expression of
α-SMA in Ang II-stimulated CFs. As presented in Fig. 2, the expression levels of α-SMA
were significantly increased following treatment with Ang II;
however, diosgenin reduced Ang II-induced α-SMA expression in
CFs.
Diosgenin inhibits Ang II-induced ECM
synthesis in rat CFs
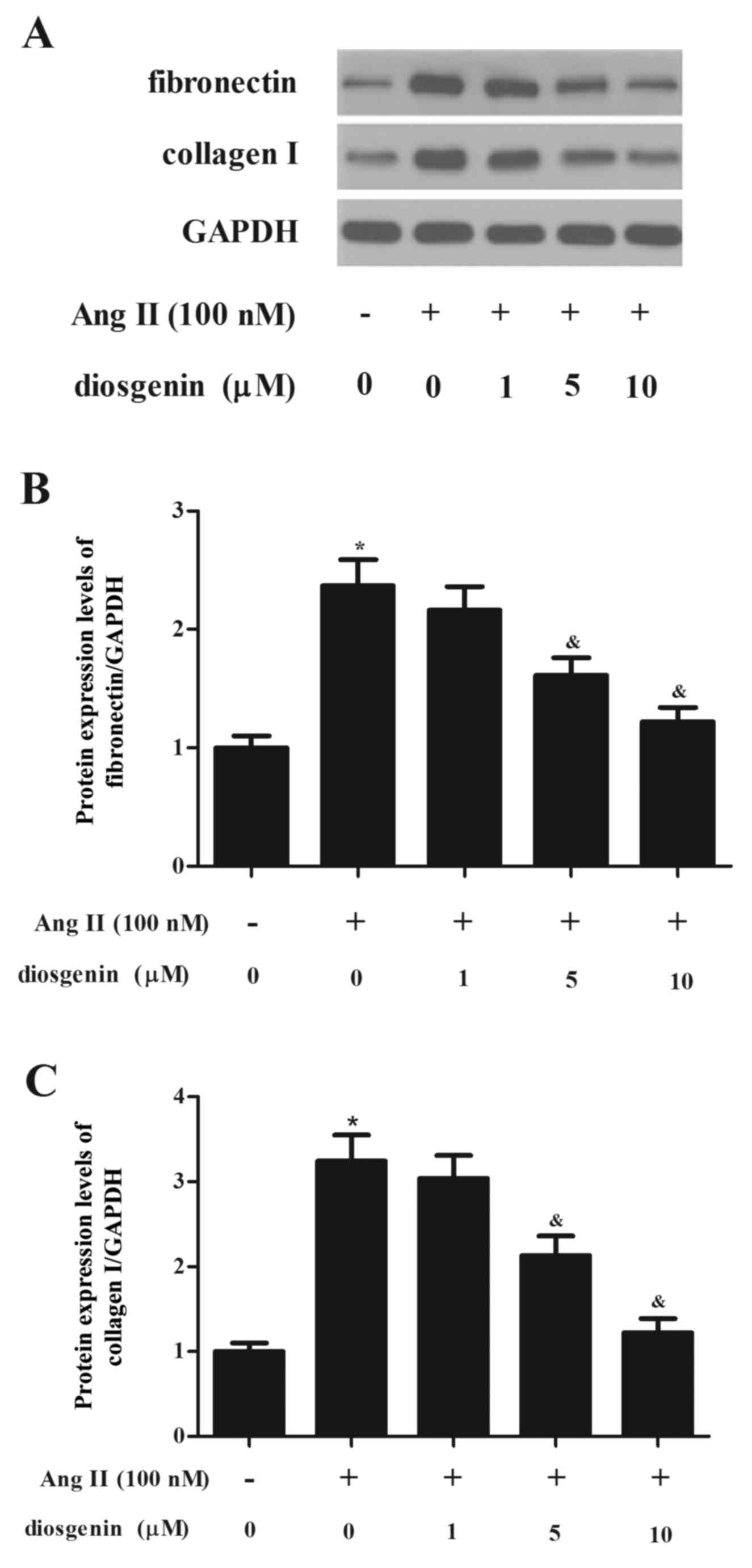
It is widely accepted that ECM accumulation serves
an important role in cardiac fibrosis; therefore, the present study
aimed to determine whether ECM was regulated by diosgenin. As
presented in Fig. 3, Ang II
stimulation significantly induced the expression of fibronectin
(FN), and this effect was reduced following treatment of CFs with
diosgenin. Similarly, the Ang II-induced enhancement of type I
collagen in CFs was significantly reversed by diosgenin.
Diosgenin inhibits Ang II-induced
TGF-β1 expression
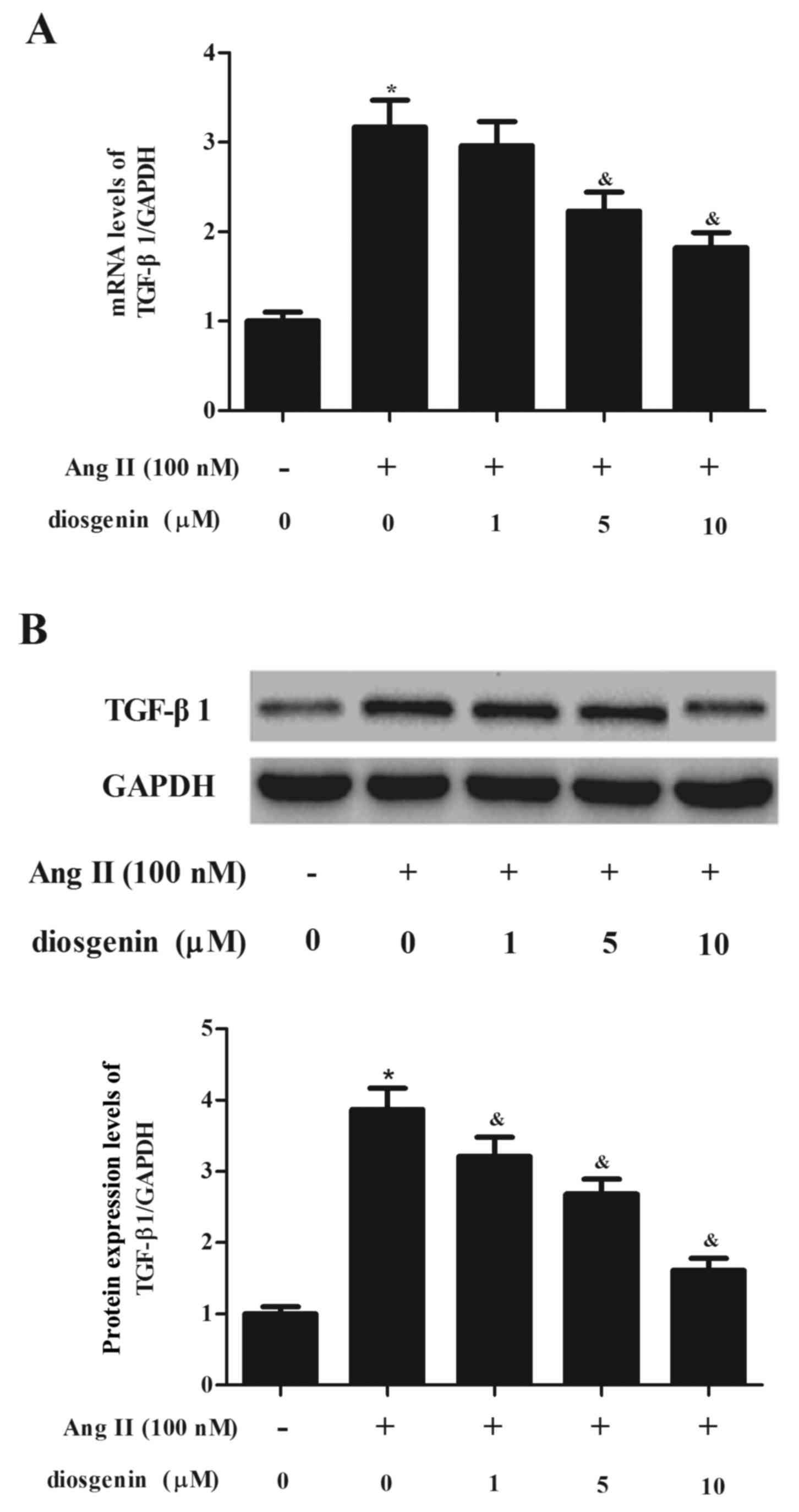
To investigate whether diosgenin has an effect of on
TGF-β1 in CFs exposed to Ang II, CFs were treated with diosgenin
prior to Ang II. As presented in Fig.
4A, Ang II treatment markedly increased the mRNA expression
levels of TGF-β1 in CFs compared with the normal group. However,
Ang II-induced TGF-β1 upregulation was prevented following
treatment of CFs with diosgenin. Similarly, the western blot
analysis demonstrated that diosgenin inhibited Ang II-induced
TGF-β1 protein expression in CFs in a dose-dependent manner
(Fig. 4B).
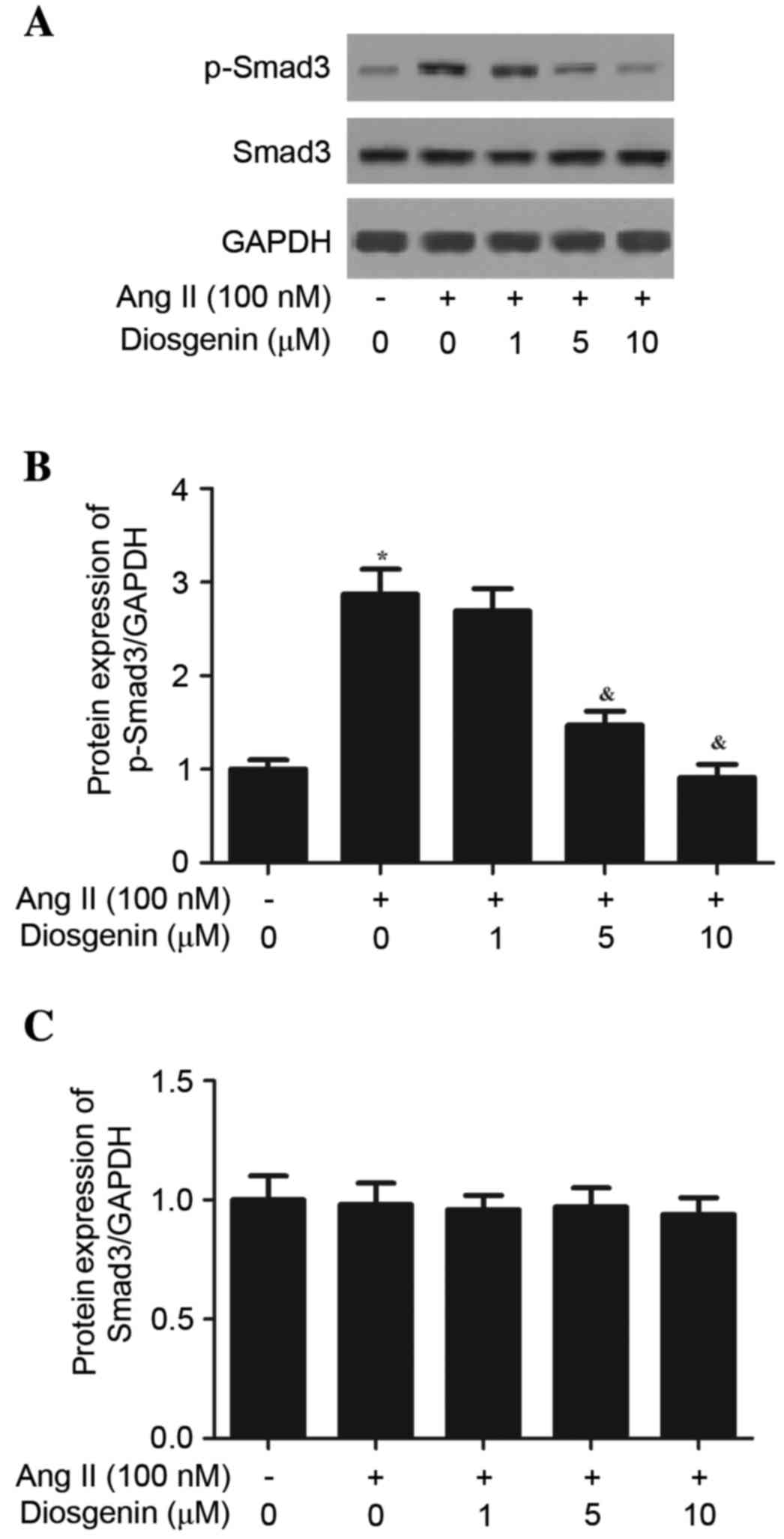
Diosgenin inhibits Ang II-induced
TGF-β1/Smad3 pathway activation
To explore the intracellular pathway that is
associated with diosgenin-induced inhibition of TGF-β1 expression
in Ang II-stimulated CFs, the present study examined the effects of
diosgenin on Smad3 phosphorylation. As presented in Fig. 5, Ang II treatment was able to
induce the phosphorylation of Smad3. Conversely, Ang II-stimulated
Smad3 phosphorylation was markedly suppressed by diosgenin. These
data indicated that diosgenin may inhibit the Ang II-mediated CF
fibrotic response by suppressing the TGF-β1/Smad3 pathway.
Discussion
The major findings of the present study were: i)
Diosgenin inhibited Ang II-induced CF proliferation and the
differentiation of CFs to myofibroblasts; ii) diosgenin inhibited
Ang II-induced ECM synthesis of rat CFs; and iii) diosgenin
inhibited Ang II-induced expression of TGF-β1 and Smad3
phosphorylation in CFs.
Cardiac fibrosis, which is partially characterized
by the proliferation of CFs, is a consequence of the cardiac
remodeling process that is initiated by pathophysiological events
(17). Previous studies have
reported that Ang II serves as a pivotal positive regulator of CF
proliferation (18–20). The results of the present study
demonstrated that CF proliferation was significantly inhibited by
treatment of Ang II-stimulated CFs with diosgenin in a
dose-dependent manner. Therefore, it may be suggested that
diosgenin has a role in cardiac remodeling via inhibiting the
proliferation of CFs.
A critical event in the progression of cardiac
fibrosis is the differentiation of CFs into myofibroblasts;
myofibroblasts are highly active cells that are characterized by
augmented expression of α-SMA (21). In addition, myofibroblast
differentiation is induced by several profibrotic factors,
including Ang II (22), TGF-β
(23), endothelin-1 and
platelet-derived growth factor (24). The present study revealed that the
expression of α-SMA was significantly increased following treatment
with Ang II; however, diosgenin prevented Ang II-induced α-SMA
expression in CFs. These data strongly indicated that diosgenin
serves a crucial role in the phenotypic transformation of CFs to
myofibroblasts.
Cardiac fibrosis is defined as a progressive
accumulation of fibrillar ECM in the myocardium. FN is a key
component of the ECM, which is upregulated in cardiac tissue during
myocardial hypertrophy and failure (25). Collagen type I, which is excreted
by CFs, is the major collagenous component of the cardiac
interstitium and represents ~80% of total collagen (26). Furthermore, previous studies have
demonstrated that collagen synthesis can be induced by Ang II
(27,28). Consistent with these results, the
present study demonstrated that Ang II stimulation significantly
induced the expression of FN and type I collagen; however,
diosgenin was able to inhibit Ang II-induced ECM synthesis in rat
CFs. These findings suggested that diosgenin has a prominent role
in regulating ECM expression during pathological cardiac
remodeling.
The TGF-β/Smad signaling pathway serves an important
role in the pathogenesis of cardiac fibrosis (29–31).
As a main downstream signal transducer of TGF-β1, Smad3 can be
phosphorylated by activated TGF-β1 type I receptor, after which it
may form a complex with Smad4, which translocates into the nucleus,
where it acts as a transcription factor to promote the expression
of target genes, including type I and type III collagen (32). Furthermore, it has been reported
that Ang II induces TGF-β1 upregulation and significant activation
of the TGF-β1/Smad3 signaling pathway, thus resulting in a marked
upregulation of type I and type III collagen expression (33). Similar to these previous results,
the present study demonstrated that Ang II treatment induced TGF-β1
expression and Smad3 phosphorylation; however, diosgenin was able
to inhibit Ang II-induced TGF-β1 expression and Smad3
phosphorylation. These results suggested that diosgenin may inhibit
Ang II-induced ECM remodeling through suppressing the TGF-β1/Smad3
signaling pathway in rat CFs.
In conclusion, the results of the present study
suggested that diosgenin inhibits Ang II-induced ECM remodeling
through suppressing the TGF-β1/Smad signaling pathway in rat CFs.
Therefore, diosgenin may be considered to possess therapeutic
potential towards the treatment of cardiac fibrosis.
References
|
1
|
Khan R and Sheppard R: Fibrosis in heart
disease: Understanding the role of transforming growth factor-beta
in cardiomyopathy, valvular disease and arrhythmia. Immunology.
118:10–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camelliti P, Borg TK and Kohl P:
Structural and functional characterisation of cardiac fibroblasts.
Cardiovasc Res. 65:40–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee AA, Dillmann WH, McCulloch AD and
Villarreal FJ: Angiotensin II stimulates the autocrine production
of transforming growth factor-beta 1 in adult rat cardiac
fibroblasts. J Mol Cell cardiol. 27:2347–2357. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim DS, Lutucuta S, Bachireddy P, Youker
K, Evans A, Entman M, Roberts R and Marian AJ: Angiotensin II
blockade reverses myocardial fibrosis in a transgenic mouse model
of human hypertrophic cardiomyopathy. Circulation. 103:789–791.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawano H, Do YS, Kawano Y, Starnes V, Barr
M, Law RE and Hsueh WA: Angiotensin II has multiple profibrotic
effects in human cardiac fibroblasts. Circulation. 101:1130–1137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hara M, Sakata Y, Nakatani D, Suna S,
Usami M, Matsumoto S, Sugitani T, Ozaki K, Nishino M, Sato H, et
al: Renin-angiotensin-aldosterone system polymorphisms and 5-year
mortality in survivors of acute myocardial infarction: A report
from the osaka acute coronary insufficiency study. Int Heart J.
55:190–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: Physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirai S, Uemura T, Mizoguchi N, Lee JY,
Taketani K, Nakano Y, Hoshino S, Tsuge N, Narukami T, Yu R, et al:
Diosgenin attenuates inflammatory changes in the interaction
between adipocytes and macrophages. Mol Nutr Food Res. 54:797–804.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park HJ, Byeon HE, Koo HJ and Pyo S:
Anti-atherosclerotic effects of diosgenin in ApoE-deficient mice.
FASEB J. 25:980–982. 2011.
|
|
11
|
Moalic S, Liagre B, Corbière C, Bianchi A,
Dauça M, Bordji K and Beneytout JL: A plant steroid, diosgenin,
induces apoptosis, cell cycle arrest and COX activity in
osteosarcoma cells. FEBS Lett. 506:225–230. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Son IS, Kim JH, Sohn HY, Son KH, Kim JS
and Kwon CS: Antioxidative and hypolipidemic effects of diosgenin,
a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol
fed rats. Biosci Biotech Biochem. 71:3063–3071. 2007. View Article : Google Scholar
|
|
13
|
Badalzadeh R, Yousefi B, Tajaddini A and
Ahmadian N: Diosgenin-induced protection against myocardial
ischaemia-reperfusion injury is mediated by mitochondrial KATP
channels in a rat model. Perfusion. 30:565–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang WC, Liu SF, Chang WT, Shiue YL, Hsieh
PF, Hung TJ, Hung CY, Hung YJ, Chen MF and Yang YL: The effects of
diosgenin in the Regulation of renal proximal tubular fibrosis. Exp
Cell Res. 323:255–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quintas LE, Pierre SV, Liu L, Bai Y, Liu X
and Xie ZJ: Alterations of Na+/K+-ATPase function in caveolin-1
knockout cardiac fibroblasts. J Mol Cell Cardiol. 49:525–531. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schnee JM and Hsueh WA: Angiotensin II,
adhesion, and cardiac fibrosis. Cardiovasc Res. 46:264–268. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bouzegrhane F and Thibault G: Is
angiotensin II a proliferative factor of cardiac fibroblasts?
Cardiovasc Res. 53:304–312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Kesteren CA, Van Heugten HA, Lamers
JM, Saxena PR, Schalekamp MA and Danser AH: Angiotensin II-mediated
growth and antigrowth effects in cultured neonatal rat cardiac
myocytes and fibroblasts. J Mol Cell Cardiol. 29:2147–2157. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hattori Y, Hattori S, Akimoto K, Nishikimi
T, Suzuki K, Matsuoka H and Kasai K: Globular adiponectin activates
nuclear factor-kappaB and activating protein-1 and enhances
angiotensin II-induced proliferation in cardiac fibroblasts.
Diabetes. 56:804–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roy S, Khanna S, Rink T, Radtke J,
Williams WT, Biswas S, Schnitt R, Strauch AR and Sen CK:
P21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle
actin expression and differentiation of cardiac fibroblasts to
myofibroblasts. Mol Biol Cell. 18:4837–4846. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu M, Han M, Li J, Xu X, Li T, Que L, Ha
T, Li C, Chen Q and Li Y: 17beta-estradiol inhibits angiotensin
II-induced cardiac myofibroblast differentiation. Eur J Pharmacol.
616:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cucoranu I, Clempus R, Dikalova A, Phelan
PJ, Ariyan S, Dikalov S and Sorescu D: NAD(P)H oxidase 4 mediates
transforming growth factor-beta1-induced differentiation of cardiac
fibroblasts into myofibroblasts. Circ Res. 97:900–907. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leask A: Potential therapeutic targets for
cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF,
partners in fibroblast activation. Circ Res. 106:1675–1680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reddy VS, Harskamp RE, Van Ginkel MW,
Calhoon J, Baisden CE, Kim IS, Valente AJ and Chandrasekar B:
Interleukin-18 stimulates fibronectin expression in primary human
cardiac fibroblasts via PI3K-Akt-dependent NF-kappaB activation. J
Cell Physiol. 215:697–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brilla CG, Zhou G, Matsubara L and Weber
KT: Collagen metabolism in cultured adult rat cardiac fibroblasts:
Response to angiotensin II and aldosterone. J Mol Cell cardiol.
26:809–820. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brilla CG, Zhou G, Rupp H, Maisch B and
Weber KT: Role of angiotensin II and prostaglandin E2 in regulating
cardiac fibroblast collagen turnover. Am J Cardiol. 76:8D–13D.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen K, Chen J, Li D, Zhang X and Mehta
JL: Angiotensin II regulation of collagen type I expression in
cardiac fibroblasts: Modulation by PPAR-gamma ligand pioglitazone.
Hypertension. 44:655–661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li P, Wang D, Lucas J, Oparil S, Xing D,
Cao X, Novak L, Renfrow MB and Chen YF: Atrial natriuretic peptide
inhibits transforming growth factor beta-induced smad signaling and
myofibroblast transformation in mouse cardiac fibroblasts. Circ
Res. 102:185–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan W, Wang P, Zhao CX, Tang J, Xiao X and
Wang DW: Decorin gene delivery inhibits cardiac fibrosis in
spontaneously hypertensive rats by modulation of transforming
growth factor-beta/Smad and p38 mitogen-activated protein kinase
signaling pathways. Hum Gene ther. 20:1190–1200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Sun SQ, Hassid A and Ostrom RS:
cAMP inhibits transforming growth factor-beta-stimulated collagen
synthesis via inhibition of extracellular signal-regulated kinase
1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol.
70:1992–2003. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen K, Mehta JL, Li D, Joseph L and
Joseph J: Transforming growth factor beta receptor endoglin is
expressed in cardiac fibroblasts and modulates profibrogenic
actions of angiotensin II. Circ Res. 95:1167–1173. 2004. View Article : Google Scholar : PubMed/NCBI
|